Abstract
Seemingly mutualistic relationships can be exploited, in some cases reducing fitness of the exploited species. In plants, the insufficient receipt of pollen limits reproduction. While infrequent pollination commonly underlies pollen limitation (PL), frequent interactions with low-efficiency, exploitative pollinators may also cause PL. In the widespread protandrous herb Campanula americana, visitation by three pollinators explained 63% of the variation in PL among populations spanning the range. Bumblebees and the medium-sized Megachile campanulae enhanced reproductive success, but small solitary bees exacerbated PL. To dissect mechanisms behind these relationships, we scored sex-specific floral visitation, and the contributions of each pollinator to plant fitness using single flower visits. Small bees and M. campanulae overvisited male-phase flowers, but bumblebees frequently visited female-phase flowers. Fewer bumblebee visits were required to saturate seed set compared to other bees. Scaling pollinator efficiency metrics to populations, small bees deplete large amounts of pollen due to highly male-biased flower visitation and infrequent pollen deposition. Thus, small bees reduce plant reproduction by limiting pollen available for transfer by efficient pollinators, and appear to exploit the plant–pollinator mutualism, acting as functional parasites to C. americana. It is therefore unlikely that small bees will compensate for reproductive failure in C. americana when bumblebees are scarce.
Keywords: bumblebee, Campanula americana, pollination effectiveness, pollen depletion, mutualism exploitation, pollen limitation
1. Background
Insect pollination is crucial for the reproduction of both wild and domesticated plant species. Pollinator declines driven by habitat fragmentation, climate change and disease have been linked to declines in plant distributions, plant reproductive success and crop yields [1]. The loss of a primary insect pollinator can negatively impact fitness of native plant populations [2]. Fortunately, most flowering plant species are visited by a variety of insect taxa [3]. However, the influence of flower-visiting insects on plant reproduction can vary widely among insect taxa due to differences in their behaviour, morphology and rates of visitation [4–6]. In some cases, flower visitors considered to be pollinators can be so inefficient at transferring pollen between plants that they may impose a cost to plant reproduction [7–9]. These ‘pollen thieves’ may exploit the apparent plant–pollinator mutualism [10]. Thus, understanding the impact of specific pollinator types on plant fitness is essential for predicting the responses of plant reproduction to fluctuations in pollinator availability.
Pollen limitation, the insufficient receipt of compatible pollen, is a pervasive feature of flowering plants that limits their reproductive success. Pollen limitation underlies key processes that shape the diversity of flowering plants, including pollinator-mediated selection and the evolution of self-fertilization [11]. Spatial and temporal variation in pollinator community composition can result in heterogeneity in floral visitation by both efficient and inefficient insect pollinators [12–14], and subsequent variation in the magnitude of pollen limitation (PL) [15,16]. While we know that insect pollinators often vary in their efficiency of pollen transfer, linking patterns of PL with direct estimates of the efficiency and visitation frequency of various pollinator taxa is rare.
The behaviour and morphology of flower visitors can explain differences in their pollination efficiency [17]. Some floral visitors primarily seek nectar, contacting pollen only in passing, whereas others predominantly seek pollen. This behavioural difference is expected to impact the capacity for both pollen export and deposition. For example, in hermaphroditic plant species that separate sexual functions spatially or temporally, pollen-collecting insects may prefer male-phase flowers or male reproductive structures, exporting large amounts of pollen but rarely affecting plant reproduction. Pollinator morphology can also influence the success of pollen transfer. For instance, larger insects, or those with pollen-collecting hairs (scopae), may export and deposit larger pollen loads than smaller insects [6]. However, pollen in the corbicula (i.e. pollen basket) of large social bees is often inviable, while that carried on scopae of solitary bees is viable [18]. Thus, knowledge about visitation behaviour and morphology can be key in predicting how different flower visitors could influence PL of plant reproduction.
The contribution of a single pollinator visit to a flower to plant reproduction is commonly used to determine pollination efficiency [19]. Studies that quantify pollinator efficiency, however, are strongly biased towards quantification of female fitness via fruit or seed set. Those that have quantified male fitness often use a proxy (e.g. pollen removal or pollen deposition) that inadequately measures total male fitness [20,21]. Quantification of both pollen removal and deposition affords an estimation of the number of grains removed from a flower that do not contribute to plant fitness (i.e. pollen depletion [9]). Inefficient ‘pollen thieves’ that deplete pollen have been described in a number of systems, but their impact on PL in natural populations is little understood [8,22]. Additionally, the relative visitation rates of efficient and inefficient pollinators can interact in complex ways to shape plant reproductive fitness because pollen removed by one pollinator type is unavailable for transfer by another [23–25]. A study that relates PL to visitation from pollinators with variable pollen-depletion capabilities can shed light on the direct effects of a given pollinator to patterns of plant reproductive success. It can further help to predict the responses of plant fitness to declines of specific pollinators.
In this study, we explore how differential efficiency of pollinators drives large-scale geographical variation in the magnitude of PL in the widespread, protandrous herb Campanula americana. The flowers of C. americana are insect-pollinated by bumblebees, the bellflower resin bee (Megachile campanulae) and small solitary bees [25,26]. Here, we determine the contribution of visitation by each pollinator type to PL across 23 populations spanning the range of C. americana. In a subset of populations, we assess the opportunity for pollen deposition and export by each pollinator class by scoring visitation rates to each floral sex phase. We then determine the contribution of each visitor type to plant fitness using single-visit efficiency assays. Finally, we measure the relationship between pollen receipt and seed production to estimate the number of visits required by each insect type to saturate seed set. With this series of experiments, we answer the following questions. (i) What is the influence of visitation by each dominant pollinator class to PL in natural populations? (ii) Does sex-biased flower visitation affect the opportunity for pollen deposition and export? (iii) Do visitors differ in single-visit efficiency for pollen deposition, contribution to seed set and pollen removal? (iv) Do pollinator taxa differ in their potential to deplete pollen from natural populations?
