Introduction
The homomeric α7 nicotinic acetylcholine receptor (nAChR) has recently been implicated as a target that modulates nicotine reward and reinforcement in preclinical models. Activation of α7 nAChRs attenuate nicotine conditioned place preference and i.v. self-administration, while blockade of the receptor enhances nicotine reward and reinforcement (Brunzell and McIntosh 2012; Harenza et al. 2014). These observations raise the question of whether there is a role of the α7 nAChR in another important aspect of nicotine dependence, nicotine withdrawal. Nicotine withdrawal, the primary negative reinforcer that strengthens nicotine dependence, is one of the primary causes of high tobacco relapse rates (Le Foll and Goldberg 2009). In humans, it consists of somatic signs such as bradycardia, as well as non-somatic signs such as anxiety and depression (Hughes et al. 1992). The physical indications of nicotine withdrawal in rodents are measured by the observation of somatic signs, hyperalgesia, and affective signs such as anxiety-like behaviors (Damaj et al. 2003; Salas et al. 2004).
Studies by our group and others have been performed utilizing null mutant α7 mice in nicotine withdrawal. α7 knockout (KO) mice undergoing nicotine withdrawal showed a reduction in hyperalgesia (Grabus et al. 2005; Jackson et al. 2008), no alterations in their somatic signs upon withdrawal (Grabus et al. 2005; Jackson et al. 2008),the absence of attentional deficits (Higa et al. 2017) and attenuated anxiety-like behavior compared to their wild type counterparts (Stoker et al. 2012). Pharmacological blockade of the α7 receptor with methyllycaconitine (MLA) has been shown in some studies to precipitate a subset of nicotine withdrawal somatic signs in rats and mice (Nomikos et al. 1999; Damaj et al. 2003; Salas et al. 2007) while in other studies, MLA was found to be ineffective at inducing nicotine withdrawal signs (Markou and Paterson 2001). Recently, the selective α7 nAChR agonist ABT-107 (Malysz et al. 2010) was shown to reduce nicotine withdrawal-induced anxiety-like behaviors in mice (Yohn et al. 2014). Collectively, these studies suggest that α7 nAChRs are not necessary for the development of nicotine withdrawal but seem to modulate the physical and affective signs of nicotine withdrawal. Therefore, there is a need for further investigation of the role of α7 nAChRs in nicotine withdrawal.
As a ligand-gated ion-channel, homomeric α7 nAChRs have unique features of high calcium permeability, rapid desensitization and low probability of channel opening (Séguéla et al. 1993; Williams et al. 2011a). The development of α7-subtype selective agonists and partial agonists (Horenstein et al. 2008) have facilitated investigations of α7 receptors as therapeutic targets. However, the interpretation of results with these agents is complicated by the fact that they will both activate and desensitize receptors and subsequently decrease the effects of endogenous agonists (Papke et al. 2011). The recent development of α7 nAChR positive allosteric modulators (PAMs) and ago-PAMs (direct allosteric channel activators) may aid in understanding significance of α7 channel properties in nicotine dependence paradigms. Additionally, silent agonists, compounds that are ineffective at channel activation but induce the conformational changes associated with desensitization, have indicated that there are likely to be effects of α7 ligands that are independent of channel activation (Papke et al. 2015).
The agents which increase α7 channel activation vary by their mechanisms and relative efficacy. PAMs classified as Type I, such as NS1738, transiently enhance the probability of α7 nAChR channel opening, while PAMs classified as Type II, the best studied of which is PNU120596, not only increase the opening probability, but slow or reverse the desensitization of the receptor which can result in prolonged bursts of channel opening (Grønlien et al. 2007; Williams et al. 2011b). To date, the impact of these α7 nAChR modulators in nicotine withdrawal is unknown.
Therefore, the current study investigated the impact of α7 nAChR pharmacological modulation on nicotine withdrawal behaviors using a well-established mouse model of nicotine dependence. We compared the effects of the orthosteric full agonist PNU282987 to the type I α7 PAM NS1738 and the type II α7 PAM PNU120596 to evaluate the effect of receptor activation, desensitization and subsequent modulation of endogenous acetylcholine(ACh)/choline tone in mecamylamine-precipitated nicotine withdrawal in the mouse.
