Abstract
Objective
Older patients with cancer suffer from chemotherapy-related toxicities more frequently than younger patients. As novel agents are being used more commonly in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL), toxicities of these agents in older patients have not been well studied. Further, impact of these toxicities on outcomes in the elderly is unknown. This study aimed to answer both questions.
Patients and Methods
We reviewed 14 Alliance for Clinical Trials in Oncology trials that enrolled CLL and/or NHL patients between 2004–2014. Toxicity was assessed per the NCI-CTCAE (version 3–5). Probabilities of experiencing grade three or four hematologic and non-hematologic toxicities were modeled as a function of clinical and disease-related factors using logistic regression.
Results
1199 patients (409 age ≥ 65; 790 age < 65) were analyzed; 438 received only biologic therapy (145 age ≥ 65; 293 age < 65), and 761 received biologic + chemotherapy (264 age ≥ 65; 497 age < 65). The odds of grade three or four hematologic [odds ratio (OR) 1.70; p = 0.009: 95% CI (1.57–1.84)] and non-hematologic toxicities [OR 1.47; p = 0.022; 95% CI (1.39–1.55)] were increased in older patients with CLL, as well as odds of grade three or four non-hematologic toxicities [OR 1.89; p = 0.017; 95% CI (1.64–2.17)] in older patients with NHL. Grade three or four hematologic toxicities were associated with inferior OS and PFS in older patients with NHL [HR 3.14; p = 0.006; 95% CI (2.25–4.39) for OS and 3.06; p = 0.011; 95% CI (2.10–4.45) for PFS], though not in CLL. A prognostic model predicting grade three or four toxicities was also developed.
Conclusions
CLL and NHL patients ≥ 65 year encounter more toxicities than younger patients even when treated with novel biologic agents. Development of grade three or four hematologic toxicities lead to inferior PFS and OS in NHL but not in CLL.
Keywords: Non-Hodgkin lymphoma, Chronic lymphocytic leukemia, Toxicity, Older patients
1. Introduction
Advanced age is associated with increased incidence of chronic lymphocytic leukemia (CLL) and Non-Hodgkin Lymphoma (NHL), with a median age of diagnosis >65 for both [1]. Chemoimmunotherapy has markedly improved outcomes in patients with CLL/NHL, but despite advances, older age has consistently been shown to be associated with poorer outcomes [2,3]. Older patients usually present with different characteristics, including increased cardiovascular and pulmonary comorbidities, and have worse outcomes than their younger counterparts, possibly due to an inability to tolerate cytotoxic therapy, potential under-treatment, and sequelae of aging itself [4–9].
Models have been constructed to identify the risk of chemotherapy toxicity in older patients with cancer. A model employing geriatric assessment variables, laboratory test values, histologies, and treatment characteristics was developed to predict grade three through five toxicities in patients age 65 and older treated with chemotherapy [10]. However, <6% of patients in this model had hematologic malignancies, underscoring the need for predictive models designed specifically for hematologic cancers. The increasing use of novel agents in CLL and NHL provides different toxicities than what is encountered with traditional chemotherapy and need to be studied. The frequency of these toxicities and how they might influence outcomes have not been adequately studied.
We aimed to better understand the frequency of toxicities in older patients treated with novel agents for CLL and NHL. Moreover, we sought to assess the degree to which toxicity affected overall survival (OS) and progression free survival (PFS) among these patients.
2. Patients and Methods
We utilized data collected by the Alliance for Clinical Trials in Oncology to: 1) compare the frequency of toxicities between older (≥65 years old) and younger (<65 years old) patients, 2) model OS and PFS of patients as a function of toxicity of treatment with novel drugs including biologic combinations, monoclonal antibodies, cell cycle inhibitors, chemoimmunotherapy, and immunomodulators, and 3) identify characteristics which may help determine a patient’s risk of experiencing toxicities when receiving novel therapies. Age ≥ 65 was chosen as a cut-off based on traditional definitions of older adults, under-representation in clinical trials [11–12], and a demonstrated higher risk of chemotherapy toxicity [10,13].
2.1. Between-age Comparisons of Impact of Treatment on Toxicity
The probability of experiencing grade three or four hematologic and non-hematologic toxicities was modeled separately as a function of age (≥65 years vs. <65), specific study, time on study, treatment (novel agents only vs. novel agents plus chemotherapy), gender, race, lactate dehydrogenase (LDH), performance status (PS), stage, and an age-by-treatment interaction using logistic regression. These analyses were conducted separately for patients with CLL and NHL.
22. Analysis of Relationship Between Toxicity and OS/PFS in Older Patients
To assess the extent to which these toxicities were associated with OS and PFS in patients ≥65 years, several landmark analyses were performed. Specifically, patients ≥65 years were classified based on whether they experienced at least one grade three or four hematologic toxicity before the landmark time, which we defined as three months after beginning treatment. For patients who were still on study after the landmark time, OS was redefined as the time from the landmark (three months) to death. PFS was redefined in a similar fashion. These endpoints were then modeled separately as a function of the landmark-based toxicity status, time on study, treatment, gender, race, LDH, PS, and stage using Cox proportional hazards models. In addition, as age effects were likely to vary across studies, a random, study-specific intercept and a random study-specific age effect were included in these models. The landmark time of three months was chosen to reflect the initial time of imaging/disease reassessment and to account for lead-time bias and under-reporting of toxicities because of early treatment discontinuation due to underlying disease or other factors.
