Abstract
Objective
To describe risk factors for scar formation and changes to fibrotic scar through five years in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT).
Design
Multicenter, prospective cohort study.
Participants
1061 subjects in CATT.
Methods
Color photographic and fluorescein angiographic images from baseline and 1, 2, and 5 years were evaluated. Incidence of scar formation was estimated with Kaplan-Meier curves. Risk factors were assessed with Cox regression models.
Main outcome measures
Scar formation, fibrotic scar area and macular atrophy associated with fibrotic scar (“atrophy”).
Results
Cumulative proportion of eyes with scar was 32%, 46% and 56% at year 1, 2 and 5, respectively. Baseline factors associated with increased risk (adjusted hazards ratio (aHR) and 95% CI) were classic CNV (aHR 4.49, 95% C.I 3.34, 6.04) vs occult, hemorrhage > 1 disc area (2.28, 95% C.I. 1.49, 3.47) vs no hemorrhage, retinal thickness >212 um (2.58, 95% C.I. 1.69, 3.94) vs <120 um, subretinal tissue complex thickness >275 um (2.64, 95% CI.1.81, 3.84) vs <=75um, subretinal fluid thickness >25um (1.31, 95% CI 0.97, 1.75) vs no fluid, visual acuity in fellow eye 20/20 (1.72, 95% CI 1.25, 2.36) vs 20/50 or worse, RPE elevation absence (1.71, 95% C.I 1.21, 2.41) and subretinal hyperreflective material (1.72, 95% CI 1.25, 2.36).
Among 68 eyes that developed fibrotic scar at year 1, visual acuity decreased by a mean of additional 13 letters between year 1 and 5. Mean scar area was 1.2, 1.2 and 1.9 DAs at 1, 2 and 5 years, respectively. Atrophy was present in 18%, 24%, and 54% of these eyes at year 1, 2 and 5, mean areas being 1.6, 2.0 and 3.1 DAs, respectively. Atrophy replaced fibrotic scar in 8 eyes at year 5. There was no significant correlation between scar growth and atrophy growth. The rate of growth for both was similar between the clinical trial and observation periods.
Conclusions
Several morphological features, including classic CNV and large hemorrhage, are associated with scar formation. Rate of new scar formation declined after two years. Most fibrotic scars and accompanying macular atrophy expanded over time, reducing visual acuity.
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) treatment for neovascular age-related macular degeneration (nAMD) has become the standard of care based on the results of several multicenter, randomized clinical trials with generally good visual and morphological outcomes.1,2 The results of treatment are derived mostly from clinical trials that have been capped at 2 years. Subjects who completed a 2 year clinical trial continue to receive care from ophthalmologists, unrestricted from the protocol of the clinical trial. Some investigators have collected follow up data from these clinical trial subjects 5 to 8 years after initiation of anti-VEGF therapy, but their reports have been focused mainly on the visual status of these subjects.3 A few studies have also reported on the long-term outcomes of patients treated with anti-VEGF therapy in a real world setting but these too have dealt primarily with visual acuity (VA) outcomes.4,5
Scar and atrophy are the two most important morphological features that influence visual outcomes in untreated nAMD as well as in patients treated with anti-VEGF intravitreal injections.6,7 Scars thicker than 0.2 mm have been associated with large scale loss of the photoreceptors overlying the scar tissue.8, 9 We have previously reported that both macular atrophy and foveal scar are the two foremost morphological outcomes associated with poor visual acuity in CATT.9 While long-term follow up of geographic atrophy after anti-VEGF therapy, more recently referred to as macular atrophy, has been described, long term follow-up of scars after the initiation of anti-VEGF therapy has not received much attention.10,11
The original Comparison of Age-related macular degeneration Treatments Trials (CATT) clinical trial was designed to assess differences between ranibizumab and bevacizumab as well as differences between monthly and PRN dosing. At the end of the 2-year clinical trial period, the subjects were released from the systematic ocular examination and treatment specified by the study protocol.12, 13 After providing consent, subjects received ocular examinations and imaging approximately 5 years after initiation anti-VEGF treatment. The results of the follow up study detailing the vision outcomes have been published.14 In this paper, we report the incidence and risk factors of scar development through 5 years of follow-up as well as the morphological changes observed in and around fibrotic scars that had developed during the first year of the clinical trial.
Methods
Enrollment and Follow-up of Subjects
From 43 clinical centers in the United States, 1185 subjects who had untreated active (leakage on FA and fluid on OCT) choroidal neovascularization (CNV) associated with AMD were enrolled in the CATT clinical trial between February 2008 and December 2009. The study eye was required to have CNV or fluid at the foveal center. Subjects were excluded if scar was located at the foveal center at enrollment but study eyes with non-foveal scars that were <50% of the total CNV lesion were included in the clinical trial. Additional eligibility criteria have been described previously.12 Subjects were randomly assigned to treatment with intravitreal injections of ranibizumab or bevacizumab and to 1 of 3 dosing regimens for the initial 2 years of the study: monthly injections, monthly evaluation with injection only when signs of active neovascularization were present (pro re nata [PRN]), or monthly injections for 1 year followed by PRN injections for 1 year.
During the clinical trial, color fundus photographs (CFP), fluorescein angiograms (FA), and optical coherence tomograms (OCT) were obtained. CFP and FA were obtained at baseline, 1 and 2 years while OCTs were obtained more frequently. The study was approved by the Institutional review boards associated with each center and all subjects provided written informed consent. The study was compliant with Health Insurance Portability and Accountability Act regulations. The CATT was registered with ClinicalTrials.gov (NCT00593450). At the end of the 2-year follow up period of the clinical trial, subjects were released from the study protocol and managed according to best medical judgment. All CATT 1117 patients alive at the end of the clinical trial were invited to participate in the CATT Follow-up Study at approximately 5 years after initiation of treatment with the anti-VEGF therapy. Among the invited patients, 203 had died by the time of Follow-up Study and among the remaining 914 living patients, 647 (71%) participated. Non-participating patients were on average 2 years older, had visual acuity 5 letters worse by the end of the clinical trial, and had 2 fewer injections during the clinical trial when they had been assigned to treatment as needed.14 The percentage of participants with scar at 2 years was 44% and was 38% among non-participants (p=0.10).14 CFP, FA and OCTs were acquired at the time of the study visit.