Materials and Methods
Animals
Drug-naive, ICR male mice (8 weeks old upon arrival; Envigo Laboratories, Indianapolis, IN) served as subjects. Mice were housed four per cage with ad libitum access to food and water on a 12-h light cycle in a humidity and temperature controlled vivarium that was approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Mice received corn cob bedding and were fed Envigo Teklad mouse/rat diet 7102 (LM-485). Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Drugs
(−)-Nicotine hydrogen tartrate [(−)-1-methyl-2-(3-pyridyl) pyrrolidine (+)-bitartrate] and mecamylamine HCl (non-selective nAChR antagonist) were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). PNU120596 [1-(5-Chloro-2, 4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)] and PNU282987 [N-(3R)-1 Azabicyclo [2.2.2] oct-3-yl-4-chlorobenzamide] were obtained from the National Institute on Drug Abuse (NIDA) supply program (Bethesda, MD). NS1738 was purchased from Tocris Biosciences (Minneapolis, MN). Nicotine, mecamylamine, and PNU282987 were dissolved in physiological saline. NS1738 and PNU120596 were dissolved in a mixture of 1:1:18 [1 volume ethanol/1 volume Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ) and 18 volumes distilled water]. Nicotine, PNU282987, and mecamylamine were injected subcutaneously (s.c.) while NS1738 and PNU120596 were administered intraperitoneally (i.p.). The nicotine solution pH was neutralized with sodium bicarbonate as needed. Freshly prepared solutions were given to mice at 10 ml/kg. Doses of nicotine and mecamylamine are expressed as the free base of the drugs.
Mecamylamine-Precipitated Nicotine Withdrawal Studies
Mice were infused with 24 mg/kg/day nicotine or saline through 14 day s.c. osmotic minipumps (model 2002; Alzet Corporation, Cupertino, CA) that were implanted under isoflurane anesthesia. The concentration of nicotine was adjusted according to animal weight and mini pump flow rate. On the morning of day 15, mice were injected with vehicle, PNU282987 (1, 3, 9 mg/kg, s.c.), NS1738 (1, 10 mg/kg, i.p.), or PNU120596 (3, 9 mg/kg, i.p.), 15 min before the challenge with the nonselective nAChR antagonist, mecamylamine (2 mg/kg, s.c.). Precipitated nicotine withdrawal assessment was performed 10 min later as described in (Jackson et al. 2008). Affective (anxiety-like behavior) and physical (somatic signs, and hyperalgesia) indications of nicotine withdrawal were evaluated in this paradigm. Mice were first evaluated for 5 min in the plus maze test for anxiety-related behavior. Time spent on the open arms of the plus maze was assessed as a measure of anxiety-related response, with a reduction in open-arm time taken to indicate increased anxiety. The number of arm crosses between the open and closed arms was also counted as a measure of locomotor activity. The plus maze assessment was immediately followed by a 20-min observation of somatic signs measured as paw and body tremors, head shakes, backing, jumps, curls, and ptosis. Mice were placed in clear activity cages without bedding for the observation period. The total number of somatic signs was tallied for each mouse and the average number of somatic signs during the observation period was plotted for each test group. Hyperalgesia was evaluated using the hot plate test immediately following the somatic sign observation period. Specifically, mice were placed into a 10-cm wide glass cylinder on a hot plate (Thermojust Apparatus, Richmond, VA) maintained at 52°C. The latency to reaction time (jumping or paw licking) was recorded. The specific testing sequence was chosen based on our prior studies showing that this order of testing reduced within-group variability and produced the most consistent results (Jackson et al. 2008). All studies were performed by an observer blinded to the experimental treatment.
Statistical analysis
Data were analyzed using the GraphPad software version 6.0 (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± S.E.M. A one-way analysis of variance (ANOVA) in conjunction with Holm-Šídák comparison tests were conducted to determine significant effects of drug treatments vs controls. Comparisons were considered statistically significant when p < 0.05.
Results
Somatic and affective mecamylamine-precipitated nicotine withdrawal signs are attenuated by α7 nAChR orthosteric full agonist PNU282987
The affective (anxiety-related behavior) and physical (somatic signs and hyperalgesia) indications of nicotine withdrawal were measured in mice following pretreatment with either PNU282987 or vehicle 15 min prior to mecamylamine administration on day 15. Nicotine withdrawn mice had a significantly increased anxiety-related behavior in the plus maze, as indicated by a reduction in the time spent in the open arms [F (5, 32) = 11.21, p< 0.0001] (Fig. 1A), increased expression of somatic withdrawal signs [F (5, 32) = 24.48, p< 0.0001] (Fig. 1B), and decreased response latencies in the hot-plate test [F (5, 32) = 17.89, p< 0.0001] (Fig. 1C). Mice implanted with saline minipumps and received vehicle expressed no withdrawal signs. PNU282987 attenuated mecamylamine-precipitated nicotine withdrawal signs in a dose related manner. The highest dose of PNU282987 tested (9 mg/kg) did not significantly affect behavioral responses in saline-infused mice in any withdrawal test. However, pretreatment of nicotine-dependent animals with PNU282987 showed a trend of reducing anxiety-like behavior (increasing time in open arms in the plus-maze test) at 3 mg/kg and reached significance at 9 mg/kg (s.c.) (p<0.05) (Fig. 1A). To examine whether the results observed in the elevated plus maze test were possibly confounded by alterations in locomotor activity induced by PNU282987 administration, the number of arm crosses were recorded. As shown in Table 1 PNU282987 had no effect on the number of arm crosses in the plus maze [F (5, 32) = 0.7950, p=0.5613]. In addition, pretreatment with PNU282987 decreased precipitated nicotine somatic withdrawal signs and was statistically significant at doses 3 and 9 mg/kg (p<0.05) (Fig. 1B). However, as the post hoc analysis showed, pretreatment with PNU282987 was ineffective at attenuating the expression of hyperalgesia (hot-plate latency) at all doses tested (p<0.05) (Fig. 1C). PNU282987 was administered within the range of doses used for other behavior studies (Vicens et al. 2013; de Moura and McMahon 2017).
