Abstract
Objective
To report the three-year assessment of feasibility and utility of microscope integrated intraoperative optical coherence tomography (iOCT) during ophthalmic surgery.
Design
Prospective, consecutive, case series
Participants
Adult subjects undergoing incisional ophthalmic surgery with intraoperative OCT imaging that consented to be enrolled in the Determination of Feasibility of Intraoperative Spectral Domain Microscope Combined/Integrated OCT Visualization During En Face Retinal and Ophthalmic Surgery (DISCOVER) study.
Methods
The DISCOVER study is a single-site, multi-surgeon, IRB-approved investigational device prospective study. Participants included patients undergoing anterior or posterior segment surgery and underwent iOCT imaging with one of three prototype microscope-integrated iOCT systems (i.e., Zeiss Rescan 700, Leica EnFocus, Cole Eye iOCT system). Clinical characteristics were documented, iOCT was directed by the operating surgeon at predetermined surgical time points, and each surgeon completed a questionnaire after surgery to evaluate the utility of iOCT during surgery.
Outcomes
1. Feasibility of iOCT based ability to obtain an OCT image during surgery. 2. Utility of iOCT based on surgeon reporting during surgery
Results
A total of 837 eyes (244 anterior segment and 593 posterior segment cases) were enrolled in the DISCOVER study. IOCT demonstrated feasibility with successful image acquisition in 820 (98.0%, CI: 96.8–98.8%) eyes. In 106 (43.4%, CI: 37.1–49.9) anterior segment cases, the surgeons indicated that the iOCT information impacted their surgical decision-making and altered the procedure. In posterior segment procedures, surgeons reported iOCT provided altered surgical decision-making during the procedure in 173 (29.2%, CI: 25.5–33.0%) cases.
Conclusions
The DISCOVER iOCT study demonstrates both generalized feasibility and utility based on the surgeon-reported impact on surgical decision-making. This large-scale study confirms similar findings of other studies on the potential value and impact of iOCT on ophthalmic surgery.
Introduction
Optical coherence tomography (OCT) is a vital diagnostic tool in the medical management of numerous ophthalmic diseases. OCT facilitates diagnosis, provides therapeutic surveillance, and enhances clinical decision-making in numerous ophthalmic conditions, including corneal, optic nerve and retinal conditions. Given the critical role that it plays in day-to-day clinical management of ophthalmic patients, it seems intuitive to integrate this technology into the operating room to provide real-time feedback through image-assisted or image-guided surgery. Though still an emerging technology, numerous studies and reports have described the utility and value of this technology as it applies to ophthalmic surgery.1–19
The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) study two-year results published in 2014 demonstrated the potential of this technology.3 The PIONEER study utilized an external portable OCT system mounted on a microscope. Although PIONEER demonstrated tremendous potential of iOCT technology in the ophthalmic operating room, the external system utilized during that study required the surgeon to pause the surgical procedure to perform imaging and did not allow for visualization of tissue-instrument interaction on OCT.
Since the PIONEER study, significant advances in integrative technology have been realized. Both research systems and commercial systems have been described that provide microscope-integrated technology. A number of smaller studies of microscope-integrated iOCT systems have provided early data suggesting the feasibility and potentially significant utility of this technology; however, results of many these studies were limited by small sample sizes.12, 14–16 The impact of microscope-integration and the overall influence of iOCT on surgical outcomes is still debated in the ophthalmic community. More information is needed on the potential role for OCT in the operating room and to help facilitate surgeon’s use of the technology in areas that maximize utility and outcomes.
The Determination of Feasibility of Intraoperative Spectral Domain Microscope Combined/Integrated OCT Visualization During En Face Retinal and Ophthalmic Surgery (DISCOVER) study was initiated in 2014 to assess the feasibility and utility of microscope-integrated iOCT during ophthalmic surgery. In this report, we present the three-year results of the DISCOVER study.
Methods
The DISCOVER study is a prospective, single-center, multi-surgeon consecutive case series evaluating the potential role of microscope-integrated iOCT during ophthalmic surgery. The DISCOVER study was an IRB-approved investigational device study. This study adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained for each patient.
The study design and methods for DISCOVER have been previously described.4 Inclusion criteria included age 18 or older, ability to provide informed consent, and planned incisional ophthalmic surgery. Consecutive surgical cases were included for all surgeon investigators when the intraoperative OCT platforms and research coordinator were available. All posterior cases procedure types were included. Anterior case enrollment particularly focused on lamellar keratoplasty procedures and lamellar dissection procedures. Briefly, the study involved a prespecified intraoperative protocol for imaging patients during and/or after surgical milestones as determined by the operating surgeon. Data collected included indication for surgery, type of procedure, ocular comorbidities, specific surgical maneuvers performed and instrumentation used, type of OCT images obtained, and adverse events (AEs).
The DISCOVER study included three microscope-integrated OCT prototypes: the RESCAN 700 prototype (Carl Zeiss Meditec, Inc., Oberkochen, Germany), the EnFocus prototype (Bioptigen/Leica Microsystems, Wetzlar, Germany), and an integrated prototype internally developed at the Cleveland Clinic Cole Eye Institute (Figure 1). As previously described, all systems included a microscope-integrated OCT system thus allowing for real-time OCT imaging during surgery and providing immediate feedback.10,11,12 Imaging data was reviewed by the surgeon intraoperatively and also reviewed independently postoperatively in select cases. Display options for imaging data included heads-up display incorporated in the microscope oculars and/or external screen-based display. System ergonomics feedback was collected following each case, including display preference and any difficulties with system operation. Real-time acquisition of images was defined as continuous iOCT image acquisition during the surgical maneuver. Static iOCT image acquisition was defined as image acquisition before and/or after the maneuver. A trained research coordinator assisted intraoperatively with OCT image acquisition and collecting surgeon feedback and data.
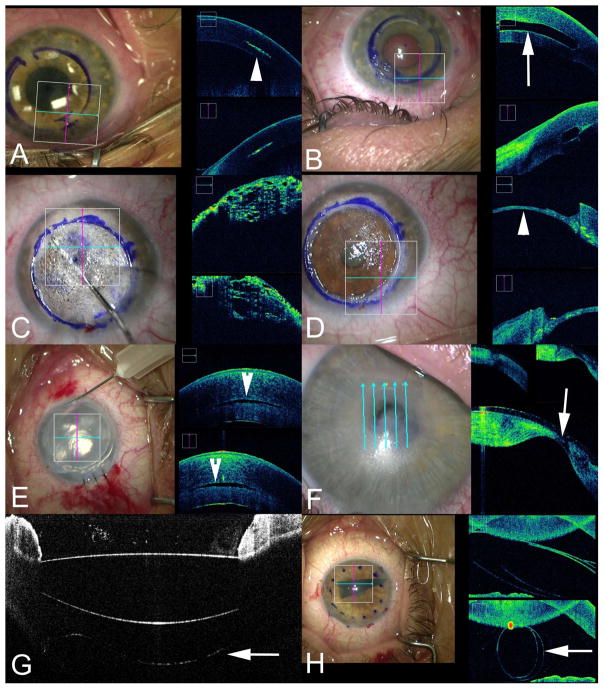
Figure 1.
