Abstract
The well-developed Achilles tendon in humans is generally interpreted as an adaptation for mechanical energy storage and reuse during cyclic locomotion. All other extant great apes have a short tendon and long-fibred triceps surae, which is thought to be beneficial for locomotion in a complex arboreal habitat as this morphology enables a large range of motion. Surprisingly, highly arboreal gibbons show a more human-like triceps surae with a long Achilles tendon. Evidence for a spring-like function similar to humans is not conclusive. We revisit and integrate our anatomical and biomechanical data to calculate the energy that can be recovered from the recoiling Achilles tendon during ankle plantar flexion in bipedal gibbons. Only 7.5% of the required external positive work in a stride can come from tendon recoil, yet it is delivered at an instant when the whole-body energy level drops. Consequently, an additional similar amount of mechanical energy must simultaneously dissipate elsewhere in the system. Altogether, this challenges the concept of an energy-saving function in the gibbon's Achilles tendon. Cercopithecids, sister group of the apes, also have a human-like triceps surae. Therefore, a well-developed Achilles tendon, present in the last common ‘Cercopithecoidea–Hominoidea’ ancestor, seems plausible. If so, the gibbon's anatomy represents an evolutionary relict (no harm–no benefit), and the large Achilles tendon is not the premised key adaptation in humans (although the spring-like function may have further improved during evolution). Moreover, the triceps surae anatomy of extant non-human great apes must be a convergence, related to muscle control and range of motion. This perspective accords with the suggestions put forward in the literature that the last common hominoid ancestor was not necessarily great ape-like, but might have been more similar to the small-bodied catarrhines.
Keywords: gibbon's Achilles tendon, functional anatomy, hominoid evolution
1. State of the art
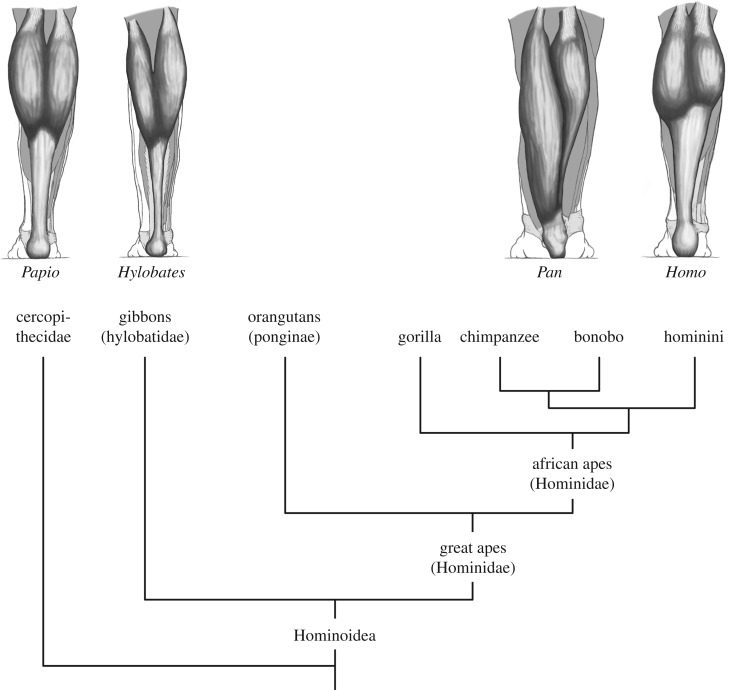
When looking at the gross morphology of the triceps surae in the extant great apes,1 the difference between humans and the other species is conspicuous (figure 1). In humans, the bellies of the gastrocnemius muscle are short-fibred and pennate, and insert together with the soleus via a well-developed Achilles tendon onto the calcaneus (e.g. [1–3]). By contrast, in non-human great apes, an Achilles tendon is externally barely visible and the bellies of the gastrocnemius muscle are extended with a more parallel orientation of the long muscle fibres (e.g. [1,2,4–7]). These contrasting morphologies correlate with differences in locomotor repertoire: while orangutans, gorillas, chimpanzees and bonobos share a wide range of, often arboreal, locomotor behaviours such as orthograde (i.e. upright trunk) suspension and clambering, quadrupedalism, vertical climbing and hand-assisted bipedalism (see [8,9] for reviews), modern humans are primarily terrestrial habitual bipeds.
Figure 1.
Phylogenetic tree of the Catarrhini, with dorsal views of the baboon, gibbon, chimpanzee and human lower leg showing the Achilles tendon and the gastrocnemius muscle (anatomical drawings: courtesy of Timo van Leeuwen).
The well-developed human Achilles tendon is considered to be an adaptation for energy-efficient cyclic locomotion and is assumed to have originated at some point after 3 million years ago (Ma) in the genus Homo [10]. The tendon is stretched and loaded with strain energy during initial dorsiflexion (decreasing ankle angle) of the stance phase and recoils during the plantar flexion (increasing ankle angle) later in stance to power the foot push-off. As such, the Achilles tendon is a component of the spring element in the SLIP mechanism (spring-loaded inverted pendulum; [11,12]) that is optimally functioning during running: the kinetic and potential energy that must be extracted from the system at the whole-body level early in stance (the so-called negative external work) is converted—at least partly—to strain energy in the spring element and this is recycled to power part of the subsequent push-off (e.g. [13,14]). In humans, at a running speed of 4.5 m s−1, 35% of the required external positive work per stride can thus be recovered from the recoil of the Achilles tendon (e.g. [13,15]).