2. Material and methods
(a) System
Campanula americana L. (Campanulastrum americanum Small) is a widespread herb that grows on forest edges in the Eastern United States. It is self-compatible and flowers display protandry. Flowers open mid-morning in male phase, presenting pollen along the style which is held by pollen-collecting hairs (figure 1b). Pollinators typically remove all or most pollen on the first day. Flowers then transition to female phase with the curling open of stigmatic lobes. Campanula americana is predominantly outcrossing [27], but has the capacity to self-fertilize [28]. Flower visitors are predominantly bumblebees, the bellflower resin bee (M. campanulae) and various species of solitary bees, including those in the Halictidae and Apidae (hereafter, ‘small bees’) [25,26,29]. Megachile campanulae is one of the most common visitors across populations [26]. Though not directly measured, two studies implicate bumblebees as efficient pollinators and small bees as inefficient [25,29].
Figure 1.
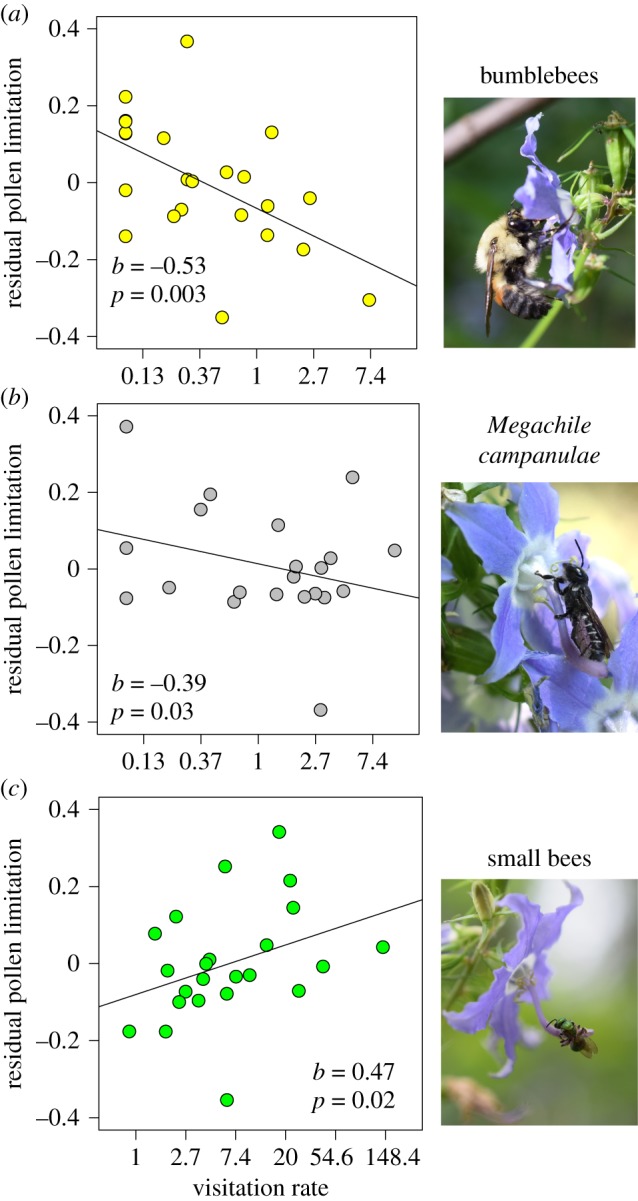
Direct effects of pollinator visitation rate on PL across 23 populations of Campanula americana for (a) bumblebees, (b) Megachile campanulae and (c) small bees. Values on the y-axis are residual PL from linear models with visitation rates (visits per flower per hour) of other pollinator types as predictor variables.
(b) Pollinator visitation rates and pollen limitation
In 2016, we measured PL and pollinator visitation rates in 23 populations spanning the range of C. americana (electronic supplementary material, figure S1). We selected six focal plants per population to score pollinator visitation rates. For each focal plant, we counted open flowers and noted every insect that visited a flower during a single 15 min interval between 11:00 and 14:45. We recorded insect identity as bumblebee, M. campanulae, or small bee (figure 1). These groups were established after extensive pollinator observations in 2015, and are based on size and behaviour. Bumblebees are large and orient their head to the base of the style to probe for nectar. Megachile campanulae is intermediate in size to bumblebees and small bees, and either orients its head to the style base to probe for nectar while pulsating its abdomen on the pollen-bearing style, or it collects pollen without seeking nectar. It is easily identified by its black-/silver-striped abdomen and abdominal scopae laden with pollen of C. americana (figure 1b). Bees smaller than M. campanulae largely collect pollen. These were identified as Halictidae (e.g. Lasioglossum, Augochlora and Augochloropsis; figure 1c) and Apidae (e.g. Ceratina). Owing to difficulty identifying these in the field and their similarity in terms of visitation behaviour (mostly pollen-collecting) and size, small bees were grouped for analyses.
We observed pollinators for 36 h across the 23 populations. Visitation rates for bumblebees, M. campanulae and small bees were calculated as visits per flower per hour at the plant level and were then averaged across plants within a population to estimate population-level visitation rates.
On the same day as pollinator observations, we conducted hand-pollinations on an average of 25 plants per population to measure outcross PL (see [26]). Specifically, on each plant, we outcross pollinated a flower by hand and tagged a control flower that was left unmanipulated. One month later, we collected fruits to score seed production. A smaller seed set in the control group relative to the outcross supplemented group indicates that seed set is limited by outcross pollen receipt. In three populations, PL fell below zero, but we bound these to zero for analyses.
To assess the contribution of visitation by each pollinator group to variation in outcross PL among populations, we modelled PL as a function of bumblebee, M. campanulae and small bee visitation rates using a multiple linear regression (pollen limitation = bumblebee rate + Megachile rate + small bee rate). Pollen limitation was square-root transformed, which improved normality. Visitation rates were ln + 0.1 transformed. We compared the magnitude of the effect of each pollinator on PL with standardized regression coefficients. Analyses were performed in R (v. 3.3.2).
(c) Visitation based on floral sex phase
We scored sex-specific floral visitation rates for each pollinator class in five populations (electronic supplementary material, figure S1). In each, we selected five to nine 1 m2 plots (n = 33 plots) and counted the number of male-phase (pollen present, stigma closed) and female-phase flowers (no pollen, stigmatic lobes open). We recorded the type of pollinator and the sex phase of each flower visited for 15 min per plot. We calculated the sex-specific visitation rate for each pollinator type as the number of visits to a given floral sex divided by the number of flowers of that sex in each plot. Four arrays were observed for less than 15 min due to inclement weather. In total, pollinators were observed for 7.83 h.
