Abstract
Bumblebees are among the world's most important groups of pollinating insects in natural and agricultural ecosystems. Each spring, queen bumblebees emerge from overwintering and initiate new nests, which ultimately give rise to workers and new reproductives later in the season. Nest initiation and survival are thus key drivers of both bumblebee pollination services and population dynamics. We performed the first laboratory experiment with the model bumblebee species Bombus impatiens that explores how early nesting success is impacted by the effects of temporary or more sustained exposure to sublethal levels of a neonicotinoid-type insecticide (imidacloprid at 5 ppb in nectar) and by reliance on a monofloral pollen diet, two factors that have been previously implicated in bumblebee decline. We found that queens exhibited increased mortality and dramatically reduced activity levels when exposed to imidacloprid, as well as delayed nest initiation and lower brood numbers in the nest, but partially recovered from these effects when they only received early, temporary exposure. The effects of pollen diet on individual queen- and colony-level responses were overshadowed by effects of the insecticide, although a monofloral pollen diet alone was sufficient to negatively impact brood production. These findings speak to the sensitivity of queen bumblebees during the nest initiation phase of the colony cycle, with implications for how queens and their young nests are uniquely impacted by exposure to threats such as pesticide exposure and foraging habitat unsuitability.
Keywords: bumblebees, neonicotinoids, queens, nesting, nutrition
1. Introduction
Worldwide, many pollinator populations appear to be declining, a trend that threatens the security of both natural and agricultural ecosystems, given the invaluable ecosystem services that pollinators provide in these systems [1–4]. Bumblebees (genus Bombus, family Apidae) are among the world's most ecologically and economically important pollinators [5] and are also among the pollinator groups for which there is the greatest evidence of decline. Species losses, extirpations and declines have now been detected in North America, Europe and other regions of the world [5–10]. The decline of bumblebees is being driven by the deleterious effects of pesticides, pathogens and floral resource unavailability, among other proximate factors resulting largely from agricultural intensification and global change (reviewed in [10–12]).
Pesticides have been broadly implicated in the decline of wild bee populations [13,14], and particularly strong evidence has emerged that neonicotinoids, the most widely used class of insecticides, are having strong negative impacts on wild bees [15]. These systemic insecticides, which act primarily as nicotinic acetylcholine receptor agonists [16], are most often applied as seed coatings, but the majority of the substance is not incorporated into the treated seeds and instead enters the surrounding environment [17,18]. Neonicotinoids can then be taken up by non-target plants from the soil or other routes of exposure, and may spread broadly throughout plant tissues, including into floral rewards (pollen and nectar) consumed by pollinators [19–22]. Although the concentrations of neonicotinoids typically detected in floral rewards are not sufficient to cause rapid mortality in pollinators [18], there is considerable evidence that field-realistic levels of these insecticides can have sublethal negative impacts on bees. In bumblebees, this evidence originates from a variety of laboratory-based manipulative studies (e.g. [23]) and also semi-wild (e.g. [24]) and field (e.g. [25–27]) studies. These studies have primarily focused on the social phase of the colony cycle (i.e. when workers are present), and collectively they suggest that neonicotinoids impact individual bumblebee workers (e.g. reducing foraging and homing abilities [28,29]; reducing feeding [30]; immune effects [31]) in ways that ultimately negatively impact colony growth and reproductive capacity [23,32–34].
Despite the current breadth of knowledge on how sublethal exposure to neonicotinoid insecticides impacts workers during the social phase of the bumblebee colony cycle, effects on other bumblebee castes and life-history stages are less well understood. Each spring in temperate regions, bumblebee queens that eclosed the previous season emerge from overwintering to initiate new colonies. During this period, queens forage frequently to provision their nests and may encounter neonicotinoid residues, which can persist in the soil and be taken up into plant tissues for several years following application [35]. The ability of individual queens to successfully initiate new nests has considerable consequence for bumblebee population dynamics [36], as each nest can produce up to several hundred workers as well as new reproductives (queens and males) later in the season. As it takes several weeks for the first brood to emerge in newly established nests, early nest-founding queens must perform all work-related tasks (such as foraging and nest construction) without the assistance of workers. The simultaneous physiological demands placed on early nesting queens related to egg production and brood care [37,38] may make queens during this stage particularly susceptible to the effects of neonicotinoids in ways that threaten their nesting success, a hypothesis that is supported by recent studies. In early-nesting queens of four species (B. terrestris, B. lucorum, B. pratorum and B. pascuorum), exposure to sublethal levels of the neonicotinoid thiamethoxam causes a reduction in oocyte size [39], which may translate to reduced brood production, and in B. terrestris, sublethal thiamethoxam exposure reduces the likelihood (by 26%) that queens will successfully initiate nests [36]. Similar effects have also been demonstrated in nest-founding queens of B. impatiens, where exposure to imidacloprid at sublethal levels causes increased mortality and delayed nest initiation and brood production [40].
An additional stressor that bumblebees face is declining floral resource abundance and diversity, which is being driven by land use change (including a trend towards monocultural practices), phenological mismatches between bee flight and plant flowering periods, and other global changes (reviewed in [41,42]). Bumblebees are largely floral generalists that collect pollen from a wide variety of plant species [43], and a breadth of recent studies suggest that a lack of diversity in their pollen diet, or relying solely on pollen from particular plant species, can have negative effects on egg production and larval development in small groups of queenless workers (termed ‘microcolonies’) [30,44–48]. Recent studies have also found additive, negative effects of neonicotinoid exposure and monofloral pollen diet on microcolony growth [23]. However, the combinatorial effects of these two stressors have not yet been examined in nest-founding bumblebee queens, and may differ from workers, given the unique biology of the castes and the extreme physiological demands placed on queens during this life stage.
Here, we provide the first examination of how the singular and combined effects of sublethal, field-realistic neonicotinoid exposure (imidacloprid in nectar at a level of 5 ppb) and pollen diet diversity (either one of two monofloral diets or a polyfloral mix) impact early nest-founding bumblebee (Bombus impatiens) queens. With the goal of differentiating between direct effects of temporary imidacloprid exposure by the queen versus more sustained exposure by the entire social nest (the queen and also the brood, of which larvae actively feed on nectar), 10-day-old queens receiving the insecticide treatment were exposed either (1) only until around the time eggs were present in the nest (17 days) or (2) for the duration of the experiment (37 days), and responses at the queen level (mortality, activity, and nectar consumption) and colony level (timing and total production of brood) were examined.
