Abstract
Background
Indirubin, a constituent of the Chinese herbal medicine “Qing-Dai,” has anti-cancer and anti-inflammatory activities. We aimed to evaluate the efficacy of indirubin for ameliorating colonic inflammation in a mouse model of inflammatory bowel disease.
Methods
Mice with dextran sulfate sodium (DSS)-induced acute and chronic colitis were treated with indirubin in their diet. Clinical and histologic changes were evaluated. In addition, colon levels of interleukin-6, a critical pro-inflammatory mediator, was detected by enzyme-linked immunosorbent assay.
Results
In the model of acute colitis, indirubin treatment improved the loss of body weight. Histology of colonic tissue revealed that indirubin treatment improved the histology grading of colitis (P = 0.02), the extent of submucosal fibrosis (P = 0.018), the number of mucosal toluidine blue-positive cells (P = 0.004) and colon length (P = 0.01). In the model of chronic colitis, indirubin treatment had no significant effect on pathologic findings except for colon length (P = 0.003). However, indirubin administration significantly reduced colon levels of interleukin-6 in the chronic-colitis model (P = 0.001).
Conclusion
Our study clearly showed that oral intake of indirubin can improve murine DSS-induced colitis (which mimics human inflammatory bowel disease).
Keywords: Chinese herbal medicine, DSS-induced colitis model, indirubin, inflammatory bowel disease, Qing-Dai
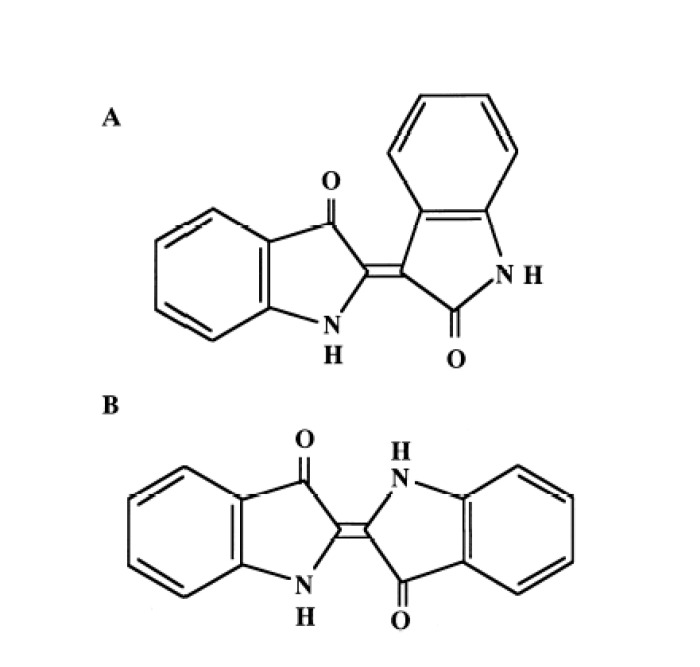
Indirubin is an active ingredient of a traditional Chinese medicine (TCM) named “Danggui Longui Wan,” which has potent activity against myelocytic leukemia.1, 2 Indirubin is the purple component of the dye indigo blue, which is extracted from plants such as Polygonum tinctorium Lour and Indigofera tinctoria. Indirubin is a 3,2’-bisindole, a stable isomer of indigo (Fig. 1).
Fig. 1.
Chemical structures of indirubin (A) and indigo (B).
Indirubin has anti-inflammatory and anti-cancer activities. Kunikata et al. reported that indirubin inhibits inflammatory reactions in delayed-type hypersensitivity in mice.3 They described the effects of indirubin on cytokine production by immunocompetent cells and on the delayed-type hypersensitivity reaction in vitro, in which interferon-γ has a crucial role. Mak et al. reported that indirubin suppresses expression of the influenza virus-induced chemokine RANTES in human bronchial epithelial cells.4 Moreover, indirubin has shown potent anti-proliferative activity in various human cancer cells.5 In a recent prospective study, Sugimoto et al. reported that the Chinese herbal medicine “Qing-Dai,” (which contains indole compounds, including indirubin) was effective for patients with ulcerative colitis (UC), which is involved in inflammatory bowel disease (IBD).6
Oral administration of the sulfated polysaccharide dextran sulfate sodium (DSS) induces colitis in mice,7, 8rats,9 guinea pigs,10 and hamsters.11 Okayasu et al. reported a UC model involving oral administration of DSS in BALB/c and CBA/J mice.7 Depending on the time-course of oral administration of DSS in drinking water, it could induce acute or chronic colitis in mice.7 DSS-induced colitis exhibits several morphologic and pathophysiologic features similar to those observed in human IBD, including production of cytokines and other inflammatory mediators, as well as leukocyte infiltration.7, 8, 12 A DSS-induced model of colitis in animals has been used to examine the effect of various anti-inflammatory agents against IBD.13–17
Herein, we examined the effect of indirubin on DSS-induced colitis in mice. We showed, for the first time, that indirubin treatment can improve murine DSS-induced colitis.