23. Exploratory Classification and Regression Tree (CART) Analyses
Classification and regression trees were used to identify patient characteristics that might be associated with the probability of experiencing toxicities [14]. These models work by recursively partitioning the data into subgroups (defined using patient characteristics), across which the average response (i.e., the probability of experiencing a grade three or four toxicity) differs. More specifically, a brief overview of the CART algorithm we used is as follows:
Beginning with the entire data set, consider all possible partitions of the data based on a single covariate, e.g. X > 5 vs X ≤ 5. For each such partition (i.e. for each observed value of each covariate), calculate the value of the splitting criterion, denoted s.
In our case, s is a measure of the predictive accuracy, with smaller values indicating “better” prediction, so we next select the partition that gives the smallest value of s, this results in the data being divided into two parts, referred to as “child” nodes.
Within each child node, repeat steps 1 and 2.
Continue in this fashion until pre-defined stopping criteria are met. In our case, we set the maximum depth to be two (meaning the data can only be divided into a maximum of four parts), and required a minimum of 20 patients to be in any partition.
Once this initial tree is constructed, we reduce it in size by eliminating partitions which don’t appear to have a strong impact on the overall fit of the tree (a process referred to as “pruning”). In particular, we collapsed any partitions that did not improve the overall fit of the model (as measured by the R-Squared) by a factor of 0.01. It should be noted that pruning often results in the elimination of one or more patient characteristics from the tree, meaning the pruned tree often only includes a subset of the patient characteristics which were considered. Thus, though we consider age, sex, race, LDH, PS and stage when constructing our trees, our final trees contain only a subset of these covariates.
Patients of all ages were included in these analyses, and age, sex, race, LDH, PS, and stage were the variables considered for defining partitions. These analyses were performed separately for patients with NHL and CLL, and for hematologic and non-hematologic toxicities. In addition, since the toxicity probabilities associated with novel agents were of specific interest given the increased use of these agents in patients with CLL/NHL, patients who received a combination of novel agents and chemotherapy were excluded from these analyses. The number of patients treated with novel agents alone resulted in a relatively small number of patients in these analyses, particularly for the CLL cohort, in which only patients from CALGB (Cancer and Leukemia Group B) 19,805 received novel agents alone. Consequently, as noted above, the maximum depth of the trees was restricted to two and the minimum number of patients in any subgroup was restricted to 20 to ensure that these data were not over partitioned. Statistical analyses were performed using R version 3.2.3 (R Project for Statistical Computing) and SAS versions 9.3 and 9.4 (SAS Institute Inc.). It should be noted that, as the number of patients treated with novel agents alone is relatively small, there is very little power to identify subgroups. Moreover, we were unable to obtain independent, external data with which to validate the results of the CART analyses. Thus, these CART analyses should be viewed as exploratory. We chose to use CART for these exploratory subgroup analyses because they require few assumptions, are able to capture complex interactions, and are generally easy to interpret.
2.4. Missing Data
Due to some missing data in baseline race (2% missing), PS (1.2% missing), LDH (8.3% missing) and stage (5.4% missing), multiple imputations were performed for missing values in these covariates for the logistic regression and Cox models [15]. We used the predictive mean-matching method proposed by Little [16] and created 30 imputed data sets. Imputations were performed using the R package mice using default settings, with the exception of the number of imputations.
Prior to data collection/analysis, we received Institutional Board Review approval of this protocol from the University of Chicago and submitted the necessary data sharing forms from the Alliance for Clinical Trials.
3. Results
3.1. Patients’ Characteristics
A total of 1199 patients (409 age ≥ 65; 790 age < 65) were analyzed. Among these patients, 438 received only novel agents therapy (145 age ≥ 65; 293; age < 65), and 761 received novel agents + chemotherapy (264 age ≥ 65; 497 age < 65). Baseline characteristics for patients with CLL and NHL are described in Tables 1 and 2, respectively.
Table 1.
Baseline characteristics by age group for patients with CLL.