To determine whether pollinators displayed sex-biased flower visitation, we modelled plot-level visitation rate as a function of pollinator type, sex phase and their interaction, with the duration of observation as a covariate using ANOVA. Population and plot nested within the population were random effects. The visitation rate was ln+1 transformed to ensure the normality of residuals. Because of a significant sex × pollinator interaction, we tested whether each pollinator type displayed visitation bias to a given sex using the SLICE statement in SAS.
(d) Single-visit efficiency: pollen deposition and seed set
We measured single-visit pollination efficiency in two natural populations in Ohio (electronic supplementary material, figure S1) and in arrays of potted plants at the College of Wooster's Fern Valley Field Station. Potted plants were grown from seed at the University of Virginia, transported to Wooster, OH in June and kept in a greenhouse at Ohio State University Agricultural Technical Institute. Bumblebee visitation is low in natural populations relative to other bees (see results), so we maintained a colony of Bombus impatiens (Natupol, Koppert Biological Systems, The Netherlands), a common pollinator of C. americana, at Fern Valley to obtain larger sample sizes for bumblebee efficiency metrics.
To estimate pollen deposition per visit, we selected male-phase flowers on naturally occurring plants that were stripped of pollen by pollinators and covered them with a drinking straw, stapled at one end to block insect visitation. The same was done on potted plants at Fern Valley, but we emasculated flowers by hand by brushing pollen off the style with a wet paintbrush. We measured pollen deposition and seed set on emasculated flowers because in natural populations, and all or most pollen is removed from the flower during male phase [30]. Moreover, if flowers were not emasculated, it would be difficult to distinguish whether pollen deposited autonomously or via pollinators. We uncovered flowers after they transitioned to female phase and collected stigmas following a single insect visit. A visit was scored if the pollinator contacted the stigma and/or style. We collected stigmas from 32 flowers visited by bumblebees, 30 by M. campanulae and 31 by small bees, and placed them in water-filled microcentrifuge tubes. We collected unvisited stigmas (n = 17) haphazardly throughout the season. We mounted stigma lobes using fuschin jelly [31] and counted pollen grains adhered to each under a light microscope (400×). We scored slides blindly and in random order. The mean number of grains on unvisited stigmas was 6.88 (range 2–14) which we subtracted from each visited sample to score the number of grains deposited.
To determine seed set following a single pollinator visit, we emasculated flowers on potted plants in the greenhouse and transported them to natural populations. Following a single visit, we protected the flowers from further visitation and then returned plants to the greenhouse. After fruit maturation, we counted seed production. If a visited flower did not set fruit, seed set was scored as zero. Seed set was scored on 31 flowers visited by bumblebees, 54 by M. campanulae and 33 by small bees.
(e) Single-visit efficiency: pollen removal
To measure pollen grains removed by a single visit to a male-phase flower, we observed visits to newly open, unvisited flowers in two natural populations and Fern Valley Field Station. We then placed the pollen-bearing style into a microcentrifuge tube with 200 µl of water. We collected 18 unvisited styles haphazardly to estimate the number of grains in an unvisited flower. To score samples, we vortexed each tube for 30 s and counted the pollen grains in an 0.8 µl aliquot using a haemocytometer [31]. We multiplied the number of grains counted by 250 to estimate the number in the entire sample. We calculated the number of grains removed for each flower by subtracting grains remaining in a visited flower from the average grains in unvisited flowers (23 432 grains). We scored 33 flowers visited by bumblebees, 59 by M. campanulae and 37 by small bees.
To evaluate the reproductive consequences of a single visit, we modelled pollen grains deposited, seeds produced and grains removed as a function of the pollinator type. We used the site and date of collection (nested within site) as random effects. We included random terms to control for variation in pollinator activity and flower availability among sites, and the presence of a bumblebee colony at only one site. For pollen deposition, we used a normal distribution, for seed set a negative binomial distribution and for pollen removal a lognormal distribution, which optimized normality and reduced residual heteroscedasticity. We used pre-planned contrasts to test pairwise differences between pollinator types because of a priori expectations of differential efficiencies.
(f) Relationship between pollen deposition and seed production
We used greenhouse-grown plants from five populations to establish the relationship between pollen deposition and seed production. We collected a small amount of pollen from a single flower on the tip of a pin and counted the number of grains using a dissecting microscope. We applied pollen to the stigmatic lobes of an emasculated female-phase flower on a different individual from the same population, and subsequently counted the pollen grains remaining on the pin. The number of grains placed on the stigma was determined as the grains counted prior to pollination minus grains remaining on the pin after pollination. We left a number of flowers unpollinated (zero grains). We counted the seeds in each fruit after maturation. A total of 109 flowers were used in the experiment, and the range of pollen grains deposited was between 0 and 700.
We used piecewise regression (‘segmented’ package, R v. 3.3.2) to determine the point at which the number of seeds produced no longer increased as a function of grains deposited (i.e. the ‘breakpoint’). Using the piecewise regression parameters and empirical estimates of single-visit pollen deposition, we estimated the number of visits required by each pollinator type to reach the saturation point for seed production.
(g) Pollen depletion in natural populations
We combined results of mechanistic experiments with pollinator visitation rates to predict pollen depletion by each pollinator type in 23 natural populations [9,24]. Specifically, we took into account visitation rates, sex-biased floral visitation, single-visit pollen deposition and single-visit pollen removal. First, we estimated a metric of population-level pollen removal for each pollinator type using the following equation:
| 2.1 |
We then estimated population-level pollen deposition for each pollinator as:
| 2.2 |
Pollen depletion by each pollinator type in each population was calculated by subtracting population-level pollen deposition (2.2) from population-level pollen removal (2.1). This represents the population-wide number of pollen grains that are removed but not deposited on female-phase flowers each hour. The proportion of visits to male and female flowers for each pollinator was calculated by dividing the sex-specific floral visitation rates by the total visitation rate (see ‘Visitation based on floral sex phase’ above). We tested whether pollinators differ in their contribution to pollen depletion across populations by modelling pollen depletion as a function of pollinator type with population as a random effect using a general mixed linear model. We used pre-planned contrasts to compare depletion between each pollinator type. Depletion was log-transformed to ensure the normality of residuals.
