Abstract
Background
We investigated the distinguishing pathological features of bilateral ovarian tumors using magnetic resonance (MR) imaging
Methods
Eighty-six patients with bilateral ovarian tumors on MR imaging were evaluated. The pathological diagnosis was investigated, and the results were subjected to statistical analysis using Mann-Whitney U test, Fisher’s exact test, Chi-squared test and receiver operating characteristic (ROC) curve to determine the features useful for the differentiation of distinct types of lesions.
Results
The diagnosis of bilateral ovarian tumors was confirmed in eighty-one patients and the majority of the lesions were further classified into serous carcinoma (n = 36), mature teratoma (n = 20) and metastasis (n = 12). We assessed the existence of factors useful for the MR imaging differentiation between metastasis and serous carcinoma or primary malignant ovarian tumors. Cancer antigen (CA) 125 serum level and maximum tumor diameter were significantly different between metastasis and serous carcinoma and similarly, between metastasis and primary malignant ovarian tumors. MR imaging morphology, ascites and peritoneal implants did not show any significant difference between the different types of lesions.
Conclusion
Within our patient cohort, most bilateral ovarian tumor lesions were determined to be serous carcinoma, mature teratoma or metastasis. CA 125 serum level and maximum tumor diameter are useful markers for the differentiation between metastasis and serous carcinoma or primary malignant ovarian tumors.
Keywords: bilateral, cancer antigen 125, magnetic resonance imaging, maximum tumor diameter, ovarian tumor
Ovarian tumors are commonly occurring gynecological disorders. Among these, ovarian cancer is the seventh most common form of cancer in women globally.1 On the other hand, 5% to 10% of malignant tumors involving the ovaries are metastasis.2 The preoperative differentiation between metastasis and primary ovarian tumors is important in selecting the most appropriate treatment.3 However, the diagnosis is sometimes challenging, because metastatic ovarian tumors are sometimes detected before the primary tumor is diagnosed.
Most studies have reported that the differentiation between primary and metastatic ovarian tumors, including Krukenberg tumors, was often not made preoperatively, due to their very similar appearance.3–5 Some studies reported that metastatic ovarian tumors were usually bilateral,5, 6 and the bilaterality was a useful feature to differentiate primary from metastatic ovarian tumors.4, 7 However, other authors reported differently.3, 8 Some common primary tumors such as serous and undifferentiated carcinomas are also known to involve bilateral ovaries in a high proportion of cases.2 In reality, bilateral primary ovarian tumors are sometimes encountered in daily practice on magnetic resonance (MR) imaging examination. These conflicting reports demanded further investigations on the distinguishing pathological and morphological features of bilateral ovarian tumors. On the other hand, there are no sufficient data about the way to differentiate the bilateral ovarian tumors detected on MR imaging. The aim of this study is thus to investigate the distinct pathological types of bilateral ovarian tumors detected on MR imaging, and to determine the existence of useful markers for the differentiation between the distinct types of bilateral ovarian tumors, including primary ovarian tumors and metastasis.
MATERIALS AND METHODS
Patient Population
In this retrospective study, 457 consecutive patients presenting ovarian tumors on MR imaging examination were included. They were all pathologically diagnosed. These patients underwent MR examinations between January 2005 and March 2015. Endometriotic cysts and functional cysts were excluded on MR findings and ultrasound examination for follow-up. Of the patients presenting ovarian tumor lesions, 371 patients were excluded due to unilateral tumor on MR imaging. A total of 86 patients were enrolled. Approval by the ethics committee of Tottori University was obtained for the study, and the requirement for informed consent was waived (1606A027).
MR Imaging Technique
MR imaging was performed using three 3.0-T MR systems (Signa EXCITE HD or Discovery MR750w; GE Medical Systems, Milwaukee, WI, or Skyra; Siemens Health Care, Erlangen, Germany) and two 1.5-T MR systems (Achieva; Philips Medical Systems, Best, Netherlands or Symphony; Siemens Health Care), with phased array coils.
