Abstract
Brood parasitic cuckoos lay their eggs in other birds' nests, whereafter the young cuckoo hatches, ejects its nest-mates and monopolizes the care of the host parents. Theory predicts that hosts should not evolve to recognize and reject cuckoo chicks via imprinting because of the risk of mistakenly imprinting on a cuckoo chick in their first brood and thereafter always rejecting their own chicks. However, recent studies have revealed that some hosts do reject cuckoo chicks from the nest, indicating that these hosts’ recognition systems either do not rely on first brood imprinting, or use cues that are independent of chick phenotype. Here, we investigate the proximate mechanisms of chick rejection behaviour in the large-billed gerygone (Gerygone magnirostris), a host of the little bronze-cuckoo (Chalcites minutillus). We find that gerygones use true template-based recognition based on at least one visual chick trait (the number of hatchling down-feathers), and that this is further mediated by experience of adult cuckoos at the nest during egg-laying. Given the theoretical constraints of acquiring recognition templates via imprinting, gerygones must possess a template of own-chick appearance that is largely innate. This true recognition has facilitated the evolution of very rapid hatchling rejection and, in turn, striking visual mimicry of host young by little bronze-cuckoo chicks.
Keywords: brood parasitism, bronze-cuckoo, gerygone, host defence, chick discrimination, chick rejection
1. Introduction
Brood parasitic cuckoos impose heavy costs on their hosts, selecting for the evolution of host defences against parasitism [1–3]. The most widespread defence is egg rejection, and many hosts have evolved highly refined abilities to detect and eject eggs that differ in appearance from their own [4,5]. Curiously, however, these same hosts typically fail to reject the parasitic chicks once hatched, despite the imposters having a clearly distinct phenotype from the host's own young [2,6]. Several theoretical solutions to this long-standing puzzle have been proposed (reviewed in [7]). One explanation is that the costs of recognition errors may constrain the evolution of learned cuckoo chick discrimination in hosts wherever cuckoos evict the host eggs from the nest soon after hatching [8]. Lotem suggested that if hosts learn the appearance of their own chicks through imprinting on their first brood, a host parasitized during its first breeding attempt would falsely imprint on the lone foreign chick as its own young and thereafter reject its own offspring for the rest of its life [8]. The same problem would not impede the evolution of egg rejection, because even parasitized hosts are exposed to some of their own eggs during the egg-laying and incubation phases.
Lotem's hypothesis provides an explanation for the lack of true learned recognition of cuckoo chicks (assessment of the match between the template for a hosts' own young and the phenotype of the parasite chick) by hosts [8]. However, some hosts have evolved the ability to discriminate cuckoo chicks using ‘recognition-free’ mechanisms [9–11]. Recognition-free discrimination involves identifying the parasite chick from cues other than chick phenotype, thereby avoiding the risk of mis-imprinting [7,10,12]. It has been shown to be the primary process operating in two hosts of evicting cuckoos. Hosts of Horsfield's bronze-cuckoo (Chalcites basalis) use the presence of a lone chick in the nest and the presence of adult cuckoos in the population as cues for abandoning parasitized nests [9]. Similarly, reed warbler (Acrocephalus scirpaceus) hosts of common cuckoos (Cuculus canorus) cue into the duration of parental care, abandoning chicks that remain in the nest for longer than the typical host nestling period [10]. These studies demonstrate that recognition-free discrimination provides hosts with a pathway for cuckoo chick rejection that circumvents the costs of mis-imprinting. Our aim is to test whether cuckoo chick discrimination can also evolve through true recognition, despite the theoretical costs of mis-imprinting proposed by Lotem [8]. One plausible way in which this could occur is if discrimination is largely innate, rather than learned [11]. In theory, true recognition has a significant advantage over some recognition-free mechanisms, because it can take place immediately upon hatching of the parasite chick, allowing the host to remove the cuckoo before it evicts host young. To date, no studies have demonstrated true recognition of parasite young. However, indirect evidence for this mechanism in some hosts stems from the apparent nestling or fledgling mimicry of host young by cuckoos [13–16]. Just as occurs at the egg stage, selection for mimicry might arise through host rejection of chicks with non-matching phenotypes [7,15].