| <65 (N = 477) | ≥ 65 (N = 259) | Total (N = 736) | p value | |
|---|---|---|---|---|
| Gender | 0.755a | |||
| Unknown | 1 (0.2%) | 0 (0.0%) | 1 (0.1%) | |
| Male | 324 (67.9%) | 175 (67.6%) | 499 (67.8%) | |
| Female | 152 (31.9%) | 84 (32.4%) | 236 (32.1%) | |
| Race | 0.390a | |||
| Unknown | 8 (1.7%) | 4 (1.5%) | 12 (1.6%) | |
| White | 422 (88.5%) | 235 (90.7%) | 657 (89.3%) | |
| Black or African American | 41 (8.6%) | 16 (6.2%) | 57 (7.7%) | |
| Asian | 1 (0.2%) | 1 (0.4%) | 2 (0.3%) | |
| American Indian or Alaska Native | 0 (0.0%) | 2 (0.8%) | 2 (0.3%) | |
| Not reported | 1 (0.2%) | 0 (0.0%) | 1 (0.1%) | |
| More than one race | 4 (0.8%) | 1 (0.4%) | 5 (0.7%) | |
| Ethnicity | 0.122 | |||
| Hispanic or Latino | 1 (0.2%) | 4 (1.5%) | 5 (0.7%) | |
| Non-Hispanic | 459 (96.2%) | 245 (94.6%) | 704 (95.7%) | |
| Unknown | 1 (0.2%) | 2 (0.8%) | 3 (0.4%) | |
| Not reported | 16 (3.4%) | 8 (3.1%) | 24 (3.3%) | |
| Performance score | 0.036a | |||
| Missing | 1 (0.2%) | 1 (0.4%) | 2 (0.3%) | |
| 0 | 284 (59.6%) | 131 (50.6%) | 415 (56.4%) | |
| 1 | 179 (37.5%) | 114 (44.0%) | 293 (39.8%) | |
| 2 | 13 (2.7%) | 13 (5.0%) | 26 (3.5%) | |
| LDH | 0.460b | |||
| N | 468 | 258 | 726 | |
| Mean (SD) | 306.4 (228.9) | 306.0 (229.6) | 306.3 (229.0) | |
| Median | 223.0 | 232.5 | 225.5 | |
| Q1, Q3 | 169.5, 371.5 | 175.0, 333.0 | 171.0, 349.0 | |
| Range | (6.0–1820.0) | (93.0–1877.0) | (6.0–1877.0) | |
| Stage | <0.001a | |||
| Missing | 9 (1.9%) | 6 (2.3%) | 15 (2.0%) | |
| Stage 0 | 27 (5.7%) | 21 (8.1%) | 48 (6.5%) | |
| Stage 1/2 | 297 (62.3%) | 111 (42.9%) | 408 (55.4%) | |
| Stage 3/4 | 144 (30.2%) | 121 (46.7%) | 265 (36.0%) |
CLL-chronic lymphocytic leukemia.
PS-performance status.
LDH-lactate dehydrogenase.
SD-standard deviation.
Q-quartile.
Chi-square.
Kruskal Wallis.
Table 2.
Baseline characteristics by age group for patients with NHL.
| <65 (N = 313) | ≥65 (N = 150) | Total (N = 463) | p value | |
|---|---|---|---|---|
| Gender | 0.027a | |||
| Male | 164 (52.4%) | 95 (63.3%) | 259 (55.9%) | |
| Female | 149 (47.6%) | 55 (36.7%) | 204 (44.1%) | |
| Race | 0.288 | |||
| Unknown | 8 (2.6%) | 3 (2.0%) | 11 (2.4%) | |
| White | 280 (89.5%) | 140 (93.3%) | 420 (90.7%) | |
| Black or African American | 20 (6.4%) | 4 (2.7%) | 24 (5.2%) | |
| Asian | 3 (1.0%) | 2 (1.3%) | 5 (1.1%) | |
| Native Hawaiian or Pacific Islander | 2 (0.6%) | 0 (0.0%) | 2 (0.4%) | |
| More than one race | 0 (0.0%) | 1 (0.7%) | 1 (0.2%) | |
| Ethnicity | 0.763a | |||
| Hispanic or Latino | 10 (3.2%) | 3 (2.0%) | 13 (2.8%) | |
| Non-Hispanic | 290 (92.7%) | 141 (94.0%) | 431 (93.1%) | |
| Not reported | 13 (4.2%) | 6 (4.0%) | 19 (4.1%) | |
| Performance score | 0.007a | |||
| Missing | 8 (2.6%) | 4 (2.7%) | 12 (2.6%) | |
| 0 | 210 (67.1%) | 80 (53.3%) | 290 (62.6%) | |
| 1 | 86 (27.5%) | 56 (37.3%) | 142 (30.7%) | |
| 2 | 9 (2.9%) | 10 (6.7%) | 19 (4.1%) | |
| LDH | 0.021b | |||
| N | 260 | 114 | 374 | |
| Mean (SD) | 296.6 (325.6) | 302.3 (261.2) | 298.3 (307.1) | |
| Median | 182.5 | 212.0 | 188.5 | |
| Q1, Q3 | 151.0, 288.0 | 164.0, 365.0 | 154.0, 312.0 | |
| Range | (14.0–2576.0) | (75.0–2297.0) | (14.0–2576.0) | |
| Stage | 0.848a | |||
| Missing | 42 (13.4%) | 8 (5.3%) | 50 (10.8%) | |
| Stage 1/2 | 27 (8.6%) | 15 (10.0%) | 42 (9.1%) | |
| Stage 3/4 | 244 (78.0%) | 127 (84.7%) | 371 (80.1%) | |
| Treatment goal | 0.983a | |||
| Curative | 54 (17.3%) | 26 (17.3%) | 80 (17.3%) | |
| Palliative | 259 (82.7%) | 124 (82.7%) | 383 (82.7%) |
NHL-non-Hodgkin lymphoma.
PS-performance status.
LDH-lactate dehydrogenase.
SD-standard deviation.
Q-quartile.
Chi-square.
Kruskal Wallis.
3.2. Toxicities
Overall, 68% of patients with CLL and 35% of patients with NHL experienced at least one grade three or four hematologic toxicity. For non-hematologic toxicities, these percentages were 48% and 54% for CLL and NHL, respectively. Among patients with CLL, older patients were found to have significantly higher odds of experiencing a grade three or four hematologic toxicity than younger patients, with an adjusted odds ratio (OR) of 1.70 [p = 0.009 95% CI (1.57, 1.84)] Comparable results were seen for non-hematologic toxicities [OR 1.47; p = 0.022; 95% CI (1.39, 1.55)]. Among patients with NHL, the odds of experiencing a grade three or four hematologic toxicity were not different between age groups, but the odds of experiencing a grade three or four non-hematologic toxicity were significantly higher for older patients [OR 1.89; p = 0.017; 95% CI (1.64, 2.17)]. The number of patients included in this analysis is included in Table 5.