MATERIALS AND METHODS
Animals
Female BALB/c mice (6 weeks; 20–22 g) were obtained from Charles River Japan (Kanagawa, Japan). The mice were treated in accordance with the Guidelines for Animal Experimentation set by Tottori University (06-S-76) (Tottori, Japan). Animals were housed in rooms at a controlled temperature of 24 ± 2 °C and light-dark cycle with a maximum of six mice per cage. Mice were fed with standard pellets (MF; Oriental Yeast, Tokyo, Japan) and had free access to drinking water.
Chemicals
DSS (molecular weight: 36,000–50,000) was purchased from ICN Biomedicals (Eschwege, Germany). Indirubin was synthesized by Asahi Kasei Finechem (Osaka, Japan).18
Induction of colitis and treatment with indirubin
Acute colitis was induced with 2% DSS given in drinking water for ≤ 6 days (n = 12). Mice were treated with indirubin (0.05 μg/g/day) in their diet (n = 6). Mice not given DSS or indirubin were used as the control group (n = 6). Chronic colitis was induced by administration of 2% DSS in three cycles for 6 days, interspersed with 8 days of giving drinking water (n = 12). Mice were treated with indirubin (0.05 μg/g/day) in their diet (n = 6). Mice not given DSS or indirubin were used as the control group (n = 6).
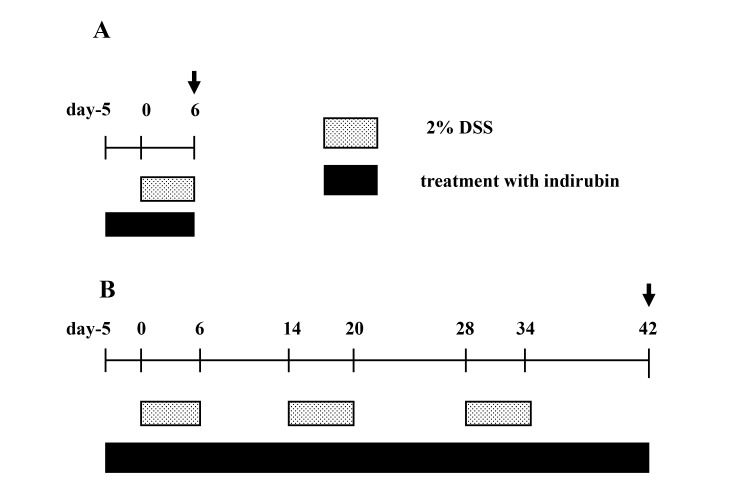
Indirubin treatment was started before the 5 days of DSS exposure and continued throughout the observation period in acute and chronic models of colitis (Fig. 2).
Fig. 2.
Experimental protocols for DSS-induced acute and chronic colitis experiments. (A) In acute-colitis studies, mice were subjected to one, 6-day DSS cycle. Mice were fed a normal or indirubin-supplemented diet, commencing 5 days before the start of the experiment and continuing until the end of the experiment. Mice were killed on day-6 (arrow). (B) For the study of chronic colitis, mice were subjected to three consecutive DSS cycles. Each cycle consisted of a DSS-administration period followed by a recovery period in which water was administrated. Mice were fed a normal or indirubin-supplemented diet commencing 5 days before the start of the experiment and continuing until the end of the third DSS cycle. All surviving mice were killed at the completion of the third DSS cycle (arrow). DSS, dextran sulfate sodium.
Evaluation of colitis and drug effects
General parameters recorded in the experiments were body weight and food consumption. After the end of the experiment, all mice were killed by cervical dislocation. Immediately, large intestines were resected between the ileocecal junction and proximal rectum. The length of the large intestine was measured to evaluate the preventive effect of indirubin treatment on shortening of the large intestine accompanied by colitis. Large intestines were placed on filter papers to measure their length, after which they were opened longitudinally so that their contents could be removed.
The colon was divided into two equal segments (proximal and distal) and fixed in neutral-buffered 10% formalin. All slides were stained with hematoxylin and eosin. For histopathologic evaluation of tissue damage, areas of inflammatory lesions were evaluated under light microscopy and quantified using the method of Cooper et al.,8 with minor modifications (Table 1). Grading was based on pathologic changes: 0, intact crypt; 1, loss of the basal one-third of the crypt; 2, loss of the basal two-thirds of the crypt; 3, loss of the entire crypt with the surface epithelium remaining intact; 4, loss of the entire crypt and surface epithelium (erosion). These changes were quantitated according to percentage involvement by the disease: (1) 1–25%; (2) 26–50%; (3) 51–75%; (4) 76–100%. Each section was scored with a grade and percentage area involvement, with the product of the two being a “crypt score.” The scores for each tissue were summed and divided by the number of pieces of tissue to give a “histology score.” Colitis was not observed in the proximal colon, so only the distal colon was evaluated in all cases. The distal colon was divided into 4 sections and evaluated.
Table 1.
Histology grading of colitis
| Feature | Score | Description |
| Damage | 0 | None |
| 1 | Loss of the basal 1/3 of the crypt | |
| 2 | Loss of the basal 2/3 of the crypt | |
| 3 | Loss of entire crypt but intact surface epithelial cells | |
| 4 | Loss of both the entire crypt and the surface epithelial cells | |
| Percentage area involvement | 1 | 1–25% |
| 2 | 26–50% | |
| 3 | 51–75% | |
| 4 | 76–100% |
For histologic evaluation of the extent of fibrosis in the submucosa, all slides were stained with Masson’s trichrome. The extent of fibrosis per 1-cm of the distal colon was calculated as “percentage involvement.”