Assessment of Images
The methods used to grade the digital CFP and FA images, and the OCT scans have been described previously.15,16 At baseline, photographic images were evaluated at the fundus photograph reading center, University of Pennsylvania for: CNV type; contiguous hemorrhage, serous pigment epithelial detachment or blocked fluorescence; presence of scar or macular atrophy in both the study eye and the fellow eye. The CNV area and the total CNV lesion area were measured using Image J ((available at http://rsbweb.nih.gov/ij/; Rasband WS, ImageJ, US National Institutes of Health, Bethesda, MD, 1997e2012). Grading of year 1, 2 and 5 visit images was performed applying the same methods. Each image set was dual-Reader graded for the various morphological outcomes by trained non-physician Readers and the CATT fundus photographic Reading Center Director (ED), all of whom were masked to demographic and clinical details. Discrepancies were adjudicated between the graders and the director of the reading center and unresolved discrepancies were reviewed by the principal investigator (JEG) to complete a final consensus grading form. Similarly OCT evaluation was performed at the Duke University reading Center where a reader team, composed of 2 independent readers and a senior reader, evaluated each scan. Grading included the CATT OCT end points of total thickness at the foveal center point and intraretinal fluid (IRF), subretinal fluid (SRF), and subretinal pigment epithelium (RPE) fluid. The Director of Grading (CAT) and the Reading Center Director (GJJ) remained masked to subject identifiers and made final decisions on reader disagreements that remained controversial after arbitration.17
Scar was identified by CFP and the FA and described previously.16 Fibrotic scars were defined as obvious white or yellow mounds of fibrous-appearing tissue that were well defined in shape and appeared solid on color stereo images. Hyperfluorescence due to tissue staining or blocked fluorescence of the underlying choroid was identified from FA. Nonfibrotic scars were typically flat, small, well-circumscribed areas of pigmentation with varying degrees of central hypopigmentation on CFP images. The hypopigmented area was flat, and choroidal vessels were not visible. Hyperfluorescence of the depigmented area appeared early on FA and persisted or increased in intensity in the late phase. Hypofluorescence on FA surrounding the hyperfluorescence corresponded to the pigmented borders apparent on CFP.
Assessment of the Fibrotic Scar at 1, 2 and 5 years
Additional evaluation was done only for fibrotic scars among eyes with images available for all visits (baseline, 1, 2 and 5 years). The reading center director assessed all year 1 CFP and FA images that also had year 2 and year 5 visit images to identify fibrotic scars. Indeterminate or uncertain fibrotic scars were subjected to evaluation by a retina specialist and a senior reader and consensus was obtained. Using Image J, measurements of area were obtained for the optic disc; fibrotic scar; and macular atrophy associated with fibrotic scar (atrophy) either contiguous with or amidst the fibrotic scar. Atrophy had well-defined hypopigmented areas with exposed choroidal vessels observed on color images without visible fibrosis. The areas of fibrotic scar and atrophy were mutually exclusive with fibrotic scar taking precedence over atrophy in instances when it was difficult to differentiate the two morphological features. Hyperpigmentation on the fibrotic scar was also documented. These measurements and assessments were performed on the 2- and 5-year images for eyes that had fibrotic scar identified at 1 year (Figure 1).
Figure 1.
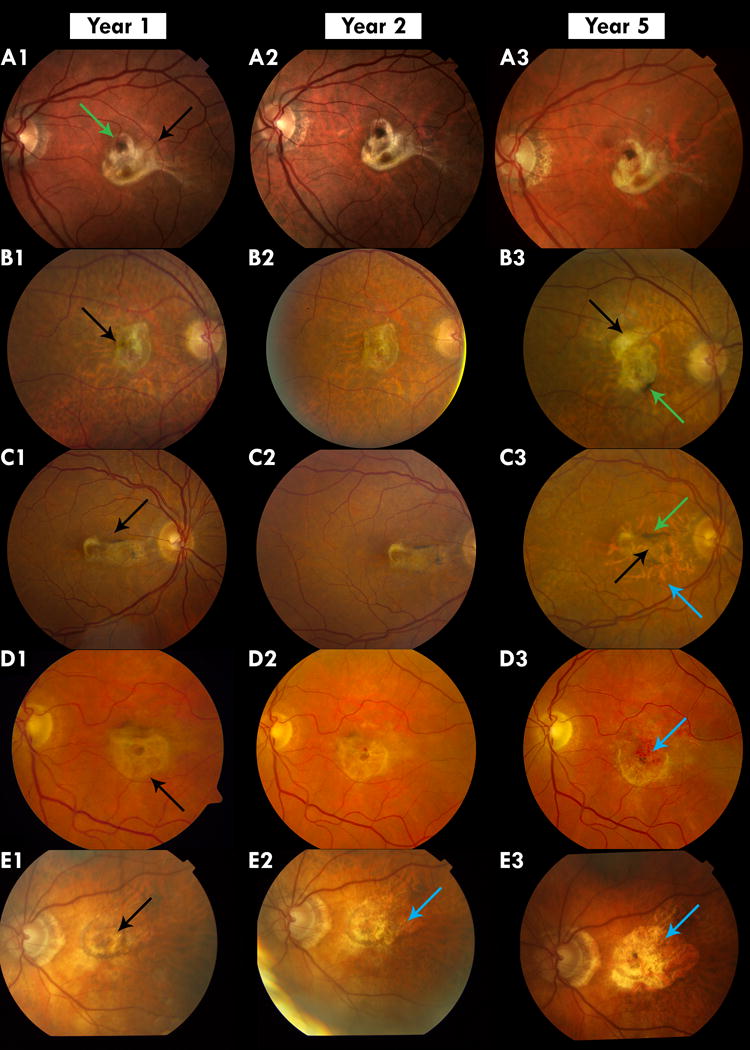
Color images of fibrotic scar and macular atrophy associated with fibrotic scar (atrophy)
A: Stable Fibrotic Scar: A1 shows a fibrotic scar that has developed at year 1 (black arrow). It remains stable at Year 2 and 5. There is hyperpigmentation on the scar (Green arrow)
B Scar increasing in size: B1 shows a fibrotic scar that shows no change in area from Year 1 (B1) to year 2 (B2) but has a larger area in year 5 (B3) when there is growth superiorly (black arrow). There is also pigmentation on the scar (Green arrow) at year 5
C Scar with Peripheral Macular Atrophy: C1 shows a fibrotic scar at year 1 that undergoes thinning at year 2 (B3 Black arrow) with an area of macular atrophy associated with fibrotic scar (atrophy) surrounding it in year 5 (B3 Green arrow). Increased hyperpigmentation is also seen at year 5 (B3 Blue arrow).