Table 5.
Number of patients included in analyses.
| Analyses | CLL | NHL |
|---|---|---|
| OS/PFS landmark | 259 | 150 |
| Logistic Reg. | 736 | 463 |
| CART models | 46 | 286 |
OS-overall survival.
PFS-progression-free survival.
REG-regression.
CART-classification and regression tree.
CLL-chronic lymphocytic leukemia.
NHL-non-Hodgkin lymphoma.
3.3. Outcomes
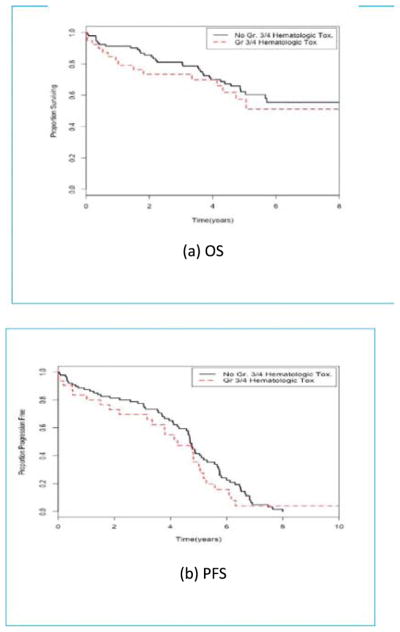
Among older patients with CLL, those who experienced at least one grade three or four hematologic or non-hematologic toxicity within the first three months were found to have a similar OS and PFS as those who did not experience such a toxicity. Older patients with NHL who experienced at least one grade three or four hematologic toxicity within the first three months were found to have worse OS and PFS than patients without such toxicity [HR 3.14; p = 0.006; 95% CI (2.25, 439)] for OS and [3.06; p = 0.011; 95% CI (2.10, 4.45)] for PFS (Fig. 1). In addition, experiencing at least one grade three or four non-hematologic toxicity within the first three months was not found to be significantly associated with subsequent OS or PFS among patients with NHL Treatment characteristics and outcomes are described in Tables 3 and 4 for patients with CLL and NHL, respectively. The number of patients included in this analysis is included in Table 5.
Fig. 1.
Kaplan-Meier curves for older (≥65) NHL patients by grade three or four hematologic toxicity status. (A) OS in NHL patients who did not experience a grade three or four hematologic toxicity at or before landmark time (black line) and patients who did experience such a toxicity (red line). (B) PFS in NHL patients who did not experience a grade three or four hematologic toxicity at or before landmark time (black line) and patients who did experience such a toxicity (red line). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Treatment characteristics and outcomes by age (CLL).
| <65 (N = 477) | ≥65 (N = 259) | Total (N = 736) | p value | |
|---|---|---|---|---|
| Treatment | 0.635a | |||
| Concurrent FLU/Ritux + Consolidation | 85 (17.8%) | 54 (20.8%) | 139 (18.9%) | |
| Concurrent FLU/Rituximab | 91 (19.1%) | 47 (18.1%) | 138 (18.8%) | |
| FLU/Cy/Rituximab | 90 (18.9%) | 50 (19.3%) | 140 (19.0%) | |
| Flavopiridol | 29 (6.1%) | 20 (7.7%) | 49 (6.7%) | |
| Fludarabine IV + Alemtuzumab- | 63 (13.2%) | 22 (8.5%) | 85 (11.5%) | |
| High risk early intervention: Ritux + FLU | 13 (2.7%) | 4 (1.5%) | 17 (2.3%) | |
| High Risk Observ. + Later treatment: Ritux + FLU | 7 (1.5%) | 4 (1.5%) | 11 (1.5%) | |
| Low Risk Observ. + Ritux. + FLU | 34 (7.1%) | 21 (8.1%) | 55 (7.5%) | |
| Rituximab/Fludarabine + Alemtuzumab | 65 (13.6%) | 37 (14.3%) | 102 (13.9%) | |
| Treatment type | 0.485a | |||
| Biologic | 29 (6.1%) | 20 (7.7%) | 49 (6.7%) | |
| Biologic + Chemo | 448 (93.9%) | 239 (92.3%) | 687 (933%) | |
| Treatment delay | 0.494a | |||
| Missing | 177 (37.1%) | 108 (41.7%) | 285 (38.7%) | |
| No | 86 (18.0%) | 48 (18.5%) | 134 (18.2%) | |
| Yes | 214 (44.9%) | 103 (39.8%) | 317 (43.1%) | |
| Blood transfusion | 0.335a | |||
| Missing | 95 (19.9%) | 39 (15.1%) | 134 (18.2%) | |
| No | 369 (77.4%) | 209 (80.7%) | 578 (78.5%) | |
| Yes | 13 (2.7%) | 11 (4.2%) | 24 (3.3%) | |
| Days to progression/last follow-up | 0.579b | |||
| N | 462 | 250 | 712 | |
| Events | 137 | 72 | 209 | |
| Median survival days | 2626.0 | 2178.0 | 2372.0 | |
| 5 year survival rate | 61.1% (55.2%–66.9%) | 56.8% (47.8%–65.8%) | 59.7% (54.8%–64.6%) | |
| Year 5 N at risk | 71 | 31 | 102 | |
| Days to death/last follow-up | <0.001b | |||
| N | 434 | 244 | 678 | |
| Events | 51 | 55 | 106 | |
| Median survival days | 3427.0 | 3371.0 | 3418.0 | |
| 5 year survival rate | 87.9% (84.0%–91.7%) | 72.6% (65.4%–79.9%) | 82.4% (78.8%–86.1%) | |
| Year 5 N at risk | 106 | 52 | 158 |
CLL-chronic lymphocytic leukemia.