The morphology of the non-human great apes, on the other hand, is generally considered to represent the ancestral state [10], being beneficial when moving about in an arboreal environment. Movement patterns are less cyclic and less uniform, and the long-fibred muscles facilitate muscular control over a large range of motion, which is essential to deal efficiently with the high three-dimensional complexity of the habitat and to respond to the compliance of the substrate [5,7,16].
Remarkably, given their phylogenetic position and their largely arboreal lifestyle, the gibbon's triceps surae has a more human-like appearance (figure 1). There is a long and well-developed Achilles tendon, firmly attaching onto the heel bone, and the muscle bellies of the gastrocnemius are short-fibred and pennate [1,6]. Questioning the adaptive meaning of this morphology in the primarily brachiating lesser apes seems essential to understand the evolution of locomotor diversity in the apes.
Because of the high resemblance between the gibbon and human Achilles tendon, it is tempting to search for an explanation for gibbons that follows a similar line of thought to that assumed for the human Achilles tendon. When on the ground to cross gaps between trees that are too large to cross arboreally, or when moving on large tree branches, gibbons most often use a bipedal gait [17–21]. Despite the presence of a double support phase (i.e. both feet on the ground simultaneously; there is no aerial phase in the bipedal cycle), this gait must be classified as ‘grounded running’ (cf. [22,23]) as is also found for terrestrial locomotion in birds (e.g. [24,25]): at the whole-body level, kinetic and potential energy fluctuations accord to the dynamics of running (i.e. in-phase decrease and subsequent increase of kinetic and potential energy in each single step; [22,26]). As such, the SLIP mechanism might be functional. Moreover, the safety factor of the Achilles tendon (i.e. tendon strength over tendon loading) appears, together with that of the patella tendon, to be the lowest of all the hindlimb muscles in the gibbon [6,27,28]. A low (but safe) safety factor is required for functionally significant energy storage and recoil. Furthermore, the ratio of the tendon length over the effective muscle fascicle length (i.e. accounting for pennation angle) is rather high for the triceps surae [6,27,28]). Such muscle–tendon morphology can be expected if the tendon needs to do the work. One may also look at this from a slightly different perspective. Channon et al. [27] presented the relationship between the physiological cross-sectional area (PCSA; a measure for potential maximal load) of the limb muscles and their fascicle length (a measure for the potential shortening), thus representing a sort of concentric work space, as it expresses the potential maximal load against potential shortening. Muscle–tendon complexes that are part of the spring element of the SLIP mechanism should combine a high PCSA or a force output with short fibres, enabling the tendon to do most of the concentric work. Surprisingly, all plantar flexors occupy a rather ‘unspecialized’ region in the concentric work space where small PCSA and short fibres are combined [27]. In this respect, gibbons appear to be no different from the non-human great apes.
2. The role of the tendon revisited
The evidence provided above for the gibbon's Achilles tendon working as energy-saving device during ‘grounded’ running is indirect. Moreover, its identification as ‘unspecialized’ in the muscle–tendon workspace could be interpreted as a counter-indication for this role and former analyses [22,26] were also unable to categorically demonstrate an energy-saving role during grounded running. Here, we take a novel approach to resolve this debate. The amount of strain energy that is stored in vivo in the tendon of the white-handed gibbon (Hylobates lar) during walking steps at the onset of plantar flexion, and that can thus potentially be recovered via recoil, is calculated and compared with the mechanical work input needed at the whole-body level (the so-called positive external work) to complete a walking cycle (i.e. stride = left + right step). To do so, the kinematic, dynamic, material property and anatomical data collected by Vereecke et al. [6,17,22,23,29], Vereecke & Aerts [26], Channon et al. [27,30] and Vereecke & Channon [28] will be combined in a new synthesis. (A short synopsis of the Material and Methods of these papers is included as the electronic supplementary material.)
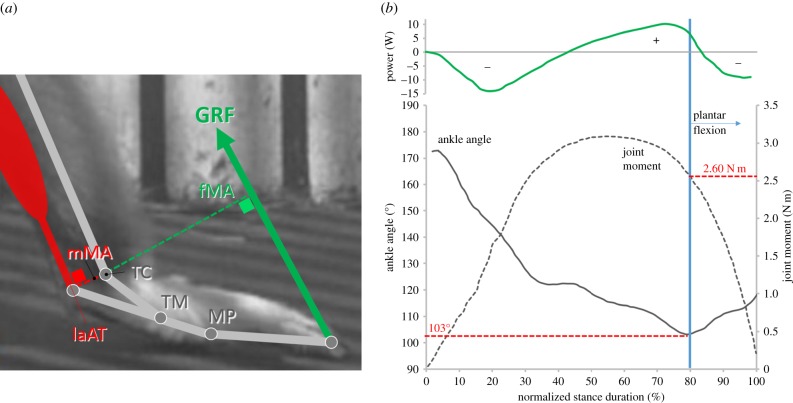
When the centre of pressure (instantaneous position of the point of application of the resultant ground reaction force (GRF) at the plantar surface of the foot) is known throughout the ground contact phase, the moment of the GRF with respect to the ankle joint can be determined quite accurately as a function of stance time by multiplying at any instant the GRF with the perpendicular distance from the joint centre to the GRF (figure 2a).2 This moment (dashed curve) is presented together with the ankle joint kinematics (thin curve) as a function of normalized stance time in figure 2b. For the larger part of stance (approx. 79%), the ankle joint dorsiflexes and only during the last 21% of stance does it plantar flex again to power the foot push-off. At the onset of plantar flexion, the (minimal) ankle joint angle reaches 103° and the in vivo moment of the GRF that tends to dorsiflex the joint equals 2.60 N m (figure 2b).
