Abstract
Urban habitats are drastically modified from their natural state, creating unique challenges and selection pressures for organisms that reside in them. We compared locomotor performance of Anolis lizards from urban and forest habitats on tracks differing in angle and substrate, and found that using artificial substrates came at a cost: lizards ran substantially slower and frequently lost traction on man-made surfaces compared to bark. We found that various morphological traits were positively correlated with sprint speed and that these same traits were significantly larger in urban compared to forest lizards. We found that urban lizards ran faster on both man-made and natural surfaces, suggesting similar mechanisms improve locomotor performance on both classes of substrate. Thus, lizards in urban areas may be under selection to run faster on all flat surfaces, while forest lizards face competing demands of running, jumping and clinging to narrow perches. Novel locomotor challenges posed by urban habitats likely have fitness consequences for lizards that cannot effectively use man-made surfaces, providing a mechanistic basis for observed phenotypic shifts in urban populations of this species.
Keywords: performance, Puerto Rico, urbanization, Anolis cristatellus, adaptation, urban evolution
1. Introduction
Adaptation to a novel habitat involves not only phenotypic change, but a functional benefit resulting in increased fitness. This phenotype–performance–fitness paradigm provides a framework for understanding adaptive phenotypic differentiation [1–3]. In short, natural selection acts on performance in a given habitat. If performance is correlated with heritable phenotypic variation in morphology, selection can result in morphological change [2].
Novel habitats, such as urbanization, present new opportunities to study functional consequences of phenotypic change under altered selective conditions. Research has shown that urban populations of the lizard Anolis cristatellus exhibit morphological shifts compared to forest populations. Urban lizards have relatively longer limbs and more subdigital scales called lamellae, used in adhesion to smooth surfaces [4]. Although agents of selection responsible for these shifts have not been shown, Winchell et al. [4,5] documented substantial differences in habitat openness, perch width and perch roughness between forest and urban sites and showed that urban A. cristatellus commonly use anthropogenic structures.
We propose that locomotor performance is the functional link underlying this observed pattern. Prior research on Anolis lizards (anoles) has established a strong connection between limb morphology and sprinting [6–8]. In general, lizards with longer limbs run faster, although this effect depends on perch diameter with the greatest benefit on flat surfaces [9]. On inclined perches body width also influences sprint speed by lowering the centre of mass and increasing stability [10,11]. In addition, both toepad area and lamella number are correlated with clinging performance and habitat use: lizards with larger toepads and more lamellae exert stronger cling forces and perch higher [12–15].
These morphology–performance relationships seem likely to persist in urban areas, but with a selective landscape modified such that phenotypes conferring optimal performance differ compared to forests. Urban areas are more open than forest and perches tend to be broad and flat [5]. Travelling between perches in urban areas also requires substantial over-ground movement with little refuge, potentially favouring phenotypes enabling rapid locomotion on the ground. Moreover, perches in urban areas are commonly man-made and tend to be vertical, unbranching and substantially smoother than those in forests [4,5]. On these surfaces, traits enhancing clinging ability and stability, by enabling a more sprawled posture, will be beneficial as they should reduce falls and facilitate locomotion.
Though urban and forest habitats differ dramatically, the extent to which urban lizards are exposed to novel selection will depend on their behaviour [2]. Individuals may avoid habitat in which they perform sub-optimally, a phenomenon known as the habitat constraint hypothesis [3]. Alternatively, lizards that perform well across a variety of conditions may be indiscriminate in their habitat use (habitat breadth hypothesis [3]). Winchell et al. [5] showed that individual A. cristatellus use anthropogenic and vegetative elements of the urban habitat to differing degrees, which suggests some urban lizards may be constrained in their habitat use, while others capitalize on the novel habitat and expand their resource utilization. If discriminatory habitat use (constraint) is related to performance, then lizards with less-than-optimal phenotypes may behaviourally avoid novel urban selection pressures and impede evolution. Conversely, if individuals with the most appropriate phenotypes tend to expand into novel urban spaces (breadth), evolutionary change may be promoted.
Here, we test the following hypotheses related to locomotor performance and habitat use in A. cristatellus: (1) locomotor performance should differ between man-made and natural substrates, and we predict a decrease in performance associated with man-made surfaces. (2) Lizard morphology will be correlated with sprint performance, and urban lizards should exhibit phenotypic shifts in functionally relevant traits. (3) Consequently, urban and forest lizards will differ from each other in locomotor ability, particularly on man-made surfaces. Finally, (4) urban lizards should use a broader range of perches than forest lizards, although habitat use may be correlated with locomotor performance (habitat constraint and breadth hypotheses). Addressing these four hypotheses will help us quantify the role of locomotor performance in urban habitats as a mechanism driving morphological divergence.
2. Material and methods
(a). Study system
Anolis is a diverse neotropical lizard genus containing approximately 400 species. They are perhaps best known for repeatedly converging in ecology and morphology on different Caribbean islands, and for rapid adaptation in response to changing environmental conditions (reviewed in [8]). Adaptive trait–environment relationships are well understood for the group because of a rich literature on ecological and evolutionary dynamics in natural systems [8]. The Puerto Rican Crested Anole, A. cristatellus, is a small (adult male snout–vent length (SVL) 50–75 mm) arboreal lizard abundant in forest and urban habitats where it extensively uses anthropogenic resources [4,5]. Previous research demonstrated that this species has undergone morphological shifts in urban versus forest environments in limb lengths and toepad morphology, and that these changes may be genetically based [4].
(b). Data collection
We constructed a 2.5 m long, 9 cm wide racetrack with three surfaces: wood bark, painted concrete and aluminium sheeting (electronic supplementary material S1). We set the track at 37° (gradual) and 60° (steep) inclines. At angles less than 37° lizards tend to jump rather than sprint [3], and at angles greater than 60° the majority of lizards we tested could not consistently climb the smooth substrates.
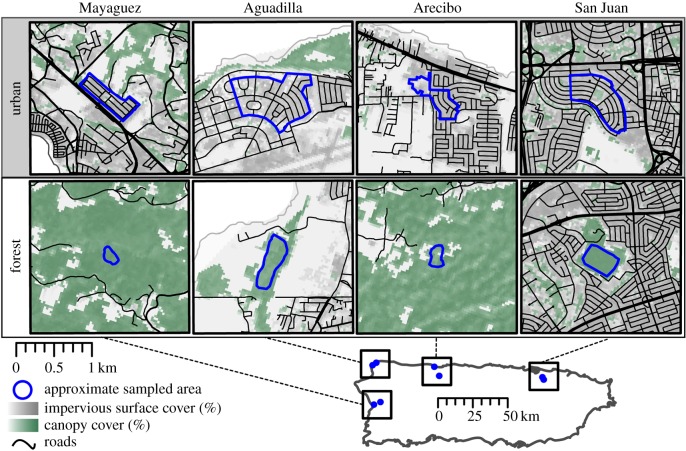
We sampled lizards from paired urban and forest sites in four Puerto Rican municipalities (figure 1). Urban sites were dominated by impervious surface and developed land with minimal canopy cover. Forest sites included managed reserves and restricted-access secondary natural forest. Paired urban and forest sites were 3.2–7.5 km apart. The forest site in San Juan was unusual in that it is completely surrounded by urbanization. Because San Juan is a sprawling urban region, identifying suitable urban and forest sites with minimal distance between them is challenging as not much forest remains in the greater metropolitan area. We also sampled this forest site in previous work [4] and found consistent phenotypic shifts between forest and urban populations.