Ritux-rituximab.
Flu-fludarabine.
Cy-cyclophosphamide.
Observ-observation.
Chi-square.
Log-rank.
Table 4.
Treatment characteristics and outcomes by age (NHL).
| <65 (N = 313) | ≥65 (N = 150) | Total (N = 463) | p value | |
|---|---|---|---|---|
| Treatment | <0.001a | |||
| Bortezomib + Lenalidomide | 21 (6.7%) | 33 (22.0%) | 54 (11.7%) | |
| Epoch Rituximab | 49 (15.7%) | 24 (16.0%) | 73 (15.8%) | |
| Epratuzumab + Rituximab | 47 (15.0%) | 13 (8.7%) | 60 (13.0%) | |
| Fludarabine IV + Alemtuzumab | 0 (0.0%) | 1 (0.7%) | 1 (0.2%) | |
| Galiximab | 28 (8.9%) | 2 (1.3%) | 30 (6.5%) | |
| Lenalidomide | 26 (8.3%) | 19 (12.7%) | 45 (9.7%) | |
| Lenalidomide + Rituximab | 55 (17.6%) | 8 (5.3%) | 63 (13.6%) | |
| Rituximab | 2 (0.6%) | 1 (0.7%) | 3 (0.6%) | |
| Rituximab + Galiximab | 41 (13.1%) | 20 (13.3%) | 61 (13.2%) | |
| Rituximab + Lenalidomide | 23 (7.3%) | 19 (12.7%) | 42 (9.1%) | |
| Rituximab + Ibritumomab | 5 (1.6%) | 2 (1.3%) | 7 (1.5%) | |
| Thalidomide | 16 (5.1%) | 8 (5.3%) | 24 (5.2%) | |
| Treatment type | 0.781 | |||
| Biologic | 264 (84.3%) | 125 (83.3%) | 389 (84.0%) | |
| Biologic + Chemo | 49 (15.7%) | 25 (16.7%) | 74 (16.0%) | |
| Treatment delay | 0.007a | |||
| Missing | 87 (27.8%) | 66 (44%) | 153 (33.0%) | |
| No | 120 (38.3%) | 30 (20.0%) | 150 (32.4%) | |
| Yes | 106 (33.9%) | 54 (36.0%) | 160 (34.6%) | |
| Blood transfusion | 0.484 | |||
| Missing | 247 (78.9%) | 118 (78.7%) | 365 (78.8%) | |
| No | 65 (20.8%) | 32 (21.3%) | 97 (21.0%) | |
| Yes | 1 (0.3%) | 0 (0.0%) | 1 (0.2%) | |
| Days to progression/last follow-up | 0.826b | |||
| N | 284 | 135 | 419 | |
| Events | 74 | 33 | 107 | |
| Median survival days | 3213.0 | NA | NA | |
| 5 year survival rate | 73.5% (68.0%–79.1%) | 71.7% (63.0%–80.5%) | 73.0% (68.4%–77.7%) | |
| Year 5 N at risk | 117 | 43 | 160 | |
| Days to death/last follow-up | <0.001b | |||
| N | 308 | 147 | 455 | |
| Events | 35 | 55 | 90 | |
| Median survival days | NA | NA | NA | |
| 5 year survival rate | 87.6% (83.6%–91.6%) | 60.8% (52.1%–69.6%) | 78.7% (74.6%–82.9%) | |
| Year 5 N at risk | 143 | 51 | 194 |
NHL-non-Hodgkin lymphoma.
Chi-square.
Log-rank.
3.4. Classification and Regression Trees Analyses
We used CART models that recursively partition the data using patient characteristics, with the goal of dividing the data into subgroups of similar patients with respect to the toxicity outcome. When constructing the tree, we considered all possible covariates, and for each, we considered partitioning based on each unique observed value. The specific covariates and thresholds which ultimately construct the final trees were selected as they were found to fit the data best
The CART analyses for patients with CLL identified several potential risk subgroups. Data were partitioned into two subgroups, based upon baseline PS. In total, 62% of patients with a PS of ≥1 at baseline experienced a grade three or four hematologic toxicity, while 35% of those with a PS of 0 at baseline experienced such a toxicity. For non-hematologic toxicities, 62% of patients with a PS of ≥1 at baseline experienced a grade three or four non-hematologic toxicity, while 30% of those with a PS of 0 at baseline experienced such a toxicity (Fig. 2).
Fig. 2.

CART results for patients with CLL receiving only biologic therapy. Patients with CLL were partitioned into subgroups (based on PS ≥1 vs. PS <1) across which the probability of experiencing a grade three or four toxicity may differ.