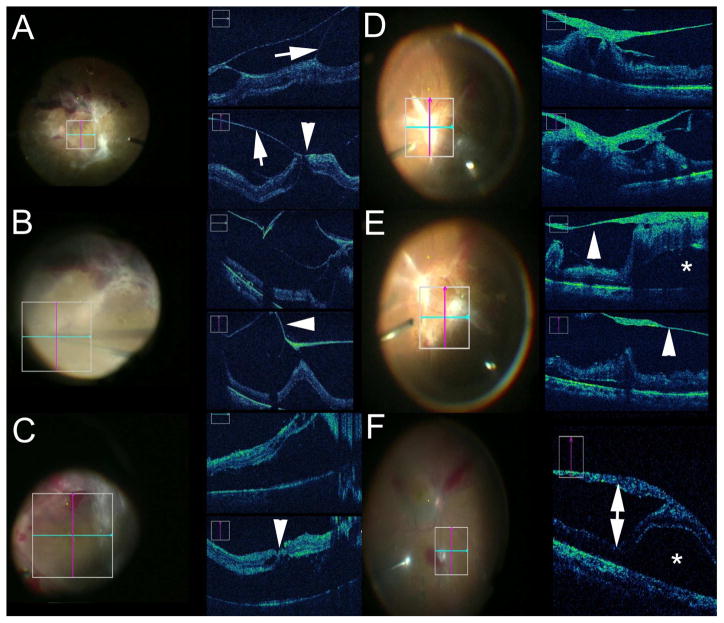
Intraoperative Optical Coherence Tomography (iOCT) in Anterior Segment Surgery. (A) iOCT during corneal inlay procedure evaluating channel dissection depth (arrowhead). (B) iOCT following corneal inlay placement (arrow) within the cornea. (C) iOCT during deep anterior lamellar keratoplasty (DALK) following injection of air bubble for big bubble creation. (D) iOCT during DALK following anterior stromal removal revealing minimal stromal bed and Descemet membrane (arrowhead). (E) iOCT during Descemet stripping automated endothelial keratoplasty (DSAEK) following graft placement an dair infusion demonstrating small persistent fluid interface visible on iOCT (arrowhead) but subclinical on microscope view. (F) Intraoperative evaluation of corneal thickness prior to initating the surgical procedure demonstrating severe central corneal thinning on iOCT (arrowhead). (G) iOCT confirmation of intraocular lens placement with visualization of the posterior capsule (arrow). (H) iOCT during Descemet membrane endothelial keratoplasty (DMEK) procedure providing visualization of the graft within the anterior chamber (arrow).
Standardized surgeon feedback questionnaires were completed immediately following surgery for all cases, focusing on several specific areas of interest related to the microscope-integrated system and surgical procedure. This included value of iOCT to the procedure and specific impact on surgical decision-making based on surgeon perspective. Additionally, procedure-specific (e.g. membrane peeling, retinal detachment repair, deep anterior lamellar keratoplasty [DALK]) questionnaires were completed to assess the value of iOCT as it pertained to that specific procedure. As an example, surgeons reported “residual membranes” if a membrane was identified with iOCT following initial membrane peeling that was thought to be anatomically/visually significant and required additional membrane removal that was not apparent on conventional microscope view. In addition, given potential changes in data feedback between early technology adoption and more experience utilization of the technology, a comparison of year 1 results to years 2–3 was performed. Given that not all posterior segment surgeons were included as investigators for the duration of the study, posterior segment surgeon experience was also stratified based on duration of experience with iOCT, (i.e., 1-year of experience with iOCT in the DISCOVER study compared to subsequent years). The participating anterior segment surgeons did not significantly change between the various years and did not require stratification. Confidence intervals were calculated. Chi-square test was used to compare categorical data points.
Results
A total of 837 eyes were enrolled during the first 36 months of the DISCOVER study (Table 1). A total of 244 eyes were enrolled in the anterior segment arm and 593 eyes were enrolled in the posterior segment arm. Successful image acquisition was obtained in 820 (97.8%; 95%CI: 96.8–98.8%) eyes. Of the 17 cases not imaged, software or hardware malfunction accounted for 5 cases, poor view precluding image acquisition accounted for 4 cases, surgeon decision to not image accounted for 4 cases, and other factors accounted for 5 cases.
Table 1.
Baseline Demographics and Clinical Characteristics of the DISCOVER Study
| Anterior | Posterior | ||||
|---|---|---|---|---|---|
| Enrollment | 244 | 593 | |||
| Eye | Right | 122 | 316 | ||
| Left | 122 | 277 | |||
| Pseudophakic | 114 | 240 | |||
| Lens | Phakic | 125 | 342 | ||
| Status | Aphakic | 5 | 11 | ||
| Preoperative Diagnosis | Fuchs Dystrophy | 120 (49%) | Proliferative diabetic retinopathy sequelae | 132 (22%) | |
| Bullous Keratopathy | 34 (14%) | ||||
| Keratoconus | 10 (4%) | Epiretinal membrane | 121 (20%) | ||
| Failed PK | 16 (7%) | Retinal detachment | 120 (20%) | ||
| Cataract | 11 (5%) | Full-thickness macular hole | 77 (13%) | ||
| Glaucoma | 5 (2%) | Panuveitis | 39 (7%) | ||
| Failed DSAEK | 14 (6%) | Chorioretinal biopsy | 8 (1%) | ||
| Other | 30 (12%) | Argus prosthesis | 3 1%) | ||
| Other | 93 (16%) | ||||
| Procedures | DSAEK | 123 (50%) | PPV | 547 (92%) | |
| DALK | 10 (4%) | Fluocinolone implant without PPV | 9 (2%) | ||
| CE/IOL | 9 (4%) | Scleral buckle without PPV | 7 (1%) | ||
| DMEK | 59 (24%) | Other | 27 (5%) | ||
| Tube shunt | 4 (2%) | ||||
| PK | 4 (2%) | ||||
| Other | 35 (14%) | ||||
Abbreviations: CE: cataract extraction; IOL: intraocular lens; PPV: pars plana vitrectomy; DSAEK: Descemet stripping automated endothelial keratoplasty; PK: penetrating keratoplasty; DALK: deep anterior lamellar keratoplasty; DMEK: Descemet membrane endothelial keratoplasty.
Anterior Segment IOCT
Of the 244 eyes in the anterior segment arm, frequent indications for surgery included Fuchs endothelial dystrophy (120 eyes, 49%), bullous keratopathy (34 eyes, 14%), failed penetrating keratoplasty (16 eyes, 7%), and keratoconus (10 eyes, 4%). Lamellar keratoplasty accounted for 193 (79%) of the cases. Descemet stripping automated endothelial keratoplasty (DSAEK) accounted for 123 (50%) cases, followed by Descemet membrane endothelial keratoplasty (DMEK) for 60 (25%) cases, and DALK in 10 (4%) cases (Figures 1,2). Other less common surgical procedures included tube shunt placement for glaucoma procedures in 4 (2%) cases, corneal inlay placement in 2 (1%) cases, lamellar keratectomy in 2 (1%) cases, and removal/aspiration of conjunctival cyst in 2 (1%) cases (Figure 2).
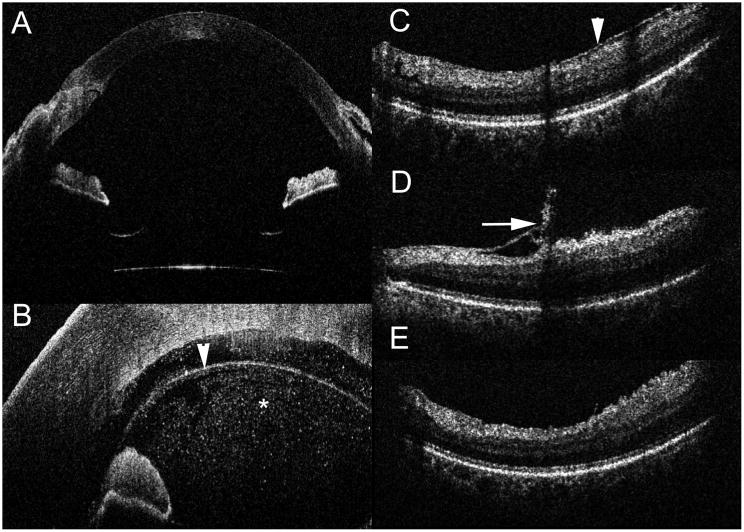
Figure 2.