The gerygone (Gerygone spp.) hosts of Australia's little bronze-cuckoos (Chalcites minutillus) are strong candidates for using true recognition of chicks. Despite typically suffering high parasitism rates, gerygones do not reject bronze-cuckoo eggs [13,14,17]. Instead, gerygones have the most effective known form of chick rejection because they reject cuckoo chicks by dragging them out of the nest within hours of hatching [13,14,18], sometimes succeeding in removing the cuckoo nestling (a nest-mate evictor) before it has a chance to evict the host young from the nest. Most Australian bronze-cuckoo species lay non-mimetic eggs, but their chicks are excellent visual mimics of host young, with each subspecies matching the colour of nestling skin, rictal flange and down-feathers of their favoured host species [15,19,20]. The little bronze-cuckoo is a particularly accurate mimic of host young [15], and it is unique among cuckoos in displaying multi-barbed nestling down-feathers, which are typical of passerine nestlings including their hosts, but are otherwise unknown in the cuckoo family [15].
Here, we use experimental manipulations to establish for the first time the mechanisms by which gerygones recognize and reject little bronze-cuckoo nestlings. We test for three non-mutually exclusive recognition-free cues (hatch order, the presence of an adult cuckoo and discordancy) and one true recognition cue (nestling down-feathers) that may be used to facilitate chick rejection. The presence of an adult cuckoo in the nest's vicinity has been shown to be an important component of chick rejection decisions in another bronze-cuckoo host [11]. Hatch order is also a possible recognition-free cue [21], because cuckoo eggs typically require a shorter incubation period and usually hatch 1–2 days before gerygone young [22]. However, this cue would only be useful in conjunction with another cue, indicating that the nest has been parasitized. Recognition by discordancy involves assessment of the differences between chick phenotypes within the same brood and rejection of the least common phenotype [23,24]. In the absence of true recognition, discordancy might favour visual mimicry of host young by cuckoos, provided that the cuckoo and host chicks are present in the nest together prior to rejection, and that host chicks typically outnumber cuckoos.
2. Material and methods
(a). Study area and species
We carried out our study from August to December 2016 along creeklines in and around Cairns, Queensland, Australia (16°55′ S, 145°46′ E) on a population of large-billed gerygones (Gerygone magnirostris) that experience high rates of parasitism by little bronze-cuckoos (63–65% [17]; this study). Little bronze-cuckoos were seen or heard, and parasitism occurred, at all creeks in the study. The large-billed gerygone builds untidy domed nests using grass, moss and spiders' egg-sacks, usually over-hanging water [25]. Gerygones lay one egg every second day over a period of 4–8 days (average clutch: mean ± s.e. = 3 ± 0.09, range: 1–5, n = 100) and start incubation when their clutch is complete [25]. Cuckoos lay a single egg per host nest, during or shortly after the hosts’ egg-laying period, and usually remove one host egg during the same visit. Two or three different females may lay in the same host nest [17,26]. Hosts mob the cuckoo if it is detected during laying, but mobbing has not been observed to prevent parasitism [17].
