Abstract
Rationale
Initial drug abstinence (modeled here as Extinction Day 1, ED1) is a critical time point in the progression of addiction that is strongly influenced by stress and sex. ED1 induces corticosterone release in both sexes, and cocaine-seeking during ED1 can be mitigated by corticotrophin releasing factor (CRF) antagonists more effectively in female rats. Oxytocin (OXT) is a neuropeptide that has several biological functions, including regulation of stress pathways.
Methods
To investigate a relationship between OXT, sex, and cocaine-seeking, we examined Fos on ED1 in OXT neurons of paraventricular (PVN) and supraoptic nuclei (SON) compared to homecage (cocaine experienced) or naïve male and female rats. We also administered OXT 30-min prior to ED1 testing or cued reinstatement testing.
Results
OXT neurons had decreased activity (as reflected by Fos protein) in PVN and SON on withdrawal day 1 (homecage) compared to naïve rats. Fos in OXT neurons was further decreased on ED1, compared to homecage controls, in both males and females even though in SON, cocaine exposure increased the number of OXT-expressing neurons. In addition, systemically administered OXT reduced cocaine seeking during ED1 and cue-induced reinstatement of cocaine seeking, but delayed extinction, similarly among male and female rats.
Conclusions
These data indicate that OXT neurons in PVN and SON may be involved in cocaine-seeking during ED1, and support OXT as a possible therapeutic to decrease cocaine seeking during initial abstinence and in response to cocaine-associated cues.
Keywords: Oxytocin, addiction, self-administration, sex differences, immunohistochemistry
Introduction
Oxytocin (OXT) is a hypothalamic neuropeptide that is critically involved in a wide array of biological functions in both males and females, including maternal behavior, pair-bond formation, sexual arousal, social interaction and memory, and anxiety reduction (Keverne and Curley, 2004, Lim and Young, 2006, Neumann, 2007). Importantly, OXT administration exerts anxiolytic and anti-stress properties in animals (Ring et al., 2006) and exerts anxiolytic and prosocial effects in humans (Keverne and Curley, 2004, Lim and Young, 2006, Marazziti and Catena Dell’osso, 2008). Central OXT attenuates stress-induced neuroendocrine and molecular responses via the hypothalamic-pituitary-adrenal (HPA) axis and OXT-sensitive brain regions such as the hypothalamus, dorsal hippocampus, and septum (Windle et al., 2004). In a general sense, OXT can be conceived of as neuropeptide that modulates stress reactivity as both direct effects on OXT receptors and secondary effects of modulating HPA axis function underlie the actions of OXT on stress (Van de Kar and Blair, 1999, Landgraf and Neumann, 2004). OXT also modulates shifts in behavioral strategies in response to environmental stimuli (Amadei et al., 2017) by engaging corticostriatal circuitry implicated in reward seeking (Ragozzino et al., 2003, Block et al., 2007). As stress and reward-seeking strategies are both substantially engaged in addiction phenotypes, OXT represents a significant neuromodulatory target for developing addiction therapeutics.
Although OXT is well characterized as a neurohormone that is released into the neurohypophysis from the paraventricular (PVN) and supraoptic nuclei (SON), there are also central OXT projections from the hypothalamus to brain nuclei implicated in addiction, including the ventral tegmental area, amygdala, nucleus accumbens, and prefrontal cortex. In behavioral studies, OXT has rewarding effects and produces a conditioned place preference (Liberzon et al., 1997). Also, OXT administration reduces cocaine-induced hyperactivity (Kovacs et al., 1990) and stereotypies (Sarnyai et al., 1991). In addition, OXT has been identified as a potential therapeutic for treatment of drug addiction. Indeed, administration of OXT decreases demand for cocaine in male rats, and demand for methamphetamine in males and females (Carson et al., 2010, Bentzley et al., 2014, Baracz et al., 2016, Cox et al., 2017). As well, OXT directly infused into the nucleus accumbens, amygdala, or the hippocampus attenuated morphine tolerance and dependence (Sarnyai and Kovacs, 1994). Thus, there is strong evidence that OXT plays a role in the rewarding effects of abused drugs.
We previously reported that Extinction Day 1 (ED1) from repeated daily cocaine self-administration is a stressful event, marked by cocaine-seeking behavior, increased Fos expression in stress-responsive brain regions, and increased serum corticosterone levels. Cocaine-seeking on ED1, Fos expression in dorsal raphe, hippocampus, locus coeruleus, and periaqueductal grey, and circulating corticosterone both during and following the completion of the session, are greater in females than in males (Cason et al., 2016, Kohtz and Aston-Jones, 2017). In addition, cocaine-seeking on ED1 can be reduced by antagonizing corticotrophin releasing factor-1 receptors to a greater extent among female rats (Cason et al., 2016). We hypothesize that these substantial sex-differences in ED1 behavior are driven by sex-differences in underlying stress and anti-stress circuitries. Hence, we propose that downregulation of the anti-stress neurohormone OXT, on ED1, may increase cocaine-seeking during initial abstinence, cocaine-seeking persistence, and reinstatement. In this study, we utilized the ED1 model of initial abstinence from cocaine to examine activity of PVN and SON (as seen by expression of Fos protein) on ED1 compared to homecage controls. We then tested the therapeutic potential of pre-treatment with systemic OXT on cocaine-seeking on ED1 and cocaine-seeking persistence tested 2 weeks later, extinction resistance, and cued reinstatement in male and female rats.
Methods
Subjects
Male (325–450g, n=52) and female (225–300g, n=54) Sprague Dawley rats (Charles River Laboratories; ~55–60 days of age) were singly housed under a reversed 12h/12h light/dark cycle (lights off 0600 h); all experiments were performed during the active cycle. Rats had free access to food and water and were housed in animal facilities at the Medical University of South Carolina (Fos and immunohistochemistry analyses) or Rutgers University (behavioral efficacy of OXT pre-treatment; both AAALAC-accredited). All experiments were approved by the Institutional Animal Care and Use Committees and conducted in accordance with the National Institutes of Health specifications outlined in their Guide for the Care and Use of Laboratory Animals: Eighth Edition (2011).
Jugular Catheter Surgeries
Animals were anesthetized with ketamine/xylazine (56.5/8.7 mg/kg) and given Carprofen (1.0 mg/kg) as an analgesic. Chronic in-dwelling catheters were constructed in-house and inserted as described previously (Smith et al., 2009). Animals were given Kefzol (10 mg, iv) and heparin (10 U, iv) starting 3 days following surgery and daily following self-administration sessions. Rats recovered from surgery for at least 1 week before self-administration training.
Drugs
Cocaine hydrochloride (NIDA, Research Triangle Park, NC) was dissolved in 0.9% sterile saline. OXT (Tocris) was dissolved in 0.9% saline and administered at 0.3 mg/kg or 1.0 mg/kg, intraperioneally (IP), 30 minutes prior to testing in all behavioral paradigms. At most, rats received 3 injections of OXT (ED1, Cued Reinstatement, Acclimated Motor Testing). There was a minimum of 3 weeks between OXT administration on ED1 and testing in cued reinstatement, and a minimum of 2 weeks between OXT injections prior to cued reinstatement and acclimated motor testing.