Figure 1.
Sites sampled in four Puerto Rican municipalities with approximate sampling areas outlined in blue. Canopy cover, impervious surface cover and roads are shown within 2 km2 from the centre of the sampled area. Grey solid lines represent coastline.
We captured 15–19 adult male A. cristatellus in each of the eight sites. We took macro photographs of the perch on which each lizard was found and recorded perch type and angle of inclination. We also took the same measurements from a randomly selected potential perch nearby. We selected potential perches using a random direction generator, identifying the closest perch (any structure ≥0.5 m high capable of supporting a lizard) in the direction indicated.
We transported lizards to a field laboratory where they were housed individually at ambient temperature. We marked lizards dorsally at the centre of the pelvis with a white mark. We measured lizard body temperature at the start of each trial as temperature affects sprint performance in ectotherms [16]. We tested each lizard on three tracks per day (7.00–20.00) with three consecutive trials per track and a minimum 3 h rest between tracks. We placed a cloth bag at the top of the track to provide lizards a refuge to run towards, and tapped lizards gently when they paused for more than 5 s. We recorded the middle 60 cm of the track with a high-speed digital camera at 120 frames per second (fps). Following sprint trials, we anaesthetised lizards with isoflurane, imaged them using an X-ray system, and obtained digital scans of toepads. We returned lizards to their point of capture when fully recovered from anaesthesia.
We quantified sprint performance using the software ImageJ [17]. We marked the location of the lizard in every frame and calculated the distance travelled between frames. We then calculated maximum velocity over a minimum of 20 cm and excluded uncooperative (frequent stops and starts) or exceptionally slow-crawling runs (less than 0.1 m s−1) as unsuccessful trials. This method is analogous to studies using timed gates (e.g. [3]). We selected the single fastest sprint across all three trials per track per individual as a representative trial. Finally, we counted the number of stops, slips and slides over the middle 50 cm of track. We defined slips as a failure of a foot to engage with the surface and slides as backward movement (≥2 mm) of the lizard when fully stopped.
From X-rays we measured SVL, pectoral and pelvic widths, and lengths of 10 bones commonly measured in Anolis lizards: third metacarpal and phalanx (the longest digit on the forefoot), fourth metatarsal and phalanx (the longest digit on the hindfoot; electronic supplementary material, S2), radius, ulna, humerus, femur, tibia and fibula. We calculated total forelimb length by summing lengths of the third phalanx, metacarpal, radius and humerus, and total hindlimb length by summing the lengths of the fourth phalanx, metatarsal, tibia and femur. We counted lamellae and measured toepad area for the third digit of forefeet and fourth digit of hindfeet. We measured all traits three times and averaged right and left values. We quantified surface roughness (Rq) from the macro surface photographs of perches in the wild and the three experimental tracks using the plugin surfacecharJ in ImageJ [4,18].
(c). Statistical analyses
We performed statistical analyses in R (v. 3.4.2) [19]. We used the package lme4 for mixed effects model analyses [20]. We estimated significance of fixed effects with the package lmerTest [21], which estimates degrees of freedom, t-statistic and p-value for lme objects using type III ANOVAs with Satterthwaite's approximation. We chose linear mixed effects models over simpler ordinary least squares (OLS) regression because our dataset contains repeat measures in nested groups: multiple trials per individual, within a site that was either urban or forest, within a municipality.
We first determined effects of possible covariates: body size (SVL), mass, body temperature, and their interactions on sprint speed across all tracks and sites, with site of origin and lizard ID as random effects. We included significant covariates in subsequent models. We examined the number of successful trials by track (electronic supplementary material, S3) with a logistic regression in which trial success was modelled as a function of track type and context (defined as urban versus forest). We quantified relative velocity on the six combinations of substrate type and inclination using a model of velocity by track type with body temperature as a covariate and site of origin and lizard ID as random effects. We determined percentage of maximum speed by comparing the regression slopes for velocity on each track to the track on which lizards ran fastest: 37° wood.
We assessed the effects of track angle and substrate type for all lizards regardless of context of origin (forest or urban). We analysed a linear mixed effects model with substrate and angle of inclination interacting as fixed effects, body temperature as a covariate, and site of origin and lizard ID as random effects. We dropped the non-significant interaction between track substrate and angle (F2,580 = 1.441, p = 0.238). We then compared the frequency of stops, slips and slides using generalized linear mixed effects models (with Poisson-distributed error). We also analysed relative effects of stops, slips and slides on velocity with body temperature, angle and substrate as covariates, and site of origin and lizard ID as random effects.
We analysed differences in sprint speed between urban and forest contexts with a linear mixed effects model of velocity by substrate type, track angle, context (urban or forest) and the following interactions: substrate × angle, substrate × context, and angle × context. We included body temperature as a covariate and municipality and lizard ID as random effects. We removed the substrate × angle interaction from the final model as it was not significant (F2,576 = 1.499, p = 0.224). We also compared differences in frequencies of stops, slips and slides between contexts with generalized linear mixed effects models (with Poisson-distributed residual error). No interactions were significant in the stops and slides models and only the angle × context interaction was significant in the slips model (χ2 = 10.761, d.f. = 1, p = 0.001). All non-significant interactions were dropped from final models.
We compared 8 log-transformed morphological traits between urban and forest lizards with MANOVA (‘manova’ in R base package stats [19]), with municipality and body size as covariates (trait correlations in electronic supplementary material S4). We examined the partial effects of each trait on sprint speed using a multivariate regression. (We also provide a bivariate trait analysis in electronic supplementary material, S5.) We used a multivariate linear mixed effects model with backwards stepwise regression based on Akaike information criterion (AIC). Because trait–performance relationships likely vary by track, we included track as an interacting factor for all traits in the full model and removed non-significant interactions during model selection. We included log-transformed body size (SVL) and body temperature as covariates and site of origin and lizard ID as random effects.
We next examined how interacting trait combinations influence sprint speed at both angles (37° and 60° inclinations analysed separately). For example, perhaps long-limbed lizards sprint faster if they also have larger toepads. We fitted a multivariate linear mixed effects model for velocity with forelimb length interacting with toepad traits and hindlimb length (based on results from the previous model), with substrate type, body size and body temperature as covariates, and lizard ID and site of origin as random effects. We also considered the relationship between limb length and slipping (on metal and concrete tracks) using a generalized linear mixed effects model (with Poisson-distributed residual error) of slip frequency interacting with angle of inclination, with body size as a covariate, and lizard ID and site as random effects.
Lastly, we evaluated habitat availability and use. We first asked if perch roughness and angle differed between forest and urban habitats, and whether these characteristics differed between man-made and natural perches (ANCOVA with municipality as a covariate). We then asked whether lizards used perches discriminately by comparing attributes of actual and potential perches within forest and urban habitats using logistic regression of perch choice by perch roughness, angle of inclination and type, with municipality as a covariate. Finally, to test the habitat constraint and breadth hypotheses we first divided urban lizards into thirds to compare lizards performing in the top and bottom terciles (accounting for body temperature) on the steeply inclined concrete track. These groups were thus composed of individuals that sprinted fastest and slowest on the most challenging track. We then compared randomly available and used habitat in the wild for the fastest and slowest animals with two logistic regressions of perch choice by perch characteristics with municipality as a covariate.