(b). General experimental methods
We located 54 large-billed gerygone nests during the nest-building phase by searching along creeks, rivers and lakes. Of these nests, 35 (65%) were subsequently parasitized by one (n = 30) or two (n = 5) cuckoos, and 19 were not parasitized. We checked nest contents daily to allow clutch manipulation as soon as eggs appeared and before incubation began. From hatching day, we monitored all 54 nests to determine whether nestling rejection occurred. Parasitized nests were filmed or observed continuously from hatching until host chick eviction (by cuckoo chicks) or cuckoo chick rejection (by host parents) occurred. When cuckoo chicks evicted host young and became the sole occupant of the nest, we continuously monitored the nest for at least a further 2 days during daylight hours to document any chick rejection or nest predation. In total, 19 nests (16 parasitized and three unparasitized) were monitored from 06.00–07.00 to 17.00–18.00 by an observer in a hide (approx. 5 m from nest) using binoculars, 17 nests (16 parasitized and one unparasitized) were filmed continuously with a video camera (Panasonic, HC-VX870M) and the remaining 20 nests (three parasitized and 15 unparasitized) were monitored with daily nest checks to determine whether any chicks were missing. If a host chick was missing from an unparasitized nest, we concluded that the host had rejected the chick. This conclusion is based on the lack of observations of partial predation in our study site (other than when an egg was stuck to or embedded in the lining of a depredated nest) and the fact that nest predation usually results in nest damage as the predator forces entry into the dome nest. If a nest check revealed that all chicks in the nest were missing, we concluded that the nest had been predated. We excluded three unobserved disappearances of cuckoo chicks from our analyses, because in each case the cuckoo chick was alone in the nest so we could not determine whether it disappeared due to ejection or predation. Three host chicks that died in the nest on the day after hatching day were excluded from the analysis because we have no evidence either for or against the idea that dead chicks were rejected. We calculated ‘time to ejection’ to the nearest day (hatch day = 0 days). In our experiments, we first manipulated exposure of hosts to an adult cuckoo using a cross-fostering experiment, and then randomly assigned nests to (i) a hatch-order manipulation experiment, (ii) a feather trimming experiment, or (iii) both the hatch order and the feather trimming experiment (see electronic supplementary material, figure S1).
(c). Manipulation of opportunity to observe an adult cuckoo at the nest
To assess whether hosts' exposure to an adult cuckoo at the nest influenced chick rejection rates, we cross-fostered cuckoo eggs from some parasitized nests to unparasitized nests to create two conditions among nests containing one or two cuckoo eggs: naturally parasitized, such that parents had the opportunity to observe a cuckoo lay at their nest (nests: n = 22, chicks: n = 25), and artificially parasitized nests where adults did not see a cuckoo lay in their nest (nests: n = 13, chicks: n = 15). Two sources of evidence suggest that parents of naturally parasitized nests are likely to have had the opportunity to observe a cuckoo entering the nest. First, we filmed parasitism of the nest on three occasions and, in every case, the gerygone parents mobbed the cuckoo [17]. Second, although we cannot be certain that all naturally parasitized hosts observed the cuckoo during parasitism, it is certain that more gerygones in the ‘naturally parasitized’ group will have seen or interacted with a cuckoo at their nest than did gerygones in the ‘artificially parasitized’ group.
(d). Manipulation of hatch order and discordancy
Cuckoo eggs usually hatch 1–2 days before host eggs [22]. To determine the effect of hatch order on chick rejection, we delayed the hatching of cuckoo eggs (n = 12) by 5 days. We removed each freshly laid cuckoo egg and stored it in a cool, dark place. We replaced it temporarily with a non-viable gerygone egg, which had been collected from a depredated or abandoned nest (depredated nests sometimes contained intact eggs, if they were stuck to the nest lining). After 2 days of incubation, we removed the dummy egg and returned the cuckoo egg to the nest, such that any host eggs in the nest hatched 1–2 days before the cuckoo chick. As a control, we used the same procedure to remove and later replace a single gerygone egg from unparasitized nests (n = 14). Five cuckoo chicks also hatched later than host young naturally and these were included in the dataset.
When a cuckoo chick or chicks are the minority species in the brood, hosts may discriminate via discordancy and reject the most dissimilar chick or chicks. This recognition-free mechanism requires that cuckoo and host chicks are present in the nest at the same time, and that host chicks reliably outnumber cuckoos. In combination, our cross-fostering and hatch-order manipulations varied the composition of chicks in the nest at the same time, and thus allowed us to test for evidence of discordancy as a rejection cue by comparing rejection rates when the cuckoo chick was (n = 8) or was not (n = 32) the brood's minority species.