Cocaine self-administration
Self-administration sessions were conducted in standard operant conditioning chambers housed in sound attenuating cubicles and controlled via MED-PC IV software (Med-Associates, St Albans, VT) as described previously (Smith and Aston-Jones, 2011). Rats were trained in daily 2h sessions to press an active lever for intravenous cocaine (0.2 mg/50 ul infusion for males, 0.16 mg/50 ul infusion for females). Different doses in infusions allowed females and males to achieve the same average number of lever presses, and cocaine-cue pairings, across the last 3 days of self-administration by adjusting for weight differences and high cocaine intake in female rats, as per prior reports (Kohtz & Aston-Jones, 2017). Rats trained for at least 10 days on a fixed ratio 1 (FR1) schedule of reinforcement to reach a criterion of >10 cocaine infusions/day wherein light+tone cocaine-associated cues were presented with active lever presses. Each cocaine infusion was followed by a 20s time-out period in which lever pressing produced neither cocaine nor cues. An inactive lever was also present; presses on it were tabulated but had no consequence.
Cocaine-seeking during ED1
Twenty-four hours after the final self-administration session, rats were tested in an initial extinction session (ED1). In this session rats were exposed to the operant conditioning chamber for 90 min during which time presses on either lever had no consequence. Active lever pressing in the absence of cocaine reward or cues on ED1 was taken as a measure of cocaine-seeking during early abstinence, as previously reported (Feltenstein et al., 2011, Cason et al., 2016, Kohtz and Aston-Jones, 2017). Rats were injected with saline, 0.3 mg/kg or 1.0 mg/kg OXT 30 min prior to testing.
Persistence, Extinction, and Cued Reinstatement
Following the ED1 test, rats were returned to the homecage for 14 days of abstinence. Then, rats were returned to the context as in ED1 procedures, for a second extinction session (ED2) 14 days later on withdrawal day 15 (WD15). Active lever pressing during this session was taken as a measure of cocaine seeking persistence following abstinence. Following this persistence test, rats were subjected to daily extinction sessions (90 min in the drug self-administration context in which lever presses had no consequence) for a minimum of 7 days, until they reached a criterion of <25 lever presses on either lever. Once extinction criterion was met for 3 consecutive days, rats were tested with one dose of OXT (0.3 mg/kg or 1.0 mg/kg) and a control injection of saline, in a counterbalanced manner 30 min before a test for cued reinstatement of cocaine-seeking, wherein light+tone cocaine-associated cues were presented with lever presses, but no cocaine was delivered. Rats were counterbalanced across test order in a latin-square design to control for carry-over effects of repeated OXT administration.
Locomotor Behavior
One week following cued reinstatement testing, acclimated locomotor activity was measured in clear acrylic motor testing chambers (approximately 40 × 40 × 30 cm) equipped with Digiscan monitors (AccuScan Instruments, Inc., Columbus, OH) containing a 16 × 16 photobeam array. Photobeam breaks were recorded by DigiPro software (Version 1.4). Rats (n=6/sex) were tested twice each; saline or OXT (1.0 mg/kg) was administered ip 30 min prior to testing in a counterbalanced fashion, with tests at least 2 days apart in the acclimated group (3 days of habituation in the locomotor testing chamber). A separate group of rats without prior cocaine experience was used to test the effects of OXT between-subjects on novel locomotor behavior testing.
Sucrose Progressive Ratio
Male (n=8) and female (n=8) rats that were trained to lever press on an FR1 schedule for sucrose pellets in an operant conditioning chamber. Following a minimum of 7 days of FR1 for sucrose, rats were tested in a counterbalanced fashion for sucrose progressive ratio with the following ratio schedule within a session: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603 (Richardson and Roberts, 1996). Rats were administered 1.0 mg/kg OXT or saline vehicle ip 30 min prior to testing.
Tissue Collection
Fifteen min following the ED1 session, some rats were deeply anesthetized and transcardially perfused using 0.9% saline followed by 4% paraformaldehyde. Homecage control rats were given ip injections of saline 22h after the final self-administration session and returned immediately to their home cage. Two h later those rats were sacrificed. Homecage controls and ED1 rats were paired for euthanasia date, such that each ED1-tested rat had a paired non-tested home cage control with similar FR1 experience. Homecage rats were perfused first, accounting for 15 min difference in euthanasia times between homecage- and ED1-tested rats. As Fos expression nominally is maximal at 90–120 min after neuronal stimulation, the ED1 rats were euthanized 135 min post-injection (105 min after the beginning of the ED1 test), and the homecage rats were euthanized 120 min post-injection (at the 24h withdrawal timepoint). Euthanasia at 105 min after the beginning of ED1 captured the first 25 min of the ED1 session during which maximal responding occurs (Kohtz and Aston-Jones, 2017). An additional group of rats without cocaine experience were handled daily for 10d and euthanized directly from their home cages. Brains were collected, post-fixed for 24h in 4% paraformaldehyde, transferred to a 20% sucrose solution, and stored at 4° C. Coronal sections (40 um thick) were cut using a cryostat and processed for double-label immunohistochemistry for Fos and OXT, as described below.