2. Material and methods
(a). Bee rearing and experimental design
Five queen-producing B. impatiens colonies (with an egg-laying queen, greater than 50 workers, and few or no males in the nest) were supplied for the experiment by Biobest USA, Inc. (Romulus, MI, USA). Callow queens (less than 24 h old; identified by their silvery appearance) were removed from natal colonies on the day of eclosion and maintained individually in small, plastic queen rearing boxes (approximately 15 × 8 × 8 cm) stored in either a temperature- and humidity-controlled incubator (at 28 ± 2.5°C, 60 ± 5% RH) or a queen-rearing room supplied with a humidifier (at 28 ± 2.5°C, 60 ± 10% RH); a subset of queens from all treatment groups (equal in number) was stored in the latter. Queens were not mated, in order to minimize effects of mating variation on the experiment and at ages 12 and 13 days were treated with CO2 (30 min per day) to cause them to bypass diapause and initiate laying of haploid, male-destined eggs [49]. Queens were kept under constant darkness for the duration of the experiment except when food was replenished. Prior to the start of the treatments, queens were provided with 50% (w/v) sucrose solution (ad libitum, replaced every other day) and a wax-coated pollen ball (honeybee-collected, mixed-source pollen from Brushy Mountain Bee Farm, Moravian Falls, NC); this initial pollen ball is typically eaten by the first brood of larvae and not by the queen (described in detail in [50]).
(b). Pesticide and diet treatment administration
A total of 180 queens from five colonies were included in the experiment, with 15 queens per treatment group (insecticide exposure×pollen diet, table 1) and equal representation from each colony across groups. For the imidacloprid-treated queens (hereafter, ‘IMD’), the 50% (w/v) sucrose solution was treated with imidacloprid (Pestanal analytical standard, Sigma-Aldrich) at a level of 5 ppb. Imidacloprid has been detected at this level in wildflowers near agricultural fields in the southern US (early-season [51]) and southeastern UK (in early summer [20]), and in worker bumblebee pollen loads in urban areas in the UK [21], and thus 5 ppb is a field-realistic exposure level for experimental analyses, including with queens. A summary of studies on imidacloprid exposure is provided in the electronic supplementary material. To provide either temporary or sustained exposure to the insecticide, 10-day-old IMD queens (2–3 days prior to CO2 treatment) were either treated only for a period of 17 days and then removed from the treatment and fed untreated nectar for the remainder of the experiment (IMD-A; n = 45), or were treated for the duration of the experiment, which lasted for an additional 20 days (IMD-B; n = 45). The age at which to begin pesticide exposure was chosen to allow queens time to mature before starting the treatment, and the IMD-A queens were removed from the treatment around age 27 day because this is around the time that CO2-treated B. terrestris queens [24] and B. impatiens queens (in this experiment) begin to have larvae present in the nest. IMD-A queens were probably still exposed to residual traces of imidacloprid within the nest-box and from feeding from stored nectar in honey pots, but were not actively feeding themselves or developing brood on fresh nectar containing the insecticide beyond day 17. An additional set of 90 control queens were not treated with IMD. Queens in each of the three pesticide treatment groups also received one of three unique pollen diet treatments: primarily Erica (heather) or Cistus (rockrose) pollen (purchased fresh from Pollenergie and stored at −80°C), or a polyfloral mixture (50% each by weight) of the two pollens. Previous studies have found that microcolonies subsisting solely on Cistus and Erica pollens have reduced larval weights and colony development rates versus when provided with some other pollen species [30,46,47,52]. These effects may be due to the relatively low protein contents of these pollens (e.g. both less than 15% in [31]), although these pollens probably also differ in regard to other nutritional constituents, such as micronutrients. In the wild, bumblebees may preferentially collect pollens with high protein contents [53], or may collect a mix of pollens with varying protein contents [54], depending upon the species, but there is also evidence that profiles of individual amino acids may drive foraging decisions [48]. Thus, these monofloral diets were intended to mimic scenarios where bumblebees are highly limited in their foraging choices, and the polyfloral diet treatment represented a slightly more nutritionally complex foraging environment. Additional information (from previously published studies) about the nutritional content of the commercially available honeybee-collected pollens used in this study is provided in the electronic supplementary material. Across the duration of the experiment, nests were inspected every 1–2 days and pollen was replenished as needed.
Table 1.
Factorial design of experiment. IMD-A: queens were treated for a temporary period of 17 days; IMD-B: queens were treated for the duration of the experiment, which lasted for an additional 20 days. Monofloral Cistus (rockrose); monofloral Erica (heather) and mixed-source: mixture of Cistus and Erica.
| pesticide exposure |
||||
|---|---|---|---|---|
| untreated | IMD-A (temporary) | IMD-B (sustained) | ||
| pollen diet | mixed (Cistus and Erica) | 30 | 15 | 15 |
| Cistus | 30 | 15 | 15 | |
| Erica | 30 | 15 | 15 | |
(c). Queen responses to treatments
Queen mortality was monitored daily across the experiment. To examine the effects of neonicotinoid exposure on queen activity levels, a subset of queens from the IMD-A, IMD-B and untreated groups (n = 9 per group) were subjected to simple activity assays at ages 39–41 days; these ages equate to lengths of time on the imidacloprid treatment of 17 days for the IMD-A group (followed by 8–10 days on the control diet prior to activity assessments), 25–27 days for the IMD-B group, and 0 days for control queens. Each of the three sets of observed queens included an equal number of representatives (n = 3) from all diet treatment groups and only queens with brood located on the opposite side of the cage from the feeder were used in the analysis. Queens were observed under dim red light on 2–3 consecutive days for periods of 10 min per day (occurring between 12.00 and 16.00 h), during which the total number of times they were observed crossing the midpoint of the cage was recorded (hereafter referred to as their ‘activity score’). Observations were performed blind with respect to treatment.