3. Results
(a). Locomotor performance
We collected data on 128 animals from eight sites resulting in 713 successful trials across all tracks. The number of successful trials differed by track but not by context (track: χ2 = 30.80, p < 0.001, context: χ2 = 0.11, p = 0.92). Compared to the gradual inclination (37°) wood track (the most successful trials, n = 127), the concrete 37° and concrete and metal 60° had significantly fewer successful trials (d.f. = 761, C-37°: n = 106, z = −2.00, p = 0.046; M-60°: n = 116, z = −2.46, p = 0.014; C-60°: z = −3.17, p = 0.002).
Each measure of locomotor performance (velocity, stops, slips, slides) across all tracks was correlated with body temperature but not mass, SVL, or their interaction (n = 713). Warmer temperatures were associated with faster sprint speeds (+0.11 ± 0.016 m s−1/°C; ANOVA type III, F1,497 = 42.3, p < 0.001), along with fewer stops (−0.092 ± 0.042 stops/°C; ANOVA type III Wald, χ2 = 4.39, d.f. = 1, p = 0.028), slips (−0.32 ± 0.052 slips/°C; ANOVA type III Wald, χ2 = 37.1, d.f. = 1, p < 0.001) and slides (−0.30 ± 0.084 slides/°C; ANOVA type III Wald, χ2 = 13.2, d.f. = 1, p < 0.001). Consequently, we included body temperature as a covariate in all analyses.
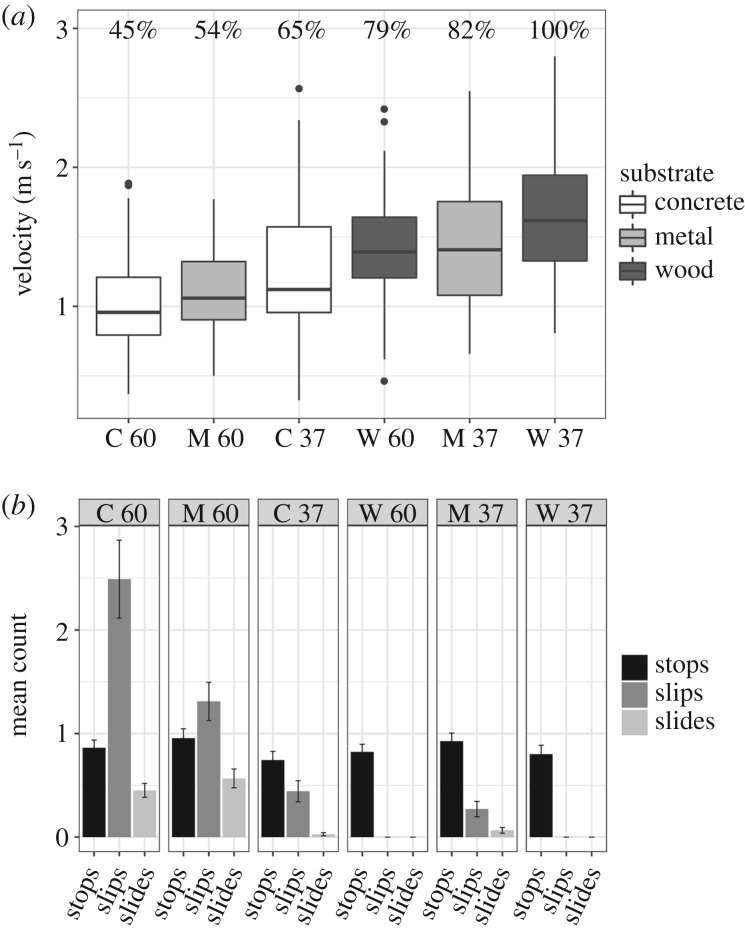
With respect to hypothesis 1—that locomotor performance should vary by track type—we found maximum velocity across all lizards and sites ranged from 0.33 to 2.80 m s−1 and differed by track type (n = 713; F5,583 = 61.3, p < 0.001; figure 2). Both angle and substrate significantly impacted sprint speed (ANOVA type III; angle: F1,586 = 138, p < 0.001; substrate: F2,588 = 91, p < 0.001). Overall, the steep concrete track was the most difficult for both urban and forest lizards with mean velocities substantially slower than on other tracks and only 45% of maximum speed. Lizards ran on average 0.26 ± 0.023 m s−1 slower on steep compared to gradual inclinations, resulting in velocities 21–34% slower depending on the substrate (t = −11.80, d.f. = 586, p < 0.001). Regardless of track inclination, lizards ran slower on metal (−0.24 ± 0.028 m s−1, 18–32% reduction; t = −8.72, d.f. = 586, p < 0.001) and concrete (−0.39 ± 0.029 m s−1, 35–44% reduction; t = −13.3, d.f. = 594, p < 0.001) compared to wood bark.
Figure 2.
(a) Decrease in sprint speed by track. Percentages above each represent the per cent of maximum sprint speed compared to performance on the 37° wood track, on which lizards sprinted the fastest. (b) Mean number of stops, slips, and slides on each track, with whiskers showing ± s.e. of each (there were no slips or slides on wood tracks). C, concrete; M, metal; W, wood.
Frequency of stops did not differ by track, angle or substrate (ANOVA type III Wald; angle : substrate: χ2 = 4.81, d.f. = 22, p = 0.86; angle: χ2 = 0.098, d.f. = 1, p = 0.75; substrate: χ2 = 3.58, d.f. = 2, p = 0.17; figure 2). Frequency of slips differed by substrate and angle but not their interaction (n = 713; ANOVA type III Wald; angle : substrate: χ2 = 0.79, d.f. = 1, p = 0.37; angle: χ2 = 206.6, d.f. = 1, p < 0.001; substrate: χ2 = 54.6, d.f. = 1, p < 0.001; figure 2). Slips were more common on concrete than on metal, and were not observed on wood tracks (concrete: +0.74 ± 0.10 slips, z = −7.39, p < 0.001). Frequency of slides differed only by angle and not by substrate or angle and substrate interaction (n = 713; ANOVA type III Wald; angle : substrate: χ2 = 1.11, d.f. = 1, p = 0.29; angle: χ2 = 56.2, d.f. = 1, p < 0.001; substrate: χ2 = 2.21, d.f. = 1, p = 0.14; figure 2). Both slipping and sliding were more common on steep compared to gradual tracks (slips: +1.91 ± 0.13, z = 14.4, p < 0.001; slides: +2.39 ± 0.32, z = 7.50, p < 0.001). Unsurprisingly, the tracks on which lizards ran slowest were also the ones on which they slipped, slid and stopped the most. Across all tracks and sites, velocity was negatively correlated with all three (ANOVA type III; stops: −0.17 ± 0.014 m s−1/stop, F1,677 = 154.7, p < 0.001; slips: −0.047 ± 0.007 m s−1/slip, F1,619 = 51.8, p < 0.001; slides: −0.084 ± 0.024 m s−1/slide, F1,626 = 12.3, p < 0.001).
