Abstract
Background
Time-to-treatment-failure (TTF) is the interval from chemotherapy initiation to premature discontinuation. We evaluated TTF based on age.
Methods
Pooled analyses were conducted with, first-line chemotherapy trials for advanced non-small cell lung cancer (CALGB 9730, 30203, and 30801). Comparisons -- with age 65+ and 70+ years -- were performed for TTF (primary endpoint), reasons for early chemotherapy cessation, grade 3+ adverse events, and overall survival.
Results
Among 1006 patients, 460 (46%) were 65+ years of age. 145 older patients (32% of this age cohort) completed all six, planned chemotherapy cycles, as did 170 (32%) younger patients. Median TTF was 2.9 months (95% confidence interval (CI) = (2.7, 3.2)) in older and 3 months (95% CI= (2.9, 3.5)) in younger patients; adjustment for performance status and stratification by chemotherapy by trial yielded no statistically significant age-based difference in TTF. However, reasons for early chemotherapy cessation differed between age groups (multivariate p = 0.004). Older patients were less likely to discontinue from cancer progression (41% versus 55%) and more likely from toxicity or patient choice (16% and 15%, respectively) compared to younger patients (13% and 6%, respectively). Older patients were more likely to experience grade 3+ adverse events (86% versus 79%) with no statistically significant difference in survival. An age cut point of 70+ showed no difference in TTF, a lower trend of early cessation due to cancer progression, and somewhat shorter older patient survival.
Conclusion
TTF was comparable between older and younger patients; but different, age-based, and potentially modifiable reasons account for it.
Keywords: geriatric, time-to-treatment failure, chemotherapy cessation, non-small cell lung cancer, adverse events
INTRODUCTION
Time-to-treatment-failure (TTF) is the interval from initiation of chemotherapy to its premature discontinuation. Since early discontinuation can occur due to various reasons – such as, cancer progression, adverse events, patient choice, or patient death – TTF can be influenced by factors unrelated to chemotherapy efficacy and is therefore seldom used for regulatory drug approval.[1] A compilation of endpoints from 144 consecutively-published randomized controlled trials found that TTF ranked fifth in usage among six time-related endpoints, ahead of only by a time-to-tumor-response endpoint.[2]
In older cancer patients, TTF can be an invaluable endpoint. TTF can potentially explain why multiple earlier studies show worse chemotherapy toxicity in older patients but comparable survival.[3–7] Older patients have well-documented, higher rates of severe adverse events, which can prompt the premature discontinuation of chemotherapy. Furthermore, many older patients may opt to transition to palliative care sooner.[8] Thus, comparing TTF between older patients and their younger counterparts is an important step in exploring treatment-related outcomes in geriatric oncology; it allows us to probe into the reasons for chemotherapy discontinuation, thereby potentially enabling us to intervene to make chemotherapy more tolerable, especially for older patients.
This study relied on a 1000+ patient cohort to compare TTF in older versus younger patients and to better understand factors that contribute to comparative age-based reasons for the premature discontinuation of chemotherapy. This cohort consisted of patients who had been diagnosed with metastatic non-small cell lung cancer, a malignancy associated with notable morbidity and a limited life expectancy and who were at risk for early discontinuation of chemotherapy for many reasons. This cohort provided a platform for understanding age-based duration of chemotherapy, reasons for stopping chemotherapy early, and associated outcomes.
METHODS
Overview
Individual patient data from all arms of three, first-line, multi-institutional, prospectively-conducted clinical trials from the Cancer and Leukemia Group B (CALGB), now the Alliance for Clinical Trials, were pooled. A deliberate decision was made to focus on non-small cell lung cancer patients who were receiving conventional chemotherapy because these patients are at high risk for chemotherapy toxicity with a limited survival and because previous studies show a finite number of initial conventional chemotherapy cycles is appropriate.[9]
CALGB 9730, 30203 and 30801 were included. Patients had signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines. These three trials had similar patient eligibility criteria that included 1) no prior chemotherapy, 2) stage IIIB/IV incurable non-small cell lung cancer, 3) Eastern Cooperative Oncology Group performance score of 0–2, and acceptable organ function.