D Macular Atrophy Associated with Fibrotic scar: D1 shows a thick round fibrotic scar at year 1 (Black arrow). In year 5 the superior part of the fibrotic scar has been replaced by macular atrophy (D3 Blue arrow)
E. Scar completely replaced by macular atrophy: E1 shows a fibrotic scar (Black arrow) that develops atrophy in year 2 (E2 Blue arrow) and eventually at year 5 the area of the fibrotic scar is replaced by an expanding macular atrophy (E3 Blue arrow).
Candidate Risk Factors
Candidate risk factors for scar formation included baseline patient characteristics such as age, gender, cigarette smoking, hypertension, diabetes, body mass index, dietary supplement use, cancers, hypercholesterolemia, and osteoarthritis. Ocular candidate risk factors were baseline visual acuity in each eye; baseline morphological features observed on color and fluorescein angiography such as the type of CNV, hemorrhage, blocked fluorescence, non-foveal scar and macular atrophy, serous pigment epithelial detachment; baseline features observed in OCT images such as intraretinal, subretinal and sub- retinal pigment epithelial (RPE) fluid, vitreo-macular adhesion and traction, subretinal hyper-reflective material (SHRM), RPE elevation, subretinal tissue complex thickness (any combination of SHRM, PED, drusen material and RPE), subretinal fluid thickness and central retinal thickness; and the anti-VEGF drug and regimen used in the clinical trial period.
Statistical Analysis
We used Kaplan-Meier estimates for the cumulative incidence of scar through 5 year follow-up. Subjects who did not participate in the CATT follow-up study were censored at 2 years. For subjects missing images during the first 2 years, the previous scar status was carried forward. We used univariate and multivariate Cox proportional hazards models to identify the baseline risk factors for scar development.
Risk factors were first evaluated by univariate analysis (without adjustment for any other risk factors) using a discrete time Cox proportional hazard model for time to scar formation. The factors with a p<0.20 in the univariate analysis were included in a multivariate Cox proportional hazard model so that the independent effect of each risk factor could be assessed. The final multivariate model was created by applying a backward selection procedure that retained only those risk factors with a p<0.05, with the exception of drug and regimen groups, which were included in all multivariate models. Adjusted hazard ratios (aHRs) for scar development during 5 years and their 95% confidence intervals (CIs) were calculated on the basis of the final multivariate models.
Among subjects who were identified with fibrotic scar at year 1 and followed up to year 5, we performed descriptive analysis for VA change over time, growth of fibrotic scar area over time, and hyperpigmentation changes over time, by using mean (SD) for continuous measures, and using proportion for categorical measures. Fisher exact test was used for low count frequency comparisons. All the statistical analyses were performed in SAS v9.4 (SAS Institute Inc., Cary, NC), and p<0.05 was considered to be statistically significant.
Results
After excluding eyes with scar at baseline, ungradeable scar status at baseline, or no gradeable images after baseline, there were 1061 patients with at least one gradeable set of images during follow-up (Figure 2 available at www.aaojournal.org). After excluding subjects who had missing values in one or more variables cited in the footnote of Table 1, there were 1020, 965 and 510 complete image sets available for scar assessment at 1, 2 and 5 years, respectively. The Kaplan-Meier estimates of the cumulative rate of scar was 32.0% at 1 year, 46.3% at 2 years and 56.4% at 5 years (Figure 3).
Table 1.
Multivariable model of factors associated with incidence of scar through year 5
| Baseline Characteristics | Eyes at Risk, N | Scar, n (%) | Hazard Ratio (95% CI) | P-value |
|---|---|---|---|---|
| Drug group | 0.23 | |||
| Ranibizumab | 526 | 253 (48%) | 1.00 | |
| Bevacizumab | 484 | 256 (53%) | 1.15 (0.92, 1.44) | |
| Dosing regimen during first 2 years | 0.17 | |||
| Pro re nata (PRN) for 2 years | 504 | 248 (49%) | 1.00 | |
| Switched from monthly to PRN at 1 year | 253 | 138 (55%) | 1.29 (0.98, 1.69) | |
| Monthly for 2 years | 253 | 123 (49%) | 1.02 (0.77, 1.35) | |
| Baseline VA in fellow eye | 0.04 | |||
| 20/50 or worse | 308 | 146 (47%) | 1.00 | |
| 20/25-20/40 | 398 | 186 (47%) | 0.97 (0.74, 1.28) | |
| 20/20 or better | 304 | 177 (58%) | 1.34 (1.00, 1.79) | |
| Lesion type | <0.001 | |||
| Occult only | 614 | 224 (36%) | 1.00 | |
| Minimally classic | 175 | 121 (69%) | 2.74 (2.04, 3.69) | |
| Predominantly classic | 221 | 164 (74%) | 4.49 (3.34, 6.04) | |
| Hemorrhage associated with the lesion | <0.001 | |||
| None | 401 | 186 (46%) | 1.00 | |
| <=1 Disc Area | 519 | 259 (50%) | 0.92 (0.73, 1.18) | |
| >1 Disc Area | 90 | 64 (71%) | 2.28 (1.49, 3.47) | |
| Retinal thickness at foveal center (μm) | <0.001 | |||
| <120 | 103 | 44 (43%) | 1.00 | |
| 120 to 212 | 541 | 252 (47%) | 1.50 (1.00, 2.24) | |
| >212 | 366 | 213 (58%) | 2.58 (1.69, 3.94) | |
| Subretinal fluid thickness at foveal center (μm) | 0.01 | |||
| =0 | 668 | 339 (51%) | 1.00 | |
| >0 to <=25 | 83 | 49 (59%) | 1.84 (1.21, 2.80) | |
| >25 | 259 | 121 (47%) | 1.31 (0.97, 1.75) | |
| Subretinal tissue complex thickness at foveal center (μm) | <0.001 | |||
| >0 to <=75 | 246 | 89 (36%) | 1.00 | |
| >75 to <=160 | 244 | 130 (53%) | 1.74 (1.23, 2.46) | |
| >160 to <=275 | 257 | 143 (56%) | 1.98 (1.38, 2.82) | |
| >275 | 263 | 147 (56%) | 2.64 (1.81, 3.84) | |
| Retinal pigment epithelial elevation | 0.002 | |||
| Yes | 878 | 419 (48%) | 1.00 | |
| No | 132 | 90 (68%) | 1.71 (1.21, 2.41) | |
| Subretinal hyperreflective material | <0.001 | |||
| No | 236 | 71 (30%) | 1.00 | |
| Yes | 774 | 438 (57%) | 1.72 (1.25, 2.36) |
P value from Cox proportional hazards model.