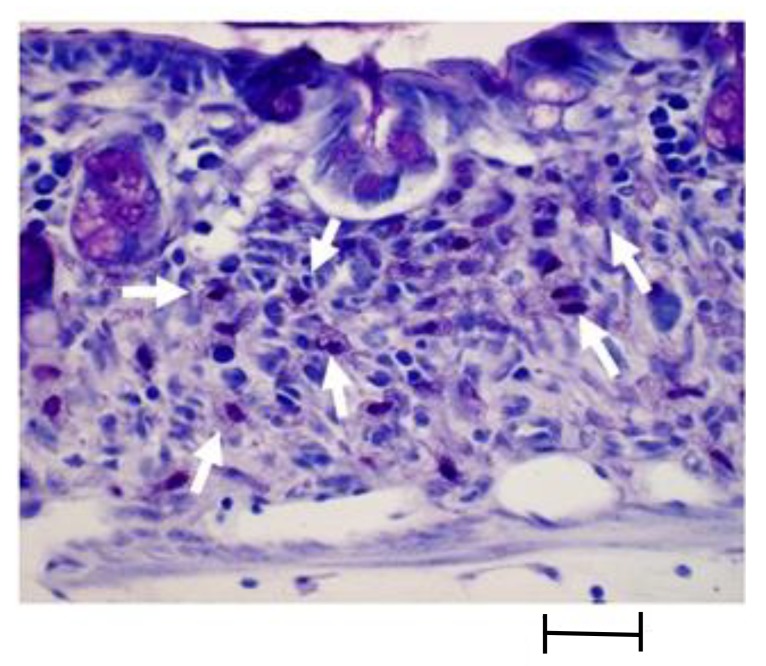
Mast cells were identified by staining (toluidine blue) (Fig. 3). The number of mucosal toluidine blue-positive cells in the distal colon was counted, and number per 1-cm was calculated.
Fig. 3.
Intracellular granules of mast cell were stained with toluidine blue (arrow). It shows metachromasia and shows reddish purple color. Bar = 0.2 mm.
Enzyme-linked immunosorbent assay (ELISA)
After killing, the rectum (5 mm × 5 mm) was excised. Feces were removed by gentle washing in physiologic (0.9%) saline. The rectum tissue was homogenized and the concentration of the interleukin (IL)-6 level in the supernatant was measured by sandwich ELISA using paired antibodies according to manufacturer (RayBio, Norcross, GA) recommendations.
Statistical analyses
Data are the mean ± standard deviation (SD). Data comparisons were made using the Student’s t-test and Pearson correlation to test the correlation between variables. P < 0.05 was considered significant.
RESULTS
Mice administered only indirubin (0.05 μg/g/day) in their diet for 1 month suffered no pathologic change, compared with the control group, in terms of body weight, colonic histology, and IL-6 content in the rectum (data not shown).
General features
Acute colitis
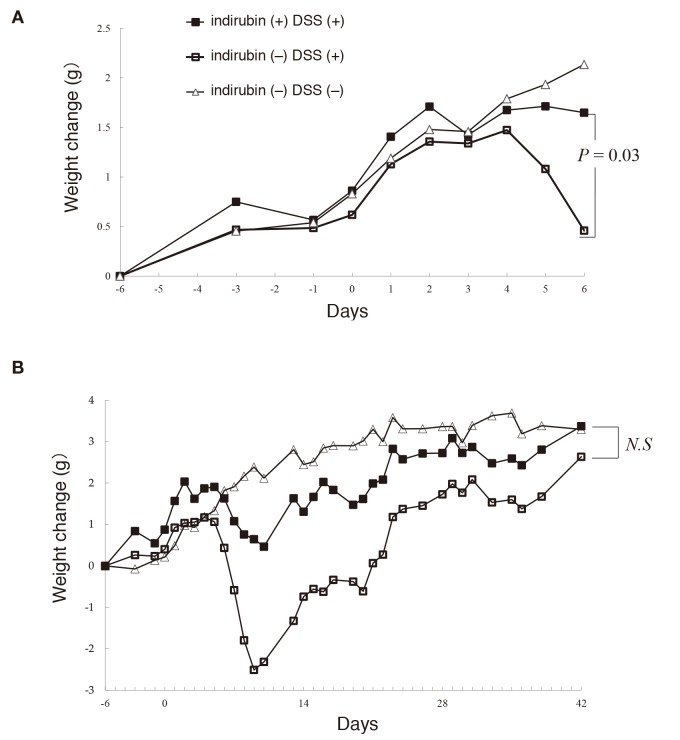
There was no difference in consumption of DSS–water between the groups given and not given indirubin in acute and chronic models of colitis. Administration of 2% DSS for 6 days resulted in severe colitis with a significant reduction in body weight and food intake from day-4. On the final day of observation, the mean increase in body weight was 0.44 ± 2.1 g in mice not given indirubin, and 1.67 ± 0.5 g in mice given indirubin, and this difference was significant (P = 0.03) [Fig. 4A]. The mean food intake per mouse per day was 4.80 ± 1.0 g in mice not given indirubin, and 4.36 ± 0.58 g in mice given indirubin, but the difference was not significant (P = 0.31).
