Abstract
New mosquito-borne diseases have emerged on multiple occasions over the last several decades, raising fears that there are yet more poorly understood viruses that may emerge in the United States. Here, we provide a data-driven ‘watch list’ of viruses in the Flaviridae family with high potential to emerge in the US, identified using statistical techniques, to enable the public health community to better target surveillance. We suggest public health authorities further incorporate predictive modeling techniques into disease prevention strategies.
Keywords: emerging infectious diseases, arboviruses, disease surveillance
The start of the 21st century was marked by multiple emerging mosquito-borne diseases. West Nile virus was introduced to the eastern United States (US) in 1999 and has since spread across the continental US, infecting tens of thousands of people and causing widespread mortality in bird populations [1]. A mutation in a strain of chikungunya virus increased overall infectiousness in the invasive mosquito vector, Aedes albopictus (Asian tiger mosquito), increasing the risk of autochthonous disease transmission in temperate zones, with outbreaks occurring in Italy and France [2]. More recently, Zika virus spread from Oceania to South America, and has continued north at a rate exceeding 15,000 km/year [3], causing severe neurological diseases including Zika congenital and Guillain-Barré syndromes. In each case, the science and public health communities lacked basic knowledge about these previously geographically limited viruses, and interventions were put in place only after the pathogen had become established. Our question is not if another mosquito-borne pathogen will emerge in the US, but which will be next?
The sheer number of potential emerging pathogens underscores the infeasibility of comprehensive surveillance. Of over 530 known arboviruses, viruses transmitted by arthropod vectors such as ticks or mosquitoes (as listed in the International Catalogue of Arboviruses [https://wwwn.cdc.gov/arbocat/]) that infect humans or domestic animals, most are poorly studied and knowledge gaps include information as basic as which species of mosquito transmit which pathogens. Without a complete list of possible vectors, health agencies are unable to adequately plan for outbreaks or target virus surveillance. It is also difficult to predict whether an imported case of disease will initiate local transmission without knowing the efficiency at which mosquito species in the area can transmit the virus. Unfortunately, development of diagnostic tools, and studies to assess vector competence and vectorial capacity (which are very labor intensive) are often undertaken only after disease is endemic. But, without this information, health agencies are ill-equipped to prevent outbreaks, and are limited to economically costly, reactive strategies to disease emergence.
To narrow the list of potential emerging diseases, we have created a ‘watch list’ of flaviviruses (the family that includes many well-known arboviruses including West Nile, dengue, yellow fever and Zika viruses) that might emerge in the US, based on known associations of flaviviruses with mosquito species currently found in the US (Box 1). We ordered this watch list by the number of predicted vectors of each virus. Transmission dynamics of mosquito-borne diseases are determined by many additional factors (e.g. mosquito abundance, human exposure, biting preference, genetic lineage), and our ranking should therefore not be interpreted as a measure of risk of emergence. Rather, this watch list provides a starting point to guide future empirical and modeling efforts in the emergence of mosquito-borne pathogens.
Box 1.
Drawing on traits of known vector-virus pairs, in recent work, we developed a statistical model of trait combinations associated with the propensity of a virus to be vectored by a mosquito species, referred to as a link [4]. Known links were compiled from the literature on experiments showing which mosquito species can transmit the virus from an infected to a susceptible host. Fifteen traits of mosquitoes and twelve traits of viruses were used to construct a predictive model (Box 1, Table I). We then applied the resulting model to data on all flaviviruses known to infect humans and the mosquito species found in the US. Our resulting list prioritizes viruses based on the number of predicted mosquito vectors found in the US and do not include other factors known to be important to transmission dynamics (e.g. population density, host preference, larval habitat type). We provide an exhaustive list of viruses, from which public health professionals can further prioritize candidate viruses based on entomological and epidemiological factors that influence transmission dynamics. For example, Rocio virus, which circulates in wild bird populations in Brazil and caused a human epidemic in Sao Paulo in 1975 [15], may be more highly prioritized due to the mobility of the zoonotic reservoir population and history of epidemic risk.
Box 1 Table I.