Axial and sagittal T1-weighted images, and axial and sagittal T2-weighted images were obtained in all patient cases. Scan parameters and sequences varied because the study was conducted over a period of 10 years. Representative pelvic MR images were acquired using the following sequences and scan parameters: axial and sagittal T1-weighted spoiled gradient recalled (SPGR) images [repetition time (TR), 250 ms; echo time (TE), 2.1 ms; flip angle (FA), 75°; acquisition time, 1 min 30 s], axial and sagittal T2-weighted fast SE images (TR, 6500 ms; TE, 100 ms; section thickness, 5 mm; intersection gap, 1.5 mm; acquisition time, 3–3.5 min), and axial and/or sagittal SPGR images with fat suppression (TR, 320 ms; TE, 2.1 ms; FA, 75°; acquisition time, 1 min 50 s). Axial diffusion weighted (DW) images were then obtained. Imaging parameters for DW imaging were as follows: TR, 5500–6000 ms; TE, 60–62 ms; inversion time (TI), 200 ms; b factors, 0 and 1000 s/mm2; 112 × 128 matrix; field of view (FOV), 400 mm; section thickness, 4–6 mm with no gap; SENSE reduction factor, 2; signals acquired, 4; acquisition time, 3.5 min. The DW imaging sequence was used for fat suppression in short TI inversion recovery (STIR) -echo planar imaging sequence, with free breathing during acquisition. Motion-probing gradient pulses were placed in the three orthogonal planes. Isotropic DW imaging was generated using three orthogonal-axis images.
In 73 patient cases, contrast-enhanced MR images were obtained. Except for one patient case, dynamic MR images were also obtained. Images were acquired before and immediately after rapid intravenous injection of the contrast agent (0.2 mL/kg of gadolinium injection), and then repeated at 25 or 30, 60, 90, and 120 seconds during examination.
Prior to the examination, patients whose images were obtained in 3.0T Signa EXCITE HD received intramuscular administration of 20 mg butyl-scopolamine (Buscopan; Nippon Boehringer Ingelheim, Tokyo, Japan) to prevent peristalsis artifacts, unless contraindicated.
Image Analysis
MR images were independently analyzed by two radiologists (S.F. and N.M., 16 and 5 years of experience in gynecological MR imaging, respectively) to evaluate the bilaterality of the lesions. Consensus between the 2 radiologists was reached after careful individual examination. MR images of patients presenting bilateral ovarian tumors were analyzed by a radiologist (N.M.) on the basis of the following findings: i) morphology - predominantly cystic (less than half of solid component) or predominantly solid (more than half of solid component); ii) maximum tumor diameter - calculated by summing the bilateral of each maximum tumor diameter. When bilateral tumors were fused, the maximum diameter of the fused mass was measured; iii) ascites - none, not beyond the pelvic cavity or beyond the pelvic cavity; and iv) peritoneal implants. The reader was blinded to the clinical patient data and pathological reports. The solid component included thickened septa, vegetation (papillary projection) and solid portions showing enhancement on contrast-enhanced T1-weighted imaging. Morphology, ascites and peritoneal implants were identified on these images including DW imaging.
Statistical Analysis
All statistical analyses were performed for comparison between serous carcinoma and metastasis, and between primary malignant ovarian tumors and metastasis. The differences in morphology and peritoneal implants were compared using Fisher’s exact test and Chi-squared test. Maximum tumor diameter, ascites and CA 125 serum levels were analyzed using Mann-Whitney test. CA 125 serum levels were not available for two of the patients presenting metastatic ovarian tumors. Receiver operating characteristic (ROC) curve analysis was performed in order to assess diagnostic performance. Statistical analyses were performed using SPSS version 21.0 for windows (SPSS, Chicago, IL). By maximizing the Youden index (defined as sensitivity plus specificity minus 1), we determined the optimal cutoff values and corresponding sensitivity and specificity values.