Figure 2.
(a) Schematic of the lower leg and foot of the white-handed gibbon (dark grey sticks) at the instant of initial foot contact during bipedal ‘grounded’ running (background: still frame of a video sequence). The ankle (talocrural) (TC), tarsometatarsal (TM) and metatarsophalangeal (MP) joints are shown. The moment arm (fMA) of the GRF (green arrow) and the moment arm (mMA) of the balancing force acting along the Achilles tendon, both with respect to the ankle joint, are indicated. The triceps surae and its Achilles tendon are represented in red. The fMA is the perpendicular distance from the TC to the GRF; the mMA from the TC to the line of action of the Achilles Tendon (laAT). (b) Upper panel: instantaneous mechanical power of the BCOM during stance. When positive, energy is being added to the BCOM. When negative, BCOM energy dissipates. The vertical blue line indicates the instant of transition from ankle dorsiflexion to ankle plantar flexion (i.e. extension of the ankle joint). For the largest part, plantar flexion (recoil eventually adding energy) occurs when, overall, whole-body energy decreases (see the text). Lower panel: average ankle joint angle (solid curve; left vertical axis) and average joint moment of the GRF at TC (dashed curve; right vertical axis) are given as a function of normalized stance time (0% = initial foot contact; 100% = toe-off) in the white-handed gibbon. This plantar flexion represents the foot push-off which can partly be powered by the release of strain energy stored in the Achilles tendon. At this transition, the ankle joint angle equals 103°, while the according joint moment is 2.60 N m (for more explanation: see the text) (based on the study of Vereecke & Aerts [26]; see also electronic supplementary material for more details of methods).
At any instant in the stride, the moment of the GRF with respect to the ankle must be balanced by the muscle–tendon systems that cross the joint. It is therefore possible to estimate the in vivo tensile force acting along the Achilles tendon at the onset of ankle plantar flexion, provided that the moment arm of the Achilles tendon (i.e. perpendicular distance from the joint centre to the tendon; figure 2a) at the coinciding joint angle (103°) is known.3,4 Muscle moment arms were accurately determined by Channon et al. [30]. For an ankle joint of 103°, the moment arm of the Achilles tendon in H. lar recalculates to 1.48 cm. Consequently, the in vivo tensile force along the tendon at the onset of ankle plantar flexion equals 175.67 N (i.e. 2.60 N m/(1.48 m 10−2)).
Cyclic tensile load–deformation tests on the Achilles tendon were carried out by Vereecke & Channon [28]. The tendon's behaviour conforms to that of the textbook examples (e.g. [13]): apart from a toe region at low loads, the load–deformation relationship is rather linear at higher loading; at recoil, a hysteresis of, on average, 13.5% (mean ± s.d. = 3.4%; n = 14) is observed (i.e. difference between loading and unloading energy). The slope of the linear loading part gives the stiffness, which is, on average, 99.6 N mm−1 (mean ± s.d. = 42.7 N mm−1; n = 14). As the tensile force divided by the stiffness equals the extension of the tendon, the in vivo stretch of the Achilles tendon at the onset of the ankle plantar flexion amounts to 1.76 mm (i.e. 175.67 N/99.6 N mm−1).
Finally, the amount of strain energy that is stored in the Achilles tendon at the onset of ankle plantar flexion (and which is available to power the plantar flexion) is represented by the area under the load–deformation curve. Because of the largely linear behaviour when loaded, this area is given by (175.67 N × 1.76 m 10−3)/2, which equals 0.15 J.
At the whole-body level, the positive work needed to complete a stride amounts on average to 3.56 J [22].5 Taking the 13.5% hysteresis into account, 0.26 J [i.e. (0.15 J left + 0.15 J right) × (1 − 0.135)] of this can theoretically be recovered from the recoiling left and right Achilles tendons during the push-off of the feet. This amounts to merely 7.5% of the required external positive work per stride. This amount can directly be compared with the 35% mentioned above for humans (but see also6). More importantly, however, plantar flexion seems to come at the wrong instant. To be effective, tendon recoil should happen when the mechanical whole-body energy level increases (i.e. mechanical energy is added to the system). However, most often (step-to-step variability is observed) plantar flexion just occurs when, at the whole-body level, mechanical energy must be extracted from the system (i.e. negative work must be performed; figure 2b). Consequently, Achilles tendon recoil in plantar flexion during ground contact (which means that mechanical energy is added to the system) could eventually come at the extra cost for energy dissipation by eccentric muscle contraction. Moreover, the foot is only partly plantar flexed at the end of stance when the joint torque is zero again (figure 2b). This means that either the recoil energy is dissipated by extending the triceps surae muscle belly during that final stance phase or, because of the biarticular arrangement of the gastrocnemius, that energy is transferred to the knee to assist further active knee flexion observed final in stance (see [22]).7
If not for energy storage and recoil during grounded running locomotion, what could the explanation of the well-developed Achilles tendon in gibbons be? Clearly, other, even rare behaviours may entail selective pressure and morphological adaptation. Gibbons also engage, for instance, in bipedal and tripedal gallops and a sort of half bound (crutching gallop) (cf. [17]), and show excellent leaping performance. The potential use of tendon recoil for energy recovery (while galloping) or power amplification (for leaping) cannot be excluded, but biomechanical results presented by Channon et al. [37–39] do not support this for leaping.