The only notable differences were CALGB 9730 excluded patients with brain metastases or HIV positive while these patients were eligible for CALGB 30203 and 30801. The latter two trials excluded patients with cardiovascular events in the preceding 6 months; in both of these latter two trials, patients with chronic steroid or non-steroidal anti-inflammatory agent use must have had these agents discontinued one week prior to registration.
CALGB 9730 randomly assigned patients to receive carboplatin plus paclitaxel (carboplatin area under the curve (AUC) 6 mg/ml/min on day 1 and paclitaxel 225 mg/m2 on day 1) versus paclitaxel alone. CALGB 30203 randomly assigned patients to carboplatin and gemcitabine (carboplatin AUC 5.5 mg/ml/min on day 1 and gemcitabine 1000 mg/m2 on days 1 and 8) with or without zileuton or celecoxib. CALGB 30801 randomly assigned patients to carboplatin plus pemetrexed (carboplatin AUC 6 on day 1 and pemetrexed 500 mg/m2 on day 1) for non-squamous histology or carboplatin plus gemcitabine (carboplatin AUC 5.5 on day 1 with gemcitabine 1000 mg/m2 on days 1 and 8) for squamous cell histology with or without celecoxib.[10–12] Patients in all three trials were to complete a maximum of 6 cycles of chemotherapy; these instructions were in keeping with findings from Socinski and others that discourage continuous chemotherapy of longer duration [9].
All three of these previously-conducted clinical trials contributed to lung cancer therapeutics, concluding, respectively, that combination chemotherapy improves outcomes in age-unspecified patients, that the addition of a 5-lipoxygenase inhibitor (zileutin) does not improve outcomes, and that the addition of a COX-2 inhibitor (celecoxib) to chemotherapy in a population selected for COX-2 expression also does not improve outcomes. Individual patient data from these three trials were used in the current study with the goal of examining age-based TTF, reasons for the early discontinuation of chemotherapy, and other clinically germane outcomes, such as adverse event rates and overall survival.
Definition of Endpoints
The primary endpoint was TTF. Treatment failure was defined as chemotherapy discontinuation prior to completion of the planned 6 cycles for any reason, including cancer progression, adverse events, patient choice, or death. Secondary endpoints were chosen to probe into reasons behind premature chemotherapy discontinuation, specifically, cancer progression versus all other reasons. During the conduct of each trial, the healthcare team had reported on each patient’s reasons from premature discontinuation. Because previous studies had reported higher rates of adverse events in older as opposed to younger patients and because adverse events were anticipated to be a major reason for chemotherapy discontinuation, age-based adverse event rates were selected as another secondary outcome. Finally, overall survival was also evaluated as a secondary outcome to provide context to the above endpoints and because survival is regarded as one of the most meaningful clinical outcomes in cancer care.[13]
Statistical Analyses
Two sets of analyses were conducted; the main set used an age cut point of 65 years and the other an age cut point of 70 years. Unless otherwise reported, conclusions from analyses based on these age cut points were similar. For the primary study endpoint, TTF is defined as the time from trial registration to treatment discontinuation or last follow-up. The event was discontinuation of chemotherapy for any reason other than completion of six cycles. The cumulative incidence of premature chemotherapy discontinuation was estimated separately by age group using the cumulative incidence function, treating chemotherapy completion as the competing risk. TTF was compared between age groups using the Cox proportional hazards model with age as the main effect and was adjusted for potential confounding baseline factors using a model selection procedure. Baseline factors including sex, performance status, smoking history, tumor histology, cancer stage, and chemotherapy by trial (paclitaxel alone in CALGB 9730 versus paclitaxel + carboplatin in CALGB 9730 versus CALGB 30203 versus CALGB 30801 to account for potential differences in outcomes based on chemotherapy regimen and time era) were considered in the model building. Variables that reached the 0.05 significance level were kept in the final model. The proportional hazards assumption was tested for all variables. The frequencies of various reasons for chemotherapy discontinuation (cancer progression versus toxicity versus death versus all other reasons) as well as grade 3 or worse adverse events were summarized separately by age group and were compared between age groups using a chi-square test or the Fisher’s exact test, as appropriate. Overall survival was summarized by age group using the Kaplan-Meier estimator and compared using Cox regression models as described for TTF. Two-way interactions between age and other significant variables that remained in the model were evaluated; none were found to be statistically significant.