RESULTS
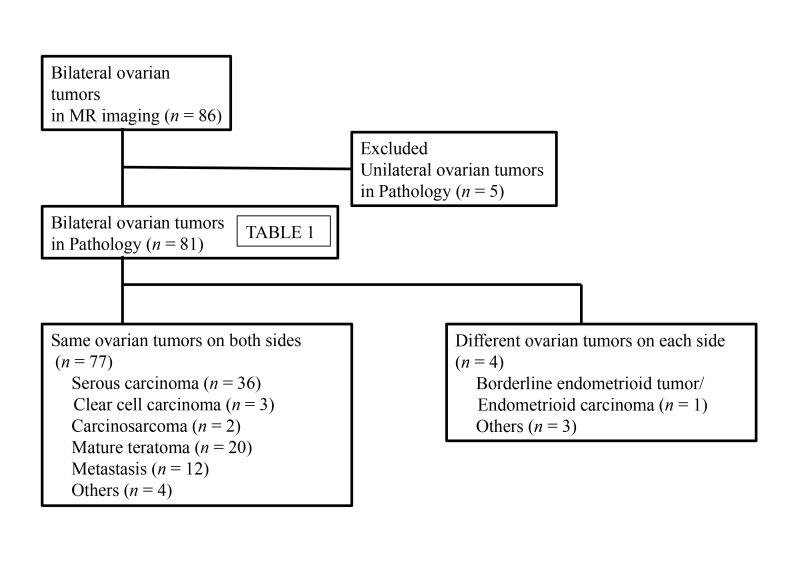
A total of 86 patients (mean age, 54 years; range, 15-86 years) presented bilateral ovarian tumors on MR imaging examination. Five of the 86 patients showed pathologically unilateral tumors, though bilateral tumors were observed on MR imaging. The pathological diagnoses of the remaining 81 patients are shown in Table 1. In 77 of the patient cases, the bilateral tumor lesions presented the same pathology on both sides. In the 4 remaining patients, the bilateral tumors were pathologically different on each side (Fig. 1). Most of the bilateral tumors were classified as serous carcinoma (n = 36), mature teratoma (n = 20) and metastasis (n = 12). The primary site of metastasis included appendiceal cancer (mucinous carcinoma, n = 2; signet ring cell carcinoma, n = 1), colon cancer (n = 2), uterine cervical cancer (n = 2), bile duct cancer (n = 1), gastric cancer (n = 1), kidney cancer (n = 1), peritoneal mesothelioma (n = 1) and uterine malignant lymphoma (n = 1) (Figs. 2–4). Mature teratomas are not generally problematic for a differential diagnosis on MR imaging. Therefore, further investigations were performed to differentiate serous carcinoma, primary malignant ovarian tumors and metastasis.
Table 1.
Pathological diagnosis of bilateral ovarian tumors
| Pathological type | N | |
| Primary benign | Total 26 | |
| Mature teratoma | 20 | |
| Mature teratoma/Serous adenoma | 1 | |
| Mature teratoma/Struma ovarii | 1 | |
| Serous adenoma | 3 | |
| Fibroma | 1 | |
| Primary benign and malignant | Total 1 | |
| Serous adenoma/Mucinous borderline tumor | 1 | |
| Primary malignant | Total 42 | |
| Serous carcinoma | 36 | |
| Clear cell carcinoma | 3 | |
| Carcinosarcoma | 2 | |
| Borderline endometrioid tumor/Endometrioid carcinoma | 1 | |
| Metastasis | Total 12 | |
| Appendiceal cancer | 3 | |
| Cervical cancer | 2 | |
| Colon cancer | 2 | |
| Bile duct cancer | 1 | |
| Gastric cancer | 1 | |
| Kidney cancer | 1 | |
| Peritoneal mesothelioma | 1 | |
| Uterine malignant lymphoma | 1 | |
Fig. 1.
Flow diagram of patients presenting bilateral ovarian tumors on MR imaging examination. MR, magnetic resonance.
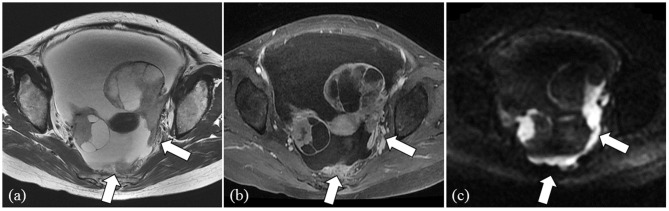
Fig. 2.