Traits used in predictive model, listed in order of importance.
| Vector Traits: | Subgenus, continental range, number of viruses vectored, geographic area, larval habitat, habitat discrimination, biting time, host breadth, urban preference, anthropophily, endophily, artificial container breeder, permanent habitat, salinity tolerance |
| Virus Traits: | Year isolated, vector breadth, disease severity, host range, genome length, viral group, viral clade, host breadth, symptoms, vectored by other arthropods, mutated envelope |
The resulting network of potential virus-vector pairs, or predicted links, in the US (Figure 1) contains thirty-seven mosquito species and twenty viruses, only five of which are currently or have historically been found in the US. Two viruses, Japanese encephalitis virus and Wesselsbron virus, have many more predicted vectors than the other viruses (36 and 29, respectively). Closely related to West Nile Virus, Japanese encephalitis causes over 67,000 reported cases annually, with the majority in Asia [5]. While only 1% of infected people develop Japanese encephalitis, there is a 20 – 30% mortality rate among cases that progress to that stage [6]. In its native Asian range, Japanese encephalitis virus requires a non-human host, domestic pigs, to amplify the virus. In North America, there are several bird species, that are competent reservoirs for the virus [7]. Much less is known about Wesselsbron virus, which has been historically restricted to the geographic region of sub-Saharan Africa, where it most commonly infects sheep and other livestock [8]. While symptoms of Wesselsbron virus in humans are relatively mild, it causes abortion in goats and cerebellar hypoplasia (a form of microcephaly) in calves [9–10], which is a significant concern for the livestock industry.
Figure 1.
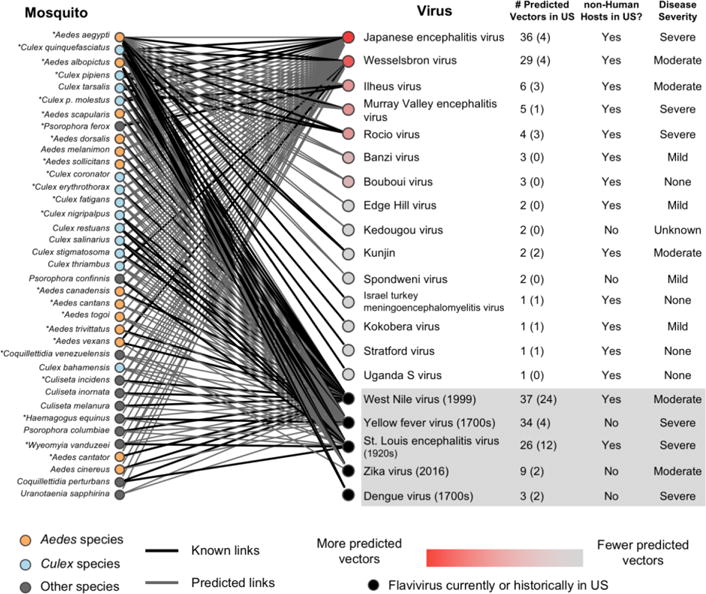
Network of flaviviruses known (black) or predicted (grey) to be transmitted by mosquito species found in the United States. Starred mosquitoes readily bite humans. Mosquitoes and viruses are rank ordered from top to bottom by the predicted associations of flaviviruses with mosquito species (not taking into account other relevant epidemiological characteristics), with the number of known US vectors in parentheses. The presence of a nonhuman host in the US, which could potentially serve as a zoonotic reservoir, is noted, as well as disease severity as defined by Mackenzie et al. [16]. Viruses that are currently or have been historically found in the US are shown in the gray box at the bottom of the list, with their approximate year of introduction, or first known case, in parentheses, and are not included in the original ranking.
The virus with the most predicted links is West Nile virus (37 links). Other US-endemic viruses have between three and 34 predicted mosquito vectors. Yellow fever virus, which caused an outbreak in New Orleans as recently as 1905, is predicted to have 34 mosquito vectors. Since the introduction of the yellow fever vaccine in the mid-20th century, transmission has been mostly restricted to Africa and South America [11]; however, the 2016 epidemic in Angola demonstrates the inadequacy of current immunization regulations in some areas. By contrast, dengue virus, which is locally transmitted in Puerto Rico (and, on rare occasions, states along the Gulf Coast) is only predicted to be vectored by three mosquito species endemic to the US. Despite the small number of competent vector species, serological surveys along the Texas-Mexico border found evidence of recent infection in 2% of the population, translating to an estimated 100,000 dengue cases in the continental US annually [12]. The high number of cases highlights the importance of not just the number of predicted vectors, but their identity, in disease transmission, in this case, Aedes aegypti, a mosquito species that is well-adapted to live with humans and is a highly efficient vector of many human pathogens. Notably, the top ranked viruses in our watch list are predicted to be vectored by mosquitoes that prefer to feed on humans (e.g. Ae. aeypgti, Ae. albopictus, Culex quinquefasciatus) and are therefore likely to transmit pathogens between humans.