Fos Immunohistochemistry
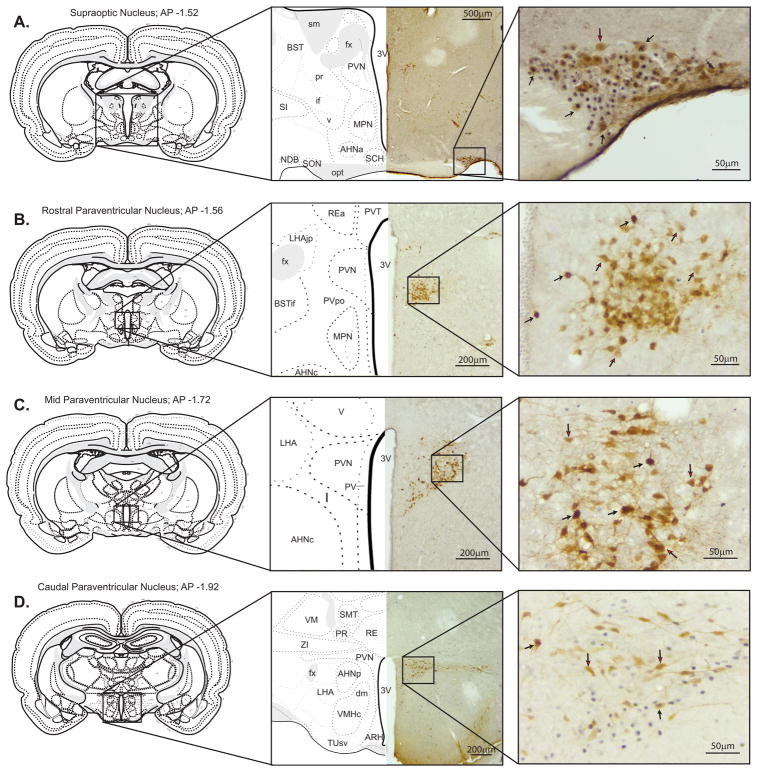
Immunohistochemistry was carried out as previously described (e.g., Mahler and Aston-Jones, 2012). Sections were quenched in 0.3% H2O2 phosphate buffered saline (PBS) plus 0.1% azide, then blocked in 2% normal donkey serum (NDS) plus PBS-Triton azide (PBST-Az) for 2h. Sections were then incubated overnight (16h) in PBST-Az-NDS with the addition of a rabbit primary antibody to Fos (1:10,000 Calbiochem, Santa Cruz, CA, Catalog# PC38, Lot #D00148958). Sections were then incubated for 2h in biotinylated donkey anti-rabbit secondary antibody (1:500, Jackson ImmunoResearch Laboratories, West Grove, PA). Sections were visualized via 32, 32-diaminobenzidine (DAB) with 0.0002% H2O2 in 0.05 M Tris buffer plus nickel ammonium sulfate for ~4.5-min to produce a dark purple reaction product (Mahler and Aston-Jones, 2012). Sections were then transferred to the second primary antibody for OXT visualization. OXT was incubated with a donkey primary antibody to OXT (1:5000 MAB5296, EMD Millipore, Burlington, MA) in PBST-NDS (16h). Sections were then incubated for 2h in biotinylated donkey anti-mouse secondary antibody (1:500, Jackson ImmunoResearch Laboratories, West Grove, PA). Sections were visualized via 32, 32-diaminobenzidine (DAB) with 0.0002% H2O2 in 0.05 M Tris buffer without nickel ammonium sulfate for ~4.5-min to produce a brown reaction product (Mahler and Aston-Jones, 2012), and then mounted, dehydrated and cover slipped for imaging. A minimum of 3 sections per rat per region were analyzed for OXT and Fos labeling in the same sections. Sections were separated into SON (Fig. 1a), PVN-rostral (PVNr; Fig. 1b), PVN-mid (PVNm; Fig. 1c), or PVN-caudal (PVNc; Fig. 1d).
Figure 1.
Representative images from frontal brain sections of OXT and Fos expression. PVN subregions were identified using landmarks established by Swanson (1979). In all images, brown staining indicates OXT-positive neurons, and purple staining indicates Fos-positive nuclei. In all photos, black arrows indicate double-labeled neurons, and red arrows indicate single labeled OXT neurons. Panel A: Supraoptic nucleus. Representative image of SON OXT/Fos dual labeling (AP: −1.52; Paxinos & Watson 2007). Panel B: PVNr. Representative image of OXT/Fos dual labeling in PVNr (AP: −1.56; Paxinos & Watson 2007). Panel C: PVNm. Representative images of OXT/Fos dual labeling in PVNm (AP: −1.72; Paxinos & Watson 2007). Panel D: PVNc. Representative images of OXT/Fos dual labeling in PVNc (AP: −1.92; Paxinos & Watson 2007).
Statistical Analysis
Independent t-tests, Pearsons-R correlations, or one- or two-way analysis of variance (ANOVA) were used to compare differences between groups for behavioral responding or for neuronal activation. Post-hoc comparisons were conducted using Bonferroni’s multiple comparisons test. All data passed the Shapiro-Wilks test of normality with W values in the range of 0.7756–0.9998, p > 0.05.
Results
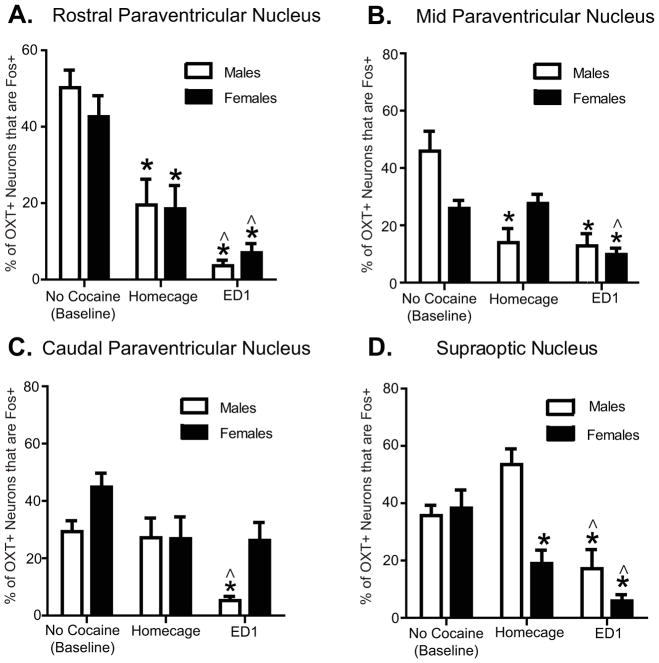
Fos in OXT neurons decreased on ED1 in a sex- and region-specific manner
To identify the response of OXT neurons in PVN and SON to cocaine withdrawal and cocaine seeking behavior, we examined Fos expression in OXT neurons in male and female rats without cocaine experience (no-cocaine baseline), 24h withdrawal from cocaine (homecage), or following engagement in ED1 cocaine-seeking (ED1).
Paraventricular Nucleus – Rostral
Cocaine self-administration followed by 24h withdrawal (homecage) decreased Fos expression in PVNr OTC+ neurons compared to no-cocaine (baseline) controls, and exposure to the context previously associated with cocaine (ED1) decreased Fos expression in OTC+ neurons compared to homecage or baseline controls (Fig. 2a); these results were similar for male and female rats. There was a significant main effect of test condition (no-cocaine baseline, 24h withdrawal, or ED1) to decrease Fos expression in OTC+ neurons [F(2,36)=42.63, p < 0.0001], but no main effect of sex nor an interaction between sex and ED1 exposure on the percentage of OTC+ neurons that were also Fos+ in PVNr.
Figure 2.