A separate experiment was performed to determine whether the presence of neonicotinoids in nectar influences queen nectar consumption. Here, an additional set of 34 young queens (24–72 h old based on light-coloured pigmentation) not included in the nesting experiment were fed untreated or IMD-treated (5 ppb) 50% w/v sucrose solution (n = 16 treated, 18 untreated bees). This set of queens received only ad libitum mixed-source honeybee-collected pollen (Brushy Mountain Bee Farm, Moravian Falls, NC). Queens were placed in individual plastic containers (as described above), stored in an incubator constantly maintained at 28 ± 2.5°C and 60 ± 5% RH and under constant darkness (except when feeders were replaced), and subjected to a CO2 treatment (30 min per day) for the first 2 days after being removed from their natal colonies. On days 1–7 all queens received untreated nectar, then on day 8 were transferred to new plastic containers (12 × 13 × 8 cm) with four horizontally positioned feeder tubes (filled with IMD-treated or untreated nectar, depending on treatment group) designed to prevent spillage. On days 8–14, the nectar tubes were weighed and replaced every 48 h and changes in weight were converted to volume based on weight of the nectar solution, in order to estimate daily nectar consumption.
(d). Colony responses to treatments
For the duration of the experiment, nests were inspected every 1–2 days for the presence of any eclosed adult males. For all queens that survived for the duration of the experiment (age 47 days for all queens), nests and their inhabitants were placed in a −80°C freezer and stored until they were dissected on dry ice. To assess colony development, brood was removed from the nest-box and the total number of eggs, larvae, pupae, and adult males were recorded. The earliest possible age at initiating egg laying (hereafter, ‘age at nest initiation’) was estimated for each queen by determining the oldest possible age of brood in the nest based on male brood development times for B. impatiens (maximum age for eggs: 5 days; larvae: 15 days; pupae: 25 days [55]). This method was deemed more feasible than using the date eggs were first observed in nests, as eggs can be difficult to detect in young nests without disturbing the queen by removing her from the cage. This estimate is conservative in that it may underestimate how early nests were initiated by ignoring any eggs that died or were eaten, and also underestimate brood ages if developmental rates were delayed in certain pesticide and/or diet treatment groups; the latter has been demonstrated in bumblebees fed certain pollen diets [23,30,46,47,52] and neonicotinoid insecticides [23]. Due to the large margin of error from this method, the estimated mean age at nest initiation was calculated for each group to provide insight into potential delays in nest initiation, but the ages were not compared using statistical analysis.
(e). Statistical analyses
All statistical analyses were performed in R v. 3.3.1 (R Core Team 2017 [56]). Effects of pesticide exposure, pollen diet and colony of origin on queen responses were examined as follows. The optimal model for survival data was selected on the basis of which model produced the lowest value of second-order Akaike information criterion corrected for small sample sizes (AICc) [57] using the MuMln package (v. 1.40.0) [58]. Using the best-fit model with fixed factors (treatment, pollen) and random factor (colony) we examined the effects of fixed factors on timing of death using a mixed-effects Cox regression model with coxme (v. 2.2-7) and the survival package (v. 2.39.5) [59] in R, and visualized using survminer (v. 0.4.0) and ggplot2 (v. 2.2.1). Survival to the end of the experiment was analysed using linear mixed effects regression (lmer) in R using lme4 package (v. 1.1-16). Age at death across treatment levels was analysed using one-way analysis of variance (ANOVA) in R. Activity level data were analysed with linear models (lm) in R following AICc model selection as above. Nectar consumption by treated and untreated queens was compared using Welch's two-sample t-test for unequal variances.
The effects of pesticide exposure (untreated, IMD-A or IMD-B), pollen diet (Cistus, Erica or mixed), and natal colony of origin on colony-level responses (including total number of eggs, larvae, and pupae or adults in the nest when collected) were examined using GAMLSS v. 5.0-6 fitted with a zero inflated negative binomial type I family using the MuMln package. GAMLSS fits regression models with skewed data, performing transformations specific to zero-inflated data while including both fixed and random effects [60]. The best-fit model for these terms was selected on the basis described above [57]. A summary of these models and their outputs is provided in table 2. Given that the effect of pollen diet was minimal compared with the effects of the insecticide treatment in our model selection, a separate analysis was performed to explore how pollen diet impacted brood production solely in untreated queens. For this, we analysed brood abundance data for control (untreated) queens only with linear model (lm) in R following model selection.
Table 2.
Summary of the models used in analysis of effects of imidacloprid and pollen diet on queen-level and colony-level responses in Bombus impatiens queens.
| analysis | model types | fixed factors | random factors | R packages used | transformations | parameters | estimate | s.e. | t-value | Pr (>|t|) |
|---|---|---|---|---|---|---|---|---|---|---|
| queen survival | lmer | treatment | colony | MuMIn, lme4, survival | none | intercept | 0.041 | 0.047 | 0.866 | |
| treatment: CTL versus IMD-A | 0.078 | 0.062 | 1.253 | |||||||
| treatment: CTL versus IMD-B | 0.369 | 0.062 | 5.949 | |||||||
| queen activity | lm | treatment | — | MuMIn, lm in stats | none | intercept | 14.667 | 3.17 | 4.626 | 0.000107 |
| treatment: CTL versus IMD-A | −12.389 | 4.483 | −2.763 | 0.010812 | ||||||
| treatment: CTL versus IMD-B | −10.593 | 4.483 | −2.363 | 0.027 | ||||||
| queen feeding | — | treatment | — | — | none | — | — | — | −0.703 | 0.488 |
| colony no. eggs | treatment | — | GAMLSS | zero-inflated negative binomial type I | intercept | 2.804 | 0.090 | 31.18 | <2 × 10−16 | |
| treatment: CTL versus IMD-A | −0.811 | 0.185 | −4.385 | 2.27 × 10−5 | ||||||
| treatment: CTL versus IMD-B | −1.141 | 0.295 | −3.864 | 0.00017 | ||||||
| colony no. larvae | — | treatment; pollen | colony | GAMLSS | zero-inflated negative binomial type I | intercept | 2.373 | 0.156 | 15.236 | <2 × 10−16 |
| treatment: CTL versus IMD-A | −0.419 | 0.295 | −1.420 | 0.158 | ||||||
| treatment: CTL versus IMD-B | −3.432 | 0.828 | −4.146 | 6.06 × 10−5 | ||||||
| pollen: Erica versus Mixed | 0.028 | 0.221 | 0.128 | 0.898 | ||||||
| pollen: Erica versus Cistus | −0.597 | 0.251 | −2.379 | 0.019 | ||||||
| colony no. pupae and adults | treatment; pollen | colony | GAMLSS | zero-inflated negative binomial type I | intercept | 0.954 | 0.283 | 3.375 | 9.72 × 10−4 | |
| treatment: CTL versus IMD-B | −1.239 × 1016 | 4.129 × 1015 | −3.00 | 0.003 |
3. Results
(a). Effects of pesticide and pollen diet on individual queen responses
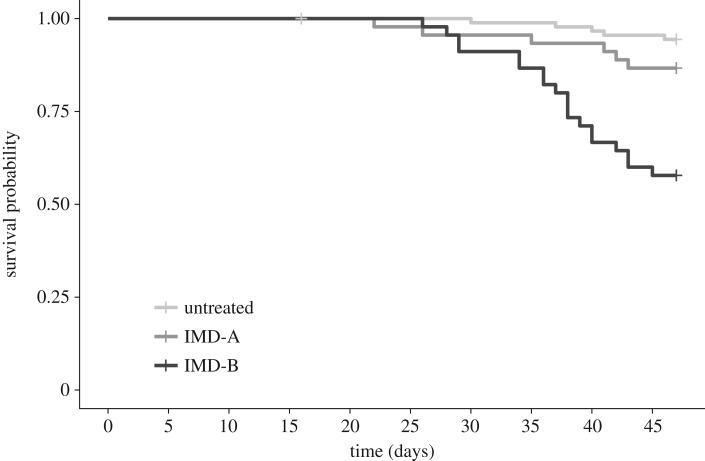
Queen mortality was only strongly influenced by pesticide exposure (figure 1), with nearly six times higher mortality among IMD-B queens (40%) relative to the untreated queens (7%) (p < 0.001, estimate = 0.369, 95% CI [0.246, 0.490]), and three times higher mortality in IMD-B relative to the IMD-A queens (13%). There was no difference in mortality between the IMD-A and untreated queens (estimate = 0.078, 95% CI [−0.044, 0.199]). Colony of origin, pollen diet treatment, and the interaction of pollen and pesticide treatment did not significantly affect queen mortality. Mean ages at death were not significantly different across pesticide treatments, at 35.2, 35 and 37.1 days for untreated, IMD-A and IMD-B groups, respectively (p = 0.77).