Here, we propose an alternative perspective. Available information from the literature and our own observations suggests that the Achilles tendon is also well developed and firmly attached to the heel bone in extant Cercopithecoidea (e.g. [1,2]; own dissections on Theropithecus gelada (gelada baboon), Papio anubis (olive baboon), Macaca maura (Moor macaque), Macaca mulatta (rhesus monkey), Semnopithecus entellus (Hanuman langur), Colobus guereza kikuyensis (mantled guerza), Colobus sp., Trachypithecus francoisi (François' leaf monkey); see electronic supplementary material and figure 1). It seems therefore conceivable that this morphological character was also present in the basal ancestor of this superfamily, hence also in the basal representative of the sister taxon, the Hominoidea (figure 1). Is it plausible that the Achilles tendon is retained as a relict in the branch leading to the Hylobatidae (figure 1)?
If selection acted predominantly on the principal locomotor mode in gibbons, i.e. on brachiation (e.g. [19,40–49]), adaptations can be expected primarily at the level of the forelimbs. During brachiation, hindlimb movements are potentially useful to modulate whole-body rotational inertia and to avoid hitting lower lying branches, a role which, most likely, did not imply important adaptive modifications of the lower hindlimb muscles. As such, it can be hypothesized that the triceps surae, with its long tendon, was not under selective pressure (no harm and no benefit) and could keep its ancestral appearance during hylobatid evolution.
3. Consequence for the evolution of the great apes
If the above hypothesis is supported, then the short-fibred gastrocnemius muscle with a long Achilles tendon should also be ancestral for the Hominidae. Given that this represents also the extant human morphology, it seems most parsimonious that this ancestral morphology was retained rather than re-acquired in the evolutionary lineage leading to the habitually bipedal, terrestrial modern humans. This lends weight to Thorpe et al.'s [50] conclusion that human bipedalism is less an innovation than an exploitation of a locomotor behaviour retained from the common great ape ancestor. To be effective, the recoiling Achilles tendon should work against a stiff lever as it is functionally present during the push-off phase in the modern human foot. Considerable evidence exists that early hominins had more mobile feet and, therefore, probably a less complete toe-off function compared with modern humans (e.g. [51]). Thus, although the performance of the Achilles tendon as an energy-saving device might well have been further improved during human evolution,8 considering the emergence of the human Achilles tendon as a key adaptation for economical cyclic bipedal locomotion is probably no longer appropriate.
This also implies that the ‘long-fibred–short tendon’ appearance of the triceps surae in the orangutan, the gorilla, the chimpanzee and the bonobo does not represent the retained ancestral state as it is generally considered (cf. above). Rather, it might represent further convergent evolution from an above-branch quadrupedal ancestor (cf. [59,60]) with short-fibred gastrocnemius muscles and a long Achilles tendon towards the long-fibred muscles facilitating the muscular control and a large range of motion that is beneficial for the arboreal lifestyles of each of the large-bodied extant non-human Hominidae (see, for instance, [5,7,16]).9 In this context, it is remarkable that lorisines also have a short Achilles tendon comparable to great apes [4], which is interpreted as a convergent feature (next to others) related to selection for slow, cautious arboreal clambering (see, for instance, [61]). This view conforms to the suggestion by Alba et al. [60] that the last common hominoid ancestor was not necessarily great ape-like and that small-bodied catarrhines could have played a remarkable role in ape evolution. It also accords with the suggestions by Almécija et al. [59] (based on their analysis of hominoid forelimbs) that above-branch quadrupedalism inherited from stem hominoids constituted a significant component of the locomotor repertoires of different hominoid lineages at least until the Late Miocene. And finally, it also supports the suggestion by Lovejoy et al. [62,63] that the last common ancestor of the African apes probably had feet that functioned like those of living monkeys rather than like those of apes. On the basis of the present revision, it seems plausible to include the evolution of the Achilles tendon in their functional perspective and to extend this to all extant great apes.
Supplementary Material
Acknowledgements
We thank the staff of the Animal Park Planckendael for access and support in studying the gibbons. We also acknowledge Timo van Leeuwen for the artwork in this manuscript. R. Lacoste (CNRS Primatology Station, Rousset, France), Dr M. Herbin (Collection in fluid of Comparative Anatomy, National Museum of Natural History, Paris, France), Dr François Druelle (University of Antwerp) and Dr Mélanie Berthet (zoo of Besançon, FR) were very helpful in collecting additional cadaver material of cercopithecoids and in assisting with the dissections.
Endnotes
Here, orangutans, gorillas, chimpanzees, bonobos and humans are considered the extant great apes (i.e. extant Hominidae).
Inertial effects can safely be neglected in this account, given the small mass (1.2% of total body mass) and the low accelerations of the slender foot segments (cf. [22,26]).
In this approach it is assumed that the balancing activity is taken entirely by the triceps surae, hence solely acting along the Achilles tendon. As such, the estimate for the tensile force along the tendon at the onset of ankle plantar flexion represents a maximal estimate, as co-contraction of the digital flexors would result in a reduction of the Achilles tendon stress.