Analyses were conducted using SAS, version 9.4 on a dataset locked May 15, 2017. Data collection, analyses, and quality assurance was done by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
RESULTS
Demographics
A total of 1006 patients comprise the cohort from all three trials.[10–12] Four hundred sixty patients (46%) were 65 years of age or older at trial enrollment, and 546 (54%) were younger than 65 years of age; 270 (27%) patients were 70 years of age or older. The age distributions were similar across the three trials. Patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or better; all had normal organ and marrow function at trial enrollment. Demographics appear in Table 1.
Table 1.
Baseline Demographics (n=1006)*
| >/= 65 Years of Age N=460 (%) |
< 65 Years of Age N=546 (%) |
p-value | >/= 70 Years of Age N=270 (%) |
< 70 Years of Age N=736 (%) |
p-value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Sex | ||||||
| Male | 346 (63) | 290 (63) | 0.09 | 178 (66) | 458 (62) | 0.28 |
| Female | 200 (37) | 170 (37) | 92 (34) | 278 (38) | ||
|
| ||||||
| Performance Status | ||||||
| 0 | 156 (34) | 168 (31) | 0.23 | 89 (33) | 235 (32) | 0.65 |
| 1 | 235 (51) | 308 (56) | 140 (52) | 403 (55) | ||
| 2 | 69 (15) | 70 (13) | 41 (15) | 98 (13) | ||
|
| ||||||
| Tumor Histology | ||||||
| Adenocarcinoma | 247 (54) | 330 (61) | 0.20 | 141 (53) | 436 (59) | 0.11 |
| Other | 211 (46) | 215 (39) | 127 (47) | 299 (41) | ||
|
| ||||||
| Cancer Stage | ||||||
| IIIB | 63 (14) | 85 (16) | 0.68 | 41 (16) | 107 (15) | 0.84 |
| IV | 359 (81) | 413 (79) | 208 (80) | 564 (79) | ||
|
| ||||||
| Chemotherapy | ||||||
| carboplatin + gemcitabine | 123 (27) | 167 (31) | 0.4 | 69 (26) | 221 (30) | 0.53 |
| carboplatin + pemetrexed | 76 (17) | 80 (15) | 46 (17) | 110 (15) | ||
| carboplatin + paclitaxel | 138 (30) | 146 (27) | 77 (29) | 207 (28) | ||
| paclitaxel | 123 (27) | 153 (28) | 78 (29) | 198 (27) | ||
|
| ||||||
| Smoking Status | ||||||
| Smoked (current or past) | 373 (92) | 441 (96) | 0.01 | 218 (92) | 596 (95) | 0.15 |
| Never | 34 (8) | 19 (4) | 19 (8) | 34 (5) | ||
Percentages may not sum to 100 because of either missing data or rounding.
TTF
Approximately 30% (315 of 985 patients, TTF was missing for 21 patients) of patients across all age groups completed the planned 6 cycles; 70% (670/985) discontinued prematurely. One hundred forty-five patients who were 65 years of age or older (32% of 452 of this age cohort) completed all six cycles of chemotherapy, whereas 170 of 533 (32%) younger patients did the same. With a cut point of 70 years, the number of older and younger patients were 78 of 265 (29%) and 237 of 720 (33%), respectively.