Fig. 4.
Changes (0, baseline) in body weight (mean ± SE) of mice receiving water (control), DSS, or DSS and indirubin in (A) acute colitis and (B) chronic colitis. DSS, dextran sulfate sodium; N.S, not significant.
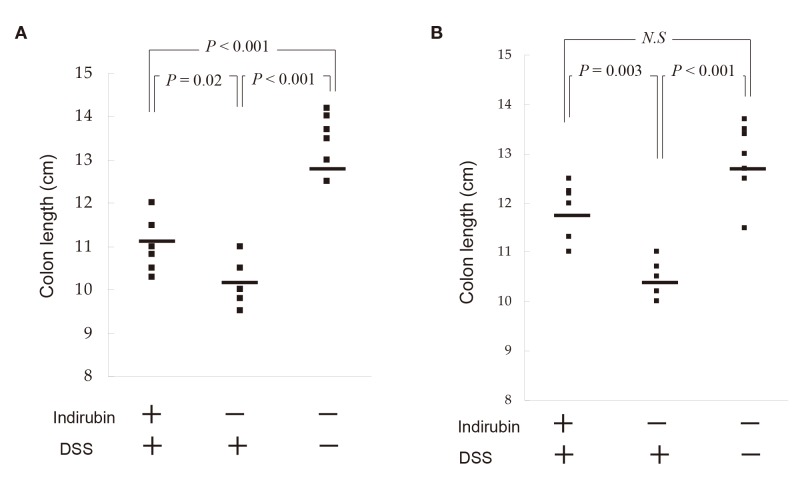
The length of the large intestine was 13.4 ± 0.58 cm in mice not given DSS or indirubin (control), 10.2 ± 0.52 cm in colitis mice not given indirubin, and 11.1 ± 0.58 cm in colitis mice given indirubin (Fig. 5A). Thus, indirubin showed a significant preventive effect on shortening of the large intestine in our model of acute colitis (P = 0.02) [Fig. 5A].
Fig. 5.
Effect of indirubin on the length of the large intestine of mice with DSS-induced colitis. (A) Acute-colitis model on day-6. (B) Chronic-colitis model on day-42. The large intestine was isolated, and its length measured. Median scores are indicated by horizontal lines. DSS, dextran sulfate sodium; N.S, not significant.
Chronic colitis
Stable chronic colitis could be achieved by three repeat 6-day cycles of DSS interrupted by 8-day recovery periods using water not containing DSS. Initially, mice manifested diarrhea and blood in feces and body-weight loss in the first cycle of administration of 2% DSS. However, these signs improved after drinking water for the next 8 days. On subsequent administrations of DSS (three cycles), these symptoms improved during the 8-day period of water consumption (Fig. 4B). Only one mouse receiving 2% DSS without indirubin died with severe loss of body weight (16.1 g) on day-18. On the final day of observation, the mean body weight increased from 2.76 ± 0.4 g in mice not given indirubin to 3.53 ± 1.1 g in mice given indirubin, and this difference was not significant (P = 0.18) [Fig. 4B]. The mean food intake per mouse per day was 5.31 ± 1.7 g in mice not given indirubin, and 4.64 ± 1.6 g in mice given indirubin, and this difference was not significant (P = 0.11).
The length (mean ± SD) of the large intestine was 12.9 ± 0.75 cm in mice not given DSS and indirubin (control group), 10.4 ± 0.42 cm in the chronic-colitis model without indirubin, and 11.8 ± 0.68 cm in the chronic-colitis model with indirubin (Fig. 5B). Thus, indirubin prevented a significant reduction in colon length in the chronic-colitis model (P = 0.003).
Histology
Acute colitis
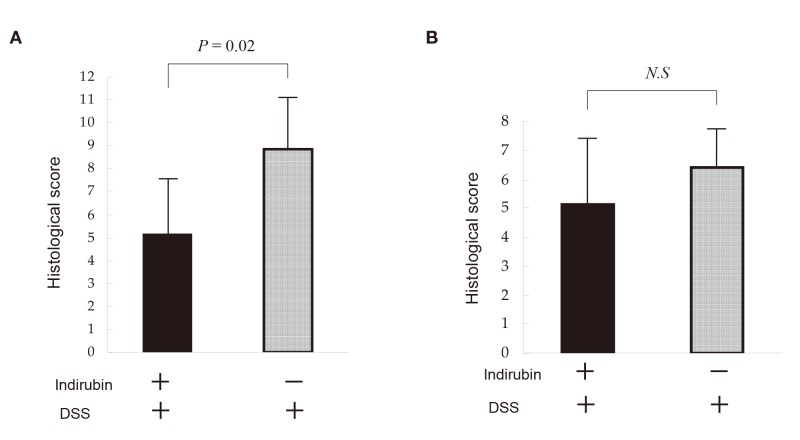
Representative histologic findings are shown in Figs. 6A–C. DSS induced erosive colitis in a variable manner (Fig. 6B). The histology score was 8.83 ± 2.2 in acute-colitis mice not given indirubin and 5.16 ± 2.4 in acute-colitis mice given indirubin, and this difference was significant (P = 0.02) [Fig. 7A]. The extent of submucosal fibrosis was 40.4 ± 8.9% in mice not given indirubin and 22.2 ± 13% in mice given indirubin, and this difference was significant (P = 0.018). The histology score correlated well with body-weight reduction according to the Pearson’s correlation coefficient test (r = 0.62).