(e). Manipulation of chick morphology
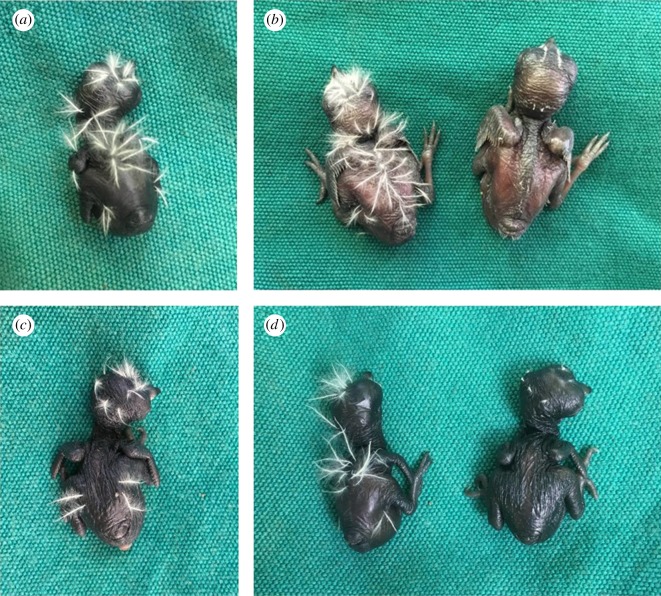
To assess whether gerygones' rejection of cuckoo chicks is based on true recognition, and specifically on the recognition of nestling down-feathers, we manipulated hatchlings’ feathers in a subset of nests (n = 32). On the day of hatching, we used nail scissors to trim the down-feathers of either one cuckoo chick (n = 13, including one naturally naked cuckoo chick) or one gerygone chick (unparasitized nests: n = 16, parasitized nests: n = 4) in the nest (figure 1). We compared the rate of rejection of trimmed chicks with that of chicks that were handled on hatching day, but did not have their feathers trimmed. We also counted the number of down-feathers of all chicks on hatch day, prior to the manipulation, to quantify natural variation in feather density and weighed chicks four times (hatching day, and 3, 7, and 13 days old) to test whether the manipulation otherwise affected chick growth.
Figure 1.
Photographs of large-billed gerygone and little bronze-cuckoo chicks. (a) An untrimmed gerygone on hatching day. (b) An experimental brood comprising one untrimmed host chick (left) and one trimmed host chick (right, both 3 days old). (c) An untrimmed cuckoo on hatching day. (d) An experimental brood comprising one untrimmed host chick (left) and one trimmed cuckoo chick (right, both on hatching day).
(f). Statistical analyses
We used a generalized linear model (GLM) with a binomial distribution and a logit link using all chicks (full dataset) to assess parental responses to the chicks (accept/reject) according to (i) the presence or absence of down-feathers, (ii) hatching order, (iii) whether or not host was exposed to adult cuckoo and/or (iv) whether or not a cuckoo chick was in the minority in the nest. The independent variables were species (cuckoo or host), the four manipulations (all scored as yes/no: hatched first, feathers trimmed, naturally parasitized cuckoo visited the nest and cuckoo chick in the minority), hatching date and the two-way interactions between these variables. We also tested the quadratic term for the hatching date because seasonal trends are often nonlinear, but the result was the same. Initially, we attempted to run a mixed model controlling for nest identity as a random effect because there were multiple chicks in each nest, but this made the model unstable due to the small number of replicates in each nest. Instead, we ran a binomial GLM with a logit link function on a reduced dataset comprising only one experimental chick per nest (reduced dataset) and then compared the results from the full dataset and the reduced dataset. Where there was only one manipulated chick in the nest, this was included in the reduced dataset. If there were two experimental chicks or there was no manipulated chick in the nests, we selected one chick randomly. In addition, to identify which of these factors contributed significantly to the time to rejection, we used a GLM with a binomial distribution depending on whether or not they were rejected on the day of hatching using all rejected chicks. The independent variables were the same as in the former GLM analysis. Owing to the controversy over whether null hypothesis testing or information theoretic approaches are better for analysis of experimental studies [27], we used both methods. We applied a backward-elimination procedure (tables 1 and 2), and the Akaike information criterion (AIC) was also used to support selection of the final model (best-fit model) (electronic supplementary material, tables S1 and S2). The results did not differ depending on the approach used; the significant effects as identified by the backward-elimination procedure were the same as the best model using AIC. We also evaluated multicollinearity using the variance inflation factor (VIF) in the models, and all VIF values were lower than the suggested threshold (greater than 10 [28]). All statistical analyses were performed using R software v. 3.4.3 [29].