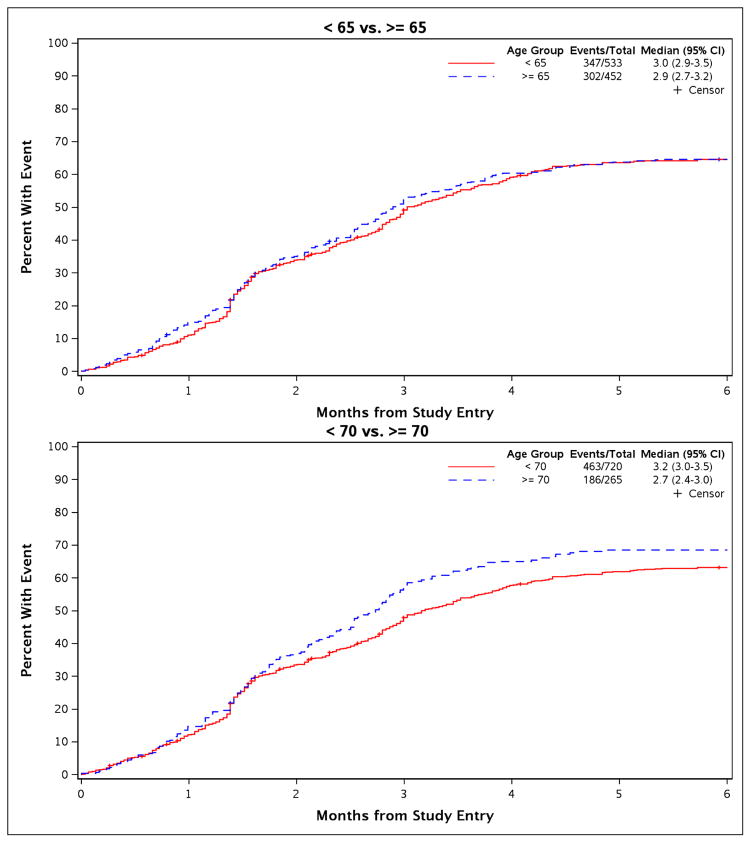
Based on the cut point of 65 years, 302 of 452 (67%) premature chemotherapy discontinuation events occurred in patients 65 years of age or older, and 347 of 533 (65%) in younger patients. The median TTF was 2.9 months (95% confidence interval (CI=(2.7, 3.2) months) in patients 65 years of age or older and 3 months (95% CI=(2.9, 3.5) months) in younger patients (Figure 1). Using the cut point of 70 years, 186 of 265 (70%) of older patients and 463 of 720 (64%) discontinued chemotherapy prematurely. The median TTF was 2.7 months (95% CI=(2.4, 3.0) months) in patients 70 years of age or older and 3.2 months (95% CI=(3.0, 3.5) months) in younger patients (Figure 1).
Figure 1.
Cumulative incidence curves for TTF show no statistically significant differences in age-based comparisons.
In the multivariate analysis, performance status was the only baseline factor that attained a significance level to merit inclusion in the final model. With adjustment for performance status and with stratification by chemotherapy by trial (categorized as CALGB 9730 paclitaxel alone versus CALGB 9730 paclitaxel + carboplatin versus CALGB 30203 versus CALGB 30801, which did not meet the proportional hazards assumption – in this context, it means that our model did not assume that the relative risks of treatment failure among types of chemotherapy were constant over time), no statistically significant difference in TTF was observed based on age; comparisons between patients 65 years of age or older versus younger generated a hazard ratio (HR) of 1.060 (95% CI= (0.908, 1.238); p = 0.46). Poorer performance status was associated with a higher risk of premature chemotherapy discontinuation. Age-based comparison with an age cut point of 70 years confirmed this finding (HR = 1.156; 95% CI = (0.97, 1.37); p=0.10).