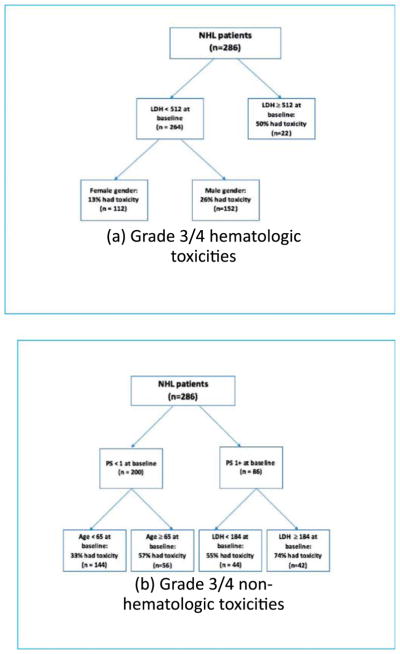
CART models were also fit for patients with NHL. For hematologic toxicities, data were partitioned into three subgroups based on LDH and gender. Among these patients, 50% of those with a baseline LDH of 512 or greater experienced a grade three or four hematologic toxicity. Among patients with a baseline LDH of <512, 13% of women experienced a grade three or four hematologic toxicity, while 26% of men experienced such a toxicity. For non-hematologic toxicities, data were partitioned into four subgroups based on PS, age and LDH. Among patients with a baseline PS of 1 or higher, 74% of those with an LDH of 184 or higher experienced a grade three or four non-hematologic toxicity and 55% of those with an LDH of <184 experienced such a toxicity. Among patients with a baseline PS of 0, 57% of those who were 65 years of age or older experienced a grade three or four non-hematologic toxicity, whereas 33% of those who were under the age of 65 experienced such a toxicity (Fig. 3). The number of patients included in these analyses is included in Table 5.
Fig. 3.
CART results for patients with NHL receiving only biologic therapy. (A) NHL patients were partitioned into subgroups based on LDH ≥ 512 vs. LDH < 512 and male gender vs. female gender as predictors of grade three or four hematologic toxicity across which the probability of experiencing a grade three or four toxicity differed. (B) NHL patients were partitioned into subgroups based on PS ≥ 1 vs. PS < 1, age ≥ 65 vs. age < 65, and LDH ≥ 184 vs. LDH < 184 as predictors of grade three or four non-hematologic toxicity across which the probability of experiencing a grade three or four toxicity differed.
4. Discussion
The results of this multicenter analysis of prospective studies conducted by the Alliance for Clinical Trials in Oncology Group suggest that, compared to younger patients, older adults with CLL treated with novel agents may have increased odds of both grade three or four hematologic and non-hematologic toxicities and that older adults with NHL treated with novel agents may have greater odds for grade three or four non-hematologic toxicity. Also, hematologic, but not non-hematologic, toxicities in older adults with NHL were associated with inferior OS and PFS. Moreover, despite higher toxicities in older patients with CLL, there was no association between observed hematologic or non-hematologic toxicity and PFS and/or OS. These findings have clinical implications for future clinical trials and can impact how clinicians manage toxicities.
The observed associations between hematologic toxicity and OS/PFS among older patients with NHL require further investigation. These findings could represent a direct effect of toxicity due to decreased physiologic reserve, decreased drug clearance, or an increased sensitivity of tissue to novel agents. As far as treatment delays, there was no significant difference between younger and older patients, though we do not have data regarding dose reductions. Prior studies have shown that primary changes in relative dose intensity, or the amount of drug delivered per unit of time, is affected by both dose delays and reductions, and has been correlated with worse outcomes in breast cancer, colon cancer, and NHL [16–18]. Several retrospective studies in older patients with large cell lymphoma have shown a correlation between reduced dose intensity of cyclophosphamide and/or anthracycline and inferior OS [19–23], though RDI has also been correlated with advanced age, worse performance status, and multiple comorbidities, confounding any survival analysis. Formal assessment in prospective clinical trials will help further delineate the role of dose delays and reductions in NHL and CLL.
To elucidate the risks of developing novel agent-related toxicities, CART models were used to identify clinical and disease-related factors as potential predictors of developing toxicities. These included LDH, sex, PS, and age in patients with NHL and PS in patients with CLL. LDH was also recognized as a risk factor in our predictive model. Higher LDH activity at the time of lymphoma diagnosis reflects increased tumor bulk [24] and LDH serves as a marker of tissue damage [25]. These insults may predispose patients to hematologic toxicities and diminished drug tolerance. It should also be noted that, unlike treatments, patient characteristics cannot be randomly assigned. Though the results of our CART analyses suggest potential associations between a number of patient characteristics and increased risk of toxicity, it is impossible to determine the exact cause of these toxicities in individual patients. The CART analyses should be viewed as exploratory, and these results need to be validated using external data before being used in practice.
Previous studies have shown that functional status predicts mortality in older patients [26–28], and it affects the prognosis of patients with CLL and NHL as well as their tolerance to therapy [29–32]. PS was likewise a risk factor for toxicity in our model. Our tree model also suggests that male sex may be associated with increased hematologic toxicity in NHL, though underlying reasons are uncertain. Previous reports have suggested that women diagnosed with lymphoma have favorable response when treated with novel agents [33–35], with some evidence that the benefit may result from decreased clearance of rituximab compared to their male counterparts. However, this toxicity risk would need further exploration of sex effects on drug metabolism.