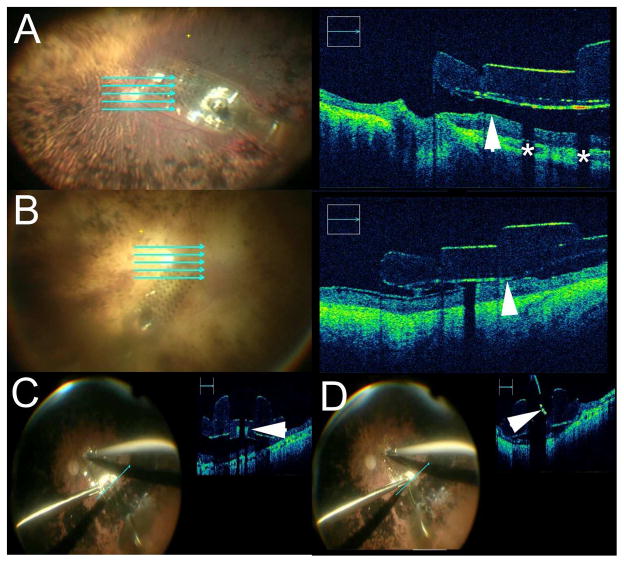
Intraoperative Optical Coherence Tomography (iOCT) with Deep Range Systems and Real-time Feedback. (A) Deep range system provides excellent visualization of the entire anterior chamber from anterior cornea to the anterior surface of the intraocular lens. (B) iOCT during a Descemet membrane endothelial keratoplasty (DMEK) procedure demonstrating excellent visualization of the graft (arrowhead) as well as extensive debris (asterisk) in the anterior chamber. (C–E) iOCT during membrane peeling procedure that demonstrates the pre-peel OCT with epiretinal membrane (arrowhead, C), post-peel with residual membrane (arrow, D) and post-second peel iOCT (E) demonstrating complete membrane removal.
Anterior Segment IOCT Value and Impact on Surgical Procedure
In 216 (88.5%; 95%CI: 83.8–92.2%) cases, microscope-integrated iOCT was reported to provide valuable feedback. The most frequently cited area of value of iOCT feedback was to evaluate graft/host apposition and assess the extent of interface fluid in DSAEK and DMEK (Figures 1,2). Verification of graft orientation during DMEK procedures utilizing iOCT was also commonly reported (Figures 1,2). The value added from iOCT in the identification of graft orientation in DMEK allowed surgeons to abandon the use of the S-stamp on corneal tissue. In DALK, the most frequently cited area of value was to evaluate the needle depth within the corneal stroma, the big bubble dissection plane, and residual stromal depth in the cornea bed (Figure 1).
In 106 (43.4%; 95%CI: 37.1–49.9%) cases, iOCT information resulted in changes to surgical decision-making or surgical maneuvers. The primary reason for surgical modification based on iOCT information in DSAEK and DMEK was graft malposition and persistent interface fluid. For example, in DSAEK, the surgeon noted that the graft appeared to be clinically apposed in 84 (68.3% of DSAEK cases; 95%CI: 59.3–76.4%) cases. However, persistent interface fluid was visualized on iOCT in 46 (54.8%; 95%CI: 43.5–65.7%) of these cases (FIGURE 1). Based on the iOCT information, additional sweeping/corneal massage, graft reposition, peeling of residual host Descemet membrane or re-bubbling was performed. In DMEK, the surgeons reported iOCT-enabled graft orientation feedback in 38 (63%; 95%CI: 49.9–75.4%) cases based on the scrolling configuration of the tissue (FIGURE 1,2). Orientation was frequently confirmed with iOCT prior to visualization of the orienting “S” stamp when the “S” stamp was utilized. Additionally, the surgeons reported clinical graft apposition in 54 (90.0%; 95%CI: 79.5–96.2) cases after initial placement. Intraoperative imaging identified persistent interface fluid in four (7%; 95%CI: 2.1–17.9%) cases allowing for additional corneal sweeping prior to completing the procedure.
Intacs corneal inserts were placed in 2 patients with keratoconus. In both cases, iOCT was utilized to judge depth of the corneal incision and corresponding channel (Figure 1). In one case, iOCT identified suboptimal incision depth and was subsequently employed to verify appropriate incision/channel depth. In addition, the iOCT information was able to utilized to verify IOL position (Figures 1,2). Utilizing the extended range systems, the entire anterior segment was able to be imaged during surgery (Figure 2). In 2 cases, image-guided lamellar keratectomy was performed for the surgical treatment of Salzmann nodular degeneration. IOCT was noted to be particularly helpful for visualizing the cleavage plane between the nodule and underlying corneal tissue. The iOCT was also utilized in cases of severe corneal thinning to provide immediate feedback to surgeons regarding the corneal anatomy (Figure 1).
Anterior Segment System Ergonomics Feedback
Real-time iOCT was the preferred mode of image acquisition in 191(78.3%; 95%CI: 72.6–83.3%) cases during anterior segment surgery. In 42 (17%; 95%CI: 12.7–22.5%) cases, static iOCT images were preferred. In 11 (4.5%; 95%CI: 2.3–7.9%) cases, the surgeons did not report an imaging acquisition preference. When viewing the images, the surgeons preferred looking through the oculars in 38 (15.6%; 95%CI: 11.3–20.7%) cases, preferred the display screen in 154 (63.1%; 95%CI: 56.7–69.2%) cases, or preferred a combination of both in 50 (20.5%; 95%CI: 15.6–26.1%) cases. In 10 (4.1%; 95%CI: 2.0–7.4%) cases, the surgeon did not have a preference for visualization. Of the 192 cases with real-time acquisition of OCT images, the surgeon preferred utilizing the display screen in 114 (59%; 95%CI: 52.1–66.4%) cases rather than utilizing the oculars in the microscope. No surgeon reported interference of the iOCT with the surgical procedure. No iOCT-related AEs occurred.
Dynamic Trends in Anterior Segment IOCT Use with Experience
A comparison of year 1 data to subsequent years was assessed for variations in surgical procedures and surgeon trends. While DSAEK remained the most commonly performed procedure, the percentage of DMEK cases increased from 8% after year 1 to 24% after years 2 and 3. The percentage of surgical cases modified based on iOCT findings was 44% for year 1 and 42% for subsequent years. Real-time OCT image feedback increased slightly from 69% in year 1 to 84% in subsequent years (p < 0.01). The preferred review strategy also shifted over time. In the first year, surgeons utilized the screen in 30% of cases, the heads-up display in the oculars in 25%, and both forms of visualization in 42%. In years 2–3, there was a significant shift towards utilizing the screen in 82% of cases compared to oculars in 10% and both systems in 8% (p < 0.001) (Table 2).
Table 2.