Fig 1. Effects of Full Orthosteric Agonist PNU282987 on Physical and Affective Signs of Precipitated Nicotine Withdrawal.
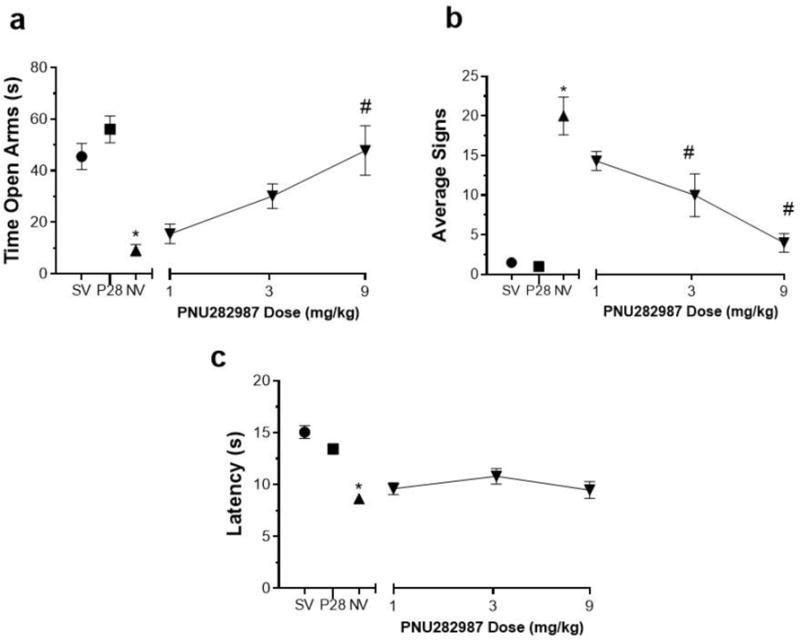
Mice were chronically infused with saline or nicotine (24 mg/kg/day) for 14 days. On day 15 mice received s.c. injection of PNU282987 (1, 3 and 9 mg/kg) or vehicle. Mice then were administered mecamylamine (2mg/kg; s.c.) 10 min prior to behavioral assessment of A) anxiety-like behaviors (Time spent in the open arm), B) somatic signs, and C) hyperalgesia (hot plate latency). Nicotine induced withdrawal symptoms: increased anxiety related behavior and somatic signs, but decreased hot plate latency. Compared to vehicle, pretreatment with PNU282987: A) attenuated the anxiety-like behavior at 9mg/kg; B) reduced somatic signs at 3 and 9mg/kg; and C) and no effect on hot plate latency in nicotine withdrawn mice. Each point represents the mean ± S.E.M. of n=6–8 mice per group. * Denotes p< 0.05 vs. Saline minipump group, # Denotes p< 0.05 vs. Nicotine minipump group. (MP) minipump. SV = Saline minipump/vehicle treatment; NV = Nicotine minipump/vehicle treatment; P28 = PNU282987 at 9 mg/kg.
Table 1. PNU282987 does not have an effect on the average number of arm crosses in the elevated plus maze test.