It should be noted that co-contraction of the dorsiflexors (which could lead to higher tensile stress in the Achilles tendon) at that instant in stance is highly unlikely.
This must be considered as a minimum estimated for the required positive work input, as this concerns the external work only (i.e. whole-body level; movements of the body centre of mass or BCOM). Swinging the limbs with respect to the BCOM can represent a considerable extra cost (see, for instance, [31]).
Similar approaches on human running (4.5 m s−1) show that 35% of the external positive work (mechanical energy) comes from elastic recoil of the Achilles tendon during the second half of the ground contact phase in each cycle, i.e. energy stored during the first half of stance [13,15,32]. This is probably even a conservative estimate. Lai et al. [33] show that at comparable running speeds, energy recovery from the triceps surae tendon can amount to more than 50 J per step (which is approx. 140% of what was determined from the former ex vivo experiments; see [33] and references therein). Clearly, the eccentric–concentric work of the triceps surae during running steps will still require metabolic energy, even when the entire strain cycle of the muscle tendon unit (MTU) is taken by the tendon. Cross-bridge cycling is needed to prevent extension of the muscle belly and enable loading of the tendon (e.g. [34]). For this purpose, short-fibred (pennate) bellies are the best option: these can deliver the required force at a minimal metabolic cost. At the MTU level, in humans, up to 75% of the positive work output comes from the tendon (e.g. [33,35]). Given an efficiency of 0.2–0.25 for concentric work in muscle [36], similar MTU stress–strain cycles for ‘long-fibred–short tendon’ triceps surae muscle would become very expensive.
It should be noted that this conclusion refers to the role of the Achilles tendon during plantar flexion of the foot only. It may well be that other muscle–tendon systems (for instance, the digital flexors or the knee extensors) do act as functional energy-saving mechanisms.
Given that modern humans gain up to 35% of the positive BCOM work required for running from tendon recoil (e.g. [13]; approx. 5× more than what can potentially be recovered in gibbons), it is tempting to speculate on what has changed during human evolution to improve the percentage contribution of elastic recoil. This percentage increase may be due to (relatively) lower positive BCOM work requirements, (relatively) higher energy storage in the tendon or both. The positive work requirements over a complete cycle (L + R) for human running at preferred speed are 3.4 J kg−1 (body mass; [52,53]), much higher than the 0.6 J kg−1 here calculated for the gibbon (3.6 J per cycle for a 6.3 kg animal; cf. electronic supplementary material). Consequently, elevated energy storage must be in play. Human Achilles tendon stiffness is rather variable (for instance, depending on age or training level), but an average and physiologically relevant value of 180 N mm−1 is reported in the literature (e.g. [54–56]), nearly doubling the stiffness of the gibbon's tendon. Stiffer tendons imply less elastic energy storage for a given tendon loading. Thus, tendon force at the onset of plantar flexion (enabling recoil) has to be considerably higher in humans. The ankle extension torque is maximal and equals 2.5 N m kg−1 ([52]; preferred running speed) when plantar flexion starts (about at midstance; note that this value increases further with running speeds). Taking account of the according Achilles tendon moment arm of approximately 5 cm (e.g. [57,58]; relative to the lower leg length about twice that of the gibbon), maximal tendon loading equals 50 N kg−1, actually not that much higher than what can be calculated for the gibbon (29 N kg−1 = 2.6 N m/6.3 kg/0.0148 m). In other words, size (body mass) as such (obviously coupled to the specific locomotor dynamics) seems to be an important determinant for the higher elastic energy storage. Using the above-mentioned data for human preferred running (and accounting for a hysteresis of 10%; e.g. [34,56]), the relative energy storage over a cycle recalculates to 0.7 J kg−1, which is approximately 17× more than in the gibbon (0.041 J kg−1 = 0.26 J/6.3 kg) and accounts (at this relatively slow running speed) for 21% of the positive BCOM work.
It should be noted that this alternative scenario does not necessarily imply a larger number of character-state changes (i.e. being less parsimonious) than the classic scenario in which the ‘short-fibred–long-tendon’ triceps surae evolved independently from a ‘long-fibred–short tendon’ ancestral state both in the gibbons and in humans. If it is agreed that the common ancestor of the cercopithecoids and hominoids shared the ‘long-tendon’ character state (cf. the main text and see the electronic supplementary material), this feature must first have been lost, in order to then reappear then in the stem hylobatids (as all seem to have a well-developed tendon) and in humans independently. This implies an identical number of character-state changes as for the premised convergent appearance of the ‘long-fibred–short tendon’ state of the triceps surae in the non-human great apes.
Data accessibility
This paper revises and integrates previously published data. We refer to the source publications (cf. references). No additional data were collected for the purpose of this specific contribution. On request, more information can be provided by the authors (contact corresponding author).
Authors' contributions
P.A. provided the new perspective, revisited and integrated the original data, participated in some of the original data collection, participated in the final discussion and drafted the manuscript. K.D. participated in the collection of the original data and participated in the final discussion on the manuscript. S.T. brought in the evolutionary insights and participated in the final discussion on the manuscript. G.B. provided the anatomical data on Macaca, Papio and Colobus and participated in the final discussion on the manuscript. E.V. carried out and/or supervised all the studies providing the original data and participated in the final discussion on the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interest.
Funding
Part of the original studies was funded by the Research Foundation Flanders, and part of the dissections of cercopithecoids was funded by the International Research Network (IRN, CNRS) Bipedal Equilibrium.