Surgeon Feedback For Year 1 and Year 2–3 Results
| First Year Enrollment | Second and Third Year Enrollment | P-value | |
|---|---|---|---|
| Anterior Segment | n = 91 | n = 153 | |
| iOCT provided valuable feedback | 82 (90%) | 135 (88%) | 0.20 |
| Procedure modified based on iOCT | 40 (44 %) | 66 (42 %) | 0.90 |
| Visualization Preference | |||
| Real-time iOCT | 62 (69%) | 129 (84%) | 0.03 |
| Static iOCT | 21 (23%) | 21 (14%) | 0.03 |
| Posterior Segment | n = 136 | n = 457 | |
| iOCT provided valuable feedback | 97 (71%) | 257 (56%) | 0.002 |
| Procedure modified based on iOCT | 49 (36%) | 123 (27%) | 0.04 |
| Visualization Preference | |||
| Real-time iOCT | 93 (68%) | 170 (37%) | <0.0001 |
| Static iOCT | 39 (29%) | 236 (58%) | <0.0001 |
| First Year Enrollment by Specific Surgeon* | Second and Third Year Enrollment by Specific Surgeon* | ||
| Posterior Segment | n = 203 | n = 322 | |
| iOCT provided valuable feedback | 137 (68%) | 176 (55%) | 0.004 |
| Procedure modified based on iOCT | 63 (31%) | 88 (27%) | 0.33 |
Stratifies patient enrollment based on surgeon experience with iOCT (i.e., first year of surgeon enrolling in the study compared to subsequent years when surgeon enrolled subjects).
Posterior segment iOCT
Of the 593 eyes in the posterior segment arm, the most common indications for posterior segment surgery included epiretinal membrane (ERM) (121 eyes, 20%), proliferative diabetic retinopathy (PDR) sequelae (132 eyes, 22%), rhegmatogenous retinal detachment (RD) (120 eyes, 20%), and macular hole (MH) (77 eyes, 13%). Other less common indications included panuveitis (39 eyes, 7%), chorioretinal biopsy (8, 1%), and Argus prosthesis implant (3, 1%), Table 1.
Posterior Segment IOCT Value and Impact on Surgical Procedure
In 352 (59.4%; 95%CI: 55.2–63.3%) posterior segment cases, iOCT was identified to add valuable information related to the surgical anatomy and/or surgical procedure. In 173 (29.2%; 95%CI: 25.5–33.0) cases, this information directly impacted the surgical procedure.
Following posterior hyaloid elevation, iOCT feedback was performed in 95 cases. In 15 (15.8%; 95%CI: 9.1–24.7%) cases, iOCT identified a possible full-thickness macular hole or outer retinal hole after hyaloid elevation that altered subsequent surgical management, such as additional inner limiting membrane (ILM) peeling or gas tamponade choice (Figure 3).
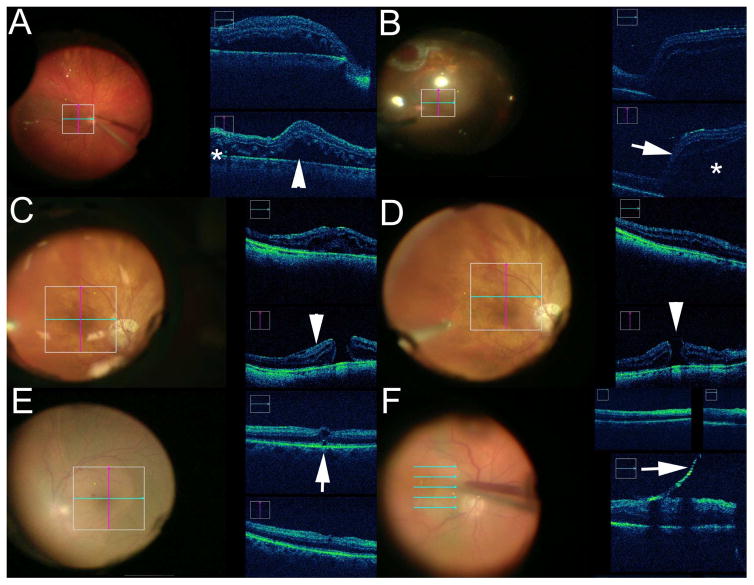
Figure 3.
Intraoperative Optical Coherence Tomography (iOCT) in Posterior Segment Surgery. (A) Real-time iOCT during retinal detachment repair visualizing perfluorocarbon liquid placement with progressive displacement of subretinal fluid (arrowhead). Small amounts of residual fluid (asterisk)remain under perfluorocarbon liquid. (B) Following air-fluid exchange, significant reaccumulation of subretinal fluid (asterisk) with associated retinal elevation (arrow) is noted on iOCT that was not clinically apparent, resulting in additional surgical maneuvers to address the subretinal fluid. (C) During macular hole surgery, iOCT is able to identify epiretinal membrane (arrowhead) at hole edge prior to peeling. (D) Following membrane peeling, iOCT confirms complete peel and also demonstrates changes in hole contour and size (arrowhead). (E) After elevating the hyaloid a small subclinical potential full-thickness macular hole was noted on iOCT (arrow). This resulted in additional internal limiting membrane peeling and gas tamponade placement. (F) Real-time iOCT-guided epiretinal membrane removal (arrow).
Membrane peeling was performed in 272 (45%) for a variety of indications (e.g., ERM, MH, proliferative vitreoretinopathy). Prior to iOCT utilization, the surgeon thought the membranes were entirely peeled in 177 (65.1%; 95%CI: 59.1–70.7%) cases. In 35 (19.8%; 95%CI: 14.2–26.4%) of these cases, the iOCT revealed residual membranes, discordant with surgeon impression from the surgical microscope view (Figure 2). In the 95 cases that the surgeons believed that residual membranes were present following maximal peeling, complete membrane removal was identified with iOCT in 38 cases (40.0%; 95%CI: 30.1–50.6%) eliminating unnecessary surgical maneuvers.
There were 120 RDs included in this series. Utilizing iOCT feedback, significant persistent subretinal fluid was identified in 65 eyes (54.2%; 95%CI: 44.8–63.3%). Subclinical macular holes were identified in 6 eyes (5.0%; 95%CI: 1.9–10.6%). In 21 eyes (17.5%; 95%CI: 11.2–25.5%) the iOCT resulted in alterations to the surgical procedure. Reported iOCT findings that enabled surgical decision-making included localization of occult membranes (e.g., subretinal, preretinal), retinal breaks, residual preretinal/subretinal perfluorocarbon liquid, significant reaccumulation of posterior fluid as well as differentiation between schisis and detachment (Figures 3,4).
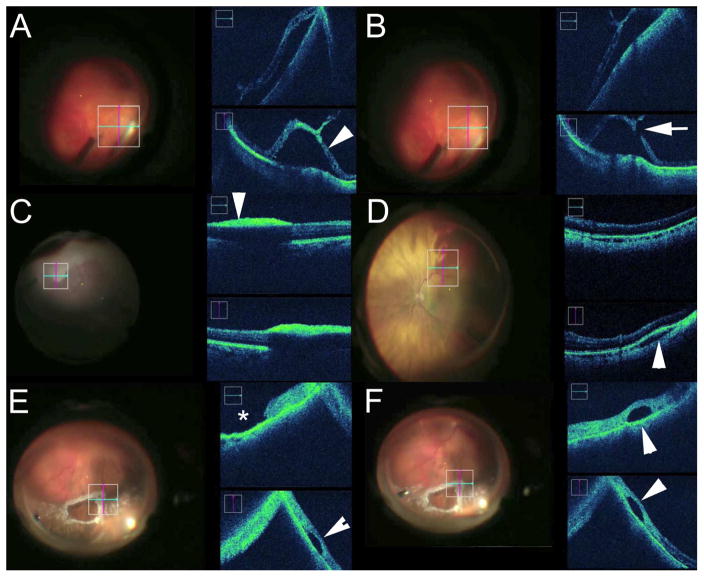
Figure 4.