Table 1.
Effects of experimental treatment on chick rejection. Acceptance or rejection of nestlings was modelled as a binomial in a GLM with a logit link function on both the full dataset and the dataset including only one manipulated chick in each nest (results in parentheses). Significant p-values are shown in italic.
| term | effect | estimate | s.e. | 95% CI | deviance | p-values | |
|---|---|---|---|---|---|---|---|
| LCI | UCI | ||||||
| included | species (cuckoo) | −1.578 (−0.264) | 0.777 (0.506) | −3.454 (−1.122) | −0.239 (0.565) | 31.667 (5.969) | <0.0001 (0.015) |
| species (host) | −6.020 (−3.170) | 1.370 (1.080) | −9.520 (−5.498) | −3.861 (−1.702) | |||
| trim (y) | 6.252 (3.174) | 1.441 (1.084) | 3.893 (1.675) | 9.840 (5.500) | 41.478 (16.372) | < 0.0001 (< 0.0001) | |
| exposure to adult cuckoo (y) | 2.269 (1.273) | 0.924 (0.939) | 0.601 (−0.187) | 4.355 (2.994) | 2.108 (2.037) | 0.146 (0.154) | |
| trim (y): exposure to adult cuckoo (y) | −4.109 (−3.069) | 1.62 (2.267) | −7.798 (−7.131) | −1.1690 (0.581) | 3.920 (−1.924) | 0.005 (0.165) | |
| excluded | hatch order (first) | −0.0144 (−0.828) | 0.6391 (0.794) | −1.094 (−2.197) | 1.027 (0.444) | −0.001 (−1.130) | 0.982 (0.288) |
| discordancy (y) | 0.388 (0.837) | 0.764 (0.844) | −0.868 (−0.526) | 1.673 (2.287) | −0.259 (−1.011) | 0.6111(0.315) | |
| hatching date | 0.013 (0.011) | 0.012 (0.014) | −0.006 (−0.013) | 0.035 (0.037) | −1.287 (−0.572) | 0.256 (0.450) | |
| species (h) : hatch order (f) | −2.864 (3.100) | 3831.454 (1.780) | −80.844 (0.343) | 75.116 (6.331) | −0.000 (−3.458) | 1 (0.063) | |
| species (h) : trim (y) | 17.168 (34.63) | 2045.330 (9300) | −77.750 (−) | 515.633 (−) | −1.859 (−0.826) | 0.173 (0.364) | |
| species (h) : exposure to adult cuckoo (y) | −2.332 (16.335) | 3488.828 (2655.399) | −67.924 (−) | 59.085 (−) | 0.000 (−0.676) | 1 (0.411) | |
| species (h): discordancy (y) | −17.307 (−18.476) | 2327.470 (2711.847) | −634.712 (−) | 84.847 (−) | −1.693 (−2.094) | 0.193 (0.150) | |
| trim (y) : hatch order (y) | −1.510 (−19.058) | 1.702 (4091.637) | −4.614 (−) | 1.166 (−) | −0.839 (−1.690) | 0.360 (0.194) | |
| trim (y) : discordancy (y) | 1.659 (−) | 17970 (−) | −327.640 (−) | 344.594 (−) | −0.000 (−) | 1 (−) | |
| hatch order (y) : exposure to adult cuckoo (y) | −1.326 (14.62) | 1.370 (15490) | −3.679 (403.428) | 0.886 (360.749) | −0.963 (0.000) | 0.326 (1) | |
| hatch order (y) : discordancy (y) | 3.115 (0.967) | 1.991 (1.822) | −0.026 (−2.071) | 6.700 (4.015) | −2.659 (−0.281) | 0.103 (0.596) | |
| exposure to adult cuckoo (y) : discordancy (y) | −1.258 (11.97) | 1.918 (30940) | −4.531 (−804.412) | 2.061 (780.835) | −0.422 (−0.000) | 0.516 (1) | |
Table 2.