Premature Discontinuation of Chemotherapy and Underlying Descriptive Reasons
Descriptive reasons for the premature discontinuation of chemotherapy varied in frequency based on age groups (Table 2). Cancer progression was the most common reason. In older patients, 41% (123/302) discontinued chemotherapy due to cancer progression, 16% (49/302) due to chemotherapy toxicity, 13% (40/302) related to death, 15% (45/302) as per patient choice, and 15% (45/302) due to other reasons. In younger patients, 55% (189/347) discontinued chemotherapy because of cancer progression, 13% (44/347) due to toxicity, 11% (39/347) related to death, 6% (22/347) as per patient choice, and 15% (53/347) due to other reasons. In the multivariate analysis, adjusting for performance status and stratifying by chemotherapy by trial, the risk of early chemotherapy discontinuation due to cancer progression was lower in older patients compared to younger ones: HR = 0.72 (95% CI=(0.57, 0.90); p=0.004). Age-based comparison with an age cut point of 70 years showed a similar trend (HR = 0.79; 95% CI = (0.61, 1.02); p=0.07).
Table 2.
Reasons for Stopping Chemotherapy*
| >/= 65 Years of Age N= 302 (%) |
< 65 Years of Age N= 347 (%) |
p-value | >/= 70 Years of Age N= 186 (%) |
< 70 Years of Age N=463 (%) |
p-value | |
|---|---|---|---|---|---|---|
| Cancer progression | 123 (41) | 189 (55) | 0.0006 | 78 (42) | 234 (51) | 0.07 |
| Chemotherapy Toxicity | 49 (16) | 44 (13) | 34 (18) | 59 (13) | ||
| Death | 40 (13) | 39 (11) | 23 (12) | 56 (12) | ||
| Patient Choice | 45 (15) | 22 (6) | 26 (14) | 41 (9) | ||
| Other** | 45 (15) | 53 (15) | 25 (13) | 73 (16) |
Includes reasons only among the 302, 347, 186, and 463 patients who stopped chemotherapy within the above respective age groups prior to the completion of 6 cycles.
Includes also a small subgroup of patients who had long therapy delays that precluded timely completion of 6 cycles of chemotherapy.
Grade 3+ Adverse Events and Overall Survival
A higher percentage of older patients experienced grade 3 or worse adverse events, 395 of 460 (86%) older patients compared to 429 of 546 (79%) younger patients. After adjusting for chemotherapy by trial, tumor histology, and performance status, the odds ratio (OR) of developing a severe adverse event (grade 3–5) was higher in older patients 1.77 (95% CI=(1.24, 2.51); p = 0.0015).
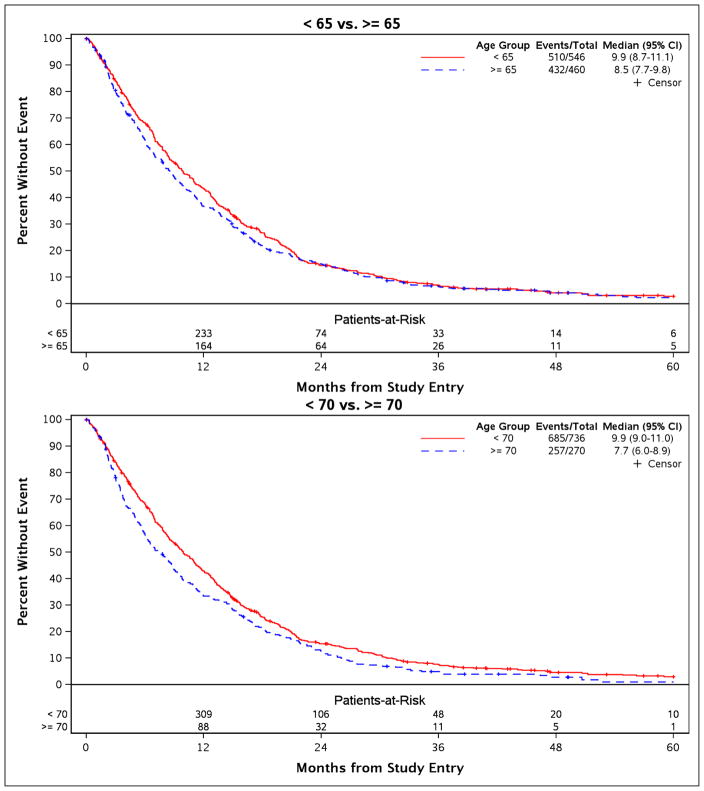
In patients 65 years of age or older, the median overall survival was 8.5 months (95% CI= (7.7, 9.8)), and in younger patients 9.9 months (95% CI=(8.7, 11.1)). In the multivariable analysis, performance status did not meet the proportional hazards assumption and hence was included in the Cox model as a stratification factor. Other baseline variables that were retained in the final model include sex, tumor histology, cancer stage, chemotherapy by trial, and smoking status. After adjusting for these baseline factors and after stratifying by performance status, no statistically significant difference in overall survival was discernible (HR = 1.08; 95% CI=(0.95, 1.22); p = 0.25) (Figure 2). Age-based comparison with a cut point of 70 years yielded conservatively comparable findings with admittedly somewhat shorter survival among older patients (HR = 1.16; 95% CI= (1.00, 1.34); p = 0.04) (Figure 2).