Mice undergoing nicotine withdrawal received PNU282987 (1, 3 and 9 mg/kg; s.c.) or vehicle. The average number of arm crosses was recorded in the plus maze test. The numbers are presented as the total number of arm crosses ± SEM (n=8). MP = minipump
| Treatment | Average number of arm crosses ±SEM |
|---|---|
| Saline MP-vehicle | 6.8± 0.6 |
| Saline MP-PNU282987 (9) | 7.2±0.7 |
| Nicotine MP-vehicle | 7.5±0.9 |
| Nicotine MP-PNU282987 (1) | 6.2±0.7 |
| Nicotine MP-PNU282987 (3) | 6.5 ± 0.6 |
| Nicotine MP-PNU282987 (9) | 5.8±0.5 |
Only mecamylamine-precipitated somatic nicotine withdrawal signs are attenuated by α7 nAChR Type I PAM NS1738
Physical and affective signs of nicotine withdrawal were measured in mice following pretreatment with either NS1738 or vehicle 15 min prior to mecamylamine administration on day 15. NS1738 was used at pretreatment times and at the lower range of doses previously described (Freitas et al. 2013a, b). Nicotine withdrawn mice had a significantly increased anxiety-related behavior in the plus maze [F (4, 35) = 21.86, p<0.0001] (Fig. 2A), increased expression of somatic withdrawal signs [F (4, 35) = 37.32, p<0.0001] (Fig. 2B), and decreased response latencies in the hot-plate test [F (4, 35) = 5.208, p=0.0021] (Fig. 2C). Pretreatment with NS1738 had no effect on the expression of anxiety-related behaviors (time in open arms in the plus-maze test) (p>0.05) (Fig. 2A). As shown in Table 2 NS1738 had no effect on the number of arm crosses in the plus maze [F (4, 35) = 0.7950, p=0.9962]. However, NS1738 reduced nicotine precipitated somatic withdrawal signs at 10mg/kg (p<0.05) (Fig. 2B). Pretreatment with NS1738 15 min prior to mecamylamine injection did not reverse hot plate latencies (measure of hyperalgesia) at either of the doses tested (p>0.05) (Fig. 2C). The highest dose of NS1738 (10 mg/kg) did not significantly affect behavioral responses in saline-infused mice in any withdrawal test.
Fig 2. Effects of Type I PAM NS1738 on Physical and Affective Signs of Precipitated Nicotine Withdrawal.
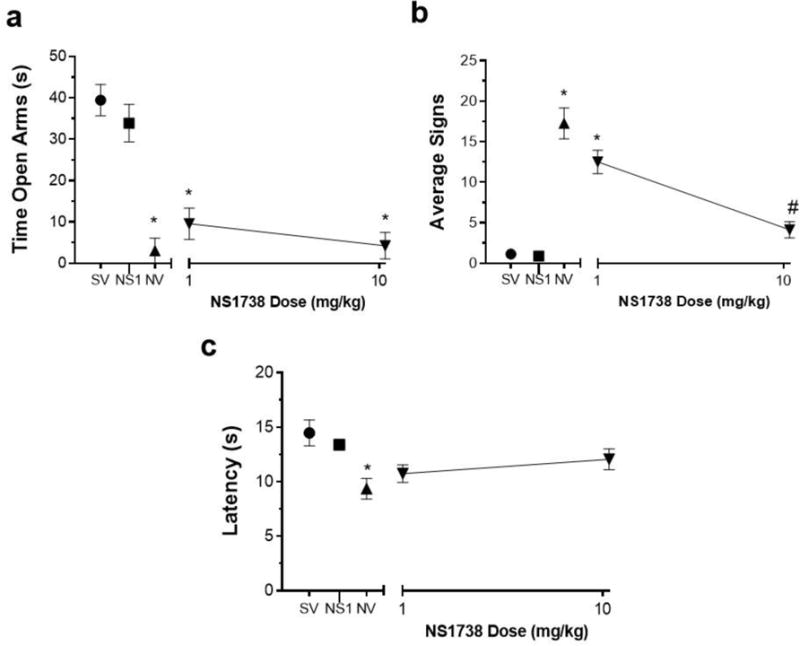
Mice were infused with saline or nicotine (24 mg/kg/day) for 14 days. On day 15 mice received s.c. injection of NS1738 (1 and 10 mg/kg) or vehicle. Mice then were administered mecamylamine (2mg/kg; s.c.) 10 min prior to behavioral assessment of A) anxiety-like behaviors (Time spent in the open arm), B) somatic signs, and C) hyperalgesia (hot plate latency). Nicotine induced withdrawal symptoms: increased anxiety related behavior and somatic signs, but decreased hot plate latency. Compared to vehicle, pretreatment with NS1738: A) did not attenuate the anxiety-like behavior at any dose tested; B) reduced somatic signs at 10mg/kg; and C) and had no effect on hot plate latency in nicotine withdrawn mice. Each point represents the mean ± S.E.M. of n=6–8 mice per group. *Denotes p< 0.05 vs. Saline minipump group, # Denotes p< 0.05 vs. Nicotine minipump group. (MP) minipump. SV = Saline minipump/vehicle treatment; NV = Nicotine minipump/vehicle treatment; NS1 = NS1738 at 10 mg/kg.
Table 2. NS1738 does not have an effect on the average number of arm crosses in the elevated plus maze test.