Fos expression in OXT neurons of PVN and SON nuclei is decreased in males and females on ED1 compared to no cocaine (baseline) and homecage controls. Panel A: Percentages of OXT+ neurons that are also Fos+ in rostral PVN for males (no cocaine/baseline n=8, homecage n=5, ED1 n=6) and females (no cocaine/baseline n=5, homecage n=7, ED1 n=11). ED1 exposure decreased the percentages of OXT neurons that are Fos+ in both males and females, compared to homecage controls; * indicates significant differences from respective baseline, p <0.05. ^ indicates significant differences from respective homecage, p <0.05. Panel B: PVNm. Percentages of OXT+ neurons that are also Fos+ in PVNm for males (no cocaine/baseline n=4, homecage n=5, ED1 n=11) and females (no cocaine/baseline n=5, homecage n=6, ED1 n=12). ED1 exposure decreased the percentage of OXT neurons that are also Fos+ in females only, compared to female homecage controls; * indicates significant differences from respective baseline, p <0.05. ^ indicates significant differences from respective homecage, p <0.05. Panel C: Caudal PVN. Percentages of OXT neurons that are Fos+ in PVNc for males (no cocaine/baseline n=4, homecage n=6, ED1 n=6) and females (no cocaine/baseline n=6, homecage n=4, ED1 n=11). ED1 exposure decreased the percentage of OXT neurons that are Fos+ in males only, compared to male homecage controls; * indicates significant differences from male baseline, p <0.05. ^ indicates significant differences from male homecage, p <0.05. Panel D: Supraoptic nucleus. Percentages of OXT neurons that are Fos+ in SON for males (no cocaine/baseline n=7, homecage n=4, ED1 n=10) and females (no cocaine/baseline n=5, homecage n=6, ED1 n=11). ED1 exposure decreased the percentage of OXT neurons in SON that are Fos+ in both males and females compared to homecage or to no-cocaine controls; * indicates significant differences from respective baseline, p <0.05. ^ indicates significant differences from respective homecage, p <0.05.
The percentage of PVNr OXT+ neurons that were also Fos+ significantly correlated to ED1 cocaine-seeking in males [R2=0.53, p < 0.05], but not in females or when collapsed across sex. However, it is notable that all of the values for numbers of Fos+/OXT+ neurons in the ED1 group were less than 10, limiting the ability to measure differences in Fos expression in OXT neurons among groups.
There were no significant effects of test condition (no-cocaine baseline, homecage 24h withdrawal, or ED1) or sex (male, female) on the number of OXT+ neurons in rostral PVN (Table 1).
Table 1.
Total average number of oxytocin-positive cells (mean ± S.E.M.) in paraventricular nucleus and supraoptic nucleus from sections examined. Three sections per animal were analyzed per side for each area, and cell numbers were averaged across sections. Animals were only included that had 3 sections available for analysis. The number of oxytocin neurons reported in the table are the average number of cells identified per 40-micron section under each condition.
| Total number of Oxytocin positive cells | No Cocaine Baseline (Naïve Rats) | Homecage (24 Hr Cocaine Withdrawal) | Extinction Day 1 (24 Hr Cocaine Withdrawal + Cocaine-Seeking Test) | ||||
|---|---|---|---|---|---|---|---|
| Male (n=5–8) | Female (n=4–6) | Male (n=5–6) | Female (n=4–7) | Male (n=6–11) | Female (n=11–12) | ||
| PVN | PVNr (Rostral; AP −1.56, Fig. 1b) | 48.1 ± 7.9 | 43.13 ± 5.8 | 62.2 ± 10.8 | 64.4 ± 16.0 | 68.2 ± 21.2 | 54.3 ± 7.9 |
| PVNm (Mid; AP −1.72, Fig. 1c) | 99.2 ± 11.0 | 127.31 ± 26.7 | 103.3 ± 10.0 | 71.7 ± 19.4 | 77.6 ± 11.7 | 70.9 ± 14.0 | |
| PVNc (Caudal; AP −1.92, Fig. 1d) | 35.31 ± 6.6 | 45.05 ± 6.9 | 40.2 ± 4.6 | 28.9 ± 6.1 | 26.4 ± 3.7 | 39.6 ± 7.2 | |
| Supraoptic Nucleus (AP −1.52; Fig. 1a) | 38.49 ± 4.7 | 31.10 ± 5.0 | 59.21 ± 10.5* | 47.7 ± 5.3* | 64.5 ± 4.1* | 57.7 ± 5.8* | |
p < 0.05, difference from respective no cocaine controls.
Paraventricular Nucleus – Mid
Our results indicate an interaction between sex and test condition [F(2,37)=5.818, p < 0.005] wherein cocaine experience (i.e. homecage or ED1) significantly reduced Fos in PVNm OXT+ neurons in males compared to no-cocaine baseline controls. In contrast, only ED1 exposure decreased Fos in OXT+ neurons in females (Fig. 2b). Notably, the percentage of PVNm OXT+ neurons that were also Fos+ significantly correlated to ED1 cocaine-seeking among females [R2=0.29, p < 0.05] and when collapsed across sex [R2=0.21, p < 0.05], but did not significantly correlate among males, indicating that PVNm may correspond more to cocaine-seeking behaviors in females than males.
There were no significant effects of test condition (no-cocaine baseline, homecage 24h withdrawal, or ED1) or sex (male, female) on the number of OXT+ neurons in PVNm (Table 1).
Paraventricular Nucleus - Caudal
In males, ED1 exposure decreased Fos in PVNc OXT+ neurons [post-hoc F(2,15)=7.676, p < 0.01] compared to 24h withdrawal or no cocaine baseline controls, reflecting a significant main effect of sex [F(1,31)=5.040, p < 0.05] and of test condition [F(2,31)=5.794, p < 0.01]. There were no effects of cocaine withdrawal or ED1 exposure on Fos in PVNc OXT+ neurons of female rats. These results indicate that PVNc OXT+ neurons may respond to engagement in cocaine-seeking behavior to a greater extent in males compared to females, however the percentage of PVNc OXT+ neurons that were also Fos+ did not correlate to ED1 cocaine-seeking.
There were no significant effects of test condition (no-cocaine baseline, homecage 24h withdrawal, or ED1) or sex (male, female) on the number of OXT expressing neurons in PVNc (Table 1).
Supraoptic Nucleus
Sex differences emerged among SON OXT+ neurons dependent on test condition. There was a significant interaction between sex and test condition on the percentage of SON OXT+ neurons that were also Fos+ [F(2,37)=4.905, p < 0.01; Fig. 2d]. In females, cocaine self-administration followed by 24h withdrawal (homecage) decreased Fos expression in OXT+ neurons compared to no-cocaine (baseline) controls, and exposure to the context previously associated with cocaine (ED1) decreased Fos expression in OXT+ neurons further. In contrast, in males, only ED1 significantly reduced Fos in OXT+ neurons. The percentage of SON OXT+ neurons that were also Fos+ did not significantly correlate to ED1 cocaine-seeking among males, females, or when collapsed across sex. However, it is notable that the majority of values for numbers of Fos+/OXT+ neurons in the ED1 group were less than 10, limiting the ability to measure differences in Fos expression in OXT neurons among groups.
There was a significant main effect of test condition (no-cocaine baseline, homecage 24h withdrawal, or ED1), but not sex, on the number of OXT+ neurons in SON (Table 1) wherein cocaine exposure increased the number of OXT-expressing neurons in SON [F(1,37)=8.822; p < 0.001]. The number of SON OXT+ neurons negatively correlated to the percentage of OXT+ neurons that were also Fos+ in females [R2=0.48; p < 0.05], but not males. However, there were significantly less total Fos+/OXT+ neurons on ED1 [2.88 ± 0.91] compared to homecage [7.58 ± 2.07] or no cocaine controls [11.01 ± 1.66; F(2,22)=7.422, p < 0.05], indicating that the percentage decrease in Fos+/OXT+ neurons on ED1 was not accounted for by an increase in total OXT-expressing neurons in SON.