3. Results
Pollen limitation varied across populations from 0 to 0.92 (i.e. a 92% reduction in seed production due to inadequate receipt of outcross pollen). Small bee visitation rate was the highest (mean = 15.2 visits per flower per hour; figure 1c), followed by M. campanulae (mean = 4.99, figure 1b) and bumblebees (mean = 0.80, figure 1a). Together, visitation rates of bumblebees, M. campanulae and small bees explained 63% of the variation in PL across populations (F3,19 = 10.77, p < 0.001; electronic supplementary material, table S1). Bumblebee visitation was associated with reduced PL (figure 1a), as was visitation of M. campanulae, but to a lesser degree (figure 1b). However, higher visitation rates of small bees were associated with increased PL (figure 1c). Standardized regression coefficients revealed that visitation rates by bumblebees had the strongest influence on PL (b = −0.53), followed by small bees (b = 0.47) and finally M. campanulae (b = −0.39). The sum total visitation rate of all pollinator types does not explain variation in PL (R2 = 0.10, p = 0.13; electronic supplementary material, figure S2).
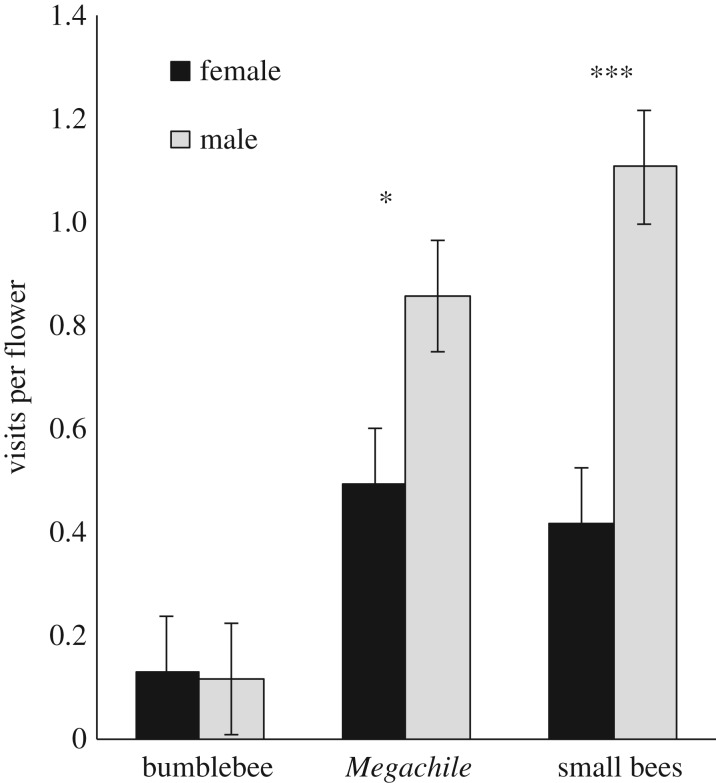
Male-phase flowers were visited more frequently by pollinators (figure 2; electronic supplementary material, table S2), but the difference in visitation rate to male- and female-phase flowers depended on the pollinator type (figure 2; electronic supplementary material, table S2). Small bees visited male flowers at a rate of 2.5 times that of female flowers. Megachile campanulae visited male flowers more frequently than female flowers, but the preference was not as strong as that of small bees. Bumblebees did not display sex-biased flower visitation (figure 2).
Figure 2.
Visitation rates (visits per flower per observation period) to female- and male-phase flowers of C. americana as a function of pollinator type. Least-squares means ± 1 s.e. from a general linear mixed-effect model are plotted. Observation periods were 15 min unless cut short by inclement weather (mean = 13.9 min). Asterisks indicate sex-biased flower visitation of a pollinator group. *p < 0.05, ***p < 0.0001.
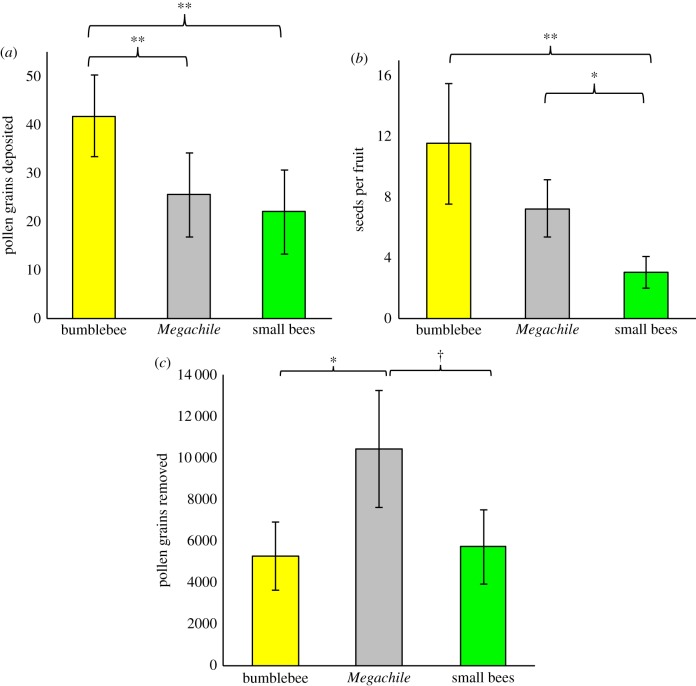
Pollinator efficiency depended on the pollinator type (table 1 and figure 3a–c). Bumblebees delivered approximately 74% more pollen grains to stigmas per visit than either M. campanulae or small bees (figure 3a). A bumblebee visit resulted in the production of three times more seeds than a small bee, and M. campanulae two times more seeds than a small bee visit (figure 3b). Pollen removal also depends on the pollinator type (table 1). Megachile campanulae removed about two times more pollen than bumblebees and small bees per visit (figure 3c).
Table 1.