(b). Morphology and performance
With respect to hypothesis 2 (morphology is correlated with sprint speed) we found strong effects of limb lengths, body width and toepad morphology (relative to body size), although this relationship varied by track (table 1; electronic supplementary material, S5). When examined together, a few key traits stood out in influencing sprint speeds. Relative hindlimb length was the most important variable, positively influencing speed across all tracks (+2.81 ± 1.40 m s−1, d.f. = 111, t = 2.01, p = 0.047). Forelimb length, front lamella number, rear toepad area and pelvic width were also correlated with sprint speed on some tracks (table 1). Longer forelimbs resulted in slower speeds on 60o tracks only (wood: −3.55 ± 1.55 m s−1, d.f. = 159, t = −2.29, p = 0.024; concrete: −4.08 ± 1.58 m s−1, d.f. = 167, t = −2.59, p = 0.011; metal: −3.81 ± 1.56 m s−1, d.f. = 161, t = −2.45, p = 0.016). This effect may be attributable to a positive relationship between slip frequency and forelimb length on steep tracks (+7.21 ± 2.80, z = 2.58, p = 0.01). More front lamellae was positively correlated with faster speeds on the 37o concrete track (+1.40 ± 0.64 m s−1, d.f. = 396, t = 2.18, p = 0.03), while larger rear toepads were positively correlated with sprint speeds on the 37o wood track (+0.74 ± 0.37 m s−1, d.f. = 251, t = 2.01, p = 0.046). Lastly, wider pelvises were correlated with faster speeds on the 60o concrete track (+1.75 ± 0.806, d.f. = 456, t = 2.17, p = 0.034).
Table 1.
Trait performance relationship between running speed (m s−1) and morphological traits estimated from mixed effects model with body size and body temperature as covariates. Shaded values are positive. Slopes significantly different from zero (at p < 0.05) are italicized and noted with ‘*’. Marginally significant effects (p < 0.1) are noted with ‘.’, and non-significant effects (p > 0.1) with ‘n.s.’. W, wood; C, concrete; M, metal.
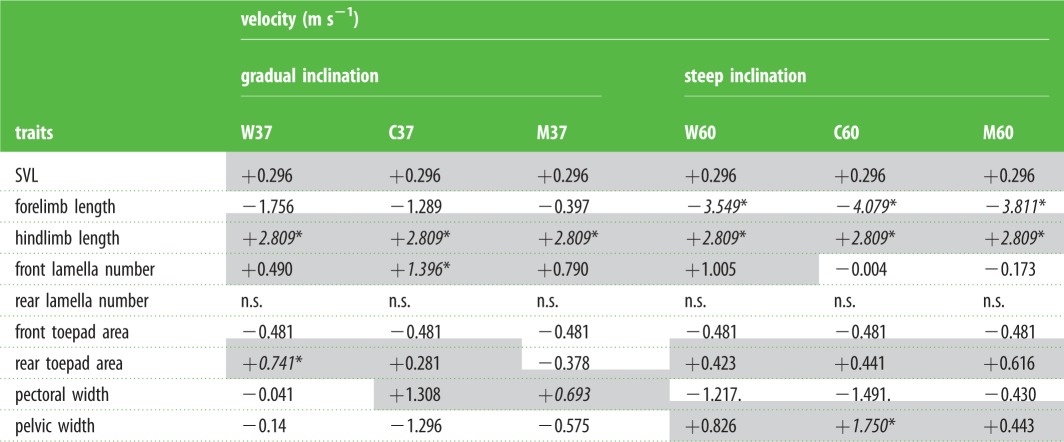 |
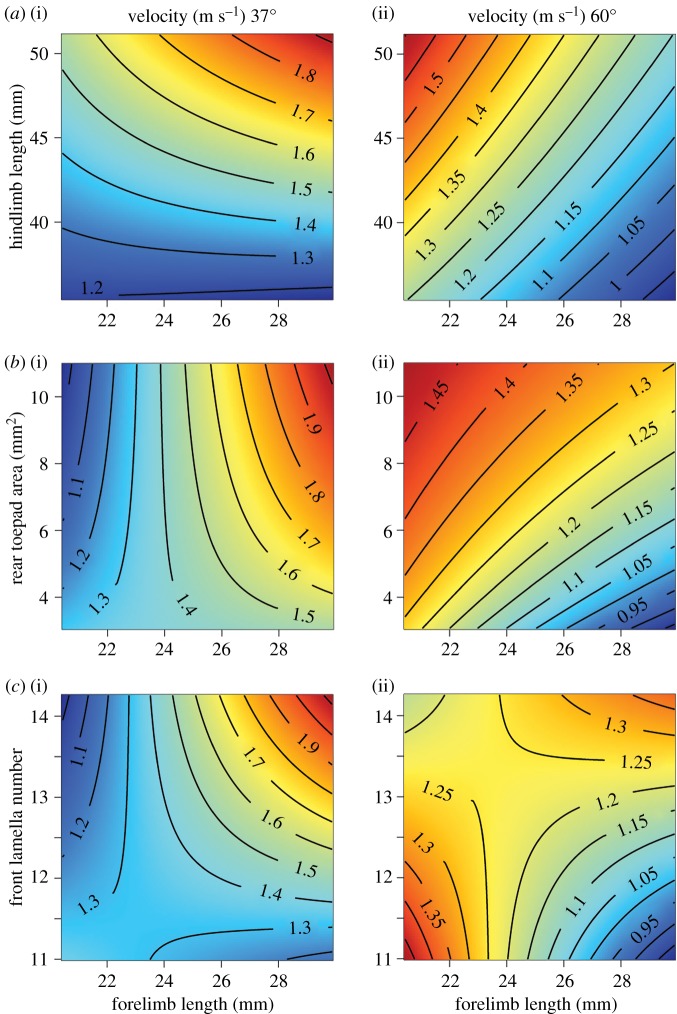
There appear to be conflicting costs and benefits of some traits depending on the angle of inclination, most notably for forelimb length. Consequently, we examined interactions with forelimb length and toepad morphology. We found significant interactions between forelimb length and each of these traits, though the effect varied depending on track angle. On gradually inclined tracks lizards with longer forelimbs were faster if they also had longer hindlimbs and larger rear toepads (forelimb × hindlimb: F1,119 = 4.53, p = 0.035; forelimb × rear toepad: F1,116 = 4.76, p = 0.031; figure 3). On steep tracks, lizards with longer forelimbs performed the slowest unless they also had long hindlimbs or more front lamellae (forelimb × hindlimb: F1,117 = 7.77, p = 0.006; forelimb × rear toepad: F1,114 = 3.47, p = 0.065; forelimb × front lamella number: F1,111 = 4.64, p = 0.033; figure 3). These favourable trait combinations appear to compensate for longer forelimbs, particularly at steeper inclinations.
Figure 3.
Trade-offs of morphology at different inclinations and the interaction of traits in producing different velocities (across all substrates). Darker blue shades are slower velocities, brighter red shades are faster velocities and contour lines are labelled with the average velocity (in m s−1) across the three substrates. On gradually inclined tracks (a(i)–c(i)), velocities are greater with relatively longer forelimbs (x-axis) only when combined with long hindlimbs (a(i)), larger rear toepads (b(i)) or more front lamellae (c(i)). On steeply inclined tracks (a(ii)–c(ii)), relatively longer forelimbs have a consistently negative effect counteracted to varying extents by other traits: lizards with long limbs and larger toepads or more lamellae still attain fast velocities.