Representative case of a 50-year-old female patient presenting serous carcinoma (CA 125 serum level: 5066 U/mL). Bilateral multilocular cystic ovarian tumors with solid components, massive ascites and peritoneal implants (arrows) in the cul-de-sac are demonstrated on T2 weighted image (a) and contrast-enhanced T1 weighted image with fat-suppression (b). Solid components of ovarian tumors and peritoneal implants (arrows) show high intensity on DW image (c). CA, cancer antigen; DW, diffusion weighted.
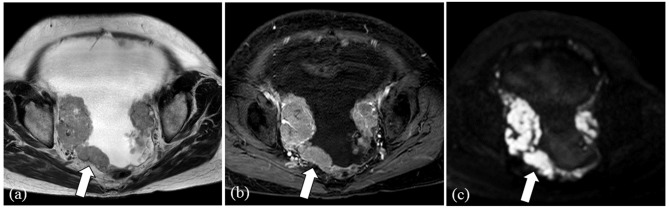
Fig. 4.
Representative case of a 57-year-old female presenting metastasis from sigmoid colon cancer (CA 125 serum level: 651 U/mL). Bilateral multilocular ovarian cystic tumors with thick internal septae (arrows) are shown on T2 weighted image (a) and contrast-enhanced T1 weighted image with fat-suppression (b). CA, cancer antigen.
Results in CA 125, maximum tumor diameter, morphology, ascites and peritoneal implants are summarized in Tables 2 and 3. The first analyses aimed to differentiate serous carcinoma from metastasis. CA 125 serum levels in serous carcinomas were significantly higher compared to metastasis (P < 0.01, AUC = 0.86, sensitivity 72%, specificity 90%, the optimal cutoff value = 683.3 U/mL), and maximum tumor diameters were significantly smaller (P < 0.05, AUC = 0.69, sensitivity 58%, specificity 83%, the optimal cutoff value = 164 mm). However, no significant difference was determined in morphology (P = 0.32), ascites (P = 0.26) and peritoneal implants (P = 0.09). When CA 125 was lower than the cutoff value or maximum tumor diameter was larger than the cutoff value, the sensitivity and specificity of metastasis were 90% and 58%. Similar analyses were performed to differentiate primary malignant ovarian tumors from metastasis. CA 125 levels in primary malignant ovarian tumors were significantly higher compared to metastasis (P < 0.01, AUC = 0.81, sensitivity 64%, specificity 90%, the optimal cutoff value = 683.3 U/mL) and maximum tumor diameters were significantly smaller (P < 0.05, AUC = 0.67, sensitivity 58%, specificity 81%, the optimal cutoff value = 164 mm). No significant difference was determined in morphology (P = 0.42), ascites (P = 0.19), or peritoneal implants (P = 0.24) between the different types of lesions. When CA 125 was lower than the cutoff value or maximum tumor diameter was larger than cutoff value, the sensitivity and specificity of metastasis were 90% and 52%.
Table 2.
CA 125 and maximum tumor diameter
| Mean CA 125 (U/mL) | Mean maximum tumor diameter (mm) | |
| Serous carcinoma (N = 36) | 3261.0 (103.2–31550) | 120 (28–304) |
| Primary malignant ovarian tumor (N = 42) | 2834.1 (40.4–31550) | 124 (28–304) |
| Metastasis (N = 12) | 389.7 (23.3–1720) | 170 (68–360) |
( ) : range
CA, cancer antigen.
Table 3.
MR findings of primary malignant ovarian tumors and metastasis
| N | Morphology (n) | Ascites (n) | Peritoneal implant (n) | |||||
| Solid | Cystic | ++ | + | − | + | − | ||
| Primary malignant ovarian tumors | 42 | 23 | 19 | 21 | 14 | 7 | 37 | 5 |
| Serous carcinoma | 36 | 21 | 15 | 19 | 11 | 6 | 34 | 2 |
| Clear cell carcinoma | 3 | 1 | 2 | 2 | 0 | 1 | 2 | 1 |
| Carcinosarcoma | 2 | 1 | 1 | 0 | 2 | 0 | 1 | 1 |
| Endometrioid borderline tumor/carcinoma | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Metastasis | 12 | 5 | 7 | 8 | 4 | 0 | 9 | 3 |
| Appendiceal cancer | 3 | 1 | 2 | 3 | 0 | 0 | 2 | 1 |
| Cervical cancer | 2 | 1 | 1 | 1 | 1 | 0 | 2 | 0 |
| Colon cancer | 2 | 1 | 1 | 0 | 2 | 0 | 2 | 0 |
| Bile duct cancer | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Gastric cancer | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Kidney cancer | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| Peritoneal mesothelioma | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Malignant lymphoma | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
Ascites+: not beyond the pelvic cavity; Ascites++: beyond the pelvic cavity
MR, magnetic resonance.