Intraoperative Optical Coherence Tomography (iOCT) in the Retinal Periphery. (A–B) During scleral depression an area of lattice with mild retinal traction was noted on surgical view. However, iOCT demonstrates what was appeared to be a traction retinal detachment (arrowhead, A), but with additional iOCT inspection a definitive retinal break is noted (arrow, B) which resulted in additional laser photocoagulation and tamponade placement. (C) During vitrectomy for blunt trauma, surgical view demonstrated whitening at the retinal surface that was initially thought to be severe commotion. However, iOCT confirmed that this area of whitening was dehemoglobinized preretinal hemorrhage with underlying shadowing (arrowhead). (D) Intraoperatively, an area of hemorrhage was noted in the inferotemporal quadrant. Initially this was thought to be subretinal based on clinical appearance. iOCT demonstrated that this was actually a small suprachoroidal hemorrhage with expansion of the choroidal space (arrowhead). (E–F) Following air-fluid exchange during retinal detachment repair, a focal fluid pocket (arrowhead) is noted adjacent to the retinal tear (asterisk). iOCT confirms that the pocket is subretinal perfluorocarbon liquid (arrowheads) which was subsequently addressed during the surgery.
Microscope-integrated iOCT enabled visualization of peripheral retinal lesions during vitrectomy. Specific case examples where iOCT information altered the surgical procedure, included visualization of undetected retinal breaks, subretinal perfluorocarbon liquid, differentiation of choroidal/subretinal hemorrhage, vitreoretinal traction, and differentiation of commotion retinae from dehemoglobinized preretinal blood (Figure 4, Supplemental video 1). In chorioretinal biopsy cases, iOCT feedback impacted the surgical procedure in 6 of 8 eyes. Specifically, iOCT was utilized for targeting the location of biopsy and evaluating the tissue following biopsy.
During PDR cases, iOCT enabled identification of surgical planes and differentiated fibrovascular proliferations from underlying retinal detachments. The iOCT system provided both real-time and static feedback to the surgeon during and following membrane dissection. Examples of iOCT feedback that altered surgical decision-making during PDR surgery, included the identification of occult retinal breaks, confirmation of the absence of a retinal break, visualization of optimal dissection planes, and differentiation between schisis/traction and retinal detachment (Figure 5).
Figure 5.
Intraoperative Optical Coherence Tomography (iOCT) in Proliferative Diabetic Retinopathy. (A–C) Complex tractional retinal detachment with suspected full-thickness macular hole on iOCT with associated significant vitreomacular traction (A). Real-time iOCT was utilized during membrane removal to visualize dissection plane (B). Following maximal membrane removal, the fovea is inspected and no full-thickness macular hole is visualized (C), which impacted positioning and tamponade choice. (D–E) Extensive tractional detachment with associated thick fibrovascular multi-layer membrane at nerve (D) and throughout the macula (E). iOCT is utilized to differentiate dissection planes where detached retina is adherent to the membrane and areas where the retina is attached and separated from the membranes (E). (F) iOCT is utilized to inspect an area of fibrovascular traction with associated schisis (double arrow) and retinal detachment (asterisk).
Three Argus II implants (Second Sight Medical Products, Inc, Sylmar, California), for retinitis pigmentosa were performed utilizing iOCT feedback in the DISCOVER study. In all 3 cases, intraoperative imaging provided information regarding array positioning and provided feedback to the surgeons regarding array-tissue apposition (Figure 6). Positioning of the implant was confirmed by iOCT before and after retinal tack placement (Figure 6, Supplemental Video 2).
Figure 6.
Intraoperative Optical Coherence Tomography (iOCT) During Argus Placement. (A) iOCT visualization of Argus array following placement in the eye. Cross-sectional imaging demonstrates significant distance between inner retina and array (arrowhead). Shadowing is noted from the electrodes (asterisk). (B) Second case of iOCT visualization of Argus array following placement. Cross-sectional imaging demonstrates minimal distance between inner retina and array with good apposition to the retinal surface (arrowhead). (C) Immediately prior to tacking, iOCT demonstrates array with tack hole visible (arrowhead). (D) iOCT during tacking process demonstrates shadowing from tack. The compressed integrated springs are visible on OCT during placement (arrowhead).
Posterior Segment IOCT Ergonomics Feedback
Static iOCT capture acquisition was preferred over real-time acquisition in 234 cases (86.0%; 95%CI: 81.3–89.9%) of membrane peeling cases. In 407 (68.6%; 95%: 64.7–72.4%) cases, the surgeons preferred viewing OCT images on the external display screen rather than using the heads-up display within the oculars. In 120 (20.2%; 95%CI: 17.1–23.7) cases, the surgeons preferred viewing through the oculars. In 56 (9.4%; 95%CI: 7.2–12.1%) cases, the surgeon did not have a preference for visualizing images.
In 37 (6%) cases, the iOCT system interfered with the surgical procedure. The most frequent cited reasons for interference included microscope software failure requiring system reboot (n = 27, 5%) and delay in surgical procedure due to difficulty in image acquisition (n = 7, 1%). Beyond extending the time of the surgical procedure, no intraoperative AEs were associated with these episodes of iOCT interference.
Dynamic Trends in Posterior Segment iOCT Use with Experience
A comparison of year 1 data to subsequent years was evaluated for variations in surgeon utilization and approach (Table 2). In year 1, the iOCT modified the surgical procedure 36% of the time. In years 2 and 3, iOCT resulted in a change in surgical procedure in 27% of cases. However, when this data was stratified to surgeon experience with intraoperative OCT, there was no difference between year 1 (31%) and subsequent years (27%). Younger surgeons may have a greater change in the perceived value of OCT and perceived changes in decision-making between year 1 and year 2 (p < 0.01), but there does not seem to be an association between OCT utilization overall and surgeon experience. This may suggest that more novice surgeons may rely on OCT more initially and have a larger decrease in utilization as they gain more experience relative to more experienced surgeons. However, younger surgeons did not necessarily indicate OCT was more valuable or that it impacted outcomes more than senior surgeons.
A striking change related to a switch towards static iOCT visualization in subsequent years. In year 1, 68% of procedures were performed with real-time iOCT. This subsequently declined to 37% in years 2 and 3, indicating a shift in surgeon preference towards a “stop-and-shoot” approach to iOCT rather than feedback during actual maneuvers (p < 0.001). The preferred visualization method of the OCT data stream also shifted following more experience with the iOCT systems. In year 1, surgeons preferred the heads-up display in the ocular in 38% of cases. In years 2–3, this decreased to 14% (p<0.0001) due to significant increase in preferred viewing with the display screen (Table 2). Feasibility for surgical visualization of iOCT data through integrating the OCT datastream and surgical landscape on a 3D immersive monitor was also demonstrated (Figure 7, Supplemental Video 3)
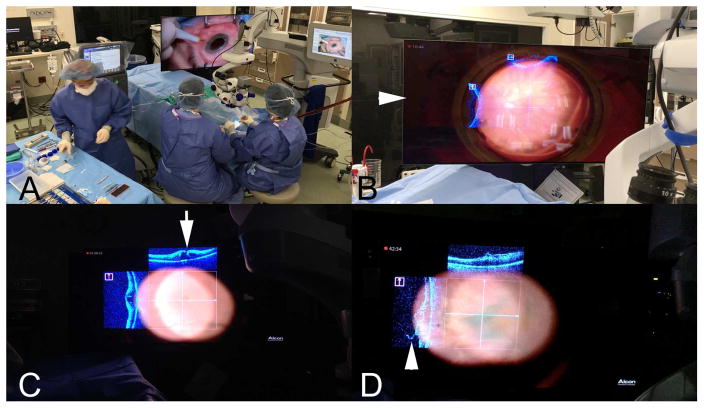
Figure 7.