These viruses illustrate, but do not exhaust, the future risk of emergence of viruses circulating elsewhere in the world. With the rise in globalization and availability of air transportation, a mosquito-borne pathogen can travel across the world in less than 24 hours, connecting countries with active transmission to countries without disease (e.g. Zika and chikungunya). While several US states, notably Florida, have large mosquito surveillance programs, most do not [13], and the accompanying virus surveillance programs screen for a limited number of pathogens. The difficulty in preventing outbreaks of Zika in the US highlights the need to shift from reactive to proactive surveillance and control strategies for mosquito-borne diseases. Focusing on the viruses with a high potential to emerge could improve the efficiency and reliability of surveillance without overly burdening public health institutions and help guide future vaccine development, such as the Coalition for Epidemic Preparedness Innovations [14]. Predictive modeling, such as the method highlighted here, is not a single solution, but does provide novel information that, in combination with conventional methods, can improve the preparedness of our public health systems. Finally, the approach we present here can be applied to other disease systems to identify groups of viruses and bacterial pathogens most likely to emerge in the US or any other country.
Acknowledgments
We would like to thank Barbara Han and Tad Dallas, who provided support in the creation and analysis of the predictive model. MVE was supported by The University of Georgia (Presidential Fellowship). JMD was funded by the National Institutes of Health (Grant No. U01GM110744). CCM was funded by the National Science Foundation (Grant No. DEB-1640780). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hayes EB, et al. Epidemiology and Transmission Dynamics of West Nile Virus Disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver SC, Forrester NL. Chikungunya: Evolutionary history and recent epidemic spread. Antiviral Res. 2015;120:32–39. doi: 10.1016/j.antiviral.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Zinszer K, et al. Reconstruction of Zika Virus Introduction in Brazil. Emerg Infect Dis. 2017;23:91–94. doi: 10.3201/eid2301.161274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans MV, et al. Data-driven identification of potential Zika virus vectors. eLife Sciences. 2017;6:e22053. doi: 10.7554/eLife.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell GL, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011;89:766–774E. doi: 10.2471/BLT.10.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur R, Vrati S. Development of a recombinant vaccine against Japanese encephalitis. Journal of NeuroVirology. 2003;9:421–431. doi: 10.1080/13550280390218454. [DOI] [PubMed] [Google Scholar]
- 7.Nemeth N, et al. North American Birds as Potential Amplifying Hosts of Japanese Encephalitis Virus. Am J Trop Med Hyg. 2012;87:760–767. doi: 10.4269/ajtmh.2012.12-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Regenmortel MHV, et al. Virus taxonomy: classification and nomenclature of viruses: seventh report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press; 2000. p. c2000. [Google Scholar]
- 9.Mushi EZ, et al. Wesselsbron disease virus associated with abortions in goats in Botswana. J Vet Diagn Invest. 1998;10:191. doi: 10.1177/104063879801000216. [DOI] [PubMed] [Google Scholar]
- 10.Coetzer JA, et al. Wesselsbron disease: a cause of congenital porencephaly and cerebellar hypoplasia in calves. Onderstepoort J Vet Res. 1979;46:165–169. [PubMed] [Google Scholar]
- 11.Frierson JG. The Yellow Fever Vaccine: A History. Yale J Biol Med. 2010;83:77–85. [PMC free article] [PubMed] [Google Scholar]
- 12.Hotez PJ. Neglected Infections of Poverty in the United States of America. PLoS Negl Trop Dis. 2008;2:e256–11. doi: 10.1371/journal.pntd.0000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamer GL. Heterogeneity of Mosquito (Diptera: Culicidae) Control Community Size, Research Productivity, and Arboviral Diseases Across the United States. J Med Entomol. 2016;53:485–495. doi: 10.1093/jme/tjw020. [DOI] [PubMed] [Google Scholar]
- 14.Butler D. Billion-dollar project aims to prep vaccines before epidemics hit. Nat News. 2017;541:444. doi: 10.1038/nature.2017.21329. [DOI] [PubMed] [Google Scholar]
- 15.Souza LO, et al. Rocio viral encephalitis. In: Beran GW, editor. Handbook of Zoonoses. 2nd. CRC Press; 2017. pp. 205–209. [Google Scholar]
- 16.Mackenzie J, et al. Japanese Encephalitis and West Nile Viruses. Springer Science and Business Media; 2012. [Google Scholar]