Effects of experimental treatment on time until rejection for those nests in which hosts rejected a chick. Time to rejection was modelled as a binomial (hatching day = 0, one or more days post-hatching day = 1) in a GLM with a logit link function and the dataset includes all the rejected cuckoo and host chicks. Significant p-values are shown in italic.
| term | effect | estimate (s.e.) | 95% CI | deviance | p-values | |
|---|---|---|---|---|---|---|
| LCI | UCI | |||||
| included | species (c) | 0.241 (0.403) | −0.418 | 0.919 | 45.780 | 0.008 |
| species (h) | −1.792 (0.764) | −3.292 | −0.682 | |||
| excluded | trim (y) | −0.942 (0.920) | −2.547 | 0.528 | −1.095 | 0.295 |
| hatch order (f) | 0.542 (0.900) | 0.282 | 0.890 | −0.368 | 0.544 | |
| exposure to adult cuckoo (y) | −1.138 (0.981) | −2.961 | 0.366 | −1.508 | 0.219 | |
| discordancy (y) | −1.212 (1.211) | −3.652 | 0.629 | −1.136 | 0.287 | |
| hatching date | 0.010 (0.017) | −0.017 | 0.039 | −0.368 | 0.544 | |
| species (h) : trim (y) | 1.005 (4696) | — | — | −0.000 | 1 | |
| species (h) : hatch order (y) | 1.854 | — | — | −2.866 | 0.091 | |
| species (h) : exposure to adult cuckoo (y) | 54.95 (21670) | — | — | 0.000 | 1 | |
| species (h) : discordancy (y) | 20.54 (4212) | — | — | −2.885 | 0.089 | |
| trim (y) : hatch order (y) | 18.820 (5628) | — | — | −1.253 | 0.263 | |
| trim (y) : exposure to adult cuckoo (y) | 15.848 (4027.416) | — | — | −0.272 | 0.602 | |
| hatch order (f) : exposure to adult cuckoo (y) | 16.920 (3810.961) | — | — | −0.624 | 0.429 | |
| hatch order (f) : discordancy (y) | 2.117 (24670) | — | — | −1.805 × 10−9 | 1 | |
| exposure to adult cuckoo (y) : discordancy (y) | 55.100 (32090) | — | — | −1.723 × 10−8 | 1 | |
3. Results
Our full dataset (all chicks in experimental nests) included 85 host chicks and 40 cuckoo chicks across 54 nests. During the course of our experiment, 36 chicks (both host and cuckoo) were rejected from 32 nests (although host chicks were only rejected following down-feather manipulations; see below). We captured nine rejection events on film at eight nests (see example in electronic supplementary material, video S1), and a further five rejection events were observed with binoculars. The remaining chick rejections by hosts were inferred from daily nest checks. In all filmed or observed cases, large-billed gerygones pulled living chicks out of the nests, and the parents then continued to care for the remaining eggs and nestlings. Ejected chicks were either dropped just under the nests or carried up to 3 m from the nest before being dropped.