Systemic administration of OXT was more effective in reducing cocaine seeking-behavior on ED1 in female than male rats
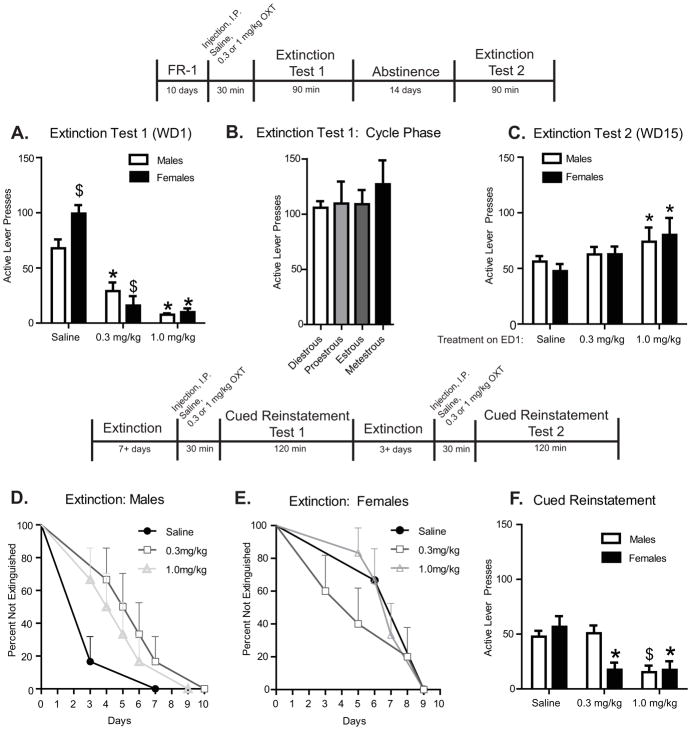
To test the effects of systemic OXT administration on cocaine-seeking behavior, rats were injected with OXT (0.3 or 1.0 mg/kg, ip) or saline vehicle 30min prior to the ED1 session in the operant conditioning box. Rats then underwent a 90min ED1 session during which ‘active’ lever presses had no outcome but were recorded as a measure of cocaine-seeking behavior. Cocaine-seeking behavior on ED1 was greater in both males [t(1,16)=2.694, p < 0.05] and females [t(1,16)=6.179, p < 0.0001] when compared to active lever presses during the last 3 days of self-administration (‘extinction burst’), as per prior reports (Kohtz and Aston-Jones, 2017). As well, females had higher active lever pressing on ED1 compared to males when administered saline vehicle [t(1,15)=3.691, p < 0.005]. These effects were independent of estrous cycle phase, as one-way ANOVA analyses reveal no significant effect of estrous cycle phase on ED1 cocaine-seeking in female rats (Fig. 3b). Systemic OXT administration had greater efficacy in females compared to males to dose-dependently reduce active lever responding OXT (Fig. 3a), as shown by two-way ANOVA analyses wherein there was a significant interaction between sex and drug condition [F(2,39)=5.384, p < 0.01]. There was a statistically significant effect of OXT dose-dependently decrease ED1 cocaine-seeking in both sexes [F(2,39)=64.42, p < 0.0001].
Figure 3.
Systemic OXT administration reduces cocaine-seeking behaviors. Panel A. Systemic administration of OXT (0.3 or 1.0 mg/kg, ip) 30 min prior to ED1 testing decreased cocaine-seeking behavior as measured by active lever presses, in males (saline n= 9, 0.3mg/kg n=8, 1.0 mg/kg, n=6) and females (saline n= 8, 0.3 mg/kg n=7, 1.0 mg/kg, n=7), compared to saline-administered controls; * p <0.05. Among saline controls, females displayed greater cocaine-seeking behavior compared to males; $ p <0.05. Panel B: ED1 responding across the estrous cycle in saline-administered rats (diestrous n=5, proestrous n=5, estrous n=10, metestrous n=4). No significant differences were observed. Panel C. Following ED1 testing, rats were returned to their homecages for 14 days of forced abstinence. When returned to the self-administration context for ED2 testing on WD15 (relapse test, no cocaine or cues), males that had been administered saline or OXT (0.3 mg/kg) on ED1 did not show extinction of the cocaine-seeking phenotype (e.g., ED2 responding was similar to ED1 saline responding), but those administered 1.0 mg/kg OXT showed increased responding on ED2 compared to ED1. In contrast, females administered saline or OXT (0.3 mg/kg) showed extinction of the cocaine-seeking phenotype on ED2 compared to ED1. However, females administered OXT (1.0 mg/kg) on ED1 had greater cocaine-seeking behavior on ED2 compared to those previously administered saline * p <0.05. Panel D. Survival curve depicting rate of extinction in male rats (n=6/group) following systemic OXT administration on ED1 and 2 weeks of abstinence. Day 0 represents responding on the first test day after 2 weeks of abstinence. * indicates significant differences from ED1 saline administered controls * p <0.05. Panel E. Survival curve depicting rate of extinction in female rats (n=6/group) following systemic OXT administration on ED1 and 2 weeks of abstinence, illustrated as for Panel D. Panel F. Systemic administration of OXT (0.3 or 1.0 mg/kg, ip) 30 min prior to cued-reinstatement testing decreased cocaine-seeking behaviors as measured by active lever presses, in males in males (saline n=19, 0.3 mg/kg n=9, 1.0 mg/kg, n=10) and females (saline n=18, 0.3 mg/kg n=8, 1.0 mg/kg, n=11) compared to saline administered controls; * p <0.05.
Following ED1, rats were returned to their homecages and underwent a period of abstinence for 2 weeks. Rats were then returned to the operant conditioning chamber and were tested for ED2 responding (lever presses had no outcome but were recorded) to determine if the effects of OXT treatment on ED1 were persistent. Our data indicate no significant sex differences in cocaine-seeking behavior on ED1, and that OXT administration on ED1 dose-dependently increased the persistence of cocaine-seeking as measured on ED2 (Fig. 3c). A two-way ANOVA comparing sex and drug condition revealed a significant main effect of ED1 OXT administration [F(2,39)=3.873, p < 0.05], but no main effect of sex nor an interaction to influence responding on ED2.
Notably, when persistent cocaine-seeking (after 2 wk abstinence as above) is compared to initial cocaine seeking behavior on ED1, males show greater initial extinction resistance (Fig. 3ac). A repeated measures ANOVA between test day (ED1 vs ED2) and treatment condition on ED1 (OXT or saline) among males revealed a main effect of day (F(1,20)=26.64, p < 0.0001), and a main effect of ED1 treatment (F(2,20)=3.851, p < 0.05). In addition, there was an interaction between test day and ED1 treatment (F(2,20)=15.70, p < 0.0001), wherein male rats administered OXT (1.0 mg/kg) on ED1 had dose-dependently greater cocaine-seeking behaviors on ED2. In females, there was a main effect of ED1 treatment, (F(1,18)=6.945, p < 0.005) and a main effect of day (F1,18)=8.475, p < 0.01) wherein females administered saline on ED1 showed decreased cocaine-seeking behaviors on ED2 (e.g., showed extinction), albeit not to criterion. In addition, there was an interaction between test day and ED1 treatment in females (F(1,18)=31.78, p < 0.0001), such that females that were administered OXT (1.0 mg/kg) on ED1 had dose-dependently greater cocaine-seeking behaviors on ED2 than those administered saline on ED1.