Figure 1.
Survival curve for queens exposed to no imidacloprid (untreated) or receiving early, temporary (IMD-A) or sustained (IMD-B) exposure to imidacloprid at 5 ppb in nectar. Group samples sizes were n = 90, 45 and 45, respectively.
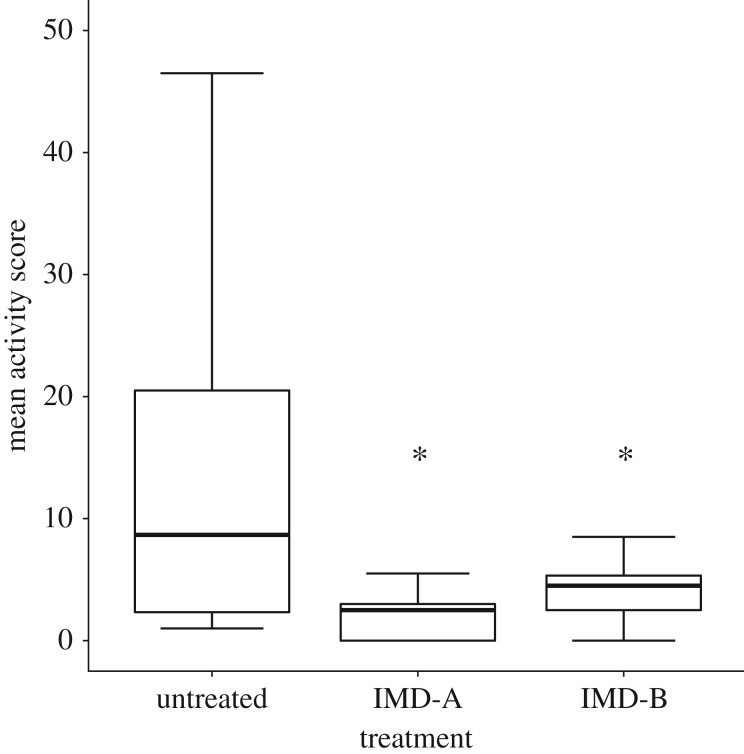
Imidacloprid exposure also negatively impacted queen activity levels (figure 2). Untreated queens (mean activity score = 14.67 ± 5.37 s.e.) were more active than both the IMD-A (mean activity score = 2.28 ± 0.69 s.e.) and IMD-B (mean activity score = 4.07 ± 0.90 s.e.) queen groups (p < 0.03 for both pairwise comparisons). There was no difference in activity between the IMD-A and IMD-B groups (p = 0.92). There was also no difference (p = 0.488) in the amount of nectar consumed by IMD-treated versus untreated queens (mean daily consumption of 0.28 g nectar ± 0.02 s.e. for treated queens, 0.31 g nectar ± 0.04 s.e. for untreated queens).
Figure 2.
Mean activity levels for queens exposed to no imidacloprid (untreated) or receiving early, temporary (IMD-A) or sustained (IMD-B) exposure to imidacloprid at 5 ppb in nectar. Bars represent group means (±s.e.) and asterisks connote a significant difference from the control (untreated) group at p < 0.05.
(b). Effects of pesticide and pollen diet on nest initiation and colony development
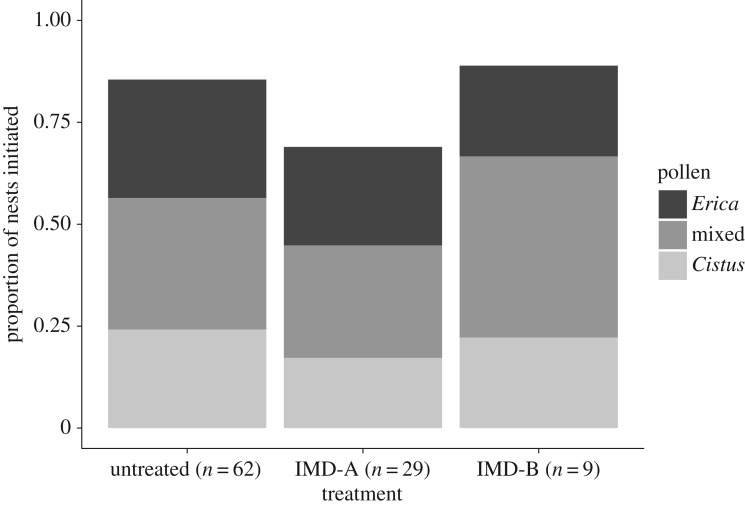
For queens in all insecticide treatment groups, more than 69% of all surviving queens initiated nests (figure 3 and electronic supplementary material); the small sample sizes of surviving queens in the IMD-B treatment group precluded statistical comparisons of nest initiation between groups. Imidacloprid-treated queens (in both the IMD-A and IMD-B groups) initiated nests on average approximately 5 days later than untreated queens (mean estimated ages at nest initiation: untreated = age 29.8 days ±1.0 s.e., IMD-A = 34.8 ± 1.1 s.e., IMD-B = 35.1 ± 2.0 s.e.).