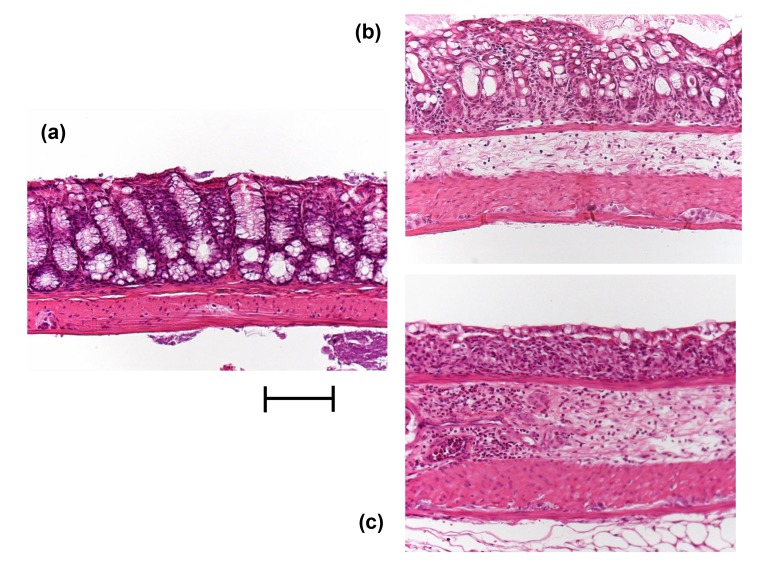
Fig. 6.
Histology (H&E stain) of colonic samples taken from mice not given DSS or indirubin (control) (A), given DSS and indirubin (B) or given DSS but not indirubin (C). Compared with that of control, the colons of DSS-induced colitis mice showed: complete destruction of epithelial architecture with loss of crypts and epithelial integrity; submucosal fibrosis and edema; intense infiltration of inflammatory cells in all layers. Indirubin treatment attenuated morphologic damage but showed mild infiltration of inflammatory cells. Bar = 1 mm. DSS, dextran sulfate sodium.
Fig. 7.
Histology score of colitis in mice not given DSS or indirubin (control), given DSS but not indirubin, or given DSS and indirubin. Mice were administered indirubin (0.05 μg/g/day, p.o.) in their diet. (A) Acute-colitis model on day-6. (B) Chronic-colitis model on day-42. The large intestine was isolated, and morphologic analyses undertaken. The histopathologic severity of colitis was calculated.
DSS, dextran sulfate sodium; N.S, not significant; p.o., per os.
Administration of 2% DSS for 6 days resulted in a marked increase in the number of mucosal toluidine blue-positive cells. The number of mucosal toluidine blue-positive cells was 334 ± 64/cm in mice not given indirubin and 163 ± 89/cm in mice given indirubin, and this difference was significant (P = 0.004).
Chronic colitis
The histology score was 6.40 ± 0.89 in chronic-colitis mice not given indirubin and 5.16 ± 2.0 in chronic-colitis mice given indirubin, and this difference was not significant (P = 0.24) [Fig. 7B]. The extent of submucosal fibrosis was 28.0 ± 11.8% in mice not given indirubin and 36.7 ± 19.2% in mice given indirubin, and this difference was not significant (P = 0.40). The histology score did not correlate well with body-weight reduction according to the Pearson’s correlation coefficient test (r = 0.21).
The number of mucosal toluidine blue-positive cells was 138 ± 109/cm in mice not given indirubin and 129 ± 77/cm in mice given indirubin, and this difference was not significant (P = 0.88).
Changes in levels of pro-inflammatory cytokines
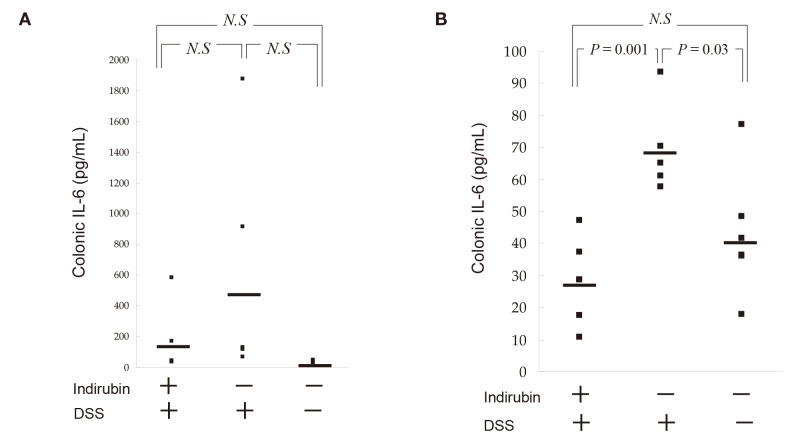
We measured IL-6 content in the rectum of mice in the presence and absence of indirubin at day-6. The IL-6 level was 541 ± 728 pg/mL in the rectum of mice not given indirubin and 154 ± 217 pg/mL in mice given indirubin, and this difference was not significant (P = 0.24) in acute colitis [Fig. 8A]. The IL-6 level was 69.6 ± 14.2 pg/mL in the rectum of mice not given indirubin and 28.4 ± 13.1 pg/mL in mice given indirubin, and this difference was significant (P = 0.001) in chronic colitis [Fig. 8B]. Serum levels of IL-6 were not detected in mice suffering from acute or chronic colitis.