51 patients were not included in the multivariate model because of missing value in one or more of the following variables: Lesion type, Hemorrhage associated with the lesion, Retinal thickness at foveal center (μm), Subretinal fluid thickness at foveal center (μm), Subretinal tissue complex thickness at foveal center (μm), Retinal pigment epithelial elevation, Subretinal hyperreflective material. VA = Visual acuity
Figure 3.
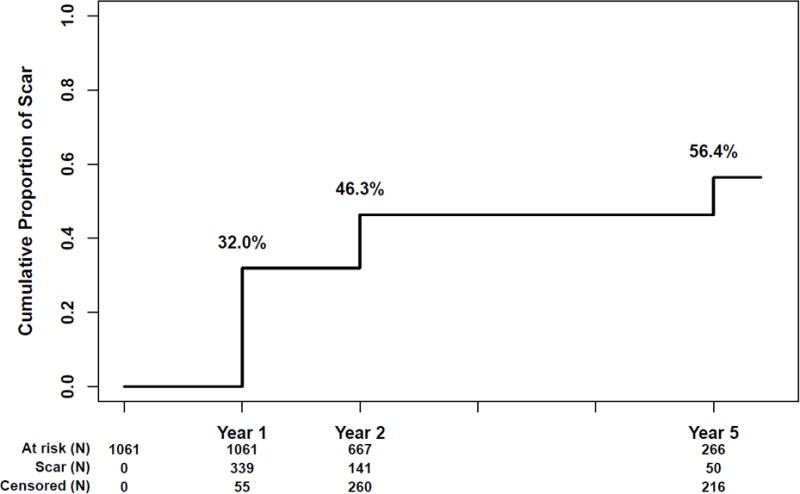
Kaplan Meier graph showing the cumulative incidence of scar through 5 years
Several baseline characteristics were associated with the development of scar through 5 years (Table 1). The presence of predominantly classic CNV was associated with 4.5 fold risk (adjusted Hazards Ratio (aHR) 4.49, 95% Confidence Interval (C.I) 3.34, 6.04, p<0.001) compared with occult CNV. Good baseline visual acuity (20/20 or above) in the fellow eye was associated with increased risk of developing scar in the study eye (aHR 1.34, 95% CI 1.00, 1.74, p=0.04) compared to visual acuity of 20/50 or worse. Large hemorrhages (>1 Disc Area (DA)) were associated with more than a two-fold risk (aHR 2.28, 95% C.I. 1.49, 3.47, p<0.001) compared to no hemorrhages associated with the CNV. Eyes with central retinal thickness (CRT) of >212 μm had increased risk (aHR 2.58, C.I. 1.69, 3.94, p<0.001) compared to eyes with CRT of <120 μm and eyes with subretinal fluid thickness of >0 to <=25 μm had increased risk (aHR 1.84, 95% C.I. 1.21, 2.80, p=0.01) compared to eyes without any subretinal fluid. Risk increased with the thickness of the subretinal tissue complex at the foveal center with more than a 2.5-fold risk for thickness >275 μm (aHR 2.64, 95% C.I. 1.81, 3.84, p<0.001) compared to a thickness ≤ 75 μm. The absence of RPE elevation (aHR 1.71, 95% C.I 1.21, 2.41, p=0.002) and subretinal hyperreflective material (aHR 1.72, 95% C.I. 1.25, 2.36, p<0.001) increased the risk of scar by 70%. Incidence of scar was distributed similarly between the two drug groups and among the three dosing regimen groups. (Table 1)
Photographic images at all three follow up visits (1, 2 and 5 years) were available for 474 subjects and 68 (14.3%) developed fibrotic scar in the study eye at 1 year. On average, the size of the scar changed slowly between year 1 and 5 (Figure 4). The change in size of fibrotic scars from year 1 to 2 and from year 2 to 5 is given in Table 2. The mean VA (SD) of these eyes was 61 [≈ 20/63] (21) letters at 1 year, 63 [≈ 20/50] (18) letters at 2 years and 49 [≈ 20/100] (26) at year 5. The mean VA (SD) was 38 [≈ 20/160] (27) letters at year 5 in 8 eyes where atrophy completely replaced the fibrotic scars. (Table 3). The mean annual expansion rate of the fibrotic scar present in 68 eyes at year 1 was 0.02 (0.41) DA (range −1.40, 2.47) (median=0.004) while it was 0.20 (0.71) between year 2 and year 5 (range −0.94, 3.56) (median=0.01). The difference in the annual rate of expansion was not statistically significant (p= 0.33 (Wilcoxon signed rank test).
Figure 4.
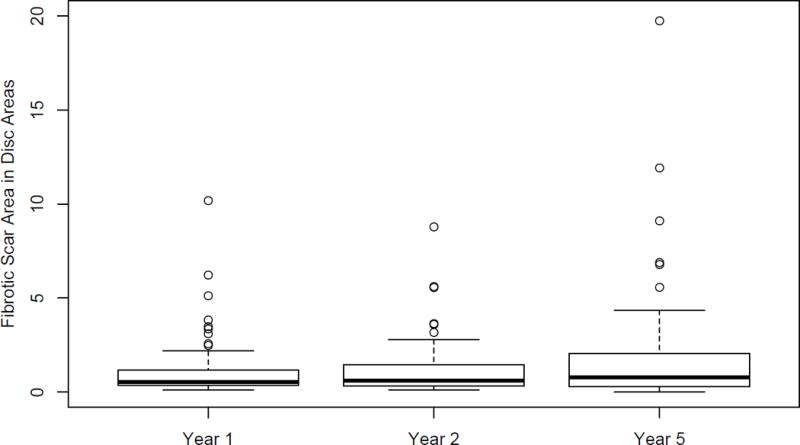
Box plot of fibrotic scar in Disc Areas over time among 68 eyes with fibrotic scar at year 1
Table 2.