OXT administration reduced cued-reinstatement of cocaine seeking to a greater extent in female than male rats
After the ED2 session, rats experienced daily 2h extinction sessions for a minimum of 7 days prior to testing for cued reinstatement of responding. Females were overall more extinction resistant (more days to extinction criterion) than males. Two-way ANOVA analyses on the number of days to reach extinction criteria revealed a main effect of sex (F(1,29)=8.604, p < 0.05) wherein females took longer to reach extinction criteria compared to males. 1.0 mg/kg, but not 0.3 mg/kg OXT administered on ED1 produced significant extinction resistance in males (Fig. 3d) and facilitated extinction in females (Fig. 3e). There was an interaction between sex and OXT administration on ED1 to influence the number of days to reach extinction criteria wherein females administered OXT on ED1 took fewer days, primarily when administered 0.3 mg/kg OXT, and males administered OXT on ED1 took more days, to meet extinction criteria irrespective of OXT dose (F(2,29)=3.826, p < 0.05).
Rats were administered OXT (0.3 mg/kg or 1.0 mg/kg) or saline vehicle 30-min prior to testing for cued reinstatement of cocaine seeking. OXT effectively decreased cued-induced cocaine seeking in both males and females. There was a significant interaction between sex and drug condition wherein OXT was effective at both 0.3 mg/kg and 1.0 mg/kg to reduce cued reinstatement in females, but in males only the 1.0 mg/kg dose was effective (F2,69)=3.343, p < 0.05) (Fig. 3f).
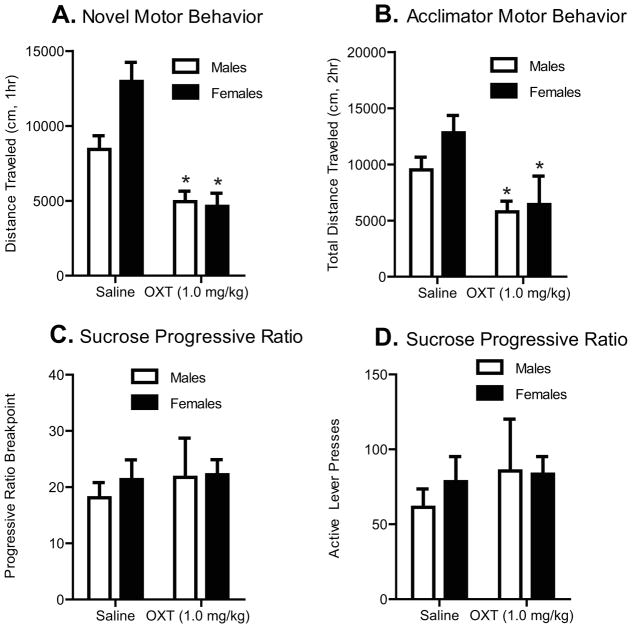
OXT administration reduced non-motivated locomotor activity, but not operant responding for sucrose reward
In naïve animals we investigated whether systemic OXT administration influenced simple, non-motivated locomotor activity in a novel, open field locomotor testing chamber. We found that systemic OXT significantly decreased total distance traveled to a greater extent in females compared to males (F1,20)=4.52, p < 0.05) (Fig. 4a). We also found that there was a significant main effect of OXT administration to reduce motor behavior in the novel locomotor testing chamber (F(1,20)=26.67, p < 0.001) independent of sex.
Figure 4.
Systemic OXT administration reduced locomotor behavior in novel and acclimated test chambers (females n=6, males n=7), but not lever responding during sucrose progressive ratio (n=8/sex). Panel A. Systemic administration of OXT (1.0 mg/kg, ip) 30 min prior to testing decreased locomotor behavior in a novel chamber as measured by total distance travelled (cm), in males and females, compared to saline controls; * p <0.05. Panel B. Administration of OXT (1.0 mg/kg, ip) 30 min prior to testing in a familiar chamber (after acclimation for 2 days), decreased locomotor behavior as measured by total distance travelled (cm), in males and females, compared to saline administered controls; * p <0.05. Panel C. OXT administration (1.0 mg/kg, ip) did not alter progressive ratio breakpoints, or the number of active lever presses made during sucrose progressive ratio testing in males or females (Panel D).
Rats that were tested in cued-reinstatement were returned to their homecages for one week. Then, these rats were acclimated to the locomotor testing chamber for 3 days prior to testing. There was a significant main effect of OXT (1.0 mg/kg) administration to reduce motor behavior in the acclimated environment (F(1,22)=10.21, p < 0.05); however, we observed no interaction between sex and drug condition, and no effect of sex on activity in the acclimated condition (Fig. 4b).
A separate group of rats was used to test whether systemic OXT (1.0 mg/kg) influenced progressive ratio lever responding for sucrose reward (Fig. 4d). We observed no effects of OXT on sucrose progressive ratio measures in either males or females. Two-way ANOVAs indicated no significant interactions between sex and OXT, no main effects of sex or OXT, on sucrose progressive ratio breakpoints or number of pellets acquired. These results show that although OXT can reduce simple, non-motivated locomotor activity, it does not affect motivated operant behavior for a natural food reward. This indicates that the effects obtained for OXT on cocaine ED1 and reinstatement behaviors were not due to general non-specific effects on lever pressing abilities.
Discussion
Our results support previous findings demonstrating that there are sex differences in operant cocaine seeking during initial abstinence, wherein females exhibit greater cocaine-seeking than males (Lynch et al., 2002, Fuchs et al., 2005, Kippin et al., 2005, Feltenstein et al., 2011, Holly et al., 2012, Cason et al., 2016). Moreover, our results indicate that the increased cocaine seeking during initial abstinence (ED1) is accompanied by decreased Fos-activation of PVN and SON OXT neurons. Pretreatment with systemic OXT decreased cocaine seeking on ED1, as well as when administered prior to cued-reinstatement, in female and male rats. Prior reports indicate that administration of OXT decreased cocaine demand in male rats, and demand for methamphetamine in males and females (Bentzley et al., 2014, Cox et al., 2017, Westenbroek et al., 2017), further supporting OXT’s therapeutic potential. However, our results also indicate that an acute administration of OXT on ED1 can delay context extinction, particularly in male rats.