Figure 2.
Overall survival showed no statistically significant differences in age-based comparisons with a trend suggestive of diminished survival in patients 70 years of age or older.
DISCUSSION
This study did not find statistically significant differences in TTF between older and younger patients, although admittedly TTF was overall short and toxicity notable. Although we had hypothesized that TTF would be shorter in older patients, such was not the case. Interestingly, this study did reveal other age-based differences in select outcomes. Although cancer progression was the most frequent reason for stopping chemotherapy prematurely irrespective of age, younger patients were more likely to discontinue chemotherapy because of cancer progression. A higher percentage of older patients stopped chemotherapy because of adverse events or choice.
These observations invite further investigation into how to make chemotherapy more tolerable and acceptable for patients, particularly for older patients. Although patients should not continue to receive chemotherapy in the face of cancer progression, adverse event profiles and unrealistic or inaccurate expectations for chemotherapy can be attenuated, could lead to a longer TTF, and thereby potentially improve clinical outcomes. First, with respect to adverse event profiles, the Cancer and Aging Research Group evaluated chemotherapy dose reductions in older cancer patients and observed first-cycle dose reductions appeared to lead to similar adverse event rates when compared to younger patients who did not receive a dose reduction [14]. Such findings suggest an intervention as simple as reducing the dose of chemotherapy with the first cycle might lead to a favorable cascade of events: better chemotherapy tolerability, longer TTF, and improved survival, particularly in older cancer patients. Recent studies seem to endorse such an approach in older patients.[15–17] Secondly, older cancer patients choose to forego or stop chemotherapy for reasons that range from fear of adverse events to logistical challenges.[18] Although older patients must undoubtedly make personal decisions about chemotherapy, efforts to counsel patients on realistic expectations, to apply an initial dose reduction to improve tolerability as described above, and perhaps even to acquire resources to help with the more quotidian aspects of chemotherapy administration, such as transportation to the chemotherapy unit, might enable older patients to remain on chemotherapy for longer and thereby derive greater benefit. Future studies should consider testing educational and practical interventions to evaluate whether they improve clinical outcomes for older cancer patients. Such could favorably influence outcomes in as many as 20% of older patients who otherwise would have stopped chemotherapy prematurely.