Figure 3.
Proportion of queens that successfully initiated nests as a function of insecticide treatment (bars) and pollen diet (as described in figure legend). Sample sizes only included queens that survived for the duration of the experiment; n = 62, 29 and 9, respectively.
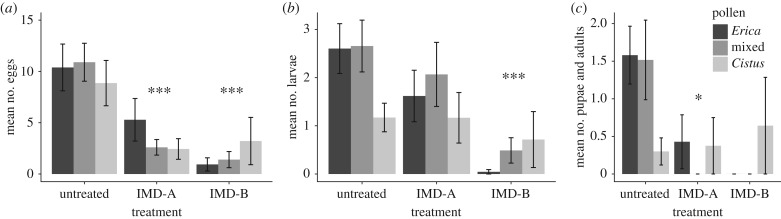
Exposure to imidacloprid had strong, negative impacts on brood production, with around three and four times greater egg and pupal abundance (respectively) in untreated queens relative to the IMD queens (figure 4). Moreover, queens with sustained exposure to imidacloprid exhibited a particularly strong reduction in the percentage of larvae and pupae produced, with IMD-B queens exhibiting more than 70% reduction in pupae as compared with untreated queens. These results are reported only for queens that lived until the end of the experiment (n = 62, 29 and 9 for untreated, IMD-A and IMD-B groups, respectively; see electronic supplementary material); only one queen that died during the experiment had brood in her nest (from the IMD-B group). Adult males eclosed in only two colonies in the experiment (in the control and IMD-A groups), and were included in the mean number of pupae and adults (figure 4c). Pollen diet was not an important driver of brood production overall, but among untreated queens, those fed monofloral Cistus pollen had significantly fewer larvae and pupae than queens fed mixed-source or monofloral Erica pollen (p < 0.03 for counts of larvae and pupae).
Figure 4.
Mean numbers of brood in the nest as a function of insecticide treatment (groups of bars) and pollen diet (bars, coloured as described in figure legend). Bars represent group means (±s.e.) and asterisks connote a significant difference from the control (untreated) group at p < 0.05 (*) or p < 0.01 (***). For (c), brood numbers were counted at the end of the experiment (day 45).
4. Discussion
Here, we demonstrate that sublethal exposure to imidacloprid, a widely used neonicotinoid-type insecticide, has a broad suite of negative effects on early-nesting bumblebee queens. These effects were observed at the level of individual queens, who were less active and exhibited greater mortality when exposed to imidacloprid, but also at the colony level, where numbers of brood in the nest were dramatically reduced in treated queens, particularly when queens experienced sustained exposure to the insecticide. We also show for the first time that the deleterious effects of this insecticide during the early nesting stage can be stronger than the negative consequences of a monofloral pollen diet, another previously identified stressor for bumblebees, under certain pesticide exposure regimes. Importantly, there may also be interactive (e.g. additive or synergistic) effects of neonicotinoid insecticide exposure and pollen diet on queens; these interactions would have been difficult to detect in our study given the high mortality and subsequent small sample sizes we observed in imidacloprid-treated queens (particularly in those that experienced sustained exposure).
We found that many of the adverse effects of imidacloprid on nesting queens occurred regardless of whether exposure is temporally restricted (to the period before larvae are present in the nest), or is sustained beyond this period. Treated queens exhibited higher mortality than untreated queens over the life of the experiment (up to 40% in the IMD-B group), laid far fewer eggs and were less active than untreated queens even when they had not received imidacloprid for 12–14 days prior to observations. Our study is the first to explore how temporally restricted versus sustained exposure to a neonicotinoid impacts reproduction and other processes in bumblebees, and our findings suggest that there are enduring behavioural and physiological effects of these insecticides that may ultimately have fitness consequences, particularly in regard to survival and brood production. The negative effects of imidacloprid on colony development were most extreme in our treatment group that received sustained exposure to imidacloprid. This is perhaps unsurprising, given that larvae in these nests also consumed treated nectar, and may have experienced negative developmental or other direct effects of the insecticide. Given that neonicotinoids are systemic insecticides that are broadly used [61] and can persist in ecosystems for up to several years [18,62], our sustained exposure paradigm probably more realistically represents the exposure that bumblebees face in the wild.
Queens did not consume less imidacloprid-treated nectar in our feeding experiment at 5 ppb, a finding that is consistent with previous bumblebee studies that have also found equal [63] or even greater [64] consumption of neonicotinoid-treated versus untreated nectar (but see [39]). Thus, the deleterious effects observed in our treated queens were likely due to the insecticide itself, rather than being a consequence of reduced feeding in avoidance of imidacloprid. Neonicotinoid insecticides are neurotoxins that interfere with nicotinic acetylcholine receptor signalling, but the full extent of their sublethal effects is currently not well understood. Negative effects on egg laying have been previously found in workers of the species B. terrestris [33,65], and in queens of B. impatiens [40] and B. terrestris [36]. These previous findings, and our similar finding here, may have been caused in part by the negative effect of the insecticide on motor activity, which may reduce egg laying, brood feeding and other activities. Recent studies have found negative impacts of neonicotinoids on several locomotor behaviours in bumblebees, such as buzz pollination [34] and thermoregulation [66]. However, a previous study in bumblebee queens of multiple species found that exposure to another neonicotinoid (thiamethoxam) caused a reduction in oocyte size [39], suggesting that these insecticides may also affect the production of eggs within the ovaries, in addition to locomotor effects.