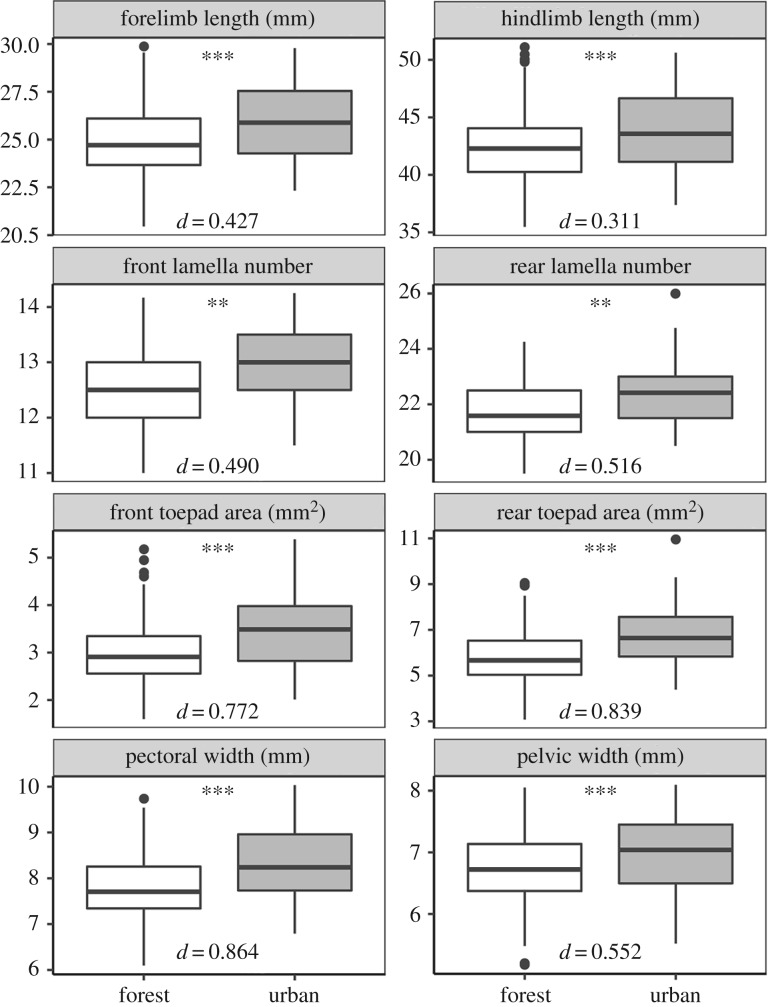
We also found, consistent with our prediction in hypothesis 2, that urban lizards had longer limbs, more lamellae, larger toepads and wider bodies (figure 4). Notably, the traits that confer some performance advantage are the same traits that have shifted in urban populations. Moreover, the trait combinations associated with faster sprint speeds—longer limbs with larger toepads and more lamellae—are typical of urban lizards.
Figure 4.
Urban lizards had relatively longer limbs, larger toepads, more lamellae and wider bodies compared to forest lizards. Significance levels were determined from MANOVA of log-transformed trait values with log-transformed body size and municipality as covariates. Effect sizes (Cohen's d) are provided for each comparison. Significance levels: **p < 0.01, ***p < 0.001.
(c). Performance in urban versus forest lizards
With respect to hypothesis 3, urban lizards sprinted faster than forest lizards in general across all tracks (mean difference: 0.18 ± 0.060 m s−1, d.f. = 323, t = 3.08, p = 0.003; electronic supplementary material S6). Differences in velocity between urban and forest lizards were unrelated to substrate (ANOVA type III for substrate × context, F3,580 = 0.261, p = 0.77) but instead to angle of inclination (ANOVA type III for angle × context, F1,583 =11.9, p < 0.001). This trend was driven primarily by differences on the 37o track (urban +0.17 ± 0.050 m s−1 faster than forest, d.f. = 180, t = −3.43, p < 0.001). Differences on the 60o track were non-significant (d.f. = 193, t = −0.351, p = 0.73).
Urban and forest lizards also consistently differed from each other in stops, slips and slides. Urban lizards stopped less (−0.42 ± 0.10 stops, z = −4.09, p < 0.001), slipped more (on steeply inclined concrete and metal tracks; +0.74 + 0.26 slips; z = 2.82, p = 0.005), but did not differ significantly from forest lizards in number of slides (z = −1.29, p = 0.20).
(d). Habitat use and performance
With respect to hypothesis 4, we found perches used by urban lizards were smoother than those used by forest lizards but did not differ in angle (roughness Rq: −4.67 ± 2.01 µm; t = −2.33, d.f. = 124, p = 0.022; angle: t = −0.97, d.f. = 109, p = 0.33; electronic supplementary material S7). In urban habitats 64% of perches used by lizards were man-made. These perches were smoother than natural perches but did not differ in inclination (Rq of man-made substrates: −17.7 ± 2.52 µm; t = −7.04, d.f. = 61, p < 0.001; angle: t = −0.339, d.f. = 47, p = 0.74).
When accounting for available perches within each habitat type, we found lizards discriminately used perches based on both roughness and angle in urban but not in forested habitats (urban: roughness z = 2.82, d.f. = 96, p = 0.005, angle z = −2.61, d.f. = 96, p = 0.009; forest roughness z = 0.474, d.f. = 120, p = 0.64, angle t = −1.12, d.f. = 120, p = 0.26; electronic supplementary material S7). Urban lizards used rougher (+5.35 ± 1.61 µm) and more gradually inclined (−18.5 ± 5.21°) perches than were randomly available, although they did not discriminate based on perch type (67% of random perches were man-made; z = −1.39, d.f. = 96, p = 0.17).
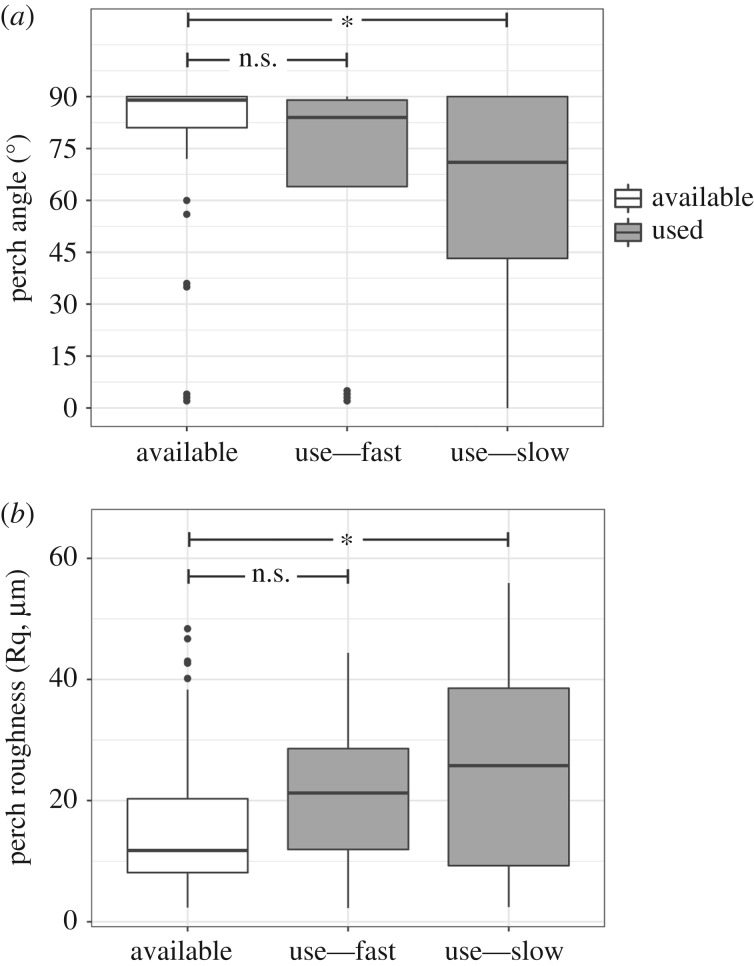
Finally, among urban lizards, the slowest sprinters on the most challenging track used perches discriminately while the best performers did not (figure 5). Poor performers used rougher (z = 2.32, d.f. = 57, p = 0.021) and less vertical perches (z = −2.05, d.f. = 57, p = 0.041; figure 5) than are available at random, but did not discriminate based on substrate (z = −1.35, d.f. = 57, p = 0.18). By contrast, perches used by the best performing lizards did not differ from random perches in any feature (d.f. = 62, roughness: z = 1.84, p = 0.066, angle: z = −1.39, p = 0.17, type: z = −0.603, p = 0.55).