Change in fibrotic scar change over time
| Fibrotic Scar Size Change | N (%) | Mean (SD) | Median (Q1, Q3) | |
|---|---|---|---|---|
| From Year 1 to 2 | Increase | 19 (27.9%) | 0.3 (0.6) | 0.10 (0.07, 0.33) |
| Decrease | 15 (22.1%) | −0.3 (0.4) | −0.13 (−0.22, −0.08) | |
| Same | 34 (50.0%) | NA | NA | |
| From Year 2 to 5 | Increase | 33 (48.5%) | 1.8 (3.0) | 0.94 (0.33, 1.48) |
| Decrease | 23 (33.8%) | −0.5 (0.6) | −0.37 (−0.65, −0.14) | |
| Same | 12 (17.6%) | NA | NA | |
| From Year 1 to 5 | Increase | 37 (54.4%) | 1.7 (2.8) | 0.71 (0.21, 1.76) |
| Decrease | 21 (30.9%) | −0.7 (0.8) | −0.38 (−0.66, −0.13) | |
| Same | 10 (14.7%) | NA | NA | |
Table 3.
Scar and macular atrophy area and visual acuity at year 1, 2 and 5 among study eyes with fibrotic scar at Year 1 (N=68).
| Size in Disc Area | Visual Acuity (letters) | |||||
|---|---|---|---|---|---|---|
| Year | N | Mean (SD*) | Median (Min, Max) | (Q1, Q3)** | Mean (SD) | |
| Fibrotic Scar | 1 | 68 | 1.2 (1.6) | 0.54 (0.11, 10.18) | (0.36, 1.19) | 61.1 (21.0) |
| 2 | 68 | 1.2 (1.5) | 0.60 (0.13, 8.78) | (0.34, 1.44) | 63.2 (18.3) | |
| 5 | 68 | 1.9 (3.1) | 0.77 (0.00, 19.74) | (0.29, 2.06) | 48.1 (26.5) | |
| Macular Atrophy Associated with Fibrotic scar | 1 | 12 | 1.6 (1.6) | 0.82 (0.14, 4.27) | (0.29, 3.07) | 69.9 (14.7) |
| 2 | 16 | 2.0 (2.0) | 1.12 (0.18, 5.62) | (0.68, 3.21) | 71.4 (13.0) | |
| 5 | 37 | 3.1 (2.6) | 2.30 (0.01, 9.66) | (0.90, 4.76) | 44.4 (27.1) | |
SD=Standard Deviation
Q=Quartile
At 1 year, macular atrophy associated with fibrotic scar was observed in 12 eyes (18%) and the mean (SD) VA was 70 [≈ 20/40] (15) while in eyes without macular atrophy associated with scar the mean VA was 59[≈ 20/63] (22) letters (p=0.11). At 2 years, atrophy was observed in 16 eyes (24%) and the mean (SD) VA was 71 [≈ 20/40] (13) while in eyes without atrophy the mean VA was 61[≈ 20/63] (19) letters (p=0.04). At 5 years, atrophy was observed in 37 eyes (54%) and the mean (SD) VA was 44 [≈ 20/125] (27) while in eyes without atrophy the mean VA was 53 [≈ 20/80] (25) letters (p=0.21). The average size of atrophy increased from 1.6 DA at year 1 to 2.0 DA in year 2 and to 3.1 DA in year 5. (Figure 5).
Figure 5.
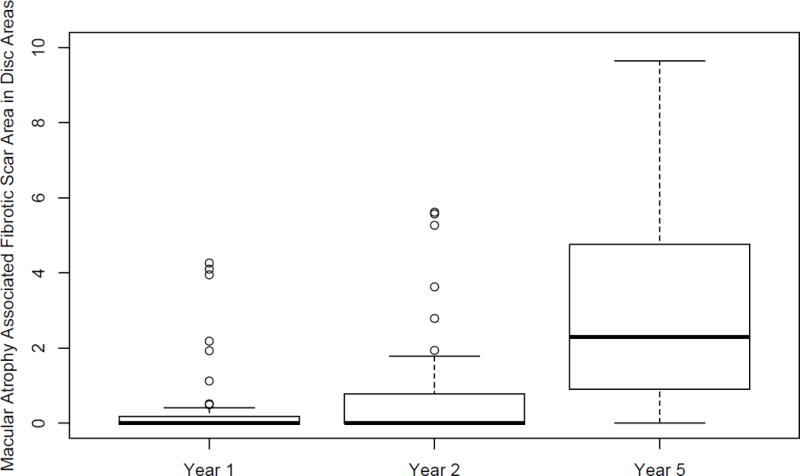
Box plot of macular atrophy associated with fibrotic scar (atrophy) in Disc Areas at 1, 2 and 5 years among 68 eyes with fibrotic scar at year 1
Among the 12 eyes with atrophy at year 1, the mean (SD) of atrophy area increase was 0.73 (0.59) DA (range 0.03 to 1.53, median 0.82 DA). Among the 16 eyes with atrophy at 2 years, the mean (SD) annual rate of area increase was 0.59 (0.53) (range 0 to 1.53, median 0.51 DA). Among eyes that had both fibrotic scar and atrophy, there was no strong correlation between the area of the scar and the area of the atrophy with correlation coefficients of 0.41 at Year 1 (N=12; p=0.18), 0.35 at Year 2 (N= 16; p=0.18); and 0.01 at Year 5 (N=37 eyes, p=0.96). The difference in the annual rate of expansion was −0.14 (0.7) DA (p= 0.79 (Wilcoxon signed rank).
There were no significant associations with demographic or ocular characteristics between eyes with atrophy and those without atrophy in years 1, 2 or 5. Similarly there were no significant associations with demographic or ocular characteristics between eyes with expanding atrophy and those without such expansion in years 1, 2 or 5. (Table 4 and 5 available at www.aaojournal.org). Pigmentation on the fibrotic scars was seen in 13% eyes at year 1, 22% at year 2 and in 53% eyes at year 5 (Figure 6).
Figure 6.
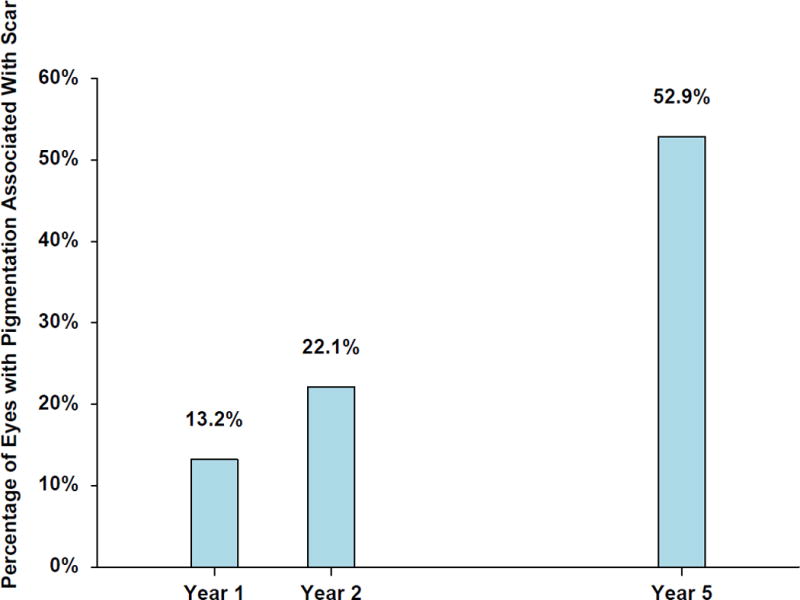
Bar diagram of percentage of eyes with fibrotic scar pigmentation in year 1, 2 and 5 among 68 eyes with fibrotic scar at year 1.