Mice undergoing nicotine withdrawal received NS1738 (1 and 10 mg/kg; i.p.) or vehicle. The average number of arm crosses was recorded in the plus maze test. The numbers are presented as the total number of arm crosses ± SEM (n=8). MP = minipump
| Treatment | Average number of arm crosses ±SEM |
|---|---|
| Saline MP-vehicle | 9.9± 1.8 |
| Saline MP-NS1738 (10) | 9.6±1.4 |
| Nicotine MP-vehicle | 10.1±1.4 |
| Nicotine MP-NS1738 (1) | 9.4±1.7 |
| Nicotine MP-NS1738 (10) | 9.4 ± 1.4 |
Only mecamylamine-precipitated nicotine withdrawal-induced hyperalgesia attenuated by α7 nAChR Type II PAM PNU120596
Physical and affective signs of nicotine withdrawal were measured in mice following pretreatment with either PNU120596 or vehicle 15 min prior to mecamylamine administration on day 15. As expected, nicotine-dependent mice had a significantly increased anxiety-related behavior in the plus maze [F (4, 29) = 3.730, p= 0.0144] (Fig. 3A), increased expression of somatic withdrawal signs [F (4, 30) = 19.92, p<0.0001] (Fig. 3B), and decreased response latencies in the hot-plate test [F (4, 30) = 6.808, p= 0.0005] (Fig. 3C). Pretreatment with PNU120596 had no significant effect on anxiety-like behaviors (p>0.05) (Fig. 3A). As shown in Table 3, PNU120596 had no effect on the number of arm crosses in the plus maze [F (5, 32) = 0.5965, p=0.6682]. In addition, PNU120596 at all doses used was ineffective at reducing precipitated nicotine somatic withdrawal signs (p>0.05) (Fig. 3B). However, pretreatment with PNU120596 exhibited a trend of reversing hot plate latencies (measure of hyperalgesia) and significantly increased hot plate latencies at 9mg/kg (p<0.05) (Fig. 3C). The highest dose of PNU120596 (9 mg/kg) did not significantly affect behavioral responses in saline-infused mice in any withdrawal test. PNU120596 was used at similar doses previously described (Freitas et al. 2013a, c).
Fig 3. Effects of Type II PAM PNU120596 on Physical and Affective Signs of Precipitated Nicotine Withdrawal.
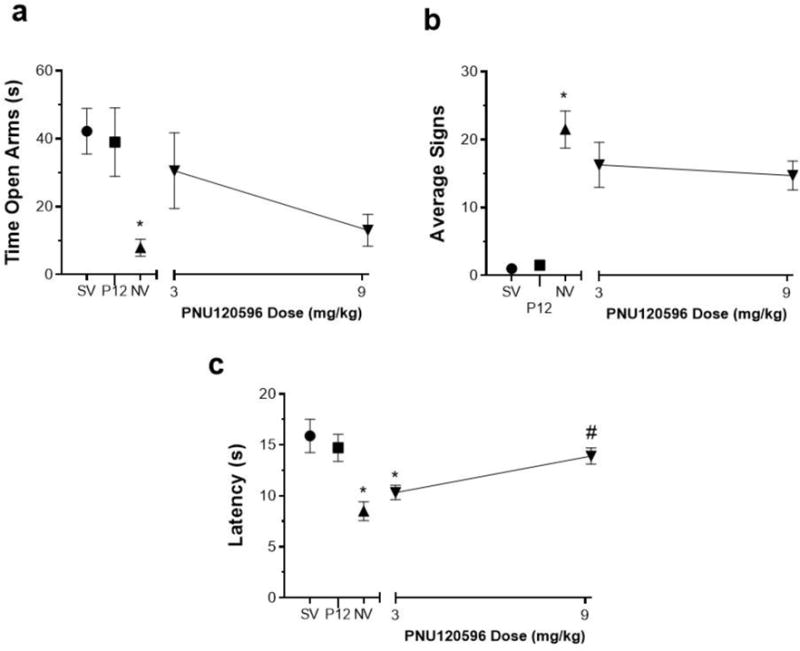
Mice were chronically infused with saline or nicotine (24 mg/kg/day) for 14 days. On day 15 mice received i.p. injection of PNU120596 (3 and 9 mg/kg) or vehicle. Mice then were administered mecamylamine (2mg/kg; s.c.) 10 min prior to behavioral assessment of A) anxiety-like behaviors (Time spent in the open arm), B) somatic signs, and C) hyperalgesia (hot plate latency). Nicotine induced withdrawal symptoms: increased anxiety related behavior and somatic signs, but decreased hot plate latency. Compared to vehicle, pretreatment with PNU120596: A) had no effect anxiety-like behavior; B) had no effect on somatic signs; and C) but significantly increased hot plate latency at 9mg/kg in nicotine withdrawn mice. Each point represents the mean ± S.E.M. of n=6–8 mice per group. *Denotes p< 0.05 vs. Saline minipump group, # Denotes p< 0.05 vs. Nicotine minipump group. (MP) minipump. SV = Saline minipump/vehicle treatment; NV = Nicotine minipump/vehicle treatment; P12 = PNU120596 at 9 mg/kg
Table 3. PNU120596 does not have an effect on the average number of arm crosses in the elevated plus maze test.