Pollinator types differ in their single-visit efficiency with respect to plant reproductive success (pollen deposition, seed set and pollen removal). Effects of general and generalized mixed linear models for each aspect of efficiency, and pairwise comparisons between the three pollinator types, are provided. F-values are reported for main effects (pollinator) and contrasts, while Z-values are reported for random effects (site and date nested in site). B, bumblebee; M, Megachile campanulae; S, small bee. Denominator d.f.: deposition = 71, seed set = 95, pollen removal = 61.
| effect | num. d.f. | pollen deposition |
seed set |
pollen removal |
|||
|---|---|---|---|---|---|---|---|
| F/Z | p | F/Z | p | F/Z | p | ||
| pollinator | 1 | 5.46 | 0.006 | 3.95 | 0.02 | 3.69 | 0.03 |
| site | 2 | 0.76 | 0.22 | 0 | — | 0.62 | 0.54 |
| date (site) | 1 | 2.18 | 0.015 | 0 | — | 0.62 | 0.54 |
| B versus M | 1 | 8.10 | 0.006 | 1.16 | 0.28 | 3.55 | 0.06 |
| B versus S | 1 | 10.42 | 0.002 | 7.57 | 0.007 | 0.02 | 0.87 |
| M versus S | 1 | 0.64 | 0.42 | 4.07 | 0.05 | 5.53 | 0.02 |
Figure 3.
The effect of a single flower visit by three dominant pollinator classes on (a) pollen deposition, (b) seed set and (c) pollen removal of Campanula americana. Least-squares means and standard errors from a general mixed-effect linear model are plotted (table 1). †p < 0.08, *p < 0.05, **p < 0.01.
Seed production increased linearly with the number of pollen grains deposited (b = 0.198) and saturated at 48 seeds with the receipt of 230 grains (electronic supplementary material, figure S3). Beyond 230 grains, the relationship between the number of pollen grains deposited and seeds produced was roughly flat (b = 0.026). The linear relationship was a good predictor of the number of seeds produced per visit for bumblebees and M. campanulae, but a poor predictor for small solitary bees (table 2). Relative to bumblebees, the estimated number of visits required to saturate seed production was 59% higher for M. campanulae and 282% higher for small bees (table 2).
Table 2.
(a) The observed number of grains deposited on stigmas from a single visit by three dominant pollinators of Campanula americana. (b) The predicted number of seeds produced based on the linear relationship between pollen grains and the number of seeds produced, see electronic supplementary material, figure S2). (c) The observed seeds produced per visit and (d) the predicted number of visits required to saturate seed set by each pollinator.
| pollinator | (a) observed grains deposited | (b) predicted seeds produced | (c) observed seeds produced | (d) visits to reach saturation (48.45 seeds) |
|---|---|---|---|---|
| bumblebee | 41.79 | 11.29 | 11.52 | 4.21 |
| Megachile | 25.53 | 8.07 | 7.24 | 6.69 |
| small bees | 22.04 | 7.39 | 3.03 | 15.99 |
Taking into account visitation rates, sex-biased flower visitation and single-visit pollen removal and deposition, pollinators differed in the predicted amount of pollen depleted from populations across the range (F2,44 = 17.86, p < 0.0001). Small bees deplete approximately 31 times more pollen from populations than bumblebees (F2,44 = 34.89, p < 0.0001) and two times more than M. campanulae (F2,44 = 4.71, p = 0.035) (figure 4).
Figure 4.
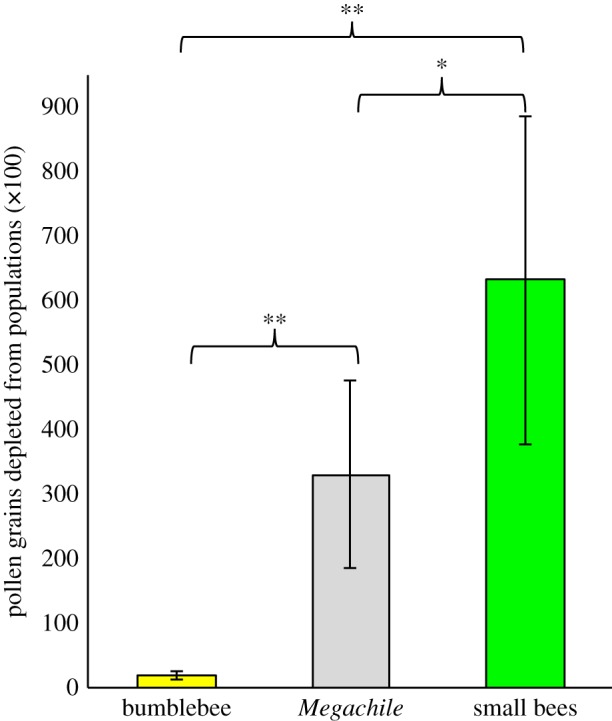
Pollinators deplete different amounts of pollen from natural populations. Depletion estimates are based on flower visitation rates in 23 populations, sex-biased flower visitation and single-visit pollen removal and deposition. They represent the predicted number of grains removed from male-phase flowers but not deposited on female-phase flowers in a single hour. Bars display mean ± 1 s.e. across 23 populations. *p < 0.05, **p < 0.01.
4. Discussion
Heterogeneity in PL among populations of C. americana was driven by visitation rates of both efficient and inefficient pollinators. While previous work has shown that bumblebee visitation alleviates PL [26], it found no effect of visitation by other, smaller bees. However, the more detailed analysis presented here that separates the common intermediate-sized resin bee, Megachile campanulae, from smaller solitary bees shows that they too reduce PL, but to a lesser degree than bumblebees. Furthermore, small solitary bees exacerbated PL. This is, to our knowledge, the first demonstration that higher visitation rates of native insects are associated with elevated PL. The differential effects by each pollinator type on PL obscure any relationship between PL and total visitation rate of all pollinator classes. Thus, it is crucial to account for taxonomic or functional diversity of pollinators when assessing the contributions of pollinator visitation to plant reproductive success.
Our study joins others that have shown that the pollinator assemblage affects PL [15,16]. For example, when large bees are more abundant, PL of Erysimum mediohispanicum is reduced, but it is elevated when beetles are abundant [16]. As pollinator abundance is not always positively correlated with actual visitation rates to flowers [32], our study provides a more direct link between insect visitation rates and PL. While non-pollinator factors may contribute to variation in PL among populations [33], the relative visitation of efficient and inefficient pollinators explained over 60% of the variation in PL in C. americana, suggesting that they are the primary drivers of PL. It is likely that pollinator visitation patterns also play an important role in determining PL in other systems.