We found that reliance on pollen from a single plant species (Cistus) was sufficient to reduce brood production in the absence of imidacloprid exposure. This finding is consistent with multiple other studies on bumblebee nutrition, which have demonstrated that a monofloral diet [23,30,45–47], and specifically reliance on Cistus pollen [23,30,44], can lead to reduced larval growth rates and/or lower numbers of brood in the nest. Cistus pollen has a low essential amino acid content (electronic supplementary material) and may be deficient in other nutrients, but is actively foraged on by bumblebees [47,48,52,67]. Many ecosystems are losing flowering plant diversity due to conversion to monocultural agricultural schemes and other processes (reviewed in [41,42]). A corresponding loss of pollen species diversity in the diet of bumblebees could have devastating consequences for bumblebee populations under scenarios where foragers collect pollen from suboptimal food sources because there is no possibility to select from among a variety of plant species. Our data suggest that neonicotinoid exposure might be a greater threat to wild bumblebee populations than monofloral diets, but, importantly, lack of pollen diet diversity also appears to limit brood development on its own, and can also interact additively with neonicotinoid exposure to limit colony growth [23]. Further, whereas our polyfloral diet treatment was a combination of pollens from just two plant species, in many ecosystems bumblebees can forage on a far greater number of plants, and we predict that greater effect size differences would be found if comparisons of monofloral and more realistically diverse polyfloral diet treatments were employed.
Our findings speak to the unique biology and sensitivity of the queen caste in bumblebees, in particular during the early nest-founding stage. The increased mortality exhibited in our imidacloprid-treated queens (fourfold) at a concentration with lesser impacts on mortality in workers of B. terrestris [32,34,68] suggests that there may be species and/or queen caste- or stage-specific sensitivity to this insecticide. The nesting success of an individual queen also has dramatic, resounding consequences for bumblebee population dynamics. Baron et al. [36] estimated a high probability of bumblebee population extinction following their observation of a 26% reduction in nest initiation by thiamethoxam-treated queens; here, we observed an even greater decline in nest initiation (69% of untreated queens initiated versus 64% in IMD-A and 22% in IMD-B, but here reductions were overwhelmingly due to queen mortality) as a consequence of sublethal imidacloprid exposure. Given that queens in our experiment were supplied with food ad libitum, confined in small cages, and maintained under stable and optimal rearing conditions, wild queens who must also forage and are exposed to fluctuating abiotic conditions and additional stressors might be even more strongly impacted beyond the degree observed in our experiment. Further, we exposed queens to a level of neonicotinoid that is below what has been detected in floral rewards in some field studies (electronic supplementary material), and only introduced the insecticide to nectar, although it can be also present in pollen. Altogether, our findings are likely to be conservative with respect to realistic exposure levels in some landscapes. Our study is one of a small but growing number of studies examining the unique effects of neonicotinoid exposure on the solitary life-history stages of bumblebee queens [36,39,40,69]. Collectively, these studies suggest that there are unique deleterious effects on queens that can have dramatic consequences for reproduction and nesting success. Thus, we posit that queens should be a target of both insecticide research and conservation efforts, given their special importance for the persistence of bumblebee populations and thus the security of their pollination services.
Supplementary Material
Acknowledgements
We thank M. Flores, N. Fischer, A. Vanecek, K. Fisher, S. Reimer, E. Sarro and other members of the Woodard lab for assistance with the experiment and for feedback on the manuscript, and Biobest USA for providing bees for the experiment.
Data accessibility
Data are available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.mm4m722) [70].
Authors' contributions
M.L. co-designed and conducted the insecticide exposure experiment; K.M.W. performed statistical analyses, generated figures for the manuscript and assisted with experiments; J.B. designed and conducted the feeding experiment; S.H.W. co-designed and assisted with the experiments; and all authors contributed to writing the publication. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the USDA National Institute of Food and Agriculture, Hatch project no. 1011665.
References
- 1.Garibaldi LA, et al. 2013. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339, 1608–1611. ( 10.1126/science.1230200) [DOI] [PubMed] [Google Scholar]
- 2.Klein A-M, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303–313. ( 10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ollerton J, Winfree R, Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos 120, 321–326. ( 10.1111/j.1600-0706.2010.18644.x) [DOI] [Google Scholar]
- 4.Biesmeijer JC, et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354. ( 10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 5.Goulson D. 2010. Bumblebees. In Silent summer: the state of wildlife in Britain and Ireland (ed. Maclean N.), pp. 415–429. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Williams PH, Osborne JL. 2009. Bumblebee vulnerability and conservation world-wide. Apidologie 40, 367–387. ( 10.1051/apido/2009025) [DOI] [Google Scholar]
- 7.Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL. 2011. Patterns of widespread decline in North American bumble bees. Proc. Natl Acad. Sci. USA 108, 662–667. ( 10.1073/pnas.1014743108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grixti JC, Wong LT, Cameron SA, Favret C. 2009. Decline of bumble bees (Bombus) in the North American Midwest. Biol. Conserv. 142, 75–84. ( 10.1016/j.biocon.2008.09.027) [DOI] [Google Scholar]
- 9.Colla SR, Packer L. 2008. Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodivers. Conserv. 17, 1379 ( 10.1007/s10531-008-9340-5) [DOI] [Google Scholar]