Discussion
Approximately one-third of the CATT eyes developed retinal scar during the first year of treatment and an additional 10% developed scar during the second year of treatment. At the completion of the 2-year clinical trial the CATT subjects were released from their treatment protocol and were free to choose their ophthalmic care. Despite this change from the CATT clinical trial protocol and a longer follow up period of 3 years, only an additional 10% of subjects developed scar, indicating that in eyes with nAMD treated with anti-VEGF therapy, the majority of scars occur within the 1st year of treatment.
We had shown earlier that during the first two years of treatment of nAMD with anti-VEGF drugs, the risk factors present at baseline for developing a scar included predominantly classic type of CNV, blocked fluorescence, thicker retina, larger foveal subretinal tissue complex thickness, foveal subretinal fluid and subretinal hyperreflective material.16 These risk factors, with the exception of blocked fluorescence, remained significantly associated with the development of scar through the additional 3 years of follow up. However two other baseline risk factors with a higher risk of scar were discovered: a better visual acuity in the fellow eye and large hemorrhages. It is not entirely clear why a better visual acuity in the fellow eye would be a risk factor for development of scar in the study eye but it is possible that it delayed the initial visit of the subject to an ophthalmologist and also might be less likely to be aggressively treated after the duration of the clinical trial.
The CATT study allowed recruitment of patients with nAMD having more than 50% hemorrhage relative to the total CNV lesion. Other major anti-VEGF clinical trials excluded such patients.18,19,20 Therefore the CATT cohort presented a unique opportunity to study eyes with hemorrhages that were more than 50% of the total CNV lesion, and many of the hemorrhages were larger than 1 disc area. The visual acuity in these eyes at the end of the 2-year clinical trial was similar to the eyes that did not have such large hemorrhages at enrollment.21 However, the 5-year CATT follow-up study results show that relatively large hemorrhages present at enrollment, if observed over longer durations, more than double the risk of developing a scar. Apart from iron toxicity to the photoreceptors due to deposition of hemosiderin and the reduced nutrient flux, large hemorrhages promote the formation of scar and fibrin meshwork contraction that can further reduce vision by involving adjacent areas of the retina.22-29
We also found the risk due to predominantly classic lesions at enrollment for developing a scar increased from 3-fold at two years to 4.5-fold through 5 years. It is possible that this increased risk is caused by treated quiescent classic CNV lesions that relapsed over time and subsequently developed a scar. It is also possible that some occult lesions converted to classic lesions and increased the risk of developing a scar.30
Once a fibrotic scar developed during the first year after initiation of anti-VEGF therapy, several changes were observed in and around the scar over a period of time. By 5 years, only 15% of the fibrotic scars at year 1 remained stable without any observable changes in the color images. There was little change in the average size of fibrotic scar between year 1 and 2 and the mean VA was maintained or was slightly better at year 2 compared with year 1. However, over the time between the Year 2 visit and the Year 5 visit, the mean size of the fibrotic scar increased with a corresponding decrease in the mean VA in these eyes at year 5. The annualized rate of growth was similar from Year 1 to 2 and Year 2 to 5, the change due to difference in scar size and VA between the two periods could be attributed to a much longer time window in the second period and the difference in the type of care given during these two periods. During the first two years, the CATT clinical trial’s protocol required a monthly examination with anti-VEGF intravitreal injections given either monthly or in a pro rata basis. At the end of two years the CATT subjects were released from the protocol and were only seen after another 3 years. During this period, it is possible that reactivation of neovascularization occurred resulting in extension of the scar area and reduction in vision.14
Macular atrophy associated with fibrotic scar develops within or adjacent to the scar and can encircle the scar partially or completely (Figure 2). Five years after enrollment, atrophy was observed in a little more than half of eyes that had developed a fibrotic scar at 1 year. As for fibrotic scar area expansion, the annualized increase in area was similar in the period of Year 1 to Year 2 and the period of Year 2 to Year 5. Mean VA in eyes that had atrophy at year 1 and 2 was good compared to eyes without atrophy but was reduced at five years. Expanding areas of atrophy are likely to produce scotomas and substantially affect vision when they involve the foveal center. Atrophy has been suggested to result from the remodeling of the choroid vasculature producing reduced blood flow around the scar and the subsequent ischemia causing RPE cell death.31 It has also been suggested that anti-VEGF therapy may induce contracture of the CNV and the subretinal fibrosis and exert a traction force on the surrounding RPE and accelerate its destruction.32 The changes within the fibrotic scar and in areas adjacent to it such as the pigment accumulation and expanding macular atrophy may be due to an aberrant wound healing process influenced by the initial anti-VEGF treatment. Myopic CNV treated with anti-VEGF have been shown to have similar development of fibrotic scars and associated progressive chorioretinal atrophy with poor visual outcome.32 However, even before the start of the anti-VEGF era Sarks et al. had described the expanding macular atrophy associated with fibrotic scars developing from nAMD. They suggested that the expansion of such atrophy was faster during the earlier periods of follow up but the study was limited by its retrospective nature with only a small number of eyes (n=20) that had undergone treatment with laser photocoagulation, radiotherapy, intravitreal triamcinolone injections, or combinations of these.31 Further the measurements of atrophy in more than half of these commenced only after two years unlike in our study that prospectively followed up fibrotic scars that had developed during the first year of therapy. It is not known whether the macular atrophy occurring as a result of nAMD without apparent fibrotic scar on color images is any different from this type of macular atrophy in terms of incidence, expansion and association with visual acuity. Reports on macular atrophy as a result of nAMD have not distinguished atrophy associated with fibrotic scar from macular atrophy without any pre-existing fibrosis.5,10, 33-35
Our study had certain limitations. Although the follow-up at 2 years among living patients was high (93%), only 71% of living patients completed a follow-up study visit. The loss to follow up may have resulted in inaccurate estimation of the incidence of scar from year 2 to year 5.