(a). True recognition
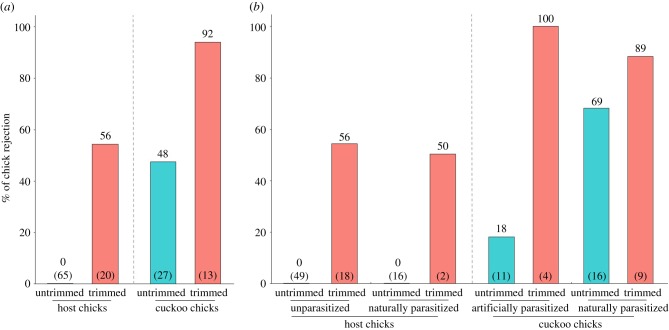
Chick species and the presence of nestling down-feathers were significant predictors of their rejection by gerygone hosts; cuckoos were more likely to be rejected than host chicks, trimmed chicks more likely to be rejected than untrimmed chicks in cuckoos and untrimmed host chicks were never rejected (table 1 and figure 2a). Hosts removed 93% of trimmed cuckoo chicks (13 of 14 chicks) and 56% of trimmed host chicks (11 of 20 chicks; figure 2a). Among untrimmed chicks, 50% of cuckoo chicks were rejected (14 of 28), while untrimmed host chicks were never removed (n = 65 chicks, figure 2a), and trimming was the only manipulation that resulted in hosts rejecting their own chicks. Where cuckoo chicks did not have their down-feathers manipulated, host parents showed a non-significant tendency to reject those that had naturally fewer down-feathers (rejected: mean ± s.e. = 13 ± 1.77, n = 14; accepted: mean ± s.e. = 17.07 ± 1.67, n = 14; Student's t-test: t = −1.67, p = 0.1068). Similarly, our results from the reduced dataset showed that chick species and the presence of down-feathers were the most significant predictors of rejection (table 1).
Figure 2.
(a) The percentage of large-billed gerygone and little bronze-cuckoo chicks that were ejected according to whether down-feathers were trimmed. (b) The percentage of untrimmed and trimmed chicks that were ejected among host and cuckoo nestlings according to whether the nest was naturally or artificially parasitized (i.e. whether an adult cuckoo visited the nest during the egg-laying period). Sample sizes are given in parentheses at the base of the bar, and numbers above bars depict the exact percentage.
The timing of nestling removal further supports a role for direct species-specific chick cues in gerygones' rejection decisions. Cuckoo chicks were more likely to be rejected on the hatch day than host chicks, but whether or not a chick was trimmed did not influence the timing of its removal (table 2). All rejected cuckoo chicks were removed by hosts within 2 days of hatching, with 56% (14 of 25) and 36% (9 of 25) rejected on hatching day and the next day, respectively. Only 8% (2 of 25) were rejected 2 days after hatching (electronic supplementary material, figure S2). By contrast, just 18% (2 of 11) of rejected host chicks were ejected on hatch day, 36% (4 of 11) were removed the day after hatching and 46% (5 of 11) were rejected 2–3 days after hatching (electronic supplementary material, figure S2). In the case of cuckoo chicks, rapid rejection was necessary to preserve the host young in the nest: when hosts removed cuckoo chicks on hatch day, none of their own nestlings had yet been evicted by the cuckoo chick, while those cuckoo chicks rejected on later days had already removed some or all gerygone young (six out of 11).
Finally, we confirmed that hatchling down-feathers vary under natural conditions in both gerygone and cuckoo young. On average, large-billed gerygone nestlings had more down-feathers on the day of hatching (mean ± s.e. = 37.35 ± 1.45, range: 23–68, n = 41, one chick per nest) than little bronze-cuckoo nestlings (mean ± s.e. = 14.40 ± 1.19, range: 0–29, n = 38, Student's t-test: t = −12.226, p < 0.0001). The variation in the number of down-feathers of gerygone chicks within the same brood was significantly less than that between broods (one-way ANOVA: F40,44 = 5.35, p < 0.0001).
(b). Recognition-free cues in host decisions to reject chicks
In naturally parasitized nests in which hatch order was not manipulated (n = 22), the cuckoo hatched before the host chicks in 77% of cases, but based on experimental nests, hatch order had no significant effect on the probability of chick rejection (table 1). Whether the cuckoo chick was in the minority in the brood also did not influence chick rejection decisions (table 1). In addition, hatch order and whether or not a chick is in the minority in the brood did not affect the timing of its removal (table 2).