Our study is not without limitations. We combined heterogeneous groups of novel agents, as well aggressive and indolent lymphomas; however, our overall goal was to assess toxicities when older patients receive novel agents for these indications. It can be acknowledged that there may be additional and different risk factors for each respective tumor and treatment type. Our sample size precluded meaningful subgroup analyses, although it is helpful to see common risk factors across multiple lymphoma types and treatment regimens. We also focused on grade three or four toxicities; however, some grade two toxicities could be relevant to an older population and may impact quality of life, but we were limited by the reported toxicities, which are often of higher grade. In addition, a relatively small number of patients in these studies received only novel agents, thus limiting the extent to which we were able to assess associations between clinical factors and toxicity among these patients.
Despite these limitations, this study identified potentially important predictors of toxicity with novel agents with CLL and NHL, and helps fill gaps in knowledge of toxicity risk. We plan to externally validate our findings, with the ultimate goal of developing a model to predict who will be at a clinically significant risk of developing grade three and/or four toxicity, as this may have an impact OS/PFS as we have shown in NHL. Predicting risk can then provide a basis to initiate early supportive care in vulnerable patients and incorporate additional safety measures for older patients who are currently under-represented in clinical trials 36–37]. Until validated in future prospective studies, our findings indicate that toxicities are prevalent, even with newer novel agents, and, in some, these toxicities adversely affect outcomes. These observations may be of considerable importance with the increasing number of novel agents combined as doublets and triplets in development for patients with CLL/NHL.
Acknowledgments
Support
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Numbers UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), U10CA180790, U10CA180833, U10CA180836, U10CA180838, U10CA180850, and U10CA180857. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
John Byrd.
Footnotes
Presented in part as an oral session at the Annual American Society of Hematology meeting in December 2016 San Diego, CA.
Author Contributions
Study Concept: J. Foster, D. Seisler, J. Lafky, S.H. Jung, M. Tallarico, C. Nabhan.
Study Design: J. Foster, D. Seisler, C. Nabhan, M. Tallarico, H. Cohen.
Data Acquisition: J. Foster, D. Seisler.
Quality Control of Data and Algorithms: J. Foster, Seizer.
Data Analysis and Interpretation: J. Foster, D. Seisler, N. Bartlett, S.H. Jung, C. Nabhan, M. Tallarico, B. Cheson, H. Cohen.
Statistical Analysis: J. Foster, H. Muss, D. Seisler, S.H. Jung, C Nabhan, M. Tallarico, H. Cohen.
Manuscript Preparation: J. Foster, H. Muss, D. Seisler, S.H. Jung, M. Tallarico, C. Nabhan.
Manuscript Editing: J. Foster, H. Muss, D. Seisler, N. Bartlett, J. Lafky, A. Jatoi, C. Nabhan, M. Tallarico.
Manuscript Review: J. Foster, H. Muss, D. Seisler, N. Bartlett, J. Lafky, S.H. Jung, C. Nabhan, M. Tallarico, H. Cohen, B. Cheson.
Disclosures and Conflict of Interest Statements
Advisory Boards: Bartlett, KITE, Pfizer, Seattle Genetics; Cheson, Roche-Genentech, Celgene, Pharmacyclics, Gilead.
Research Funding: Jatoi, NIH; Cheson, Roche-Genentech, Celgene, Pharmacyclics.
References
- 1.SEER cancer statistics review, 1975–2013. Bethesda, MD, USA: National Cancer Institute; 2015. [Google Scholar]
- 2.Nabhan C, Smith SM, Helenowski, et al. Analysis of very elderly (≥80 years) non-hodgkin lymphoma: impact of functional status and co-morbidities on outcome. Br J Haematol. 2012 Jan;156:196–204. doi: 10.1111/j.1365-2141.2011.08934.x. [DOI] [PubMed] [Google Scholar]
- 3.The Non-Hodgkin’s Lymphoma Classification Project: effect of age on the characteristics and clinical behavior of non-Hodgkin’s lymphoma patients. Ann Oncol. 1997;8:973–8. [PubMed] [Google Scholar]
- 4.Thieblemont C, Grossoeuvre A, Houot R, et al. Non-Hodgkin’s lymphoma in very elderly patients over 80 years. A descriptive analysis of clinical presentation and outcome. Ann Oncol. 2008;19:774–9. doi: 10.1093/annonc/mdm563. [DOI] [PubMed] [Google Scholar]
- 5.Aoki K, Takahashi T, Tabata S, et al. Efficacy and tolerability of reduced-dose 21-day cycle rituximab and cyclophosphamide, doxorubicin, vincristine and prednisolone therapy for elderly patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2013;54:2441–7. doi: 10.3109/10428194.2013.780654. [DOI] [PubMed] [Google Scholar]
- 6.Hoerni B, Sotto JJ, Eghbali H, et al. Non-Hodgkin’s malignant lymphomas in patients older than 80: 70 cases. Cancer. 1988;61:2057–9. doi: 10.1002/1097-0142(19880515)61:10<2057::aid-cncr2820611021>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Latta S, Cygan PH, Fried W, Nabhan C. Diffuse large B-cell non-Hodgkin lymphoma in the very elderly: challenges and solutions. Oncology. 2013;27(2):126–30. [PubMed] [Google Scholar]
- 8.Gomez H, Mas L, Casanova L, et al. Elderly patients with aggressive non-Hodgkin’s lymphoma treated with CHOP chemotherapy plus granulocyte-macrophage colony-stimulating factor: identification of two age subgroups with differing hematologic toxicity. J Clin Oncol. 1998;16:2352–8. doi: 10.1200/JCO.1998.16.7.2352. [DOI] [PubMed] [Google Scholar]