Systemic administration of OXT has poor blood-brain barrier penetrance (Ermisch et al., 1985, Lee et al., 2018). However, previous studies recapitulated effects of systemic OXT administration with intracerebroventricular or site-specific intracranial infusions across a number of behavioral tasks and species (Ring et al., 2006, Baracz et al., 2012). As well, peripheral administration of OXT induces Fos expression and activity of OXT neurons in the paraventricular nucleus, perhaps via stimulation of their peripheral terminals (Carson et al., 2010, Leong et al., 2017). We speculate that OXT administered peripherally may induce endogenous central OXT release as previously proposed by others (Rossoni et al., 2008, Neumann et al., 2013, Leong et al., 2017).
Numerous studies reveal region-, sex-, and cycle-specific differences in expression of OXT and OXT-receptors associated with stress and hormonal state. For example, OXT mRNA expression in female rats is higher during proestrous than diestrous; in fact, little or no OXT mRNA expression is observed during diestrous (Bale et al., 1995). We found that ED1 cocaine-seeking is consistently independent of the hormonal cycle in females (Fig. 3b). However, OXT activity appears to vary markedly with stress, e.g. forced swim test (Lu et al., 2015) or ED1 (Cason et al., 2016, Kohtz and Aston-Jones, 2017) as reported herein. We hypothesize that the increased circulating stress steroid levels produced by engagement in ED1 cocaine-seeking in both males and females and subsequent effects on OXT signaling obfuscates sexual dimorphisms in OXT expression.
Sex differences in OXT function in the brain in response to challenge (e.g. stress, cocaine) may relate to sexually divergent roles of OXT in estradiol-dependent behaviors. OXT administration induces maternal and sexual behaviors in females (Pedersen and Prange, 1979, Pedersen et al., 1982, Insel, 1990, Champagne et al., 2001), and the most dramatic effects of steroids on OXT in the PVN are seen post-partum during mother-infant bond formation (Insel, 1990, Keverne and Curley, 2004). These data may indicate greater responsivity of the female OXT system compared to males that confers greater efficacy of exogenous OXT on behavior.
Endogenous as well as exogenously administered OXT influences stress response mechanisms. Previous studies show that brain OXT inhibits activation of the HPA axis (Windle et al., 2004, Neumann, 2007). OXT engages a positive feedback loop via somatodendritic release of OXT in PVN (Leng et al., 1999), OXT autoreceptors, and intracellular calcium signaling independent of voltage gated calcium channels that results in increased release of OXT at terminals (Lambert et al., 1994, Dayanithi et al., 2000, Ludwig et al., 2002). Notably, OXT also interacts with corticotropin-releasing hormone (CRF) neurons, and intracranial infusions of an OXT receptor antagonist into PVN can enhance basal and stress-induced secretion of adrenocorticotropic hormone (ACTH) and corticosterone (Neumann et al., 2000). Based on these data, we speculate that OXT may exert a tonic inhibitory tone on the HPA axis. We previously found that ED1 exposure induced corticosterone secretion in male and female rats (Kohtz and Aston-Jones, 2017); this is consistent with the above hypothesis for CRF-OXT interactions. We also suggested that ED1 represents a stressful time point in the addiction cycle due to the absence of expected drug reward (Cason et al., 2016, Kohtz and Aston-Jones, 2017), and that this might engage this CRF-OXT circuit. Indeed, we found that a selective CRF1 receptor antagonist significantly decreased ED1 cocaine seeking (Cason et al., 2016). Also, previous research from our lab found that OXT substantially reduced drug-seeking in other stressful paradigms, such as primed, cued or yohimbine-induced reinstatement of methamphetamine seeking (Cox et al., 2013), or cocaine demand and cued cocaine seeking (Bentzley et al., 2014). Here, we extend those findings to show reduced cocaine seeking in ED1 following systemic administration of OXT, and that OXT’s therapeutic efficacy is greater in females compared to males. In addition, we show that activity of OXT neurons is significantly attenuated by cocaine withdrawal and engagement in cocaine-seeking, in a sex by PVN subregion specific manner.
Similar to prior reports, we observed an effect of systemic OXT to suppress non-motivated locomotor behavior, irrespective of prior OXT experience, but not active lever pressing for sucrose during progressive ratio responding (Cox et al., 2013, Zhou et al., 2015a, Zhou et al., 2015b). Repeated OXT administration during adolescence (PND 28–37) inhibits progressive ratio for methamphetamine in adulthood (Hicks et al., 2016); however repeated intermittent OXT administration (as reported herein) does not alter active lever presses or cocaine intake in female rats (Leong et al., 2016). In addition, prior reports also indicate that although FR1 sucrose is affected by OXT in naïve rats (Zhou et al. 2015), progressive ratio responding is not (Cox et al., 2013). As the amount of active lever pressing during sucrose progressive ratio responding is similar to that of ED1 after cocaine, our results indicate that the effects of OXT on ED1 and reinstatement lever responding are not due to simple motor effects; instead, motor actions of OXT appear restricted to non-motivated behavior. One possibility is that OXT is effective in reducing reward seeking under stressful contexts (eg, drug abstinence or extinction), but not when rewards are available to self-administer.
Region-specific sex differences in PVN OXT activity on ED1 may contribute to increased ED1 cocaine-seeking behavior in female rats. Here, we show that in males only, PVNr OXT neurons exhibit less Fos on ED1, and negatively correlate to cocaine-seeking behavior. Whereas, in females only, PVNm OXT neurons are less Fos-responsive on ED1 and negatively correlate to cocaine-seeking behavior. Notably, PVNm OXT neurons have stronger projections to pre-locus coeruleus (LC) and the nucleus of the solitary tract (NTS) compared to projections from PVNr/c, exerting an inhibitory tone on NE release from LC or NTS (Geerling et al., 2010, Uchoa et al., 2013). Stress-responsive norepinephrine (NE) neurons in LC and NTS are more Fos-activated on ED1 in females compared to males, and are implicated in driving the female phenotype of increased initial cocaine-seeking (Cason et al., 2016, Kohtz and Aston-Jones, 2017). Our results support and extend prior research indicating that stress-responsive neural circuitry may drive sex differences in cocaine-seeking behaviors (e.g. greater seeking in female rodents) while providing further evidence that “anti-stress” pharmacotherapies (i.e. OXT, CRF antagonists, NE antagonists) are more effective in reducing cocaine-seeking in females than in males. Investigating interactions between OXT and stress responsive signaling targets is a goal for future studies.
In summary, we found that OXT neurons are less active during initial abstinence from cocaine self-administration, and further decreased their activity during ED1 exposure. This reduction in OXT neuron activity following cocaine exposure may contribute to drug seeking during initial abstinence. Indeed, systemic administration of OXT, which we speculate may replace endogenous reductions induced by cocaine experience and ED1 exposure, decreased cocaine-seeking behavior during initial abstinence. However, these effects were transient and, in fact, high dosage OXT (1.0 mg/kg) delayed extinction. Thus, exogenously administered OXT may be a potential therapeutic target for mitigating cocaine craving during the initiation of abstinence and in the presence of cocaine-associated cues.
Acknowledgments
Funding
This research received project support from PHS grant P50 DA016511, R01 DA006214 and T32ES007148.