Figure 5.
The angle of inclination (a) and perch roughness (b) of random potential perches (white) and perches used (grey) in urban populations, contrasting perch use by fast and slow lizards as a test of the habitat breadth and constraint hypotheses. Poorly performing lizards (slowest on the most challenging track, 60° concrete) used less vertical and rougher perches than were common while lizards that performed best (fastest on the most challenging track) did not discriminate based on either factor. Significance levels of contrasts to randomly available perches: p > 0.05 ‘n.s.’, p < 0.05 ‘*’.
4. Discussion
We investigated running performance in a tropical lizard (A. cristatellus) that throughout its native range makes extensive use of urban and forested habitats. We used laboratory experiments to test running performance of urban and forest animals on natural and man-made surfaces. Overall, the cost of using man-made surfaces is high. On the most difficult track (60° concrete), lizards sprinted at only 45% of their maximum speed. Even on the man-made track on which lizards sprinted fastest (37° metal), they ran at only 82% of their maximum speed. The decline attributable to substrate type was greater than that attributable to track inclination. For a given substrate, lizards ran 21–34% slower at steep versus gradual inclinations. However, for a given angle, lizards ran 18–32% slower on metal and 35–44% slower on concrete compared to wood bark. These results support our first hypothesis that lizards experience a decrease in locomotor performance when using man-made substrates, especially at steeper inclinations.
Our results agree with previous studies showing sprint speeds in lizards decline as steepness increases and surface roughness decreases [11,22–24]. We observed a drastic loss of traction on man-made substrates, a phenomenon we did not observe on wood bark tracks and that was associated with decreased sprint speeds. Although smooth surfaces enable maximum surface contact with toepads and improve adhesion compared to rougher substrates, lizards are not able to engage their claws or rely on gripping by conformation to the surface as they do on bark, both of which might otherwise increase sprint speed [24,25].
Such a substantial decrease in performance provides insight into the mechanisms of natural selection in urban areas. If a lizard is unable to run or maintain position on smooth substrates it would be unable to use more than two-thirds of the urban habitat and would likely have trouble escaping from predators. In fact, studies have demonstrated that faster sprint speed in lizards is favoured by natural and sexual selection [26–28]. Moreover, slides and falls following loss of traction can result in injury, fatigue, and energy expenditure [10]. This is particularly true on anthropogenic structures, which typically have little structural complexity to break a fall to the hard ground below. Submaximal locomotor performance may also restrict habitat use to suboptimal perch sites or result in poor traction during competitive bouts. Given this strong relationship between fitness and sprint speed, and the cost of using man-made surfaces, urban lizards are likely subject to selective pressures to use man-made surfaces more effectively.
Morphological differences between urban and forest populations that we detected are consistent with previous studies [4] and our expectations. In particular, urban lizards had longer limbs, wider bodies, larger toepads and more lamellae than their forest counterparts. The trait–performance correlations we found support our second hypothesis and the prediction that natural selection is acting via sprint performance to shape differences between urban and forest lizards. After accounting for body size, lizards with relatively longer hindlimbs sprinted faster across all tracks, with positive effects of toepad morphology on some tracks.
Relative hindlimb length stands out as a key trait in increasing sprint speeds. This result also agrees with previous studies showing a strong positive relationship between hindlimb length and sprint speed in anoles [6,9], and between limb length, habitat openness and sprint speed in other lizards [2,29,30]. In urban habitats, where perches are more isolated, flatter and broader than in forests [4,5], long-limbed fast-sprinting lizards should have a strong fitness advantage. However, this improved performance comes at an apparent cost: on steep inclines, lizards with relatively longer forelimbs run more slowly. This suggests that in urban areas selection to run quickly on the ground may be stronger than selection for fast running on inclined surfaces. Previous studies have similarly identified trade-offs in performance involving traits beneficial for vertical, complex, arboreal habitat use versus those more appropriate for open, flat, terrestrial habitat use [31–34]. We build on this by presenting evidence suggesting a trade-off also exists for A. cristatellus limb length at gradual versus steeper inclinations.
An alternative to this trade-off hypothesis is one in which urban lizards are merely better adapted overall, perhaps due to stronger selection in urban areas. Although we cannot definitively reject this possibility, some circumstantial evidence suggests it is a less likely explanation. In particular, although weaker selection could result in lower running performance of forest compared to urban lizards, it would also predict greater phenotypic variance of performance-linked traits, such as hindlimb length, something that we do not find in our data. In addition, stronger selection in urban sites should correspond to higher mortality in urban populations, yet we have found similar adult mortality in unpublished data from one natural and two urban sites. In a related vein, the distribution of adult body sizes is similar between urban and natural samples, which suggest that at least age-specific rates of adult mortality may be similar between sites. Finally, our anecdotal observations suggest that although population densities may be slightly lower in urban compared to natural areas due to habitat patchiness, in urban areas where lizards are found they can achieve densities comparable to those observed in forest environments.
We found a positive correlation between slipping and forelimb length. This suggests that longer limbs may decrease surefootedness on smooth substrates, in turn reducing sprint speed. Longer limbs shift the centre of mass away from the surface, which may decrease stability at greater inclines and narrower perches [32]. This cost may be counteracted by other traits such as toepads. Among anoles, there is a convergence in limb length and toepad morphology within groups of species using similar microhabitat [8]. Limb length and toepad morphology may be similarly co-evolving in response to structural differences in the urban environment. Larger toepads and more lamellae improve adhesion and grip, and could enhance stability for longer-limbed lizards on the smooth, steeply inclined surfaces that typify urban environments [14,35].
Although our study design did not allow us to fully investigate correlated trait effects on sprint speed, we found that the strong negative effect of relatively long forelimbs on locomotion on steep tracks was partly counteracted by larger rear toepads and more front lamellae. Front lamellae have previously been identified as functionally important, as anole species that perch higher tend to have more lamellae on their front but not necessarily on their rear feet [13]. In general, larger toepads and toepads with more lamellae exert stronger clinging forces [12,14]. Toepads with more lamellae may enhance gripping ability on rough surfaces by increasing conformity, while greater surface area may enhance adhesion and cling strength on smooth surfaces [10,24,25].