Sex-biased flower visitation is one mechanism underlying the different relationships between visitation rates and PL for the different pollinator groups. Bumblebees largely foraged for nectar and visited male- and female-phase flowers equally, but small bees and M. campanulae preferentially visited pollen-bearing male-phase flowers. Thus, the opportunity for pollen deposition is highest for bumblebees, and the opportunity for pollen removal is greater for M. campanulae and especially small bees. Sex-specific visitation preference by pollinators has been demonstrated in sexually dimorphic plant species [34–38]; however, fewer studies evaluate its potential in hermaphroditic species with dichogamy (however, see [39]). Male-biased visitation in dichogamous species can promote pollen theft [8,40], and pollen thieves may drive the evolution of floral traits like pollen dispersal schedules [9]. In C. americana, pollen-collecting hairs hold pollen, and retract over time, governing pollen's availability to insects. There is substantial variability in the duration of pollen retention among C. americana populations [41]. Thus, selection by pollen thieves may contribute to variation in pollen retention and the pollen dispersal schedule.
Differences in pollinator efficiency are another important mechanistic component underlying relationships between pollinator visitation and PL. Bumblebees deposit the most grains per visit on stigmas and affect more seed production than small bees (figure 3a,b). This is probably due to their larger size resulting in more contact with stigmas. Megachile campanulae affects more seed production than small bees, despite the fact they deposit similar amounts of pollen per visit. This discord may be driven by different placement of pollen on the stigma between pollinator types [42]. Megachile campanulae removes substantially more grains from male-phase flowers than either bumblebees or small bees. Given the visitation behaviour of M. campanulae, whereby they actively collect pollen on abdominal scopae, this result is not surprising. However, M. campanulae also deposit significantly less pollen per visit than bumblebees. Taken together, results indicate that M. campanulae is a less efficient pollinator than bumblebees. While PL declined with M. campanulae visitation, the relationship was weak. Other studies have similarly found that large bees are more efficient pollinators than small bees [6], but the efficiency of larger bees may depend on the plant species and floral characteristics [13,43]. Our deposition and seed set efficiency metrics are based on pollen imported from other flowers because focal flowers were emasculated. Thus, our data cannot speak to the potential that pollinators could differentially contribute to within-flower self-pollination. However, within flower, pollen movement in C. americana is likely to be limited as nearly all pollen is typically removed before the onset of female phase.
By synthesizing results from multiple experiments, we show that small bees, which have the highest visitation rates in natural populations, are generally detrimental to the reproductive success of C. americana because they deplete pollen. Their high rates of pollen depletion are driven by their strong male-biased visitation. Despite M. campanulae removing more pollen per visit than small bees, it depletes less pollen overall because its male-biased visitation is less pronounced and its overall visitation rate is lower than small bees. Depletion of pollen from natural populations underlies the negative impact of small bee visitation on reproductive success of C. americana. It is likely that small inefficient pollinators limit the amount of pollen available for larger efficient pollinators (i.e. bumblebees) to transfer between plants, supporting theoretical predictions that pollinators of different efficiencies interact to shape patterns of plant reproductive fitness [24]. For example, Lau & Galloway [25] showed that the negative impacts of halictid bees to plant reproduction are only apparent when bumblebees visit at low frequency, suggesting conditionality of the effects of inefficient pollinators.
By quantifying pollen depletion in natural populations, we suggest that some apparent pollinators are not necessarily plant mutualists. While others have argued that inefficient pollinators are commensal with plants [44] or only parasitic in particular ecological contexts [25], our results suggest that inefficient small bees tend towards being consistently parasitic, effectively reducing reproductive success of C. americana. While nectar-robbing species can exploit plant–pollinator mutualisms [45], the current study provides an example of how pollen-collecting insects can also exploit their apparent mutualistic relationship with plants.
5. Conclusion
Pollen limitation of plant reproduction is a common feature among angiosperms and has important evolutionary consequences. However, the mechanisms that underlie variability in PL are not always well profiled. Spatial heterogeneity in floral visitation by efficient and inefficient pollinators explains a large proportion of variation in PL among populations of C. americana. Visitation by small bees and relatively low-efficiency M. campanulae is unlikely to make up for any reproductive failure experienced due to the decline of efficient bumblebee pollinators [46,47]. For example, Bombus affinis, a pollinator of C. americana, has seen population declines [48], while the majority of common solitary bee visitors (including M. campanulae) are considered secure [49]. Thus, this work underscores the importance of bumblebees for reproductive success of wild plant species. Additionally, variation in PL driven by pollinator composition is likely to have implications for the evolutionary trajectory of a variety of floral traits, floral sex ratios and mating systems. For example, depletion of pollen by small bees could constrain self-fertilization as a mechanism of reproductive assurance, even under strong PL [28,41]. Illuminating preferences of both efficient and inefficient pollinators for fitness-related floral traits is thus key for our understanding of pollinator-mediated selection.
Supplementary Material
Acknowledgements
A huge thanks to D. Grossenbacher for substantial fieldwork and pollinator photos, L. Kuo, S. Garcia, E. Tuan and J. Whalen for field assistance, K. Nguyen, M. Stevens and J. Roberts for pollen and seed counting, W. Crannage for plant care, T. Roulston for insect identification, and J. R. K. Forrest for insight on maintaining the bumblebee colony.
Data accessibility
Data are provided on the Dryad Data Repository (http://dx.doi.org/10.5061/dryad.5nj81nf) [50].
Authors' contributions
M.H.K. collected a subset of the PL data, analysed data and wrote the manuscript. J.L.I. developed and oversaw the collection of pollination efficiency metrics and contributed to writing. A.P. developed methodology and collected efficiency data. A.Q.P. developed and collected data on the relationship between pollen deposition and seed production. L.F.G. assisted in data interpretation and drafting the manuscript.
Competing interests
We declare no competing interests.
Funding
This work was supported by NSF DEB 1457037 and associated REU and ROA supplements.