The current study is especially important because few have evaluated the premature cessation of chemotherapy in older cancer patients. Studying a 500-patient cohort, Laurent and others observed that older patients were more likely to stop cisplatin-based chemotherapy for metastatic bladder cancer after two or fewer cycles and that this early cessation led to a higher 1-year mortality rate.[19] As another example, Kim and others observed that within a cohort of 98 older patients, slightly over 30% discontinued palliative chemotherapy after first-line treatment, and those who discontinued did not live as long.[20] These two studies underscore the main strengths of the current study: a large sample size of over 1000 patients and detailed, real time clinical information on reasons for TTF.
Of note, the current study also has limitations. First, the findings reported here were drawn from clinical trials, a fact that introduces patient selection bias. These findings might not pertain to all older cancer patients and might require confirmation among older cancer patients who had not been clinical trial participants and in other groups of cancer patients in general. Second, this study did not specifically focus on very old cancer patients, for whom we have little data here as well as in general. Future studies should perhaps strive to focus on the very old. Third, because we analyzed prospectively-collected data, it is possible that other unrecognized confounding factors might have influenced our results. Such a possibility is not unique to the current study but should be acknowledged.
Finally, we focused on TTF in patients who received conventional chemotherapy – as opposed to targeted therapy and immunotherapy – but, nonetheless, our findings remain important and relevant. Despite the emergence of targeted therapy and immunotherapy for non-small cell lung cancer, most patients with an advanced stage of this malignancy continue to be candidates for conventional, first-line chemotherapy. Importantly, some of these newer treatment approaches, such as immunotherapy, are now given in combination with chemotherapy, thus adding further to the relevance of the work presented here [21–23]. Moreover, shifting demographics with a growing population of older cancer patients also add to the impetus to study TTF in older cancer patients and prompt the need to study ways to allow older patients to remain on chemotherapy for longer and to tolerate it better.
Acknowledgments
Funding Acknowledgement: This work was supported by the Alliance for Clinical Trials in Oncology NCORP Research Base [UG1CA189823].
Footnotes
Conflict of Interest Statement: A.H reports research funding from Celgene, Novartis, and GSK, and General Consulting for Boehringer Ingelheim Pharmaceuticals, Carevive, Sanofi, GTx, Inc., Pierian Biosciences, MJH Healthcare Holdings, LLC, and AstraZeneca, outside the submitted work. All other authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pazdur R. Endpoints for assessing drug activity in clinical trials. The Oncologist. 2008;13(suppl 2):19–21. doi: 10.1634/theoncologist.13-S2-19. [DOI] [PubMed] [Google Scholar]
- 2.Arkenau H-T, Nordma I, Dobbins T, et al. Reporting time-to-event endpoints and response rates in 4 decades of randomized controlled trials in advanced colorectal cancer. Cancer. 2011;117:832–40. doi: 10.1002/cncr.25636. [DOI] [PubMed] [Google Scholar]
- 3.Muss H, Cortes J, Vahdat LH, et al. Erubulin monotherapy in patients aged 70 years and older with metastatic breast cancer. Oncologist. 2014;19:318–27. doi: 10.1634/theoncologist.2013-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahipal A, Denson AC, Djulbegovic B, et al. Effect of age on clinical outcomes in phase 1 trial participants. Cancer Control. 2015;22:235–41. doi: 10.1177/107327481502200217. [DOI] [PubMed] [Google Scholar]
- 5.Pritchard KI, Burris HA, Ito Y, et al. Safety and efficacy of everolimus with exemestane versus exemestane alone in elderly patients with HER2-negative, hormone receptor-positive breast cancer in BOLERO-2. Clin Breast Cancer. 2013;13:421–432. doi: 10.1016/j.clbc.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Graff JN, Baciarello G, Armstrong AJ, et al. Efficacy and safety of enzalutamide in patients 75 years or older with chemotherapy-naïve metastatic castration-resistant prostate cancer: results from PREVAIL. Ann Oncol. 2015 doi: 10.1093/annonc/mdv542. [DOI] [PubMed] [Google Scholar]