Integrating Intraoperative Optical Coherence Tomography (iOCT) into a New Surgical Theater. (A) Operating room layout of 3-dimensional 4K monitor for surgical manipulation (B–D) Examples of iOCT overlay on 3-dimensional monitor (arrowhead, B) for simultaneous surgical visualization of OCT datastream and surgical field, including with wide-angle viewing lens (B) and flat high magnificent contact lens (C, D). Excellent visualization of full-thickness macular hole is noted with iOCT (arrow, C) and residual membrane (arrowhead, D).
Discussion
The three-year results of the DISCOVER study reported here is the largest prospective microscope-integrated iOCT study to date of both anterior and posterior segment surgery demonstrating both the ability to reliably acquire iOCT imaging and the utility of the imaging information through the impact on surgical decision-making. In numerous disease conditions and surgical procedures, intraoperative imaging provided valuable input on anatomic configuration. Guidance in surgical decision-making resulted in various alterations to surgical procedures.
Approximately 43% of anterior segment cases underwent additional surgical steps following intraoperative imaging, most frequently due to graft malposition and interface fluid for DSAEK and DMEK cases. iOCT during DSAEK previously demonstrated that transient residual interface fluid correlates post-operatively with textural interface opacity.20 In addition, the importance of limiting this interface fluid has been supported through the identification of higher rates of early graft nonadherence based on amount of residual interface fluid on iOCT.21, 22 The role for iOCT in DMEK procedures also appears to be clear, facilitating surgical maneuvers and decision-making.2, 23, 24 For DMEK, iOCT is helpful for graft orientation to identify the scrolling pattern, which has also been previously demonstrated to possibly enable faster graft orientation and positioning, especially in the presence of severe corneal edema.2, 23, 24 The ability to properly confirm DMEK orientation with iOCT prior to visualization of the orienting “S” stamp may lead to the ability to eliminate the “S” stamp on DMEK tissue in the future, which may lead to decreased endothelial cell loss. In addition, iOCT during DMEK may help provide novice surgeons with additional feedback to enhance comfort with the procedure.
For posterior segment surgery, iOCT appears to be particularly useful during membrane assessment, such as in PDR and ERM surgery.25, 26 A recent prospective study utilizing microscope-integrated iOCT during membrane peeling procedures confirmed the ability of the technology to enhance visualization of the underlying membranes and subtle post-peel architecture changes.15 An additional study recently reported membrane peeling with iOCT feedback was able to performed without the use of adjuvant dyes through the identification of membrane edges with the OCT system.12 In fact, iOCT-assisted membrane peeling without mandated ILM peeling has been shown to have similar recurrence rates to ILM peeling, suggesting increased membrane clearance with image-guided feedback.25
In macular hole surgery, alterations in macular hole geometry on iOCT have been visualized that result in changes of macular hole shape as well as alterations in the outer retina.3, 6, 15, 18, 27–30 These subtle subclinical alterations may have important implications for patient-centered care and image-guided postoperative positioning, as these changes have been linked to macular hole closure rate and anatomic normalization.6, 29–31
Other unique opportunities for iOCT emerged from the DISCOVER study, including image-guided subretinal biopsy and iOCT-assisted placement of the Argus II implant.32, 33 In these cases, utilizing OCT surveillance of the anatomic landscape facilitated placement of either the biopsy site or confirming the optimal location for the array. More recently, reports have described the use of iOCT to confirm subretinal placement of gene therapy and stem cells during retinal surgery.34, 35
The DISCOVER 3-year results indicate that iOCT in posterior segment surgery adds valuable information in approximately 60% of cases and may alter surgical decision-making in approximately 30% of cases. This is consistent with other studies.3, 16 One recent study of 40 consecutive cases utilizing the RESCAN 700 reported that iOCT added additional information in 74% of cases and altered surgical decision-making in approximately 40% of cases.16 Interestingly, when the DISCOVER study results are stratified based on surgeon experience with iOCT, there were no significant changes from year 1 to subsequent years in the proportion of cases that were modified based on iOCT information (31% vs 27%, p = 0.33)
The OCT acquisition approach and visualization strategies employed during the study appeared to be different between the anterior and posterior segment surgeons and also appeared to change over the course of the study. Anterior segment surgeons preferred real-time iOCT that allowed for true OCT-guided tissue manipulation. There are a few major differences between anterior and posterior procedures that may result in this difference in preference for real-time approach with anterior segment surgery. A large proportion of the anterior segment procedures performed included endothelial lamellar keratoplasty procedures. In these cases, the majority of the surgical manipulations are being performed “under” the tissues of interest. This results in minimal shadowing from surgical instrumentation. This would enable continuous imaging in anterior surgery compared to posterior surgery, where metallic instruments overlying the retinal surface would result in significant shadowing. Interestingly, surgeons also preferred visualization on the display screen compared to the heads-up display in the oculars. This is of particular interest because this suggests that anterior surgeons were utilizing a 2-D display screen to perform surgical manipulations rather than the microscope view because of the enhanced visualization with OCT on the screen. This suggests that perhaps the larger tolerated movements within the anterior segment may allow for surgical manipulation with 2-dimensional feedback from a screen for corneal procedures when combined with a 3-D information of the OCT.
In comparison, posterior segment surgeons seemed to prefer static imaging over time in the subsequent years of the study. In addition, in 69% of procedures, posterior segment surgeons preferred viewing of images on the display screen as well, which increased from year 1 to the subsequent years. Multiple factors may play a role in these findings. There is a significant “wow” factor for the heads-up display in the ocular that is quite impressive initially. As the system is utilized more, the loss of OCT detail becomes apparent when using the data injection compared to the screen. It is possible that as surgeons used the technology, they began to look for more subtle changes on the OCT which required on-screen review. The lack of OCT-compatible instrumentation may have a more significant impact in vitreoretinal maneuvers that may push surgeons more towards static use of the technology rather than real-time use. The advent of new instrumentation with OCT-friendly features may facilitate transition to real-time maneuvers in posterior segment surgery.10, 36 In addition, the lack of instrument tracking may also play a role in the limited use of real-time OCT in the posterior segment. Improvements to this technology may also enable true OCT-guided maneuvers through decoupling the aiming process for the OCT from the surgeon.37 In addition, the software platforms utilized in the DISCOVER study did not allow simultaneous control of the microscope with the iOCT enabled with heads-up display. Limiting microscope control limits subtle surgeon manipulations of the microscope while performing precise maneuvers. Finally, OCT for real-time maneuvers may also be enabled with smart vibration/tremor-dampening instruments to enable more precise surgeon-reaction to image-guided surgery.
A major finding of this study is the strong preference of both anterior and posterior segment surgeons to prefer visualizing the OCT information on the screen rather than through the microscope oculars and heads-up display (e.g., image injection system). One of the current limitations of the ocular heads-up display system is the limited visualization of subtle details on the small transparent scans. This highlights the critical need to maximize the surgical theater and feedback system for ophthalmic surgeons. Potential opportunities in the future for greater integrative flexible visualization may include digital viewing technology such as the 3D screen-based visualization systems [e.g., Ngenuity (Alcon, Ft Worth, TX)], digital oculars, or even immersive 3D visors or goggles.38, 39 Utilizing a more optimal combined visualization system may also enable real-time surgical manipulations for posterior and anterior segment surgery. The DISCOVER study has already established the feasibility of the translating the OCT datastream to a 3-D digital surgical visualization system.40
There are some important strengths and limitations that should be acknowledged. The multi-surgeon, multi-platform approach to the DISCOVER study provides enhanced generalizability of the results. In addition, the large sample size and dynamic assessment of surgeon impression over time is unique. The wide-range of pathologies included and variety of surgical procedures also add strength to the report. Important limitations of this study include the inability to mask the surgeons using the iOCT during their surgical cases, which may introduce bias in terms of trends toward certain surgical maneuvers given the fact that iOCT would be available during the case. Surgical cases were also not randomized to iOCT or compared to a control group without the use of iOCT. Additionally, the impact of iOCT on outcomes was not analyzed in this study. One important feature of the DISCOVER study is the utilization of a research coordinator to facilitate imaging and operate the intraoperative OCT system. This may have specific impact on overall utility and feasibility compared to a surgeon using the system alone. For example, it is not uncommon that input at the system is needed to focus or locate the Z-location of the tissue of interest. This may be particularly important for posterior procedures.