- 10.Goulson D, Lye GC, Darvill B. 2008. Decline and conservation of bumble bees. Annu. Rev. Entomol. 53, 191–208. ( 10.1146/annurev.ento.53.103106.093454) [DOI] [PubMed] [Google Scholar]
- 11.Goulson D, Nicholls E, Botias C, Rotheray EL. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
- 12.Woodard SH. 2017. Bumble bee ecophysiology: integrating the changing environment and the organism. Curr. Opin. Insect Sci. 22, 101–108. ( 10.1016/j.cois.2017.06.001) [DOI] [PubMed] [Google Scholar]
- 13.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 14.Brown MJF, Paxton RJ. 2009. The conservation of bees: a global perspective. Apidologie 40, 410–416. ( 10.1051/apido/2009019) [DOI] [Google Scholar]
- 15.van der Sluijs JP, Simon-Delso N, Goulson D, Maxim L, Bonmatin J-M, Belzunces LP. 2013. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 5, 293–305. ( 10.1016/j.cosust.2013.05.007) [DOI] [Google Scholar]
- 16.Tomizawa M, Casida J. 2003. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu. Rev. Entomol. 48, 339–364. ( 10.1146/annurev.ento.48.091801.112731) [DOI] [PubMed] [Google Scholar]
- 17.Sur R, Stork A. 2003. Uptake, translocation and metabolism of imidacloprid in plants. Bull. Insectol. 56, 35–40. [Google Scholar]
- 18.Goulson D. 2013. Review: an overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987. ( 10.1111/1365-2664.12111) [DOI] [Google Scholar]
- 19.Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K. 2012. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 7, e29268 ( 10.1371/journal.pone.0029268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botías C, David A, Horwood J, Abdul-Sada A, Nicholls E, Hill E, Goulson D. 2015. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 49, 12 731–12 740. ( 10.1021/acs.est.5b03459) [DOI] [PubMed] [Google Scholar]
- 21.David A, Botías C, Abdul-Sada A, Nicholls E, Rotheray EL, Hill EM, Goulson D. 2016. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Int. 88, 169–178. ( 10.1016/j.envint.2015.12.011) [DOI] [PubMed] [Google Scholar]
- 22.Lentola A, David A, Abdul-Sada A, Tapparo A, Goulson D, Hill EM. 2017. Ornamental plants on sale to the public are a significant source of pesticide residues with implications for the health of pollinating insects. Environ. Pollut. 228, 297–304. ( 10.1016/j.envpol.2017.03.084) [DOI] [PubMed] [Google Scholar]
- 23.Dance C, Botías C, Goulson D. 2017. The combined effects of a monotonous diet and exposure to thiamethoxam on the performance of bumblebee micro-colonies. Ecotoxicol. Environ. Saf. 139, 194–201. ( 10.1016/j.ecoenv.2017.01.041) [DOI] [PubMed] [Google Scholar]
- 24.Stanley DA, Garratt MPD, Wickens JB, Wickens VJ, Potts SG, Raine NE. 2015. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 528, 548–550. ( 10.1038/nature16167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rundlöf M, et al. 2015. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80. ( 10.1038/nature14420) [DOI] [PubMed] [Google Scholar]
- 26.Woodcock BA, et al. 2017. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 356, 1393–1395. ( 10.1126/science.aaa1190) [DOI] [PubMed] [Google Scholar]
- 27.Tsvetkov N, Samson-Robert O, Sood K, Patel HS, Malena DA, Gajiwala PH, Maciukiewicz P, Fournier V, Zayed A. 2017. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 356, 1395–1397. ( 10.1126/science.aam7470) [DOI] [PubMed] [Google Scholar]
- 28.Yang EC, Chuang YC, Chen YL, Chang LH. 2008. Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 101, 1743–1748. ( 10.1603/0022-0493-101.6.1743) [DOI] [PubMed] [Google Scholar]
- 29.Schneider CW, Tautz J, Grünewald B, Fuchs S. 2012. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS ONE 7, e30023 ( 10.1371/journal.pone.0030023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tasei JN, Aupinel P. 2008. Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae). Apidologie 39, 397–409. ( 10.1051/apido:2008017) [DOI] [Google Scholar]
- 31.Di Pasquale G, Salignon M, Le Conte Y, Belzunces LP, Decourtye A, Kretzschmar A, Suchail S, Brunet J-L, Alaux C. 2013. Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS ONE 8, e72016 ( 10.1371/journal.pone.0072016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill RJ, Ramos-Rodriguez O, Raine NE. 2012. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491, 105–108. ( 10.1038/nature11585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laycock I, Lenthall KM, Barratt AT, Cresswell JE. 2012. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 21, 1937–1945. ( 10.1007/s10646-012-0927-y) [DOI] [PubMed] [Google Scholar]
- 34.Whitehorn PR, O 'Connor S, Wackers FL, Goulson D. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352. ( 10.1126/science.1215025) [DOI] [PubMed] [Google Scholar]
- 35.Bonmatin JM, Moineau I, Charvet R, Colin ME, Fleche C, Bengsch ER. 2005. Behaviour of imidacloprid in fields. Toxicity for honey bees. In Environmental chemistry: green chemistry and pollutants in ecosystems (eds Lichtfouse E, Schwarzbauer J, Robert D), pp. 483–494. Berlin, Germany: Springer. [Google Scholar]
- 36.Baron GL, Jansen VAA, Brown MJF, Raine NE. 2017. Pesticide reduces bumblebee colony initiation and increases probability of population extinction. Nat. Ecol. Evol. 1, 1308–1316. ( 10.1038/s41559-017-0260-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogt FD, Heinrich B, Plowright C. 1998. Ovary development in bumble bee queens: the influence of abdominal temperature and food availability. Can. J. Zool. 76, 2026–2030. ( 10.1139/z98-146) [DOI] [Google Scholar]
- 38.Woodard SH, Bloch G, Band MR, Robinson GE. 2013. Social regulation of maternal traits in nest-founding bumble bee (Bombus terrestris) queens. J. Exp. Biol. 216, 3474–3482. ( 10.1242/jeb.087403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baron GL, Raine NE, Brown MJF. 2017. General and species-specific impacts of a neonicotinoid insecticide on the ovary development and feeding of wild bumblebee queens. Proc. R. Soc. B 284, 20170123 ( 10.1098/rspb.2017.0123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu-Smart J, Spivak M. 2018. Effects of neonicotinoid imidacloprid exposure on bumble bee (Hymenoptera: Apidae) queen survival and nest initiation. Environ. Entomol. 47, 55–62. ( 10.1093/ee/nvx175) [DOI] [PubMed] [Google Scholar]
- 41.Woodard SH, Jha S. 2017. Wild bee nutritional ecology: predicting pollinator population dynamics, movement, and services from floral resources. Curr. Opin. Insect Sci. 21, 83–90. ( 10.1016/j.cois.2017.05.011) [DOI] [PubMed] [Google Scholar]