References
- 1.Frey H. 1913. Der Musculus Triceps Surae in der Primatenreiche. Morphologische Jahrbuch 47, 1–191. [Google Scholar]
- 2.Swindler D, Wood C. 1973. An atlas of primate gross anatomy. Baboon, chimpanzee and men. Seattle, WA: University of Washington Press. [Google Scholar]
- 3.Standring S. 2016. Gray's anatomy: the anatomical basis of clinical practice (ed. 41). Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 4.Hannah J, Schmitt AD. 2011. Interpreting the role of climbing in primate locomotor evolution: are the biomechanics of climbing influenced by habitual substrate use and anatomy? Int. J. Primatol. 32, 430–444. ( 10.1007/s10764-010-9479-2) [DOI] [Google Scholar]
- 5.Thorpe S, Crompton R, Günther M, Ker R, Alexander RM. 1999. Dimensions and moment arms of the hind- and forelimb muscles of common chimpanzees (Pan troglodytes). Am. J. Phys. Anthropol. 110, 176–199. ( 10.1002/(SICI)1096-8644(199910)110:2%3C179::AID-AJPA5%3E3.0.CO;2-Z) [DOI] [PubMed] [Google Scholar]
- 6.Vereecke E, D'Août K, Payne R, Aerts P. 2005. Functional analysis of the foot and ankle myology of gibbons and bonobos. J. Anat. 206, 453–476. ( 10.1111/j.1469-7580.2005.00412.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myatt J, Crompton R, Thorpe S. 2011. Hindlimb muscle architecture in non-human great apes and a comparison of methods for analysing inter-species variation. J. Anat. 219, 150–166. ( 10.1111/j.1469-7580.2011.01383.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorpe S, Crompton R. 2006. Orangutan positional behavior and the nature of arboreal locomotion in Hominoidea. Am. J. Phys. Anthropol. 131, 384–401. ( 10.1002/ajpa.20422) [DOI] [PubMed] [Google Scholar]
- 9.Hunt K. 2016. Why are there apes? Evidence for the co-evolution of ape and monkey ecomorphology. J. Anat. 228, 630–685. ( 10.1111/joa.12454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bramble D, Liebermann D. 2004. Endurance running and the evolution of Homo. Nature 432, 345–352. ( 10.1038/nature03052) [DOI] [PubMed] [Google Scholar]
- 11.Full R, Koditscheck D. 1999. Templates and anchors: neuromechanical hypotheses of legged locomotion on land. J. Exp. Biol. 202, 3325–3332. [DOI] [PubMed] [Google Scholar]
- 12.Geyer H, Seyfarth A, Blickan R. 2006. Compliant leg behaviour explains basic dynamics of walking and running. Proc. R. Soc. B 273, 2861–2867. ( 10.1098/rspb.2006.3637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander R. 2003. Principles of animal locomotion. Princeton, NJ: Princeton University Press. [Google Scholar]
- 14.Bertram J. 2016. Understanding mammalian locomotion. Concepts and applications. New York, NY: John Wiley & Sons. [Google Scholar]
- 15.Alexander R. 1991. Energy-saving mechanisms in walking and running. J. Exp. Biol. 160, 55–69. [DOI] [PubMed] [Google Scholar]
- 16.Preuschoft H, Witte H, Demes B. 1992. Biomechanical factors that influence overall body shape of large apes and humans. In Topics in primatology, vol. 3, Evolutionary biology, reproductive endocrinology and virology (eds Matano S, Tuttle RH, Ishida H, Goodman M), pp. 259–289. Tokyo, Japan: University of Tokyo Press. [Google Scholar]
- 17.Vereecke E, D'Août K, Aerts P. 2006. Locomotor versatility in the white-handed gibbon (Hylobates lar): a spatiotemporal analysis of the bipedal, tripedal, and quadrupedal gaits. J. Hum. Evol. 50, 552–567. ( 10.1016/j.jhevol.2005.12.011) [DOI] [PubMed] [Google Scholar]
- 18.Baldwin L, Teleki G. 1976. Patterns of gibbon behavior on Hall's island, Bermuda. In Gibbon and siamang, vol. 4 (ed. Rumbauch D.), pp. 21–105. Basel, Switzerland: Karger. [Google Scholar]
- 19.Fleagle J. 1976. Locomotion and posture of the Malayan siamang and implications for hominid evolution. Folia Primatol. 26, 245–269. ( 10.1159/000155756) [DOI] [PubMed] [Google Scholar]
- 20.Gittins S. 1983. Use of the forest canopy by the agile gibbon. Folia Primatol. 40, 134–144. ( 10.1159/000156095) [DOI] [PubMed] [Google Scholar]
- 21.Sati J, Alfred J. 2002. Locomotion and posture in Hoolock gibbon. Ann. For. 10, 298–306. [Google Scholar]
- 22.Vereecke E, D'Août K, Aerts P. 2006. The dynamics of hylobatid bipedalism: evidence for an energy-saving mechanism? J. Exp. Biol. 209, 2829–2838. ( 10.1242/jeb.02316) [DOI] [PubMed] [Google Scholar]
- 23.Vereecke E, D'Août K, Aerts P. 2006. Speed modulation in hylobatid bipedalism: a kinematic analysis. J. Hum. Evol. 51, 513–526. ( 10.1016/j.jhevol.2006.07.005) [DOI] [PubMed] [Google Scholar]