Contrary to expectations for our third hypothesis, we found that although urban lizards sprinted faster than forest lizards, they did so on all tracks. That urban lizards were faster on the rough, natural substrate as well as the smooth, man-made substrates of urban habitats suggests that the same functional mechanism enhances speed on both. This could be a by-product of selection for improved performance on smooth surfaces, or alternatively, there could be competing pressures to be able to use both rough and smooth surfaces effectively. Also contrary to our expectations, urban lizards slipped more than forest lizards on smooth substrates. In retrospect, this is less surprising given the correlation we found between slipping and longer limbs, as urban lizards are generally longer-limbed. Yet even though urban lizards slipped more, they stopped less often. The longer forelimbs and wider bodies of urban lizards may be more favourable for maintaining a low centre of mass with a sprawling posture [36,37]. Forest lizards, with their relatively shorter limbs and narrower bodies, may be more susceptible to the pitching effects of sustained speeds and stop more to regain their balance and grip [38].
Lastly, we found mixed support for the habitat breadth and constraint hypotheses. Despite submaximal performance on smooth man-made substrates, urban lizards occupied smoother perches than their forest counterparts. Thus it seems urban lizards are less constrained in their habitat use and are able to use a broader range of perch types compared to forest lizards, a conclusion similar to that in other studies [11]. Although urban lizards use man-made perches at a high frequency, they use them in proportion to the abundance of those perches in the habitat. Nonetheless, urban lizards used rougher and less vertical perches than are common, with habitat use associated with performance. Poorly performing lizards discriminately used the roughest and least inclined perches, suggesting they may be more constrained in their habitat use (supporting the habitat constraint hypothesis). In contrast, lizards that performed well in our study did not discriminate based on perch roughness or angle (supporting the habitat breadth hypothesis). It seems that within A. cristatellus, individuals may be segregating in the urban habitat based on their ability to use man-made perches, although we cannot rule out the alternate possibility that lizards that performed well did so because they are more experienced on man-made surfaces.
Our results shed light on the competing pressures of running on smooth vertical surfaces, in which long limbs can be disadvantageous, and flat open areas, where long limbs are unambiguously advantageous. That urban populations consistently have long limbs despite this trade-off suggests that selection to run quickly across open habitat and on flat surfaces is stronger than selection to run quickly on smooth vertical substrates. Moreover, lizards in the wild frequently do not sprint at maximum speeds identified in laboratory conditions, and maximal capabilities may differ based on substrate characteristics and complexity [34,39]. If maximum sprinting abilities are not typically employed on smooth vertical surfaces, then natural selection may instead favour surefooted locomotion on such surfaces, in which case the detrimental effects of long limbs on speed could be irrelevant.
Overall, our results paint a complex picture of how morphology, performance and habitat use shape adaptive phenotypic change, and highlight the importance of whole-organism analysis in studies of performance and adaptation. The urban habitat clearly poses novel locomotor challenges to lizards. With substantial decreases in sprint performance on man-made substrates, fitness consequences seem unavoidable for lizards that cannot navigate these surfaces or adjust their locomotor behaviour to enhance performance. Given the well-documented link between locomotor performance and fitness in lizards, including anoles, the fact that urban phenotypes are those correlated with faster sprint speeds supports the overarching hypothesis that natural selection is shaping these phenotypes via locomotor performance. However, there seems to be a complex web of trade-offs between traits depending on the type of surface and inclination, and likely many other unmeasured factors such as hardness or static-electric properties of the substrates. It seems probable that different trait combinations enhance performance under different circumstances, and that the complexity and novel pressures of living in urban habitats give natural selection great scope for shaping the phenotypes of urban organisms.
Supplementary Material
Acknowledgements
We thank Interamerican University in Arecibo for allowing us to conduct a portion of this research on their campus. We thank S. Campbell-Staton and D. Muñiz for assistance in the field, and J. Kolbe, A. Battles and K. Avilés-Rodriguez for advice in planning and conducting the study. We also thank E. Carlen and C. Donihue for providing valuable feedback on this manuscript.
Ethics
Ethical treatment of animals used in this research complies with all local and federal laws. This study was conducted under Permit #2014-IC-024 (O-VS-PVS15-SJ-00375-22042014) from the Puerto Rico DRNA, and in compliance with University of Massachusetts Institutional Animal Care and Use Committee (IACUC) protocol #2012001.
Data accessibility
Data is available on Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.5nt3045).
Authors' contributions
K.M.W. and L.J.R. conceived the project and designed methodology. K.M.W. identified study locations and secured site permissions. K.M.W., J.F. and I.M. collected the data. K.M.W., I.M. and L.J.R. analysed the data. All authors contributed to the writing of the manuscript and gave final approval for publication
Competing interests
The authors have no competing interests.
Funding
This research was funded by a grant from the National Science Foundation to LJR (DEB 1354044).
References
- 1.Arnold SJ. 1983. Morphology, performance and fitness. Am. Zool. 23, 347–361. ( 10.1093/icb/23.2.347) [DOI] [Google Scholar]
- 2.Garland T Jr, Losos JB. 1994. Ecological morphology of locomotor performance in squamate reptiles. In Ecological morphology (eds Wainwright PC, Reilly SM), pp. 240–302. Chicago, IL: University of Chicago Press. [Google Scholar]
- 3.Irschick DJ, Losos JB. 1999. Do lizards avoid habitats in which performance is submaximal? The relationship between sprinting capabilities and structural habitat use in Caribbean anoles. Am. Nat. 154, 293–305. ( 10.1086/303239) [DOI] [PubMed] [Google Scholar]
- 4.Winchell KM, Reynolds RG, Prado-Irwin SR, Puente-Rolón AR, Revell LJ. 2016. Phenotypic shifts in urban areas in the tropical lizard Anolis cristatellus. Evolution 70, 1009–1022. ( 10.1111/evo.12925) [DOI] [PubMed] [Google Scholar]