The DISCOVER study has demonstrated both the feasibility and utility of iOCT as it relates to surgical decision-making in both anterior and posterior segment surgery. This large prospective study supports the growing body of evidence that OCT in the operating room is able to detect subtle anatomic features (e.g., residual membranes) more accurately than surgeon impression. This parallels the experience of OCT in clinical use where it has been demonstrated to be superior to clinical exam in multiple situations. Additional research is needed to better understand the implications of this information for outcomes, including surgical safety (e.g., reoperation rates), anatomic results (e.g., rate of residual membranes), and the role in personalized medicine (e.g., image-guided individualized, postoperative positioning). Although there are several indicators that suggest that the technology may improve patient care (e.g., reducing unnecessary surgical maneuvers, enhancing surgical safety, improving surgical efficiency), this study was not designed to prove changes in patient outcomes. Multiple prospective randomized studies are now being planned to better answer this question. Advances in technology, including OCT-compatible instrumentation, software platforms, integrative visualization systems, and OCT technology will likely continue to expand the horizon of image-guided surgery with iOCT in ophthalmology.
Supplementary Material
Supplemental Video 1: Intraoperative OCT and the Retinal Periphery. Intraoperative OCT samples during multiple cases demonstrating various peripheral pathologies and abnormalities, including subclinical combined traction/rhegmatogenous retinal detachment, subretinal perfluorocarbon liquid, dehemoglobinized preretinal blood, choroidal hemorrhage.
Supplemental Video 2: Intraoperative OCT During Argus Placement. Integrated spring compression is visible in first subject during tacking.
Supplemental Video 3: Intraoperative OCT Integration with a Three-Dimensional Digital Display System. Video sample exhibiting combined display of OCT and surgical field, including epiretinal membrane.
Acknowledgments
Grant Support: NIH/NEI K23-EY022947-01A1 (JPE); Ohio Department of Development TECH-13-059 (JPE, WJD, SKS); Research to Prevent Blindness (Cole Eye Institutional)
Footnotes
Conflict of Interest Statement: JPE and SKS have a license agreement with Bioptigen for an external mount system related to intraoperative OCT and a license option agreement with Synergetics related to the development OCT compatible surgical instruments. WJD performs sponsored research with Zeiss related to computational modeling of laser refractive surgery that is unrelated to the current study. None of these relationships are specifically related to the contents of this report. This relationship is not specifically relevant to the findings of the study. No other specific conflicts of interest exist related to this study for any of the other authors.
Disclosures: JPE: Bioptigen (C, P), Thrombogenics (C, R), Synergetics (P), Genentech (C, R), Leica (C, P), Zeiss (C), Alcon (C, R); YM: None; PP: None; JG: None; WJD: Zeiss (R); AR: None; SS: None; AY: None; RPS: Zeiss (C), Alcon (C), Genentech (C, R), Regeneron (R)f; PKK: Zeiss (C), Topcon (C), Alcon (C), Novartis (C), Bausch and Lomb (C); JLR: None; CC: None: AW: None; SKS: Bausch and Lomb (C, R); Bioptigen (P); Allergan (R); Synergetics (P); Leica (C), Zeiss (C)
Statement Related to Financial Support: The authors had full control of study design, all data, and manuscript drafting.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ehlers JP, Ohr MP, Kaiser PK, Srivastava SK. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina. 2013;33(7):1428–34. doi: 10.1097/IAE.0b013e31828396b7. [DOI] [PubMed] [Google Scholar]
- 2.Cost B, Goshe JM, Srivastava S, Ehlers JP. Intraoperative Optical Coherence Tomography-Assisted Descemet Membrane Endothelial Keratoplasty in the DISCOVER Study. Am J Ophthalmol. 2015;160(3):430–7. doi: 10.1016/j.ajo.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehlers JP, Dupps WJ, Kaiser PK, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg With Optical CoherEncE TomogRaphy (PIONEER) Study: 2-Year Results. Am J Ophthalmol. 2014;158(5):999–1007e1. doi: 10.1016/j.ajo.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith AG, Cost BM, Ehlers JP. Intraoperative OCT-Assisted Subretinal Perfluorocarbon Liquid Removal in the DISCOVER Study. Ophthalmic Surg Lasers Imaging Retina. 2015;46(9):964–6. doi: 10.3928/23258160-20151008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers JP, Griffith JF, Srivastava SK. Intraoperative Optical Coherence Tomography during Vitreoretinal Surgery for Dense Vitreous Hemorrhage in the Pioneer Study. Retina. 2015;35(12):2537–42. doi: 10.1097/IAE.0000000000000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehlers JP, Itoh Y, Xu LT, et al. Factors associated with persistent subfoveal fluid and complete macular hole closure in the PIONEER study. Invest Ophthalmol Vis Sci. 2015;56(2):1141–6. doi: 10.1167/iovs.14-15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehlers JP, Kaiser PK, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol. 2014;98(10):1329–32. doi: 10.1136/bjophthalmol-2014-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehlers JP, McNutt SA, Kaiser PK, Srivastava SK. Contrast-enhanced intraoperative optical coherence tomography. Br J Ophthalmol. 2013;97(11):1384–6. doi: 10.1136/bjophthalmol-2012-303048. [DOI] [PubMed] [Google Scholar]
- 9.Ehlers JP, Petkovsek DS, Yuan A, et al. Intrasurgical assessment of subretinal tPA injection for submacular hemorrhage in the PIONEER study utilizing intraoperative OCT. Ophthalmic Surg Lasers Imaging Retina. 2015;46(3):327–32. doi: 10.3928/23258160-20150323-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlers JP, Srivastava SK, Feiler D, et al. Integrative advances for OCT-guided ophthalmic surgery and intraoperative OCT: microscope integration, surgical instrumentation, and heads-up display surgeon feedback. PLoS One. 2014;9(8):e105224. doi: 10.1371/journal.pone.0105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlers JP, Tao YK, Farsiu S, et al. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci. 2011;52(6):3153–9. doi: 10.1167/iovs.10-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falkner-Radler CI, Glittenberg C, Gabriel M, Binder S. Intrasurgical Microscope-Integrated Spectral Domain Optical Coherence Tomography-Assisted Membrane Peeling. Retina. 2015;35(10):2100–6. doi: 10.1097/IAE.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 13.Hahn P, Carrasco-Zevallos O, Cunefare D, et al. Intrasurgical Human Retinal Imaging With Manual Instrument Tracking Using a Microscope-Integrated Spectral-Domain Optical Coherence Tomography Device. Transl Vis Sci Technol. 2015;4(4):1. doi: 10.1167/tvst.4.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn P, Migacz J, O’Donnell R, et al. Preclinical evaluation and intraoperative human retinal imaging with a high-resolution microscope-integrated spectral domain optical coherence tomography device. Retina. 2013;33(7):1328–37. doi: 10.1097/IAE.0b013e3182831293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leisser C, Hackl C, Hirnschall N, et al. Visualizing Macular Structures During Membrane Peeling Surgery With an Intraoperative Spectral-Domain Optical Coherence Tomography Device. Ophthalmic Surg Lasers Imaging Retina. 2016;47(4):328–32. doi: 10.3928/23258160-20160324-04. [DOI] [PubMed] [Google Scholar]