References
- 1.Biesmeijer JC. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351 ( 10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 2.Brosi BJ, Briggs HM. 2013. Single pollinator species losses reduce floral fidelity and plant reproductive function. Proc. Natl Acad. Sci. USA 110, 13044 ( 10.1073/pnas.1307438110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motten AF, Campbell DR, Alexander DE, Miller HL. 1981. Pollination effectiveness of specialist and generalist visitors to a North Carolina population of Claytonia virginica. Ecology 62, 1278 ( 10.2307/1937292) [DOI] [Google Scholar]
- 4.Primack RB, Silander JA. 1975. Measuring the relative importance of different pollinators to plants. Nature 255, 143e975 ( 10.1038/255143a0) [DOI] [Google Scholar]
- 5.Ivey CT, Martinez P, Wyatt R. 2003. Variation in pollinator effectiveness in swamp milkweed, Asclepias incarnata (Apocynaceae). Am. J. Bot. 90, 214 ( 10.3732/ajb.90.2.214) [DOI] [PubMed] [Google Scholar]
- 6.Sahli HF, Conner JK. 2007. Visitation, effectiveness, and efficiency of 15 genera of visitors to wild radish, Raphanus raphanistrum (Brassicaceae). Am. J. Bot. 94, 203 ( 10.3732/ajb.94.2.203) [DOI] [PubMed] [Google Scholar]
- 7.Wilson P, Thomson JD. 1991. Heterogeneity among floral visitors leads to discordance between removal and deposition of pollen. Ecology 72, 1503 ( 10.2307/1941124) [DOI] [Google Scholar]
- 8.Hargreaves AL, Harder LD, Johnson SD. 2009. Consumptive emasculation: the ecological and evolutionary consequences of pollen theft. Biol. Rev. 84, 259 ( 10.1111/j.1469-185X.2008.00074.x) [DOI] [PubMed] [Google Scholar]
- 9.Parker AJ, Williams NM, Thomson JD. 2016. Specialist pollinators deplete pollen in the spring ephemeral wildflower Claytonia virginica. Ecol. Evol. 6, 5169 ( 10.1002/ece3.2252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronstein JL. 2001. The exploitation of mutualisms. Ecol. Lett. 4, 277 ( 10.1046/j.1461-0248.2001.00218.x) [DOI] [Google Scholar]
- 11.Ashman T-L, et al. 2004. pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85, 2408 ( 10.1890/03-8024) [DOI] [Google Scholar]
- 12.Fishbein M, Venable DL. 1996. Diversity and temporal change in the effective pollinators of Asclepias tuberosa. Ecology 77, 1061 ( 10.2307/2265576) [DOI] [Google Scholar]
- 13.Rafferty NE, Ives AR. 2012. Pollinator effectiveness varies with experimental shifts in flowering time. Ecology 93, 803 ( 10.1890/11-0967.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ison J, Prescott LJ, Nordstrom SW, Waananen A, Wagenius S. 2018. Pollinator-mediated mechanisms for increased reproductive success in early flowering plants Oikos. ( 10.1111/oik.04882) [DOI]
- 15.González-Varo JP, Arroyo J, Aparicio A. 2009. Effects of fragmentation on pollinator assemblage, pollen limitation and seed production of Mediterranean myrtle (Myrtus communis). Biol. Conserv. 142, 1058 10.1016/j.biocon.2009.01.017 [DOI] [Google Scholar]
- 16.Gómez JM, Abdelaziz M, Lorite J, Jes J, Mu JesPajares A, Perfectti F. 2010. Changes in pollinator fauna cause spatial variation in pollen limitation: pollinator assemblage and pollen limitation. J. Ecol. 98, 1243l ( 10.1111/j.1365-2745.2010.01691.x) [DOI] [Google Scholar]
- 17.Olsen KM. 1996. Pollination effectiveness and pollinator importance in a population of Heterotheca subaxillaris (Asteraceae). Oecologia 109, 114– 121. [DOI] [PubMed] [Google Scholar]
- 18.Parker AJ, Tran JL, Ison JL, Bai JDK, Weis AE, Thomson JD. 2015. Pollen packing affects the function of pollen on corbiculate bees but not non-corbiculate bees. Arthropod–Plant Interact. 9, 197 ( 10.1007/s11829-015-9358-z) [DOI] [Google Scholar]
- 19.King C, Ballantyne G, Willmer PG. 2013. Why flower visitation is a poor proxy for pollination: measuring single-visit pollen deposition, with implications for pollination networks and conservation. Methods Ecol. Evol. 4, 811 ( 10.1111/2041-210X.12074) [DOI] [Google Scholar]
- 20.Broyles SB, Wyatt R. 1990. Paternity analysis in a natural population of Asclepias exaltata: multiple paternity, functional gender, and the hypothesis. Evolution 44, 1454 ( 10.2307/2409329) [DOI] [PubMed] [Google Scholar]
- 21.Devlin B, Ellstrand NC. 1990. Male and female fertility variation in wild radish, a hermaphrodite. Am. Nat. 136, 87 ( 10.1086/285083) [DOI] [Google Scholar]
- 22.Inouye DW. 1980. The terminology of floral larceny. Ecology 61, 1251 ( 10.2307/1936841) [DOI] [Google Scholar]
- 23.Thomson JD, Thomson BA.. 1992. Pollen presentation and viability schedules in animal-pollinated plants: consequences for reproductive success. In Ecology and evolution of plant reproduction (ed. Wyatt R.), p. 1. New York, NY: Chapman & Hall. [Google Scholar]
- 24.Thomson JD, Goodell K. 2002. Pollen removal and deposition by honeybee and bumblebee visitors to apple and almond flowers: comparative pollination of apples and almonds. J. Appl. Ecol. 38, 1032l ( 10.1046/j.1365-2664.2001.00657.x) [DOI] [Google Scholar]
- 25.Lau JA, Galloway LF. 2004. Effects of low-efficiency pollinators on plant fitness and floral trait evolution in Campanula americana (Campanulaceae). Oecologia 141, 577 ( 10.1007/s00442-004-1677-1) [DOI] [PubMed] [Google Scholar]
- 26.Koski MH, Grossenbacher DL, Busch JW, Galloway LF. 2017. A geographic cline in the ability to self-fertilize is unrelated to the pollination environment. Ecology 98, 2930–2939. ( 10.1002/ecy.2001) [DOI] [PubMed] [Google Scholar]