- 42.Ogilvie JE, Forrest JRK. 2017. Interactions between bee foraging and floral resource phenology shape bee populations and communities. Curr. Opin. Insect Sci. 21, 75–82. ( 10.1016/j.cois.2017.05.015) [DOI] [PubMed] [Google Scholar]
- 43.Plowright RC, Laverty TM. 1984. The ecology and sociobiology of bumble bees. Annu. Rev. Entomol. 29, 175–199. ( 10.1146/annurev.en.29.010184.001135) [DOI] [Google Scholar]
- 44.Moerman R, Roger N, De Jonghe R, Michez D, Vanderplanck M. 2016. Interspecific variation in bumblebee performance on pollen diet: new insights for mitigation strategies. PLoS ONE 11, e0168462 ( 10.1371/journal.pone.0168462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanderplanck M, et al. 2016. Is non-host pollen suitable for generalist bumblebees? Insect Sci. 25, 259-272. ( 10.1111/1744-7917.12410) [DOI] [PubMed] [Google Scholar]
- 46.Moerman R, Vanderplanck M, Fournier D, Jacquemart A, Michez D, Schonrogge K. 2017. Pollen nutrients better explain bumblebee colony development than pollen diversity. Insect Conserv. Divers. 10, 171–179. ( 10.1111/icad.12213) [DOI] [Google Scholar]
- 47.Roger N, et al. 2017. Impact of pollen resources drift on common bumblebees in NW Europe. Glob. Chang. Biol. 23, 68–76. ( 10.1111/gcb.13373) [DOI] [PubMed] [Google Scholar]
- 48.Moerman R, Vanderplanck M, Roger N, Declèves S, Wathelet B, Rasmont P, Fournier D, Michez D. 2015. Growth rate of bumblebee larvae is related to pollen amino acids. J. Econ. Entomol. 109, 1–6. ( 10.1093/jee/tov279) [DOI] [PubMed] [Google Scholar]
- 49.Röseler P-F. 1985. A technique for year-round rearing of Bombus terrestris (Apidae, Bombini) colonies in captivity. Apidologie 16, 165–170. ( 10.1051/apido:19850206) [DOI] [Google Scholar]
- 50.Free J, Butler C. 1959. Bumblebees. London, UK: Collins. [Google Scholar]
- 51.Stewart SD, et al. 2014. Potential exposure of pollinators to neonicotinoid insecticides from the use of insecticide seed treatments in the mid-southern United States. Environ. Sci. Technol. 48, 9762–9769. ( 10.1021/es501657w) [DOI] [PubMed] [Google Scholar]
- 52.Baloglu GH, Gurel F. 2015. The effects of pollen protein content on colony development of the bumblebee, Bombus terrestris L.. J. Apic. Sci. 59, 83 ( 10.1515/jas-2015-0009) [DOI] [Google Scholar]
- 53.Kriesell L, Hilpert A, Leonhardt SD. 2017. Different but the same: bumblebee species collect pollen of different plant sources but similar amino acid profiles. Apidologie 48, 102–116. ( 10.1007/s13592-016-0454-6) [DOI] [Google Scholar]
- 54.Somme L, Vanderplanck M, Michez D, Lombaerde I, Moerman R, Wathelet B, Wattiez R, Lognay G, Jacquemart AL. 2015. Pollen and nectar quality drive the major and minor floral choices of bumble bees. Apidologie 46, 92–106. ( 10.1007/s13592-014-0307-0) [DOI] [Google Scholar]
- 55.Cnaani J, Schmid-Hempel R, Schmidt JO. 2002. Colony development, larval development and worker reproduction in Bombus impatiens Cresson. Insectes Soc. 49, 164–170. ( 10.1007/s00040-002-8297-8) [DOI] [Google Scholar]
- 56.R Core Team. 2017. R: a language and an environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 57.Brewer MJ, Butler A, Cooksley SL, Freckleton R. 2016. The relative performance of AIC, AICC and BIC in the presence of unobserved heterogeneity. Methods Ecol. Evol. 7, 679–692. ( 10.1111/2041-210X.12541) [DOI] [Google Scholar]
- 58.Bartoń K.2017. MuMIn: multi-model inference. Version 1.40.0. See http://CRAN.R-project.org/package=MuMIn .
- 59.Therneau TM. 2014. Survival: a package for survival analysis in S. R package version 2.37.7. See https://cran.r-project.org/web/packages/survival/survival.pdf.
- 60.Stasinopoulos DM, Rigby RA. 2007. Generalized additive models for location scale and shape (GAMLSS) in R. J. Stat. Softw. 23, 1–46. ( 10.18637/jss.v023.i07) [DOI] [Google Scholar]
- 61.Jeschke P, Nauen R, Schindler M, Elbert A. 2011. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 59, 2897–2908. ( 10.1021/jf101303g) [DOI] [PubMed] [Google Scholar]
- 62.Bonmatin JM, et al. 2015. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 22, 35–67. ( 10.1007/s11356-014-3332-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cresswell JE, Thompson HM. 2012. Comment on ‘A common pesticide decreases foraging success and survival in honey bees'. Science 337, 1453 ( 10.1126/science.1224618) [DOI] [PubMed] [Google Scholar]
- 64.Kessler SC, Tiedeken EJ, Simcock KL, Derveau S, Mitchell J, Softley S, Radcliffe A, Stout JC, Wright GA. 2015. Bees prefer foods containing neonicotinoid pesticides. Nature 521, 74–76. ( 10.0.4.14/nature14414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elston C, Thompson HM, Walters KFA. 2013. Sub-lethal effects of thiamethoxam, a neonicotinoid pesticide, and propiconazole, a DMI fungicide, on colony initiation in bumblebee (Bombus terrestris) micro-colonies. Apidologie 44, 563–574. ( 10.1007/s13592-013-0206-9) [DOI] [Google Scholar]
- 66.Potts R, Clarke RM, Oldfield SE, Wood LK, de Ibarra NH, Cresswell JE. 2018. The effect of dietary neonicotinoid pesticides on non-flight thermogenesis in worker bumble bees (Bombus terrestris). J. Insect Physiol. 104, 33–39. ( 10.1016/j.jinsphys.2017.11.006) [DOI] [PubMed] [Google Scholar]
- 67.De Luca PA, Vallejo-Marín M. 2013. What's the ‘buzz’ about? The ecology and evolutionary significance of buzz-pollination. Curr. Opin. Plant Biol. 16, 429–435. ( 10.1016/j.pbi.2013.05.002) [DOI] [PubMed] [Google Scholar]
- 68.Decourtye A, Devillers J. 2010. Ecotoxicity of neonicotinoid insecticides to bees. In Insect nicotinic acetylcholine receptors (ed. Thany SH.), pp. 85–95. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- 69.Fauser A, Sandrock C, Neumann P, Sadd BM. 2017. Neonicotinoids override a parasite exposure impact on hibernation success of a key bumblebee pollinator. Ecol. Entomol. 42, 306–314. ( 10.1111/een.12385) [DOI] [Google Scholar]
- 70.Leza M, Watrous KM, Bratu J, Woodard SH. 2018. Data from: Effects of neonicotinoid insecticide exposure and monofloral diet on nest-founding bumblebee queens Dryad Digital Repository. ( 10.5061/dryad.mm4m722) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Leza M, Watrous KM, Bratu J, Woodard SH. 2018. Data from: Effects of neonicotinoid insecticide exposure and monofloral diet on nest-founding bumblebee queens Dryad Digital Repository. ( 10.5061/dryad.mm4m722) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.mm4m722) [70].