- 24.Andrada E, Rode C, Blickhan R. 2013. Grounded running in quails: simulations indicate benefits of observed fixed aperture angle between legs before touch-down. J. Theor. Biol. 335, 97–107. ( 10.1016/j.jtbi.2013.06.031) [DOI] [PubMed] [Google Scholar]
- 25.Andrada E, Haase D, Sutedja Y, Nyakatura J, Kilbourne B, Denzler J, Fischer M, Blickhan R. 2015. Mixed gaits in small avian terrestrial locomotion. Sci. Rep. 5, 13636 ( 10.1038/srep13636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vereecke E, Aerts P. 2008. The mechanics of the gibbon foot and its potential for elastic energy storage during bipedalism. J. Exp. Biol. 211, 3661–3670. ( 10.1242/jeb.018754) [DOI] [PubMed] [Google Scholar]
- 27.Channon A, Günther M, Crompton R, Vereecke E. 2009. Mechanical constraints on the functional morphology of the gibbon hind limb. J. Anat. 215, 383–400. ( 10.1111/j.1469-7580.2009.01123.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vereecke E, Channon A. 2013. The role of hind limb tendons in gibbon locomotion: springs or strings? J. Exp. Biol. 216, 3971–3980. ( 10.1242/jeb.083527) [DOI] [PubMed] [Google Scholar]
- 29.Vereecke E, D'Août K, Van Elsacker L, Aerts P. 2005. Functional analysis of the gibbon foot during terrestrial bipedal walking: plantar pressure distributions and three-dimensional ground reaction forces. Am. J. Phys. Anthropol. 128, 659–669. ( 10.1002/ajpa.20158) [DOI] [PubMed] [Google Scholar]
- 30.Channon A, Günther M, Crompton R, Vereecke E. 2010. Muscle moment arms of the gibbon hind limb: implications for hylobatid locomotion. J. Anat. 216, 446–462. ( 10.1111/j.1469-7580.2009.01209.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsh R, Ellerby D, Carr J, Henry H, Buchanan C. 2004. Partitioning the energetics of walking and running: swinging the limbs is expensive. Science 303, 80–83. ( 10.1126/science.1090704) [DOI] [PubMed] [Google Scholar]
- 32.Ker R, Bennett M, Bibby S, Kester R, Alexander RMcN. 1987. The spring in the arch of the human foot. Nature 325, 147–149. ( 10.1038/325147a0) [DOI] [PubMed] [Google Scholar]
- 33.Lai A, Schache A, Lin Y, Pandy MG. 2014. Tendon elastic strain energy in the human ankle plantar-flexors and its role with increased running speed. J. Exp. Biol. 217, 3159–3168. ( 10.1242/jeb.100826) [DOI] [PubMed] [Google Scholar]
- 34.Fletcher J, MacIntosh B. 2015. Achilles tendon strain energy in distance running: consider the muscle energy cost. J. Appl. Physiol. 118, 193–199. ( 10.1152/japplphysiol.00732.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hof A, Van Zandwijk J, Bobbert M. 2002. Mechanics of human triceps surae muscle in walking, running and jumping. Acta Physiol. Scand. 174, 17–30. ( 10.1046/j.1365-201x.2002.00917.x) [DOI] [PubMed] [Google Scholar]
- 36.Woledge R, Curtin N, Homsher E. 1985. Energetic aspects of muscle contraction. New York, NY: Academic Press. [PubMed] [Google Scholar]
- 37.Channon A, Crompton R, Günther M, D'Août K, Vereecke E. 2010. The biomechanics of leaping in gibbons. Am. J. Phys. Anthropol. 143, 403–416. ( 10.1002/ajpa.21329) [DOI] [PubMed] [Google Scholar]
- 38.Channon A, Günther M, Crompton R, D'Août K, Preuschoft H, Vereecke E. 2011. The effect of substrate compliance on the biomechanics of gibbon leaps. J. Exp. Biol. 214, 687–696. ( 10.1242/jeb.046797) [DOI] [PubMed] [Google Scholar]
- 39.Channon A, Usherwood J, Crompton R, Günther M, Vereecke E. 2011. The extraordinary athletic performance of leaping gibbons. Biol. Lett. 8, 46–49. ( 10.1098/rsbl.2011.0574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertram J. 2004. New perspectives on brachiation mechanics. Yearbook Phys. Anthropol. 47, 100–117. ( 10.1002/ajpa.20156) [DOI] [PubMed] [Google Scholar]
- 41.Bertram J, Chang Y-H. 2001. Mechanical energy oscillations of two brachiation gaits: measurement and simulation. Am. J. Phys. Anthropol. 115, 319–326. ( 10.1002/ajpa.1088) [DOI] [PubMed] [Google Scholar]
- 42.Bertram J, Ruina A, Cannon C, Chang Y-H, Colema NM. 1999. A point-mass model of gibbon locomotion. J. Exp. Biol. 202, 2609–2617. [DOI] [PubMed] [Google Scholar]
- 43.Chang Y-H, Bertram J, Ruina A. 1997. A dynamic force and moment analysis system for brachiation. J. Exp. Biol. 200, 3013–3020. [DOI] [PubMed] [Google Scholar]
- 44.Chang Y-H, Bertram J, Lee D. 2000. External forces and torques generated by the brachiating white-handed gibbon (Hylobates lar). Am. J. Phys. Anthropol. 113, 201–216. ( 10.1002/1096-8644(200010)113:2%3C201::AID-AJPA5%3E3.0.CO;2-S) [DOI] [PubMed] [Google Scholar]