Mice undergoing nicotine withdrawal received PNU120596 (3 and 9 mg/kg; i.p.) or vehicle. The average number of arm crosses was recorded in the plus maze test. The numbers are presented as the total number of arm crosses ± SEM (n=8). MP, minipump.
| Treatment | Average number of arm crosses ±SEM |
|---|---|
| Saline MP-vehicle | 7.5± 0.9 |
| Saline MP-PNU120596 (9) | 6.9±0.8 |
| Nicotine MP-vehicle | 7.8±0.8 |
| Nicotine MP-PNU120596 (3) | 6.5±0.4 |
| Nicotine MP-PNU120596 (9) | 7.9 ± 0.9 |
Discussion
The α7 nAChR ligands PNU282987, NS1738, and PNU120596 varied in their ability to attenuate different signs of the nicotine withdrawal syndrome (Fig. 1, 2 and 3). These differences are likely to relate to the unique ways in which these agents modulate the function and conformational states of the α7 receptor.
In the presence of an orthosteric full agonist, the α7 nAChR is permeable to calcium but has a low probability of opening, and rapidly desensitizes (Séguéla et al. 1993; Williams et al. 2011a). These intrinsic factors may limit the usefulness of α7 nAChR ligands, especially at higher concentrations, unless nonconducting states of the receptor may themselves impact signaling. PAMs were developed as pharmacological tools to circumvent the intrinsic limitations of the α7 nAChR regarding channel activity. The probability of an α7 nAChR being open is less than one in a million (Williams et al. 2012), thus the Type I PAM NS1738 and Type II PAM PNU120596, which increase the probability of channel opening, were used to evaluate the role of this α7 nAChR feature in precipitated nicotine withdrawal. However, from the perspective of increasing channel activity, PAM effects rely on the additional effects of orthosteric agonists which could be endogenous factors like ACh and choline or exogenous agents like nicotine.
In our mecamylamine-precipitated nicotine withdrawal experiments the orthosteric agonist PNU282987 attenuated anxiety-like behaviors (Fig. 1); however, the α7 nAChR PAMs NS1738 had no effect on anxiety-like behavior as observed in the elevated plus maze (Fig. 2). PNU120596 had no significant effect, but it is important to note that there was a nonsignificant effect at the 3mg/kg dose (Fig. 3). Given the effect produced by PNU282987 administration, the results suggest that low probability of channel opening and predominant desensitization are sufficient for this effect. Our results are in agreement with a recent study of α7 nAChR activation with the α7 orthosteric agonist ABT-107 which was shown to also attenuate anxiety-like behaviors in the novelty-induced hypophagia (NIH) test (Yohn et al. 2014).
The orthosteric full agonist PNU282987 and the Type I PAM NS1738 both attenuated somatic signs, but the Type II PAM PNU120596 had no effect on somatic signs. The most reasonable/ordinary hypothesis is that PNU120596 would amplify the effects of fluctuating levels of ACh (in the brain) on α7 channel activation and to a significant degree reduces the phasic nature of that signaling. This would suggest that the reduction of somatic signs requires cycles of activation and inactivation or desensitization. It has been shown that α7 nAChR KO mice undergoing nicotine withdrawal have a reduction in somatic signs (Salas et al. 2007), consistent with the importance of α7 nAChR blockade or desensitization in nicotine dependence. However, a study from our lab that measured somatic signs did not see a reduction in somatic signs observed in α7 KO mice compared to their WT littermates (Jackson et al. 2008). This discrepancy may be contributed to the different somatic signs observed in the studies. As in our present experiments, the latter study observed somatic signs such as paw tremors, body tremors, and backing while the former study tallied more spontaneous and natural signs such as grooming, scratching, and chewing.