In our study we were unable to find a strong correlation between scar area and atrophy area at all three follow up time points where images were available for assessment. Also, limited by the small sample size, we were unable to identify any baseline demographic or ocular features that would predict the development and expansion of atrophy. It appears as though the expanding fibrotic scar as well as atrophy contribute to the decrease in VA in CATT study eyes at 5 years even though they do not seem to substantially affect VA in year 2. Regular and long term examinations of eyes to assess the development and progression of atrophy may be particularly important in clinical trials of anti-fibrotic agents to reduce the incidence of fibrotic scars after treatment of neovascular AMD with anti-VEGF agents.36-39 In a tenth of eyes with fibrotic scar at year 1, atrophy appears to completely replace the fibrotic scar by year 5. It is possible that the collagen fibers are phagocytosed by Bruch’s membrane macrophages which have been shown to be present in high counts in eyes with subclinical CNV.40
The number of treated eyes having pigmentation in or around the fibrotic scars was observed to increase proportionally with the years of follow up, starting with 14% during the first year and involving more than half the subjects at year 5. This phenotypical presentation of pigmentation may be important, as it is believed that RPE cells undergo epithelial – mesenchymal transition and the melanotic cells that are unique to CNV are associated with atrophy with and without basal laminar deposits. This trans-differentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype can cause the RPE cells to adhere to and exert traction forces on the extracellular matrix and could be one of the factors contributing to the evolution of atrophy clinically.41-43 The atrophy process increases with time and is consistent with a shift in biologic activities observed during continued passage of RPE cells in vitro.44 The phenotypic diversity of RPE and its changing biological activity seen at a molecular level needs to be understood clinically, and careful examination by different imaging modalities of the evolution of nAMD into a fibrotic scar and its gradual erosion by atrophy in eyes treated with anti-VEGF injections offers this opportunity.45-47
In summary, a relatively small percentage of CATT subjects develop scar after 2 years compared to the first two years under anti-VEGF treatment. Large hemorrhages at enrollment appear to influence scar formation at later periods of follow up. Fibrotic scars that developed at 1 year tended to change in size or be accompanied by atrophy and pigmentation with only 15% remaining stable throughout the follow-up period. We observed a slow average increase in the area of fibrotic scar probably due to ongoing organization of the collagen fibers and/or development of new areas of nAMD. Contiguous macular atrophy developed in an increasing number of eyes through 5 years and the area expanded in size. We were unable to find any significant association with atrophy incidence or expansion with any of the baseline demographic or ocular characteristics.
Supplementary Material
CATT subjects eligible for analysis through 5 years
Acknowledgments
Financial Support: This study was supported by cooperative agreements U10 EY017823, U10 EY017825, U10 EY017826, U10 EY017828, U10 EY023530 and R21EY023689 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services. ClinicalTrials.gov number, NCT00593450. The funding organization had no role in the design or conduct of this research.
Footnotes
Meeting Presentation: Part presented at ARVO 2017 and part accepted for presentation at AAO 2017
Financial Disclosure(s): The author(s) have made the following disclosure(s): Dr Jaffe has a consultancy relationship with Heidelberg Engineering, Alcon/Novartis, Genentech/Roche, and Neurotech. Dr. Ying is a consultant for Janssen R & D. Other members of the writing committee have no financial relationships to declare. Dr. Maguire serves on a data and safety monitoring committee for Genentech/Roche.
Conflict of Interest: No conflicting relationship exists for any other author than described above.
References
- 1.Sarwar S, Clearfield E, Soliman MK, et al. Aflibercept for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2016;2:CD011346. doi: 10.1002/14651858.CD011346.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014:CD005139. doi: 10.1002/14651858.CD005139.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–9. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Gillies MC, Campain A, Barthelmes D, et al. Long-Term Outcomes of Treatment of Neovascular Age-Related Macular Degeneration: Data from an Observational Study. Ophthalmology. 2015;122:1837–45. doi: 10.1016/j.ophtha.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Peden MC, Suñer IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122:803–8. doi: 10.1016/j.ophtha.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SY, Oubraham H, Uzzan J, et al. Causes of unsuccessful ranibizumab treatment in exudative age-related macular degeneration in clinical settings. Retina. 2012;32:1480–5. doi: 10.1097/IAE.0b013e318240a516. [DOI] [PubMed] [Google Scholar]
- 7.Hogg R, Curry E, Muldrew A, et al. Identification of lesion components that influence visual function in age related macular degeneration. Br J Ophthalmol. 2003;87:609–14. doi: 10.1136/bjo.87.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–35. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Toth CA, Daniel E, et al. Macular morphology and visual acuity in the second year of the Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Ophthalmology. 2016;123:865–75. doi: 10.1016/j.ophtha.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka E, Chaikitmongkol V, Bressler SB, Bressler NM. Vision-threatening lesions developing with longer-term follow-up after treatment of neovascular age-related macular degeneration. Ophthalmology. 2015;122:153–61. doi: 10.1016/j.ophtha.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 11.Grunwald JE, Pistilli M, Daniel E, et al. Incidence and Growth of Geographic Atrophy during 5 Years of Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2017;124:97–104. doi: 10.1016/j.ophtha.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The CATT Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: 2-year results. Ophthalmology. 2012;119:1388–98. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2016;123:1751–61. doi: 10.1016/j.ophtha.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grunwald JE, Daniel E, Ying GS, et al. Photographic assessment of baseline fundus morphologic features in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119:1634–41. doi: 10.1016/j.ophtha.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel E, Toth CA, Grunwald JE, et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:656–66. doi: 10.1016/j.ophtha.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeCroos FC, Toth CA, Stinnett SS, Heydary CS, Burns R, Jaffe GJ, CATT Research Group Optical coherence tomography grading reproducibility during the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2012 Dec;119(12):2549–57. doi: 10.1016/j.ophtha.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR, VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 20.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 21.Altaweel MM, Daniel E, Martin DF, et al. Outcomes of eyes with lesions composed of >50% blood in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Ophthalmology. 2015;122:391–398. doi: 10.1016/j.ophtha.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segall D, Balta F, Jackson TL. Submacular hemorrhage in neovascular age-related macular degeneration: A synthesis of the literature. Surv Ophthalmol. 2016;61:18–32. doi: 10.1016/j.survophthal.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol. 1982;94:762–773. doi: 10.1016/0002-9394(82)90301-4. [DOI] [PubMed] [Google Scholar]