Fig. 8.
Effects of indirubin treatment on colonic cytokine expression in DSS-induced colitis. (A) Acute-colitis model on day-6. (B) Chronic-colitis model on day-42. Mice were killed. A rectum sample (5 mm × 5 mm) was excised and homogenized. Tissue content of IL-6 was measured by ELISA specific for mouse IL-6. Median scores are indicated by horizontal lines. DSS, dextran sulfate sodium; ELISA, Enzyme-linked immunosorbent assay; N.S, not significant.
DISCUSSION
IBD includes the common gastrointestinal diseases UC and Crohn’s disease, and their incidence is increasing worldwide. Therapies such as mesalamine, corticosteroids, and thiopurines, in general, provide only transient or marginal relief, so the development of alternative treatments is of great interest. TCM, which is based to a large extent on the use of plant extracts, constitutes a valuable approach for the discovery of new drugs.
A recent clinical trial showed that the Chinese herbal medicine Qing-Dai (which contains indirubin) was effective for inducing remission in patients with UC.6 Dr. Amano (Hiroshima, Japan) has treated more than 4500 patients with intractable UC using Qing-Dai. The latter is extracted from the leaves and stems of plant species such as Baphicacanthus cusia (Nees) Bremek. (Acanthaceae), Polygonum tinctorium Ait. (Polygonaceae), and Isatis indigotia Fort. (Cruciferae). Qing-Dai contains several organic components such as indigo, indirubin, tryptanthrin, sterols, and amino acids.19
Indirubin is a well-known TCM used for the treatment of myelocytic leukemia. Several clinical trials using indirubin have been undertaken for the treatment of chronic myelocytic and chronic granulocytic leukemia in China.20–23 In one study undertaken by the Cooperative Group of Clinical Therapy of Indirubin, 26% of 314 patients with chronic myelocytic leukemia showed complete remission, and 33% showed partial remission, in response to indirubin treatment.21 Toxicity was low and side effects were limited to mild abdominal pain, diarrhea, nausea and vomiting. There were three cases of reversible pulmonary arterial hypertension and cardiac insufficiency.24
Indirubin exhibits only minor toxicity in animal models.25 For example, dogs given a dose of indirubin 25-times that used for human therapy (according to body weight) continuously for 6 months showed diarrhea and some damage to the liver, with no change in hematopoiesis, electroencephalographic activity or renal function.26 In our animal study, indirubin treatment for 1 month did not elicit unfavorable clinical or histologic changes in mice not given DSS (data not shown). Therefore, the therapeutic effect of indirubin can be examined without inducing toxicity in clinical studies.
Among the various models of chemically induced colitis, the DSS-induced colitis model is used widely because of its simplicity and many similarities with human UC. This model has advantages and disadvantages that must be considered when employed.27 Mice show different susceptibilities and responsiveness to DSS-induced colitis. Several mechanisms for DSS colitis have been proposed. In one representative mechanism, DSS administration blocks bacterial phagocytosis by macrophages in the lamina propria and causes direct injury to the epithelium, leading to early shortening and erosions in crypts.28, 29 Varying responses to DSS appear to be dependent on the inbred strain,30 DSS concentration,31 molecular weight of DSS,32 and duration of DSS exposure.8 In our study, significant differences in the consumption of DSS–water were not observed between groups given or not given indirubin. We, therefore, concluded that consumption of DSS–water did not affect the severity of DSS-induced colonic injury in mice. Kitajima et al. reported that the severity and primary location of colitis differ depending upon the molecular weight of DSS administered in mice. Colitis was observed predominantly in the cecum and upper colon in mice given DSS of molecular weight 5 kD. In mice given DSS of molecular weight 40 kD, colitis was more prominent in the distal colon.32 In our study, colitis was localized to the distal colon in all mice because DSS of molecular weight 36–50 kD was used for oral administration.
A histology grading system showed that indirubin improved DSS-induced acute colitis, but was not prominent in the chronic-colitis model. In the latter, the region with crypt loss had been displaced with epithelial dysplasia. Therefore, there might not be significant differences in histology grades between mice given DSS and those not given DSS. Histology demonstrated collagen accumulation in the submucosal region of intestines, which could be the cause of intestinal shortening in the colitis model. Smooth muscle cells, subepithelial myofibroblasts, and fibroblasts have been considered to be the mediators of fibrosis, but new evidence points to a role for interstitial mast cells.33 We did not observe a significant difference in collagen production, but colon length was significantly shorter in the chronic-colitis model. Graham et al. reported that the thickness of the muscularis propria is also increased in colitis,34 which might also result in shortening of the intestine wall. Indirubin might have an effect on controlling the thickness of the muscularis propria in the chronic-colitis model.