- 45.Michilsens F, D'Août K, Aerts P. 2011. How pendulum-like are siamangs? Energy exchange during brachiation. Am. J. Phys. Anthropol. 154, 581–591. ( 10.1002/ajpa.21539) [DOI] [PubMed] [Google Scholar]
- 46.Michilsens F, D'Août K, Vereecke E, Aerts P. 2012. One step beyond: different step-to-step transitions exist during continuous contact brachiation in siamangs. Open Biol. 1, 411–421. ( 10.1242/bio.2012588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preuschoft H, Demes B. 1984. Biomechanics of brachiation. In The lesser apes: evolutionary and behavioural biology (eds Preuschoft H, Chivers DJ, Brockelman WY, Creel N), pp. 96–118. Edinburgh, UK: Edinburgh University Press. [Google Scholar]
- 48.Usherwood J, Bertram J. 2003. Understanding brachiation: insight from a collisional perspective. J. Exp. Biol. 206, 1631–1642. ( 10.1242/jeb.00306) [DOI] [PubMed] [Google Scholar]
- 49.Usherwood J, Larson S, Bertram J. 2003. Mechanisms of force and power production in unsteady ricochetal brachiation. Am. J. Phys. Anthropol. 120, 364–372. ( 10.1002/ajpa.10133) [DOI] [PubMed] [Google Scholar]
- 50.Thorpe S, Holder R, Crompton R. 2007. Origin of human bipedalism as an adaptation for locomotion on flexible branches. Science 316, 1328–1331. ( 10.1126/science.1140799) [DOI] [PubMed] [Google Scholar]
- 51.Lieberman D. 2012. Those feet in ancient times. Nature 483, 550–551. ( 10.1038/483550a) [DOI] [PubMed] [Google Scholar]
- 52.Fiers P, De Clercq D, Segers V, Aerts P. 2013. Biomechanics of human bipedal gallop: asymmetry dictates leg function. J. Exp. Biol. 216, 1338–1349. ( 10.1242/jeb.074690) [DOI] [PubMed] [Google Scholar]
- 53.Willems P, Cavagna G, Heglund N. 1995. External, internal and total work in human locomotion. J. Exp. Biol. 198, 379–393. [DOI] [PubMed] [Google Scholar]
- 54.Lichtwark G, Wilson A. 2005. In vivo mechanical properties of the human Achilles tendon during one-legged hopping. J. Exp. Biol. 208, 4715–4725. ( 10.1242/jeb.01950) [DOI] [PubMed] [Google Scholar]
- 55.Lichtwark G, Wilson A. 2006. Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion. J. Exp. Biol. 209, 4379–4388. ( 10.1242/jeb.02434) [DOI] [PubMed] [Google Scholar]
- 56.Uchida T, Hicks J, Dembia C, Delp S. 2016. Stretching your energetic budget: how tendon compliance affects the metabolic cost of running. PLoS ONE 11, e0150378 ( 10.1371/journal.pone.0150378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leardini A, O'Connor J. 2002. A model for lever-arm length calculation of the flexor and extensor muscles at the ankle. Gait Posture 15, 220–229. ( 10.1016/S0966-6362(01)00153-9) [DOI] [PubMed] [Google Scholar]
- 58.Maganaris C, Baltzopoulos V, Sargeant A. 2000. In vivo measurements-based estimations of the human Achilles tendon moment arm. Eur. J. Appl. Physiol. 83, 363–369. ( 10.1007/s004210000247) [DOI] [PubMed] [Google Scholar]
- 59.Almécija S, Alba D, Moyà-Solà SS. 2009. Pierolapithecus and the functional morphology of Miocene ape hand phalanges: paleobiological and evolutionary implications. J. Hum. Evol. 57, 284–297. ( 10.1016/j.jhevol.2009.02.008) [DOI] [PubMed] [Google Scholar]
- 60.Alba D, Almécija S, DeMiguel D, Fortuny J, Pérez de los Ríos M, Pina M, Robles J, Moyà-Solà S. 2015. Miocene small-bodied ape from Eurasia sheds light on hominoid evolution. Science 350, aab2625 ( 10.1126/science.aab2625) [DOI] [PubMed] [Google Scholar]
- 61.Cartmill M, Milton K. 1977. The lorisform wrist joint and the evolution of ‘brachiating’ adaptations in the Hominoidea. Am. J. Phys. Anthropol. 47, 249–279. ( 10.1002/ajpa.1330470206) [DOI] [PubMed] [Google Scholar]
- 62.Lovejoy C, Latimer B, Suwa G, Asfaw B, White T. 2009. Combining prehension and propulsion: the foot of Ardipithecus ramidus. Science 326, 72e1–72e8. [PubMed] [Google Scholar]
- 63.Lovejoy C, Suwa G, Simpson S, Matternes J, White T. 2009. The great divides: Ardipithecus ramidus reveals the postcrania of our last common ancestors with African apes. Science 326, 100–106. ( 10.1126/science.1175833) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This paper revises and integrates previously published data. We refer to the source publications (cf. references). No additional data were collected for the purpose of this specific contribution. On request, more information can be provided by the authors (contact corresponding author).