- 7.Schild SE, Stella PJ, Geyer SM, et al. The outcome of combined modality therapy for stage III non-small cell lung cancer in the elderly. J Clin Oncol. 2003;21:3201–6. doi: 10.1200/JCO.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Sharma N, Sharma AM, Wojtowycz MA, et al. Utilization of palliative care and acute care services in older adults with advanced cancer. J Geriatr Oncol. 2016 Jan;7(1):39–46. doi: 10.1016/j.jgo.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Socinski MA, Schell MJ, Peterman A, et al. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small cell lung cancer. J Clin Oncol. 2002;20:1335–43. doi: 10.1200/JCO.2002.20.5.1335. [DOI] [PubMed] [Google Scholar]
- 10.Edelman MJ, Wang X, Hodgson K, et al. Phase III randomized, placeob-controlled, double-blind tria of celecoxib in addition to standard chemotherapy for advanced non-small cell lung cancer with cyclooxygenase-2 overexpression: CALGB 30801 (Alliance) J Clin Oncol. 2017;35:2184–2192. doi: 10.1200/JCO.2016.71.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelman MJ, Watson D, Wang X, et al. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy--Cancer and Leukemia Group B Trial 30203. J Clin Oncol. 2008;26:848–55. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
- 12.Lilenbaum RC, Herndon JE, 2nd, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730) J Clin Oncol. 2005;23:190–6. doi: 10.1200/JCO.2005.07.172. [DOI] [PubMed] [Google Scholar]
- 13.Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol. 2012;30:1030–3. doi: 10.1200/JCO.2011.38.7571. [DOI] [PubMed] [Google Scholar]
- 14.Gajra A, Klepin HD, Feng T, et al. Predictors of chemotherapy dose reduction at first cycle in patients age 65 years and older. J Geriatric Oncology. 2015;6:133–40. doi: 10.1016/j.jgo.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakaya A, Fujita S, Satake A, et al. Realistic lenalidomide dose adjustment strategy for transplant-ineligible elderly patients with relapsed/refractory multiple myeloma: Japanese Real-World Experience. Acta Haematologica. 2017;138:55–60. doi: 10.1159/000477792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quoix E, Zalcam G, Oster JP, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared to monotherapy in elderly patients with advanced non-small cell lung cancer: IFTC-0501 randomised, phase 3 trial. Lancet. 2011;378:1079–88. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 17.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomized factorial trial. Lancet. 2011;377:1749–59. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puts MTE, Tapscott B, Fitch M, et al. A systematic review of factors influencing older adults’ decision to accept or decline cancer treatment. Cancer Treatment Reviews. 2015;41:197–215. doi: 10.1016/j.ctrv.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Laurent M, Brureau L, Demery ME, et al. Early chemotherapy discontinuation and mortality in older patients with metastatic bladder cancer: the AGEVIM multicenter cohort study. Urol Oncol. 2017;34:e9–34. doi: 10.1016/j.urolonc.2016.08.003. Epub October 6. [DOI] [PubMed] [Google Scholar]
- 20.Kim JW, Kim YJ, Lee KW, et al. The early discontinuation of palliative chemotherapy in older patients with cancer. Support Care Cancer. 2014;22:773–81. doi: 10.1007/s00520-013-2033-y. [DOI] [PubMed] [Google Scholar]
- 21.Betof AS, Nipp RD, Giobbie-Hurder A, et al. Impact of age on outcomes with immunotherapy for patients with melanoma. Oncologist. 2017;22:963–971. doi: 10.1634/theoncologist.2016-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossi F, Crino L, Misino A, et al. Efficacy and safety of nivolumab in elderly patients with advanced squamous nonsmall cell lung cancer participating in the expanded access programme Italy. Annal of Oncology. 2016;27(supplement 6):1079. [Google Scholar]
- 23.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced non-squamous non-small-cell lung cancer: a randomized, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncology. 2016;17:1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]