- 16.Pfau M, Michels S, Binder S, Becker MD. Clinical Experience With the First Commercially Available Intraoperative Optical Coherence Tomography System. Ophthalmic Surg Lasers Imaging Retina. 2015;46(10):1001–8. doi: 10.3928/23258160-20151027-03. [DOI] [PubMed] [Google Scholar]
- 17.Pichi F, Alkabes M, Nucci P, Ciardella AP. Intraoperative SD-OCT in macular surgery. Ophthalmic Surg Lasers Imaging. 2012;43(6 Suppl):S54–60. doi: 10.3928/15428877-20121001-08. [DOI] [PubMed] [Google Scholar]
- 18.Ray R, Baranano DE, Fortun JA, et al. Intraoperative Microscope-Mounted Spectral Domain Optical Coherence Tomography for Evaluation of Retinal Anatomy during Macular Surgery. Ophthalmology. 2011;118(11):2212–7. doi: 10.1016/j.ophtha.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Runkle A, Srivastava SK, Ehlers JP. Microscope-Integrated OCT Feasibility and Utility With the EnFocus System in the DISCOVER Study. Ophthalmic Surgery, Lasers and Imaging Retina. 2017;48:216–22. doi: 10.3928/23258160-20170301-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juthani VV, Goshe JM, Srivastava SK, Ehlers JP. Association between transient interface fluid on intraoperative OCT and textural interface opacity after DSAEK surgery in the PIONEER study. Cornea. 2014;33(9):887–92. doi: 10.1097/ICO.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallahan KM, Cost B, Goshe JM, et al. Intraoperative Interface Fluid Dynamics and Clinical Outcomes for Intraoperative Optical Coherence Tomography-Assisted Descemet Stripping Automated Endothelial Keratoplasty From the PIONEER Study. Am J Ophthalmol. 2017;173:16–22. doi: 10.1016/j.ajo.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu D, Dupps WJ, Srivastava S, Ehlers JP. Automated volumetric analysis of interface fluid in descemet stripping automated endothelial keratoplasty utilizing intraoperative optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55(9):5610–5. doi: 10.1167/iovs.14-14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saad A, Guilbert E, Grise-Dulac A, et al. Intraoperative OCT-Assisted DMEK: 14 Consecutive Cases. Cornea. 2015;34(7):802–7. doi: 10.1097/ICO.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 24.Steven P, Le Blanc C, Velten K, et al. Optimizing descemet membrane endothelial keratoplasty using intraoperative optical coherence tomography. JAMA Ophthalmol. 2013;131(9):1135–42. doi: 10.1001/jamaophthalmol.2013.4672. [DOI] [PubMed] [Google Scholar]
- 25.Ehlers JP, Khan M, Petkovsek D, et al. Outcomes of Intraoperative OCT–Assisted Epiretinal Membrane Surgery from the PIONEER Study. Ophthalmology Retina. 2017 doi: 10.1016/j.oret.2017.05.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan M, Srivastava SK, Reese J, et al. Intraoperative OCT-assisted Surgery for Proliferative Diabetic Retinopathy in the DISCOVER Study. Ophthalmology Retina. 2017 doi: 10.1016/j.oret.2017.08.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehlers JP, Han J, Petkovsek D, et al. Membrane Peeling-Induced Retinal Alterations on Intraoperative OCT in Vitreomacular Interface Disorders from the PIONEER Study. Invest Ophthalmol Vis Sci. 2015;56(12):7324–30. doi: 10.1167/iovs.15-17526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehlers JP, Xu D, Smith GM, Srivastava SK. Assessment of macular hole dynamics utilizing intraoperative optical coherence tomography. Association for Research in Vision and Ophthalmology; Ft. Lauderdale, FL: 2012. [Google Scholar]
- 29.Xu D, Yuan A, Kaiser PK, et al. A novel segmentation algorithm for volumetric analysis of macular hole boundaries identified with optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(1):163–9. doi: 10.1167/iovs.12-10246. [DOI] [PubMed] [Google Scholar]
- 30.Itoh Y, Vasanji A, Ehlers JP. Volumetric ellipsoid zone mapping for enhanced visualisation of outer retinal integrity with optical coherence tomography. Br J Ophthalmol. 2015 doi: 10.1136/bjophthalmol-2015-307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itoh Y, Kaiser PK, Singh RP, et al. Intraoperative optical coherence tomographic factors associated with macular hole closure at early postoperative stage in the PIONEER Study. Asian-Pacific Academy of Ophthalmology Congress; Taipei. 2016. [Google Scholar]
- 32.Rachitskaya AV, Yuan A, Marino MJ, et al. Intraoperative OCT Imaging of the Argus II Retinal Prosthesis System. Ophthalmic Surg Lasers Imaging Retina. 2016;47(11):999–1003. doi: 10.3928/23258160-20161031-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Browne AW, Ehlers JP, Sharma S, Srivastava S. Intraoperative OCT-assisted chorioretinal biopsy in the DISCOVER study. Retina. 2017 doi: 10.1097/IAE.0000000000001522. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandai M, Watanabe A, Kurimoto Y, et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N Engl J Med. 2017;376(11):1038–46. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 35.Gregori NZ, Lam BL, Davis JL. Intraoperative Use of Microscope-Integrated Optical Coherence Tomography for Subretinal Gene Therapy Delivery. Retina. 2017 doi: 10.1097/IAE.0000000000001646. [DOI] [PubMed] [Google Scholar]
- 36.Ehlers JP, Uchida A, Srivastava SK. Intraoperative optical coherence tomography compatible surgical instruments for real-time image-guided ophthalmic surgery. Br J Ophthalmol. 2017 doi: 10.1136/bjophthalmol-2017-310530. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Haddad MT, Tao YK. Automated stereo vision instrument tracking for intraoperative OCT guided anterior segment ophthalmic surgical maneuvers. Biomed Opt Express. 2015;6(8):3014–31. doi: 10.1364/BOE.6.003014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adam MK, Thornton S, Regillo CD, et al. Minimal Endoillumination Levels and Display Luminous Emittance during Three-Dimensional Heads-up Vitreoretinal Surgery. Retina. 2016 doi: 10.1097/IAE.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 39.Eckardt C, Paulo EB. HEADS-UP SURGERY FOR VITREORETINAL PROCEDURES: An Experimental and Clinical Study. Retina. 2016;36(1):137–47. doi: 10.1097/IAE.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 40.Ehlers JP, Uchida A, Srivastava S. The Integrative Surgical Theater: Combining Intraoperative OCT and Digital Visualization for Vitreoretinal Surgery in the DISCOVER Study. Retina. 2017 doi: 10.1097/IAE.0000000000001999. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1: Intraoperative OCT and the Retinal Periphery. Intraoperative OCT samples during multiple cases demonstrating various peripheral pathologies and abnormalities, including subclinical combined traction/rhegmatogenous retinal detachment, subretinal perfluorocarbon liquid, dehemoglobinized preretinal blood, choroidal hemorrhage.
Supplemental Video 2: Intraoperative OCT During Argus Placement. Integrated spring compression is visible in first subject during tacking.
Supplemental Video 3: Intraoperative OCT Integration with a Three-Dimensional Digital Display System. Video sample exhibiting combined display of OCT and surgical field, including epiretinal membrane.