- 27.Galloway LF, Etterson JR, Hamrick JL. 2003. Outcrossing rate and inbreeding depression in the herbaceous autotetraploid, Campanula americana. Heredity 90, 308 ( 10.1038/sj.hdy.6800242) [DOI] [PubMed] [Google Scholar]
- 28.Koski MH, Kuo L, Niedermaier KM, Galloway LF. 2018. Timing is everything: dichogamy and pollen germinability underlie variation in autonomous selfing among populations. Am. J. Bot. ( 10.1002/ajb2.1025) [DOI] [PubMed] [Google Scholar]
- 29.Johnson SG, Delph LF, Elderkin CL. 1995. The effect of petal-size manipulation on pollen removal, seed set, and insect-visitor behavior in Campanula americana. Oecologia 102, 174 ( 10.1007/BF00333249) [DOI] [PubMed] [Google Scholar]
- 30.Evanhoe L, Galloway LF. 2002. Floral longevity in Campanula americana (Campanulaceae): a comparison of morphological and functional gender phases. Am. J. Bot. 89, 587–591. ( 10.3732/ajb.89.4.587) [DOI] [PubMed] [Google Scholar]
- 31.Kearns CA, Inouye DW.. 1993. Techniques for pollination biologists Niwot, CO: University Press of Colorado. [Google Scholar]
- 32.Herrera CM. 1989. Pollinator abundance, morphology, and flower visitation rate: analysis of the ‘quantity’ component in a plant–pollinator system. Oecologia 80, 241–248. ( 10.1007/BF00380158) [DOI] [PubMed] [Google Scholar]
- 33.Aizen MA, Harder LD. 2007. Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology 88, 271 ( 10.1890/06-1017) [DOI] [PubMed] [Google Scholar]
- 34.Delph LF, Lively CM. 1992. Pollinator visitation, floral display, and nectar production of the sexual morphs of a gynodioecious shrub. Oikos 63, 161 ( 10.2307/3545374) [DOI] [Google Scholar]
- 35.Ashman T-L. 2000. pollinator selectivity and its implications for the evolution of dioecy and sexual dimorphism. Ecology 81, 2577 ( 10.1890/0012-9658(2000)081%5B2577:PSAIIF%5D2.0.CO;2) [DOI] [Google Scholar]
- 36.Ashman T-L, Swetz J, Shivitz S. 2000. Understanding the basis of pollinator selectivity in sexually dimorphic Fragaria virginiana. Oikos 90, 347 ( 10.1034/j.1600-0706.2000.900216.x) [DOI] [Google Scholar]
- 37.Asikainen E. 2005. Preferences of pollinators and herbivores in gynodioecious Geranium sylvaticum. Ann. Bot. 95, 879 ( 10.1093/aob/mci094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waelti MO, Page PA, Widmer A, Schiestl FP. 2009. How to be an attractive male: floral dimorphism and attractiveness to pollinators in a dioecious plant. BMC Evol. Biol. 9, 190 ( 10.1186/1471-2148-9-190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klinkhamer PGL, de Jong TJ. 1990. Effects of plant size, plant density and sex differential nectar reward on pollinator visitation in the protandrous Echium vulgare (Boraginaceae). Oikos 57, 399 ( 10.2307/3565970) [DOI] [Google Scholar]
- 40.Hargreaves AL, Harder LD, Johnson SD. 2012. Floral traits mediate the vulnerability of aloes to pollen theft and inefficient pollination by bees. Ann. Bot. 109, 761 ( 10.1093/aob/mcr324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leibman L, Rowe A, Koski MH, Galloway LF. In press Populations with greater flexibility in floral traits modify mating system in response to the pollinator environment. Funct. Ecol. ( 10.1111/1365-2435.13093) [DOI] [Google Scholar]
- 42.Inouye DW, Gill DE, Dudash MR, Fenster CB. 1994. A model and lexicon for pollen fate. Am. J. Bot. 81, 1517 ( 10.2307/2445328) [DOI] [Google Scholar]
- 43.Conner JK, Davis R, Rush S.. 1995. The effect of wild radish floral morphology on pollination efficiency by four taxa of pollinators. Oecologia 104, 234 ( 10.1007/BF00328588) [DOI] [PubMed] [Google Scholar]
- 44.Schemske DW, Horvitz CC. 1984. Variation among floral visitors in pollination ability: a precondition for mutualism specialization. Science 225, 519 ( 10.1126/science.225.4661.519) [DOI] [PubMed] [Google Scholar]
- 45.Irwin RE, Bronstein JL, Manson JS, Richardson L. 2010. Nectar robbing: ecological and evolutionary perspectives. Annu. Rev. Ecol. Evol. Syst. 41, 271 ( 10.1146/annurev.ecolsys.110308.120330) [DOI] [Google Scholar]
- 46.Colla SR, Packer L. 2008. Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodivers. Conserv. 17, 1379 ( 10.1007/s10531-008-9340-5) [DOI] [Google Scholar]
- 47.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345 ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 48.Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL. 2011. Patterns of widespread decline in North American bumble bees. Proc. Natl Acad. Sci. 108, 662–667. ( 10.1073/pnas.1014743108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopek K, Burd L. 2017. Pollinators in peril: a systematic status review of North American and Hawaiian native bees. See https://biologicaldiversity.org/campaigns/native_pollinators/pdfs/Pollinators_in_Peril.pdf.
- 50.Koski MH, Ison JL, Padilla A, Pham AQ, Galloway LF. 2018. Data from: Linking pollinator efficiency to patterns of pollen limitation: small bees exploit the plant–pollinator mutualism Dryad Data Repository. ( 10.5061/dryad.5nj81nf) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Koski MH, Ison JL, Padilla A, Pham AQ, Galloway LF. 2018. Data from: Linking pollinator efficiency to patterns of pollen limitation: small bees exploit the plant–pollinator mutualism Dryad Data Repository. ( 10.5061/dryad.5nj81nf) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are provided on the Dryad Data Repository (http://dx.doi.org/10.5061/dryad.5nj81nf) [50].