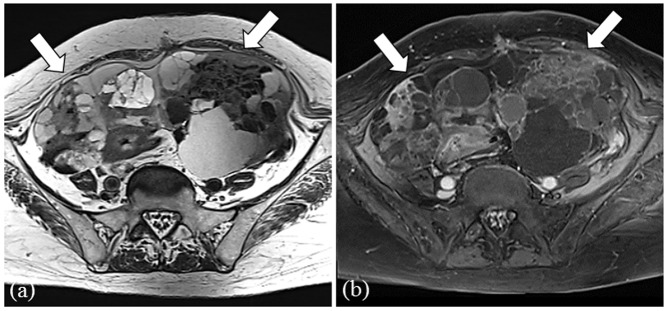
Fig. 3.
Representative case of a 73-year-old female presenting serous carcinoma (CA 125 serum level: 3532 U/mL). Bilateral solid ovarian tumors, massive ascites and peritoneal implants (arrows) in the cul-de-sac are shown on T2 weighted image (a) and contrast-enhanced T1 weighted image with fat-suppression (b). Ovarian tumors and peritoneal implants show high intensity on DW image (c). CA, cancer antigen; DW, diffusion weighted.
DISCUSSION
Our results demonstrated that most bilateral ovarian tumors detected on MR imaging consisted of serous carcinoma, mature teratoma and metastasis. These tumors should thus be considered as representative bilateral ovarian tumors. In 10 to 15% of cases, mature teratomas involve both ovaries simultaneously.9–11 Although we recognize that mature teratomas sometimes show bilateral ovarian tumors, we excluded them for further analysis as they can be easily diagnosed by detection of fat components and chemical shift artifact.12 In addition, most of the primary malignant bilateral ovarian tumors were serous carcinomas. The differentiation between primary and metastatic ovarian tumors is a clinically important issue. Therefore, we first assessed the differentiation between metastasis and serous carcinomas, and we then performed a similar analysis for the distinction between metastasis and primary malignant ovarian tumors.
Our results demonstrated that CA 125 serum level and maximum tumor diameter were useful parameters for the differentiation between metastasis and serous carcinoma or primary tumors. CA 125 antigen is a glycoprotein with a high molecular weight and is expressed by most epithelial ovarian cancers.13, 14 Approximately 80-85% of ovarian cancer patients present increased CA 125 serum levels.13, 15 In our study, a CA 125 cutoff value of 683.3 U/mL was determined to be a useful marker for the differentiation between primary malignant ovarian tumors and metastasis. Other authors reported that CA 125 levels had limited value in the diagnosis between primary and metastatic ovarian tumors.15 The reason underlying this discrepancy is not clear, but may be related to tumor extent and bias in histological types. The CA 125 serum levels of ovarian cancer patients correlate with the FIGO stages.15–17 Bilateral tumors are often characteristic of advanced stages. In addition, increased CA 125 levels in patients presenting serous carcinoma and undifferentiated carcinoma are higher than other type of tumors.18 In the present study, the most common histological type of primary tumors was serous carcinomas. Therefore, markedly high CA 125 levels are useful indicators for the prediction of primary malignant ovarian tumors.
Maximum tumor diameter was also useful for the differentiation; the maximum diameter of serous carcinoma or primary malignant ovarian tumors were smaller than metastasis. When CA 125 was lower than cutoff value in differentiation between primary ovarian tumors and metastasis, or maximum tumor diameter was larger than cutoff value, the sensitivity and specificity of metastasis were 90% and 52%. The sensitivity is high. Therefore, when these findings are shown, we can take metastasis into consideration and suggest further examinations. We consider that this strategy is cost effective. However, a previous study reported that primary ovarian tumors were larger than metastasis.7 The different result is considered to be due to the different study design and population. We included the cases showing bilateral ovarian tumors on MR imaging. On the other hand, the previous studies included the cases of ovarian carcinomas confirmed by pathology. We consider that our inclusion criterion is more practical on the basis of clinical daily practice.