Footnotes
Disclosure
The authors declare no conflict of interest.
References
- Amadei EA, Johnson ZV, Jun Kwon Y, Shpiner AC, Saravanan V, Mays WD, Ryan SJ, Walum H, Rainnie DG, Young LJ, Liu RC. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature. 2017;546:297–301. doi: 10.1038/nature22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Zhu Y, Card JP. Numerous GABAergic afferents to locus ceruleus in the pericerulear dendritic zone: possible interneuronal pool. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:2313–2321. doi: 10.1523/JNEUROSCI.5339-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM, Johnston CA. Oxytocin receptor mRNA expression in the ventromedial hypothalamus during the estrous cycle. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15:5058–5064. doi: 10.1523/JNEUROSCI.15-07-05058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracz SJ, Everett NA, McGregor IS, Cornish JL. Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats. Addiction biology. 2016;21:316–325. doi: 10.1111/adb.12198. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, Cornish JL. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behavioural brain research. 2012;228:185–193. doi: 10.1016/j.bbr.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:11822–11827. doi: 10.1073/pnas.1406324111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cerebral cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, Boucher AA, McGregor IS. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addiction biology. 2010;15:448–463. doi: 10.1111/j.1369-1600.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- Cason AM, Kohtz A, Aston-Jones G. Role of Corticotropin Releasing Factor 1 Signaling in Cocaine Seeking during Early Extinction in Female and Male Rats. PloS one. 2016;11:e0158577. doi: 10.1371/journal.pone.0158577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Bentzley BS, Regen-Tuero H, See RE, Reichel CM, Aston-Jones G. Oxytocin Acts in Nucleus Accumbens to Attenuate Methamphetamine Seeking and Demand. Biological psychiatry. 2017;81:949–958. doi: 10.1016/j.biopsych.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 2013;38:2343–2353. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayanithi G, Sabatier N, Widmer H. Intracellular calcium signalling in magnocellular neurones of the rat supraoptic nucleus: understanding the autoregulatory mechanisms. Exp Physiol. 2000;85:75s–84s. doi: 10.1111/j.1469-445x.2000.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Ermisch A, Barth T, Ruhle HJ, Skopkova J, Hrbas P, Landgraf R. On the blood-brain barrier to peptides: accumulation of labelled vasopressin, DesGlyNH2-vasopressin and oxytocin by brain regions. Endocrinologia experimentalis. 1985;19:29–37. [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology. 2011;216:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. The Journal of comparative neurology. 2010;518:1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological reviews. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Hicks C, Cornish JL, Baracz SJ, Suraev A, McGregor IS. Adolescent pre-treatment with oxytocin protects against adult methamphetamine-seeking behavior in female rats. Addiction biology. 2016;21:304–315. doi: 10.1111/adb.12197. [DOI] [PubMed] [Google Scholar]
- Holly EN, Shimamoto A, Debold JF, Miczek KA. Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology. 2012;224:179–188. doi: 10.1007/s00213-012-2846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Regional changes in brain oxytocin receptors post-partum: time-course and relationship to maternal behaviour. J Neuroendocrinol. 1990;2:539–545. doi: 10.1111/j.1365-2826.1990.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behaviour. Current opinion in neurobiology. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology. 2005;182:245–252. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Kohtz AS, Aston-Jones G. Cocaine Seeking During Initial Abstinence Is Driven by Noradrenergic and Serotonergic Signaling in Hippocampus in a Sex-Dependent Manner. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2017;42:408–418. doi: 10.1038/npp.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Barbarczi E, Szabo G, Telegdy G. The role of oxytocin-dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology. 1990;29:365–368. doi: 10.1016/0028-3908(90)90095-9. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Dayanithi G, Moos FC, Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. The Journal of physiology. 1994;478( Pt 2):275–287. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in neuroendocrinology. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lee MR, Scheidweiler KB, Diao XX, Akhlaghi F, Cummins A, Huestis MA, Leggio L, Averbeck BB. Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Molecular psychiatry. 2018;23:115–122. doi: 10.1038/mp.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Brown CH, Russell JA. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Progress in neurobiology. 1999;57:625–655. doi: 10.1016/s0301-0082(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Leong KC, Freeman LR, Berini CR, Ghee SM, See RE, Reichel CM. Oxytocin Reduces Cocaine Cued Fos Activation in a Regionally Specific Manner. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2017;20:844–854. doi: 10.1093/ijnp/pyx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KC, Zhou L, Ghee SM, See RE, Reichel CM. Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats. Experimental and clinical psychopharmacology. 2016;24:55–64. doi: 10.1037/pha0000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22:443–453. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and behavior. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu XY, Zhu QB, Li J, Shi LG, Wu JL, Zhang QJ, Huang ML, Bao AM. Sex differences in the stress response in SD rats. Behavioural brain research. 2015;284:231–237. doi: 10.1016/j.bbr.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Catena Dell’osso M. The role of oxytocin in neuropsychiatric disorders. Current medicinal chemistry. 2008;15:698–704. doi: 10.2174/092986708783885291. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc T. 2007;35:1252–1257. doi: 10.1042/BST0351252. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Kromer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regulatory peptides. 2000;96:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behavioral neuroscience. 2003;117:1054–1065. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of neuroscience methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology. 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, Moos F. Emergent synchronous bursting of oxytocin neuronal network. PLoS computational biology. 2008;4:e1000123. doi: 10.1371/journal.pcbi.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Babarczy E, Krivan M, Szabo G, Kovacs GL, Barth T, Telegdy G. Selective attenuation of cocaine-induced stereotyped behaviour by oxytocin: putative role of basal forebrain target sites. Neuropeptides. 1991;19:51–56. doi: 10.1016/0143-4179(91)90073-r. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovacs GL. Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology. 1994;19:85–117. doi: 10.1016/0306-4530(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. alpha(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biological psychiatry. 2011;70:712–719. doi: 10.1016/j.biopsych.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. The European journal of neuroscience. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchoa ET, Zahm DS, de Carvalho Borges B, Rorato R, Antunes-Rodrigues J, Elias LL. Oxytocin projections to the nucleus of the solitary tract contribute to the increased meal-related satiety responses in primary adrenal insufficiency. Exp Physiol. 2013;98:1495–1504. doi: 10.1113/expphysiol.2013.073726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Frontiers in neuroendocrinology. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Perry AN, Jagannathan L, Becker JB. Effect of social housing and oxytocin on the motivation to self-administer methamphetamine in female rats. Physiology & behavior. 2017 doi: 10.1016/j.physbeh.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, See RE, Reichel CM. Oxytocin differentially affects sucrose taking and seeking in male and female rats. Behavioural brain research. 2015a;283:184–190. doi: 10.1016/j.bbr.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sun WL, Young AB, Lee K, McGinty JF, See RE. Oxytocin reduces cocaine seeking and reverses chronic cocaine-induced changes in glutamate receptor function. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2015b:18. doi: 10.1093/ijnp/pyu009. [DOI] [PMC free article] [PubMed] [Google Scholar]