- 24.Toth CA, Morse LS, Hjelmeland LM, Landers MB., 3rd Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol. 1991;109:723–729. doi: 10.1001/archopht.1991.01080050139046. [DOI] [PubMed] [Google Scholar]
- 25.Benner JD, Hay A, Landers MB, Hjelmeland LM, Morse LS. Fibrinolytic-assisted removal of experimental subretinal hemorrhage within 7 days reduces outer retinal degeneration. Ophthalmology. 1994;101:672–681. doi: 10.1016/s0161-6420(94)31279-6. [DOI] [PubMed] [Google Scholar]
- 26.Bressler NM, Bressler SB, Childs AL, et al. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: ophthalmic findings: SST report no. 13. Ophthalmology. 2004;111:1993–2006. doi: 10.1016/j.ophtha.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grossniklaus HE, Wilson DJ, Bressler SB, et al. Clinicopathologic studies of eyes that were obtained postmortem from four patients who were enrolled in the submacular surgery trials: SST Report No. 16. Am J Ophthalmol. 2006;141:93–104. doi: 10.1016/j.ajo.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 28.Toth CA, Benner JD, Hjelmeland LM, Landers MBD, Morse LS. Ultramicrosurgical removal of subretinal hemorrhage in cats. Am J Ophthalmol. 1992;113:175–182. doi: 10.1016/s0002-9394(14)71530-2. [DOI] [PubMed] [Google Scholar]
- 29.Bhisitkul RB, Winn BJ, Lee OT, et al. Neuroprotective effect of intravitreal triamcinolone acetonide against photoreceptor apoptosis in a rabbit model of subretinal hemorrhage. Invest Ophthalmol Vis Sci. 2008;49:4071–7. doi: 10.1167/iovs.08-1892. [DOI] [PubMed] [Google Scholar]
- 30.Stevens TS, Bressler NM, Maguire MG, et al. Occult choroidal neovascularization in age-related macular degeneration. A natural history study. Arch Ophthalmol. 1997;115:345–50. doi: 10.1001/archopht.1997.01100150347006. [DOI] [PubMed] [Google Scholar]
- 31.Sarks J, Tang K, Killingsworth M, Arnold J, Sarks S. Development of atrophy of the retinal pigment epithelium around disciform scars. Br J Ophthalmol. 2006;90:442–6. doi: 10.1136/bjo.2005.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn SJ, Park KH, Woo SJ. Subretinal fibrosis after antivascular endothelial growth factor therapy in eyes with myopic choroidal neovascularization. Retina. 2016;36:2140–49. doi: 10.1097/IAE.0000000000001043. [DOI] [PubMed] [Google Scholar]
- 33.Bhisitkul RB, Mendes TS, Rofagha S, et al. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. Am J Ophthalmol. 2015;159:915–24. doi: 10.1016/j.ajo.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 34.Munk MR, Ceklic L, Ebneter A, et al. Macular atrophy in patients with long-term anti-VEGF treatment for neovascular age-related macular degeneration. Acta Ophthalmol. 2016;94:e757–e764. doi: 10.1111/aos.13157. [DOI] [PubMed] [Google Scholar]
- 35.Gillies MC, Campain A, Barthelmes D, et al. Fight Retinal Blindness Study Group Long-Term Outcomes of Treatment of Neovascular Age-Related Macular Degeneration: Data from an Observational Study. Ophthalmology. 2015;122:1837–45. doi: 10.1016/j.ophtha.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Kachi I, Yasukawa T, Kato A, et al. Combination therapy with intravitreal tissue plasminogen activator and ranibizumab for subfoveal type 2 choroidal neovascularization. Jpn J Ophthalmol. 2016;60:179–86. doi: 10.1007/s10384-016-0434-4. [DOI] [PubMed] [Google Scholar]
- 37.Zhang R, Liu Z, Zhang H, Zhang Y, Lin D. The COX-2-Selective Antagonist (NS-398) Inhibits Choroidal Neovascularization and Subretinal Fibrosis. PLoS One. 2016;11:e0146808. doi: 10.1371/journal.pone.0146808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siedlecki J, Wertheimer C, Wolf A, et al. Combined VEGF and PDGF inhibition for neovascular AMD: anti-angiogenic properties of axitinib on human endothelial cells and pericytes in vitro. Graefes Arch Clin Exp Ophthalmol. 2017;255:963–72. doi: 10.1007/s00417-017-3595-z. [DOI] [PubMed] [Google Scholar]
- 39.Jaffe GJ, Ciulla TA, Ciardella AP, et al. Dual Antagonism of PDGF and VEGF in Neovascular Age-Related Macular Degeneration: A Phase IIb, Multicenter, Randomized Controlled Trial. Ophthalmology. 2017;124:224–234. doi: 10.1016/j.ophtha.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010;94:918–25. doi: 10.1136/bjo.2009.165563. [DOI] [PubMed] [Google Scholar]
- 41.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zanzottera EC, Messinger JD, Ach T, et al. The Project MACULA Retinal Pigment Epithelium Grading System for Histology and Optical Coherence Tomography in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2015;56:3253–68. doi: 10.1167/iovs.15-16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanzottera EC, Messinger JD, Ach T, Smith RT, Curcio CA. Subducted and melanotic cells in advanced age-related macular degeneration are derived from retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2015;56:3269–78. doi: 10.1167/iovs.15-16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grisanti S, Guidry C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Invest Ophthalmol Vis Sci. 1995;36:391–405. [PubMed] [Google Scholar]
- 45.Miura M, Makita S, Sugiyama S, et al. Evaluation of intraretinal migration of retinal pigment epithelial cells in age-related macular degeneration using polarimetric imaging. Sci Rep. 2017;7:3150. doi: 10.1038/s41598-017-03529-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts PK, Baumann B, Schlanitz FG, et al. Retinal pigment epithelial features indicative of neovascular progression in age-related macular degeneration. Br J Ophthalmol. 2017 Mar 7; doi: 10.1136/bjophthalmol-2016-310004. pii: bjophthalmol-2016-310004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Balaratnasingam C, Messinger JD, Sloan KR, et al. Histologic and Optical Coherence Tomographic Correlates in Drusenoid Pigment Epithelium Detachment in Age-Related Macular Degeneration. Ophthalmology. 2017;124:644–656. doi: 10.1016/j.ophtha.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CATT subjects eligible for analysis through 5 years