Pro-inflammatory cytokines have been demonstrated to have a crucial role in IBD pathogenesis. Among these cytokines, higher expression of IL-6 has been demonstrated repeatedly.35 Naito et al. reported that DSS-induced inflammation appears to be inhibited significantly in IL-6−/− mice compared with wild-type mice. They suggested that persistent and marked blockade of IL-6 bioactivity provides some beneficial effects against intestinal inflammation.36 A clinical trial carried out recently in Japan clearly demonstrated the beneficial effects of humanized anti-IL-6 monoclonal antibody for treatment-resistant active Crohn’s disease.37 In our study, indirubin obviously suppressed the tissue level of IL-6 in acute and chronic models of colitis, with a significant effect being observed in the chronic-colitis model. This finding could imply the crucial role of IL-6 in suppressing DSS-induced colitis. Further studies are needed to clarify the precise relationship between indirubin and IL-6. Recently, Kawai et al. showed that Indigo naturalis and its major component indigo ameliorated murine DSS-induced colitis via upregulation of expression of IL-10 and IL-22 through activation of the aryl hydrocarbon receptor.38 Further studies are needed to clarify the mechanisms of action of indirubin in preventing UC pathogenesis.
We clearly proved that oral intake of indirubin can improve murine DSS-induced colitis (which mimics human IBD).39 Furthermore, we showed, for the first time, that indirubin is more effective in the acute phase than in the chronic phase.
Acknowledgments
Acknowledgments: The authors thank Mr. N. Itaki, Ms. M. Iwatani, and T. Yamasaki (Division of Organ Pathology, Faculty of Medicine, Tottori University) for their skillful technical assistance. We thank Arshad Makhdum, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
The authors declare no conflict of interest.
REFERENCES
- 1. Liu XM, Wang LG, Li HY, Ji XJ. Induction of differentiation and down-regulation of c-myb gene expression in ML-1 human myeloblastic leukemia cells by the clinically effective anti-leukemia agent meisoindigo. Biochem Pharmacol. 1996;51:1545-51. [DOI] [PubMed] [Google Scholar]
- 2. Eisenbrand G, Hippe F, Jakobs S, Muehlbeyer S. Molecular mechanisms of indirubin and its derivatives: novel anticancer molecules with their origin in traditional Chinese phytomedicine. J Cancer Res Clin Oncol. 2004;130:627-35. [DOI] [PubMed] [Google Scholar]
- 3. Kunikata T, Tatefuji T, Aga H, Iwaki K, Ikeda M, Kurimoto M. Indirubin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur J Pharmacol. 2000;410:93-100. [DOI] [PubMed] [Google Scholar]
- 4. Mak NK, Leung CY, Wei XY, Shen XL, Wong RN, Leung KN, et al. Inhibition of RANTES expression by indirubin in influenza virus-infected human bronchial epithelial cells. Biochem Pharmacol. 2004;67:167-74. [DOI] [PubMed] [Google Scholar]
- 5. Kim SA, Kim YC, Kim SW, Lee SH, Min JJ, Ahn SG, et al. Antitumor activity of novel indirubin derivatives in rat tumor model. Clin Cancer Res. 2007;13:253-9. [DOI] [PubMed] [Google Scholar]
- 6. Sugimoto S, Naganuma M, Kiyohara H, Arai M, Ono K. Clinical efficacy and safety of oral Qing-Dai in patients with ulcerative colitis: A single-center open-label prospective study. Digestion. 2016;93:193-201. [DOI] [PubMed] [Google Scholar]
- 7. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694-702. [DOI] [PubMed] [Google Scholar]
- 8. Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-49. [PubMed] [Google Scholar]
- 9. Takizawa H, Asakura H, Sasakawa T, Bannai H, Nomoto M. Immunological studies of the mucosa in colitis induced by sodium dextran sulfate in rats. Adv Exp Med Biol. 1995;371B:1383-7. [PubMed] [Google Scholar]
- 10. Iwanaga T, Hoshi O, Han H, Fujita T. Morphological analysis of acute ulcerative colitis experimentally induced by dextran sulfate sodium in the guinea pig: some possible mechanisms of cecal ulceration. J Gastroenterol. 1994;29:430-8. [DOI] [PubMed] [Google Scholar]
- 11. Ohkusa T. Production of experimental ulcerative colitis in hamsters by dextran sulfate sodium and changes in intestinal microflora. Nippon Shokakibyo Gakkai Zasshi. 1985;82:1327-36. [PubMed] [Google Scholar]
- 12. Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel diasease. Gastroenterology. 1995;109:1344-67. [DOI] [PubMed] [Google Scholar]
- 13. Egger B, Procaccino F, Sarosi I, Tolmos J, Buchler MW, Eysselein VE, et al. Keratinocyte growth factor ameliorates dextran sodium sulfate colitis in mice. Dig Dis Sci. 1999;44:836-44. [DOI] [PubMed] [Google Scholar]
- 14. Murakami A, Hayashi R, Tanaka T, Kwon KH, Ohigashi H, Safitri R, et al. Suppression of dextran sodium sulfate-induced colitis in mice by zerumbone, a subtropical ginger sesquiterpene, and nimesulide: separately and in combination. Biochem Pharmacol. 2003;66:1253-61. [DOI] [PubMed] [Google Scholar]