PNU120596 was the only α7 ligand that reduced mecamylamine-precipitated nicotine withdrawal-induced hyperalgesia in the hot plate test. It is unclear of the reason for this reduction of hyperalgesia by PNU120596 and the lack of effect of PNU282987. These results are consistent with previous studies that implicated the α7 nAChR in the reduction of hyperalgesia evidenced in nicotine withdrawn α7 KO mice (Grabus et al. 2005; Jackson et al. 2008) and the α7 nAChR antagonist MLA has been shown to precipitate hyperalgesia (Damaj et al. 2003). The lack of effect in saline minipump/vehicle/mecamylamine treatment mice in our study suggests that mecamylamine does not have an effect on its own. In addition, our lab has previously shown that chronically treated saline mice with and without mecamylamine treatment have similar behavior outcomes in the elevated plus maze, hot plate test and number of somatic signs (Jackson et al. 2008). Furthermore, it has been shown that mecamylamine has no significant effect in the elevated plus maze (Brioni et al. 1993).
Taken together, our results suggest that modulation of the α7 nAChR can play important roles in nicotine withdrawal behaviors in mice. In addition, the effects of PAMs in this study suggest that endogenous ACh/choline tone is sufficient when potentiated by the type I PAM NS1738 to attenuate some aspects of precipitated nicotine withdrawal. Unlike NS1738, which will only amplify phasic signals, the type II PAM PNU120506 may produce chronic activation, especially in the presence of the continuous delivery of nicotine from the pumps, which were not removed at the time when acute withdrawal was induced. As noted above, this suggests that the potential role for α7 nAChR in the reversal of the reduced latency measured in the hot plate test may rely on a signaling mechanism that is distinct from the cycles of channel activation/desensitization implicated for the effects of the other drugs. Another consideration is that α7 nAChRs have been shown to have a large intracellular interactome which may include G-proteins and other signal transduction pathways (Paulo et al. 2009; King et al. 2015; King and Kabbani 2016) and that signaling through these pathways may occur independent of channel activation (de Jonge and Ulloa 2007; Papke et al. 2015). Additionally, it has been suggested that PAMs and non-activating ligands, such as silent agonists, can modulate α7 signaling through non-conducting conformational states (Papke et al. 2015, 2017).
These findings highlight a beneficial effect of using α7 PAMs in some aspects of nicotine withdrawal. Different types of PAMs may provide a unique way to synergize with the endogenous cholinergic system to specifically address separate aspects of withdrawal. In addition, PAMs can provide better selectivity for α7 nAChRs than putatively selective agonists, which in many cases interact with serotonin 5-HT3 receptors due to a high homology of their ligand binding domain (Gurley and Lanthorn 1998). Working in unique ways through allosteric sites on the receptors, PAMs are likely to serve as useful tools to understand the different effects that α7 nAChR modulation may play in multiple aspects of nicotine dependence.
References
- Brioni JD, Neill ABO’, Kim DJB, Decker MW. Nicotinic receptor agonists exhibit anxiolytic-like effects on the elevated plus-maze test. Eur J Pharmacol. 1993;238:1–8. doi: 10.1016/0014-2999(93)90498-7. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, McIntosh JM. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology. 2012;37:1134–43. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–34. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura FB, McMahon LR. The contribution of α4β2 and non-α4β2 nicotinic acetylcholine receptors to the discriminative stimulus effects of nicotine and varenicline in mice. Psychopharmacology (Berl) 2017;234:781–792. doi: 10.1007/s00213-016-4514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Carroll FI, Damaj MI. The antinociceptive effects of nicotinic receptors α7-positive allosteric modulators in murine acute and tonic pain models. J Pharmacol Exp Ther. 2013a;344:264–75. doi: 10.1124/jpet.112.197871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Ghosh S, Carroll FI, et al. Effects of alpha 7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology. 2013b;65:156–164. doi: 10.1016/j.neuropharm.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Negus S, Carroll FI, Damaj MI. In vivo pharmacological interactions between a type II positive allosteric modulator of α7 nicotinic ACh receptors and nicotinic agonists in a murine tonic pain model. Br J Pharmacol. 2013c;169:567–579. doi: 10.1111/j.1476-5381.2012.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Damaj MI. Nicotine physical dependence in the mouse: involvement of the alpha7 nicotinic receptor subtype. Eur J Pharmacol. 2005;515:90–93. doi: 10.1016/j.ejphar.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Grønlien JH, Håkerud M, Ween H, et al. Distinct profiles of α7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol. 2007;72:715–724. doi: 10.1124/mol.107.035410.tamers. [DOI] [PubMed] [Google Scholar]