- 5.Winchell KM, Carlen EJ, Puente-Rolón AR, Revell LJ. 2018. Divergent habitat use of two urban lizard species. Ecol. Evol. 8, 25–35. ( 10.1002/ece3.3600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Losos JB. 1990. The evolution of form and function: morphology and locomotor performance in West Indian Anolis lizards. Evolution 44, 1189–1203. ( 10.1111/j.1558-5646.1990.tb05225.x) [DOI] [PubMed] [Google Scholar]
- 7.Vanhooydonck B, Herrel A, Van Damme R, Irschick DJ. 2006. The quick and the fast: the evolution of acceleration capacity in Anolis lizards. Evolution 60, 2137–2147. ( 10.1111/j.0014-3820.2006.tb01851.x) [DOI] [PubMed] [Google Scholar]
- 8.Losos JB. 2009. Lizards in an evolutionary tree. Berkeley, CA: University of California Press. [Google Scholar]
- 9.Losos JB, Sinervo B. 1989. The effects of morphology and perch diameter on sprint performance of Anolis lizards. J. Exp. Biol. 145, 23–30. [Google Scholar]
- 10.Cartmill M. 1985. Climbing. In Functional vertebrate morphology (eds Hildebrand M, Bramble DM, Liem KF, Wake DB), pp. 73–88. Cambridge, MA: Harvard University Press. [Google Scholar]
- 11.Kolbe JJ, Battles AC, Avilés-Rodríguez KJ. 2016. City slickers: poor performance does not deter Anolis lizards from using artificial substrates in human-modified habitats. Func. Ecol. 30, 1418–1429. ( 10.1111/1365-2435.12607) [DOI] [Google Scholar]
- 12.Irschick DJ, Austin CC, Petren K, Fisher RN, Losos JB, Ellers O. 1996. A comparative analysis of clinging ability among pad-bearing lizards. Biol. J. Linn. Soc. 59, 21–35. ( 10.1111/j.1095-8312.1996.tb01451.x) [DOI] [Google Scholar]
- 13.Glossip D, Losos JB. 1997. Ecological correlates of number of subdigital lamellae in anoles. Herpetologica 53, 192–199. [Google Scholar]
- 14.Zani PA. 2000. The comparative evolution of lizard claw and toe morphology and clinging performance. J. Evol. Biol. 13, 316–325. ( 10.1046/j.1420-9101.2000.00166.x) [DOI] [Google Scholar]
- 15.Elstrott J, Irschick DJ. 2004. Evolutionary correlations among morphology, habitat use and clinging performance in Caribbean Anolis lizards. Biol. J. Linn. Soc. 83, 389–398. ( 10.1111/j.1095-8312.2004.00402.x) [DOI] [Google Scholar]
- 16.Hertz PE, Huey RB, Nevo E. 1983. Homage to Santa Anita: thermal sensitivity of sprint speed in agamid lizards. Evolution 37, 1075–1084. ( 10.1111/j.1558-5646.1983.tb05634.x) [DOI] [PubMed] [Google Scholar]
- 17.Rashband WS. 1997–2014 Imagej. Bethesda, MD: U.S. National Institutes of Health; See http://imagej.nih.gov/ij/. [Google Scholar]
- 18.Chinga G, Johnsen PO, Dougherty R, Berli EL, Walter J. 2007. Quantification of the 3D microstructure of SC surfaces. J. Microsc. 227, 254–265. ( 10.1111/j.1365-2818.2007.01809.x) [DOI] [PubMed] [Google Scholar]
- 19.R Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org. [Google Scholar]
- 20.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 21.Kuznetsova A, Brockhoff PB, Christensen RH. 2017. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. ( 10.18637/jss.v082.i13) [DOI] [Google Scholar]
- 22.Irschick DJ, Jayne BC. 1998. Effects of incline on speed, acceleration, body posture and hindlimb kinematics in two species of lizard Callisaurus draconoides and Uma scoparia. J. Exp. Biol. 201, 273–287. [DOI] [PubMed] [Google Scholar]
- 23.Brandt R, Galvani F, Kohlsdorf T. 2015. Sprint performance of a generalist lizard running on different substrates: grip matters. J. Zool. 297, 15–21. ( 10.1111/jzo.12253) [DOI] [Google Scholar]
- 24.Vanhooydonck B, Andronescu A, Herrel A, Irschick DJ. 2005. Effects of substrate structure on speed and acceleration capacity in climbing geckos. Biol. J. Linn. Soc. 85, 385–393. ( 10.1111/j.1095-8312.2005.00495.x) [DOI] [Google Scholar]
- 25.Miles DB. 2004. The race goes to the swift: fitness consequences of variation in sprint performance in juvenile lizards. Evol. Ecol. Res. 6, 63–75. [Google Scholar]
- 26.Husak JF, Fox SF, Lovern MB, Van Den Bussche RA. 2006. Faster lizards sire more offspring: sexual selection on whole-animal performance. Evolution 60, 2122–2130. ( 10.1111/j.0014-3820.2006.tb01849.x) [DOI] [PubMed] [Google Scholar]
- 27.Calsbeek R, Irschick DJ. 2007. The quick and the dead: correlational selection on morphology, performance, and habitat use in island lizards. Evolution 61, 2493–2503. ( 10.1111/j.1558-5646.2007.00206.x) [DOI] [PubMed] [Google Scholar]
- 28.Autumn K, Hsieh ST, Dudek DM, Chen J, Chitaphan C, Full RJ. 2006. Dynamics of geckos running vertically. J. Exp. Biol. 209, 260–272. ( 10.1242/jeb.01980) [DOI] [PubMed] [Google Scholar]
- 29.Melville J, Swain RO. 2000. Evolutionary relationships between morphology, performance and habitat openness in the lizard genus Niveoscincus (Scincidae: Lygosominae). Biol. J. Linn. Soc. 70, 667–683. ( 10.1111/j.1095-8312.2000.tb00222.x) [DOI] [Google Scholar]
- 30.Sinervo B, Losos JB. 1991. Walking the tight rope: arboreal sprint performance among Sceloporus occidentalis lizard populations. Ecology 72, 1225–1233. ( 10.2307/1941096) [DOI] [Google Scholar]
- 31.Huey RB, Hertz PE. 1982. Effects of body size and slope on sprint speed of a lizard (Stellio (Agama) stellio). J. Exp. Biol. 97, 401–409. [Google Scholar]
- 32.Losos JB, Irschick DJ. 1996. The effect of perch diameter on escape behaviour of Anolis lizards: laboratory predictions and field tests. Anim. Behav. 51, 593–602. ( 10.1006/anbe.1996.0063) [DOI] [Google Scholar]
- 33.Macrini TE, Irschick DJ. 1998. An intraspecific analysis of trade-offs in sprinting performance in a West Indian lizard species (Anolis lineatopus). Biol. J. Linn. Soc. 63, 579–591. ( 10.1111/j.1095-8312.1998.tb00330.x) [DOI] [Google Scholar]
- 34.Sathe EA, Husak JF. 2015. Sprint sensitivity and locomotor trade-offs in green anole (Anolis carolinensis) lizards. J. Exp. Biol. 218, 2174–2179. ( 10.1242/jeb.116053) [DOI] [PubMed] [Google Scholar]
- 35.Goodman BA, Krockenberger AK, Schwarzkopf L. 2007. Master of them all: performance specialization does not result in trade-offs in tropical lizards. Evol. Ecol. Res. 9, 527–546. [Google Scholar]
- 36.Van Damme R, Aerts P, Vanhooydonck B. 1997. No trade-off between sprinting and climbing in two populations of the Lizard Podarcis hispanica (Reptilia: Lacertidae). Biol. J. Linn. Soc. 60, 493–503. ( 10.1006/bijl.1996.0115) [DOI] [Google Scholar]
- 37.Aerts P, Van Damme R, D'Août K, Vanhooydonck B. 2003. Bipedalism in lizards: whole-body modelling reveals a possible spandrel. Phil. Trans. R. Soc. Lond. B 358, 1525–1533. ( 10.1098/rstb.2003.1342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higham TE, Korchari P, McBrayer LD. 2010. How to climb a tree: lizards accelerate faster, but pause more, when escaping on vertical surfaces . Biol. J. Linn. Soc. 102, 83–90. ( 10.1111/j.1095-8312.2010.01564.x) [DOI] [Google Scholar]
- 39.Irschick DJ, Herrel A, Vanhooydonck B, Huyghe K, Van Damme R. 2005. Locomotor compensation creates a mismatch between laboratory and field estimates of escape speed in lizards: a cautionary tale for performance-to-fitness studies. Evolution 59, 1579–1587. ( 10.1111/j.0014-3820.2005.tb01807.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available on Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.5nt3045).