- 15. Axelsson LG, Landstrom E, Bylund-Fellenius AC. Experimental colitis induced by dextran sulphate sodium in mice: beneficial effects of sulphasalazine and olsalazine. Aliment Pharmacol Ther. 1998;12:925-34. [DOI] [PubMed] [Google Scholar]
- 16. Murthy S, Cooper HS, Yoshitake H, Meyer C, Meyer CJ, Murthy NS. Combination therapy of pentoxifylline and TNFalpha monoclonal antibody in dextran sulphate-induced mouse colitis. Aliment Pharmacol Ther. 1999;13:251-60. [DOI] [PubMed] [Google Scholar]
- 17. Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48:137-43. [DOI] [PubMed] [Google Scholar]
- 18. Russell GA, Kaupp G. Oxidation of Carbanions. IV. Oxidation of Indoxyl to Indigo in Basic Solution. J Am Chem Soc. 1969;91:3851-9. [Google Scholar]
- 19. Sugimoto S, Naganuma M, Kanai T. Indole compounds may be promising medicines for ulcerative colitis. J Gastroenterol. 2016;51:853-61. [DOI] [PubMed] [Google Scholar]
- 20. Institute of Haematology, Chinese Academy of Medical Sciences. Experimental and clinical studies of indirubin in the treatment of CML. Chinese J Intern Med. 1979;18:83-8. [Google Scholar]
- 21. Cooperative Group of Clinical Therapy of Indirubin. Clinical studies of 314 cases of CML treated with indirubin. Chinese J Intern Med. 1980;1:132-5. [Google Scholar]
- 22. Gan WJ, Yang T, Wen S, Liu Y, Tan Z, Deng C, et al. Studies on the mechanism of indirubin action in treatment of chronic myelocytic leukemia (CML). II. 5’-Nucleotidase in the peripheral white blood cells of CML. Chin Acad Med Sci Beijing. 1985;6:611-13. [Google Scholar]
- 23. Zhang ZN, Liu EK, Zheng TL, Li DG. Treatment of chronic myelocytic leukemia (CML) by traditional Chinese medicine and Western medicine alternately. J Trad. Chinese Med. 1985;5:246-8. [PubMed] [Google Scholar]
- 24. Jiang SZ, Yu G, Cao J. Adverse effects of indirubin on cardiovascular system. Chinese J. Hematol. 1986;7:30. [Google Scholar]
- 25. Ji XJ, Zhang FR, Lei JL, Xu YT. Studies on the antineoplastic action and toxicity of synthetic indirubin. Yao Xue Xue Bao. 1981;16:146-8. [PubMed] [Google Scholar]
- 26. Chang CN, Chang HM, Yeung HW, Tso W, Koo A. Advances in Chinese Medicinal Materials Research. Singapore: World Scientific Pub. Co.; 1985. p.369-76. [Google Scholar]
- 27. Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium(DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:Unit 15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chapman V, Dickenson AH. The spinal and peripheral roles of bradykinin and prostaglandins in nociceptive processing in the rat. Eur J Pharmacol. 1992;219:427-33. [DOI] [PubMed] [Google Scholar]
- 29. Hasler WL, Kurosawa S, Takahashi T, Feng H, Gaginella TS, Owyang C. Bradykinin acing on B2 receptors contracts colon circular muscle cells by IP3 generation and adenylate cyclase. J Pharmacol Exp Ther. 1995;273:344-50. [PubMed] [Google Scholar]
- 30. Mahler M, Bristol IJ, Leiter EH, Workman AE, Birkenmeier EH, Elson CO, et al. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am J Physiol. 1998;274:544-51. [DOI] [PubMed] [Google Scholar]
- 31. Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW, et al. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240-8. [DOI] [PubMed] [Google Scholar]
- 32. Kitajima S, Takuma S, Morimoto M. Histological analysis of murine colitis induced by dextran sulfate sodium of different molecular weights. Exp Anim. 2000;49:9-15. [DOI] [PubMed] [Google Scholar]
- 33. Lund PK, Zuniga CC. Intestinal fibrosis in human and experimental inflammatory bowel disease. Curr Opin Gastroenterol. 2001;17:318-23. [DOI] [PubMed] [Google Scholar]
- 34. Graham MF, Diegelmann RF, Elson CO, Lindblad WJ, Gotschalk N, Gay S, et al. Collagen content and types in the intestinal strictures of Crohn’s disease. Gastroenterology. 1998;94:257-65. [DOI] [PubMed] [Google Scholar]
- 35. Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878-82. [DOI] [PubMed] [Google Scholar]
- 36. Naito Y, Takagi T, Uchiyama K, Kuroda M, Kokura S, Ichikawa H, et al. Reduced intestinal inflammation induced by dextran sodium sulfate in interleukin-6-deficient mice. Int J Mol Med. 2004;14:191-6. [PubMed] [Google Scholar]
- 37. Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126:989-96. [DOI] [PubMed] [Google Scholar]
- 38. Kawai S, Iijima H, Shinzaki S, Hiyama S, Yamaguchi T, Araki M, et al. Indigo Naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J Gastroenterol. 2017;52:904-19. [DOI] [PubMed] [Google Scholar]
- 39. Gao W, Guo Y, Wang C, Lin Y, Yu L, Sheng T, et al. Indirubin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice through the inhibition of inflammation and the induction of Foxp3-expressing regulatory T cells. Acta Histochem. 2016;118:606-14. [DOI] [PubMed] [Google Scholar]