- Gurley DA, Lanthorn TH. Nicotinic agonists competitively antagonize serotonin at mouse 5-HT3 receptors expressed in Xenopus oocytes. Neurosci Lett. 1998;247:107–110. doi: 10.1016/S0304-3940(98)00306-1. [DOI] [PubMed] [Google Scholar]
- Harenza JL, Muldoon PP, De Biasi M, et al. Genetic variation within the Chrna7 gene modulates nicotine reward-like phenotypes in mice. Genes Brain Behav. 2014;13:213–25. doi: 10.1111/gbb.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa KK, Grim A, Kamenski ME, et al. Nicotine withdrawal-induced inattention is absent in alpha7 nAChR knockout mice. Psychopharmology (Berl) 2017;234:1573–1586. doi: 10.1007/s00213-017-4572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein NA, Leonik FM, Papke RL. Multiple Pharmacophores for the Selective Activation of Nicotinic Alpha 7-Type Acetylcholine Receptors. Mol Pharmacol. 2008;74:1496–1511. doi: 10.1124/mol.108.048892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Gulliver SB, Fenwick JW, et al. Smoking cessation among self-quitters. Health Psychol. 1992;11:331–4. doi: 10.1037//0278-6133.11.5.331. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JR, Kabbani N. Alpha 7nicotinic receptor coupling to heterotrimeric G proteins modulates RhoA activation, cytoskeletal motility, and structural growth. J Neurochem. 2016:532–545. doi: 10.1111/jnc.13660. [DOI] [PubMed] [Google Scholar]
- King JR, Nordman JC, Bridges SP, et al. Identification and characterization of a G protein-binding cluster in Alpha 7 nicotinic acetylcholine receptors. J Biol Chem. 2015;290:20060–20070. doi: 10.1074/jbc.M115.647040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb Exp Pharmacol. 2009:335–67. doi: 10.1007/978-3-540-69248-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malysz J, Anderson DJ, Grønlien JH, et al. In Vitro Pharmacological Characterization of a Novel Selective alpha 7 Neuronal Nicotinic Acetylcholine Receptor Agonist ABT-107. J Pharmacol Exp Ther. 2010;334:863–874. doi: 10.1124/jpet.110.167072. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res. 2001;3:361–73. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Hildebrand BE, Panagis G, Svensson TH. Nicotine withdrawal in the rat: role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroreport. 1999;10:697–702. doi: 10.1097/00001756-199903170-00007. [DOI] [PubMed] [Google Scholar]
- Papke RL, Stokes C, Damaj MI, et al. Persistent activation of α7 nicotinic ACh receptors associated with stable induction of different desensitized states. Br J Pharmacol. 2017 doi: 10.1111/bph.13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bagdas D, Kulkarni AR, et al. The analgesic-like properties of the alpha7 nAChR silent agonist NS6740 is associated with non-conducting conformations of the receptor. Neuropharmacology. 2015;91:34–42. doi: 10.1016/j.neuropharm.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Trocme-Thibierge C, Guendisch D, et al. Electrophysiological Perspectives on the Therapeutic Use of Nicotinic Acetylcholine Receptor Partial Agonists. J Pharmacol Exp Ther. 2011;337:367–379. doi: 10.1124/jpet.110.177485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo J, Brucker W, Hawrot E. Proteomic Analysis of an α7 Nicotinic Acetylcholine Receptor Interactome. J Proteome Res. 2009;8:1849–1858. doi: 10.1021/pr800731z.Proteomic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–9. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24:10035–9. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, et al. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Olivier B, Markou A. Role of Alpha7- and Beta4-containing nicotinic acetylcholine receptors in the affective and somatic aspects of nicotine withdrawal: Studies in knockout mice. Behav Genet. 2012;42:423–436. doi: 10.1007/s10519-011-9511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicens P, Ribes D, Heredia L, et al. Motor and anxiety effects of PNU-282987, an alpha7 nicotinic receptor agonist, and stress in an animal model of Alzheimer’s disease. Curr Alzheimer Res. 2013;10:516–523. doi: 10.2174/15672050113109990130. [DOI] [PubMed] [Google Scholar]
- Williams DK, Peng C, Kimbrell MR, Papke RL. Intrinsically low open probability of α7 nicotinic acetylcholine receptors can be overcome by positive allosteric modulation and serum factors leading to the generation of excitotoxic currents at physiological temperatures. Mol Pharmacol. 2012;82:746–59. doi: 10.1124/mol.112.080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol. 2011a;82:915–30. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. Investigation of the Molecular Mechanism of the Alpha 7 Nicotinic Acetylcholine Receptor Positive Allosteric Modulator PNU-120596 Provides Evidence for Two Distinct Desensitized States. Mol Pharmacol. 2011b;80:1013–1032. doi: 10.1124/mol.111.074302.sitized. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn NL, Turner JR, Blendy JA. Activation of α4β2*/α6β2* nicotinic receptors alleviates anxiety during nicotine withdrawal without upregulating nicotinic receptors. J Pharmacol Exp Ther. 2014;349:348–54. doi: 10.1124/jpet.113.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]