Our results also demonstrated that MR imaging findings such as general morphology, ascites and peritoneal implants are not useful indicators. These results are in agreement with previous studies.19, 20 In relation to the morphology, nearly half of the patient cases presented predominant solid patterns in serous carcinomas, primary ovarian tumors and metastasis. In other words, these tumors presented variable morphology. Serous carcinomas are classically known to present various and complex appearances including cystic and solid components.21, 22 In addition, one possible reason behind the variable morphology may be the process to serous carcinoma. Serous carcinoma are classified into low and high grades. Low grade serous carcinoma (LGSC) are considered to develop through a stepwise process going from benign serous cystadenomas/adenofibromas to serous borderline tumors and ultimately to LGSC.23, 24 Therefore, LGSC can demonstrate cystic lesions with mural nodules.22 In contrast, high grade serous carcinoma (HGSC) are considered to be de novo promoted lesions,23, 24 and are often seen as solid masses with necrosis and hemorrhage.22 These differential developmental processes may partly explain the variable appearances. In the present study, the morphology of primary malignant ovarian tumors was likely affected by that of serous carcinoma, as the majority of primary malignant tumors were serous carcinomas.
Similarly, metastasis also presented variable appearances in the present study. This may be due to differences in primary sites, as reported by previous studies. Krukenberg tumors typically show bilateral and solid appearance.20, 25–27 Metastasis from colon cancer and appendiceal cancer often appear as multicystic ovarian tumors.26, 28 Hence, morphological features are not useful determinants for the distinction between different types of bilateral ovarian tumors on MR imaging. Further subgroup analysis of metastasis is required, although it was difficult in this study because of the small number.
In the present study, we did not find any significant difference in ascites and peritoneal implants. These features were often present in both of primary malignant ovarian tumors and metastasis. Our results on the relevance of ascites is in agreement with a previous study.7 Serous carcinoma, in particular HGSC, often appears as massive ascites and peritoneal implants.29 Peritoneal implants are more frequently present in serous carcinoma compared to other histological subtypes of ovarian cancers.21 Therefore, most patient cases of primary ovarian tumors presented ascites and peritoneal implants because the majority were serous carcinoma. On the other hand, ascites and peritoneal implants are also common features in carcinomatous peritonitis, and advanced stage cancer patients with metastatic ovarian tumors often present carcinomatous peritonitis. Peritoneal spread is considered one of the underlying causes of ovarian metastasis from the primary site.4 Therefore, most patients with metastasis presented ascites and peritoneal implants.
Previous studies reported that useful factors to differentiate primary from metastatic ovarian tumors were age, multilocularity, uniformity of locules and enhancement patterns of solid component.7, 8 Ovarian tumors, particularly serous carcinomas show complex appearances and have various micro cysts with collapsed morphology and mixture of necrosis and solid component. Thus, it is difficult to precisely evaluate the multilocularity and uniformity of locules. We also did not evaluate enhancement pattern and apparent diffusion coefficient (ADC) values because distinct MR devices were used for patient examination.
The present study has a number of limitations. First, several distinct MR devices were used. Second, the study population was small, in particular metastasis and primary malignant ovarian tumors except serous carcinoma. Further investigations are required to validate the present results in a larger population cohort. Third, the patients diagnosed with serous carcinoma in the present study may include both ovarian and peritoneal carcinoma, because based on the recent study about serous tubal intraepithelial carcinoma (STIC) theory, it is said that there is no longer a reason for classifying high-grade serous carcinoma into ovarian, tubal, or peritoneal origin30. Fourth, we did not evaluate endometriotic cysts. We classified ovarian tumors in reference to the World Health Organization (WHO) histological classification of tumors of the ovary (2003),31 in which endometriotic cysts are not classified as ovarian tumors. Fifth, while ovarian tumor-like lesions were not included in the present study, it is important to note that some tumor-like lesions such as hyperreactio luteinalis, fibromatosis and stromal hyperplasia sometimes demonstrate bilateral ovarian lesions. Sixth, CA 125 serum level of metastasis is affected by primary site. The cancers of liver, pancreas and bile ducts are known to show high CA 125 level. If metastases from these primary sites are collected, a different result might be derived.