- 9.Neilly IJ, Ogston M, Bennett B, Dawson AA. High grade non-Hodgkin’s lymphoma in the elderly-12 year experience in the Grampian Region of Scotland. Hematol Oncol. 1995;13:99–106. doi: 10.1002/hon.2900130206. [DOI] [PubMed] [Google Scholar]
- 10.Arti Hurria, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–65. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 12.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–31. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 13.Repetto L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol. 2003;1(2):18–24. [PubMed] [Google Scholar]
- 14.Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference, and prediction. New York: Springer; 2009. [Google Scholar]
- 15.Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 16.Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330:1253–9. doi: 10.1056/NEJM199405053301801. [DOI] [PubMed] [Google Scholar]
- 17.Lund CM, Nielsen D, Dehlendorff, et al. Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colorectal cancer: the study. [Accessed May 16,2017];Open. 2017 1(5):e000087. doi: 10.1136/esmoopen-2016-000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanemasa Y, Shimoyama T, Sasaki Y, et al. The impacts of initial and relative dose intensity of R-CHOP on outcomes of elderly patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2017 Mar;58(3):736–9. doi: 10.1080/10428194.2016.1211279. [DOI] [PubMed] [Google Scholar]
- 19.Bosly A, Bron D, Van Hoof A, et al. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol. 2008;87:277–83. doi: 10.1007/s00277-007-0399-y. [DOI] [PubMed] [Google Scholar]
- 20.Epelbaum R, Faraggi D, Ben-Arie Y, et al. Survival of diffuse large cell lymphoma. A multivariate analysis including dose intensity variables. Cancer. 1990;66:1124–9. doi: 10.1002/1097-0142(19900915)66:6<1124::aid-cncr2820660608>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 21.Kwak LW, Halpern J, Olshen RA, et al. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol. 1990;8:963–77. doi: 10.1200/JCO.1990.8.6.963. [DOI] [PubMed] [Google Scholar]
- 22.Pettengell R, Schwenkglenks M, Bosly A. Association of reduced relative dose intensity and survival in lymphoma patients receiving CHOP-21 chemotherapy. Ann Hematol. 2008;87:429–30. doi: 10.1007/s00277-008-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terada Y, Nakamae H, Aimoto R, et al. Impact of relative dose intensity (RDI) in CHOP combined with rituximab (R-CHOP) on survival in diffuse large B-cell lymphoma. J Exp Cancer Res. 2009;28:116. doi: 10.1186/1756-9966-28-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little RJA. Missing-data adjustments in large surveys. J Bus Econ Stat. 1988;6:287–301. [Google Scholar]
- 25.William BM, Bongu NR, Bast M, et al. The utility of lactate dehydrogenase in the follow up of patients with diffuse large B-cell lymphoma. Rev Bras Hematol Hemoter. 2013;35(3):189–91. doi: 10.5581/1516-8484.20130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanford Cancer Center. Understanding cancer. Stanford Medicine; 1992. Cancer diagnosis - understanding cancer. [Google Scholar]
- 27.Reuben DB, Rubenstein LV, Hirsch SH, et al. Value of functional status as a predictor of mortality: results of a prospective study. Am J Med. 1992;93:663–9. doi: 10.1016/0002-9343(92)90200-u. [DOI] [PubMed] [Google Scholar]
- 28.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 29.Philippe Solal-Céligny, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–65. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 30.Natali Pflug, Bahlo J, Toat DS, et al. Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood. 2014;124(1):49–62. doi: 10.1182/blood-2014-02-556399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sehn Laurie H, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–61. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 32.Nicolaides C, Dimou S, Pavlidis N. Prognostic factors in aggressive non-Hodgkin’s lymphomas. Oncologist. 1998;3(3):189–97. [PubMed] [Google Scholar]
- 33.Pfreundschuh M, Muller C, Zeynalova S, et al. Suboptimal dosing of rituximab in male and female patients with DLBCL. Blood. 2014;123:640–6. doi: 10.1182/blood-2013-07-517037. [DOI] [PubMed] [Google Scholar]
- 34.Habermann TM. Is rituximab one for all ages and each sex? Blood. 2014;123:602–3. doi: 10.1182/blood-2013-12-543314. [DOI] [PubMed] [Google Scholar]
- 35.Eve HE, Carey S, Richardson SJ, et al. Single-agent lenalidomide in relapsed/refractory mantle cell lymphoma: results from a UK phase II study suggest activity and possible gender differences. Br J Haematol. 2012;159:154–63. doi: 10.1111/bjh.12008. [DOI] [PubMed] [Google Scholar]
- 36.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–9. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Konrat C, Boutron I, Trinquart L, Auleley GR, Ricordeau P, Ravaud P. Underrepresentation of elderly people in randomised controlled trials. The example of trials of 4 widely prescribed drugs. Plos One. 2012;7(3):e33559. doi: 10.1371/journal.pone.0033559. https://doi.org/l0.1371/journal.pone.0033559. [DOI] [PMC free article] [PubMed] [Google Scholar]