In conclusion, in the present study, most bilateral ovarian tumors on MR imaging were defined as serous carcinoma, mature teratoma or metastasis. CA 125 serum level and maximum tumor diameter are useful indicators for the differentiation between metastasis and serous carcinoma or primary malignant ovarian tumors.
The authors declare no conflict of interest.
REFERENCES
- 1. Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-917. [DOI] [PubMed] [Google Scholar]
- 2. Young RH, Scully RE. Metastatic tumors in the ovary: a problem-oriented approach and review of the recent literature. Semin Diagn Pathol. 1991;8:250-76. [PubMed] [Google Scholar]
- 3. Kim SH, Kim WH, Park KJ, Lee JK, Kim JS. CT and MR findings of Krukenberg tumors: comparison with primary ovarian tumors. J Comput Assist Tomogr. 1996;20:393-8. [DOI] [PubMed] [Google Scholar]
- 4. La Fianza A, Alberici E, Pistorio A, Generoso P. Differential diagnosis of Krukenberg tumors using multivariate analysis. Tumori. 2002;88:284-7. [DOI] [PubMed] [Google Scholar]
- 5. Kuhlman JE, Hruban RH, Fishman EK. Krukenberg tumors: CT features and growth characteristics. South Med J. 1989;82:1215-9. [PubMed] [Google Scholar]
- 6. Turan T, Aykan B, Koc S, Boran N, Tulunay G, Karacay O, et al. Analysis of metastatic ovarian tumors from extragenital primary sites. Tumori. 2006;92:491-5. [DOI] [PubMed] [Google Scholar]
- 7. Xu Y, Yang J, Zhang Z, Zhang G. MRI for discriminating metastatic ovarian tumors from primary epithelial ovarian cancers. J Ovarian Res. 2015;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown DL, Zou KH, Tempany CM. Frates MC, Silverman SG, McNeil BJ, et al. Primary versus secondary ovarian malignancy: imaging findings of adnexal masses in the Radiology Diagnostic Oncology Group Study. Radiology. 2001;219:213-8. [DOI] [PubMed] [Google Scholar]
- 9. Doss N, Forney JP, Vellios F, Nalick RH. Covert bilaterality of mature ovarian teratomas. Obstet Gynecol. 1977;50:651-3. [PubMed] [Google Scholar]
- 10. Posteraro AF, Mayo-Smith WW. Bilateral cystic ovarian teratomas. Med Health R I. 2001;84:19-20. [PubMed] [Google Scholar]
- 11. Sahraoui W, Hajji S, Essefi A, Haouas N, Hmissa S, Bibi M, et al. Ovary teratoma. Report of 91 cases. La Tunisie médicale. 2006;84:349-52. [PubMed] [Google Scholar]
- 12. Togashi K, Nishimura K, Itoh K, Fujisawa I, Sago T, Minami S, et al. Ovarian cystic teratomas: MR imaging. Radiology. 1987;162:669-73. [DOI] [PubMed] [Google Scholar]
- 13. Timmerman D, Van Calster B, Jurkovic D, Valentin L, Testa AC, Bernard JP, et al. Inclusion of CA-125 does not improve mathematical models developed to distinguish between benign and malignant adnexal tumors. J Clin Oncol. 2007;25:4194-200. [DOI] [PubMed] [Google Scholar]
- 14. Van Calster B, Timmerman D, Bourne T, Testa AC, Van Holsbeke C, Domali E, et al. Discrimination between benign and malignant adnexal masses by specialist ultrasound examination versus serum CA-125. J Natl Cancer Inst. 2007;99:1706-14. [DOI] [PubMed] [Google Scholar]
- 15. van der Burg ME, Lammes FB, Verweij J. CA 125 in ovarian cancer. Neth J Med. 1992;40:36-51. [PubMed] [Google Scholar]
- 16. Abulafia O, Triest WE, Sherer DM. Angiogenesis in primary and metastatic epithelial ovarian carcinoma. Am J Obstet Gynecol. 1997;177:541-7. [DOI] [PubMed] [Google Scholar]
- 17. Kim HS, Kim JW, Cho JY, Chung HH, Park NH, Song YS, et al. The role of serum CA-125 levels in early-stage epithelial ovarian cancer on preoperative CT and MRI. Eur J Surg Oncol. 2009;35:870-6. [DOI] [PubMed] [Google Scholar]
- 18. Cruickshank DJ, Fullerton WT, Klopper A. The clinical significance of pre-operative serum CA 125 in ovarian cancer. Br J Obstet Gynaecol. 1987;94:692-5. [DOI] [PubMed] [Google Scholar]
- 19. Cho KC, Gold BM. Computed tomography of Krukenberg tumors. AJR. 1985;145:285-8. [DOI] [PubMed] [Google Scholar]
- 20. Megibow AJ, Hulnick DH, Bosniak MA, Balthazar EJ. Ovarian metastases: computed tomographic appearances. Radiology. 1985;156:161-4. [DOI] [PubMed] [Google Scholar]
- 21. Tanaka YO, Okada S, Satoh T, Matsumoto K, Oki A, Saida T, et al. Differentiation of epithelial ovarian cancer subtypes by use of imaging and clinical data: a detailed analysis. Cancer Imaging. 2016;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurman RJ, Carcangiu ML, Herrington S YR, editors. WHO Classification of Tumours of Female Reproductive Organs. 4th ed Lyon: IARC press; 2014. [Google Scholar]
- 23. Kurman RJ, Shih I-M. The Origin and Pathogenesis of Epithelial Ovarian Cancer: A Proposed Unifying Theory. Am J Surg Pathol. 2010;34:433-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lalwani N, Prasad SR, Vikram R, Shanbhogue AK, Huettner PC, Fasih N. Histologic, molecular, and cytogenetic features of ovarian cancers: implications for diagnosis and treatment. Radiographics. 2011;31:625-46. [DOI] [PubMed] [Google Scholar]
- 25. Ha HK, Baek SY, Kim SH, Kim HH, Chung EC, Yeon KM, et al. Krukenberg’s tumor of the ovary: MR imaging features. AJR. 1995;164:1435-9. [DOI] [PubMed] [Google Scholar]
- 26. Choi HJ, Lee J-H, Kang S, Seo S-S, Choi J-I, Lee S, et al. Contrast-Enhanced CT for Differentiation of Ovarian Metastasis from Gastrointestinal Tract Cancer: Stomach Cancer Versus Colon Cancer. AJR. 2006;187:741-5. [DOI] [PubMed] [Google Scholar]
- 27. Koyama T, Mikami Y, Saga T, Tamai K, Togashi K. Secondary ovarian tumors: spectrum of CT and MR features with pathologic correlation. Abdom Imaging. 2007;32:784-95. [DOI] [PubMed] [Google Scholar]
- 28. Willmott F, Allouni KA, Rockall A. Radiological manifestations of metastasis to the ovary. J Clin Pathol. 2012;65:585-90. [DOI] [PubMed] [Google Scholar]
- 29. Morita H, Aoki J, Taketomi A, Sato N, Endo K. Serous surface papillary carcinoma of the peritoneum: clinical, radiologic, and pathologic findings in 11 patients. AJR. 2004;183:923-8. [DOI] [PubMed] [Google Scholar]
- 30. Saida T, Tanaka YO, Matsumoto K, Satoh T, Yoshikawa H, Minami M, et al. Revised FIGO staging system for cancer of the ovary, fallopian tube, and peritoneum: important implications for radiologists. Jpn J Radiol. 2016;34:117-24. [DOI] [PubMed] [Google Scholar]
- 31. Tavassoli F a., Devilee P, editors. World Health Organization Classification of Tumours: Pathology and genetics of tumours of the breast and female genital organs. 3rd ed Lyon: IARC press; 2003. [Google Scholar]