Host's rejection decisions were influenced in part, however, by the exposure to adult cuckoos in interaction with chick phenotype (table 1), with hosts more likely to reject a cuckoo chick if it had been laid naturally into the nest than if it had been cross-fostered there from another nest by us (table 1 and figure 2b). This was clearly evident among the sample of untrimmed cuckoo chicks; only 18% of untrimmed cuckoo chicks (two out of 11) from artificially parasitized nests were ejected, whereas parents that had the opportunity to observe adult cuckoos laying rejected 69% of untrimmed cuckoo chicks (11 out of 16; Fisher's exact probability test: p < 0.01; figure 2b). However, our results from the reduced dataset showed that the effect of whether the nest was parasitized naturally or artificially was trivial (table 1), presumably due to dataset sample size differences.
4. Discussion
Hosts that reject parasite nestlings may do so based either directly on chick phenotype (true recognition) or on recognition-free cues. True recognition is assumed to be maladaptive for cuckoo hosts if it relies on an imprinted template [8], and previous studies have found experimental support only for recognition-free mechanisms [9,10]. Our results, however, provide the first experimental evidence that hosts can use true recognition when rejecting foreign nestlings, as large-billed gerygones regularly rejected nestlings that differed from their own offsprings' phenotype due to a lack of hatchling down-feathers. Gerygones combined this use of phenotypic cues with at least one additional chick-recognition-free cue, being more likely to reject cuckoo chicks when they had the opportunity to witness an adult cuckoo laying in the nest.
(a). Chick rejection based on true recognition
At least to the human observer, the number of down-feathers present on newly hatched chicks is the most obvious morphological cue available for discriminating between own and parasitic young; most host chicks have significantly more down-feathers than cuckoo chicks. Gerygones too were confirmed to use this cue in rejection decisions, being prompted to reject cuckoos, and even some of their own young, for which down-feathers were artificially removed. However, trimmed cuckoos were rejected at far higher rates than trimmed host young, indicating that gerygones use additional, as yet unidentified phenotypic cues. The begging calls of newly hatched chicks were audible to the human ear (H.-J.N. 2016, personal observation) and parents frequently made provisioning visits to the nest before they removed the chicks, so differences in begging call structure are a possible cue that warrants further investigation.
True recognition requires that hosts possess an internal template of the acceptable chick phenotype, to which they are able to compare cuckoo chicks. Given that to acquire this template solely through experience with a first brood would lead to maladaptively high rates of recognition error in the host of an evicting cuckoo [8], a gerygone's template must have an alternative origin. One possibility is that chick templates are largely innate, driven by strong selection for correct identification of own and parasitic young. Such innate templates could still be refined through experience, in much the same way as songbirds have an innate template for their species's song that is refined through interactions with conspecifics [11]. Rejection decisions can then be further refined through the complementary use of recognition-free cues (discussed below). The resulting recognition and rejection system is certainly effective for large-billed gerygones in our study area, as we never observed the mistaken rejection of host young (other than those that were trimmed). Notably, however, Sato et al. [13] reported several cases of large-billed gerygones rejecting their own nestlings in a different study population, so it remains unclear whether low error rates are a general feature of gerygones' chick rejection behaviour. Recognition errors are most likely to occur in situations in which mimicry is highly accurate. In our study population, mimicry by little bronze-cuckoos was imperfect, because they had fewer nestling down-feathers than large-billed gerygone nestlings. However, host rejection was influenced more by the presence/absence of down-feathers than by the number of down-feathers per se. In addition, we found lower variation within than between broods in host down-feather abundance. This suggests that gerygones may be under selection for low intra-brood variation in the number of down-feathers to facilitate detection of cuckoo chicks, in much the same way as some other cuckoo hosts may experience selection for low intra-clutch variation in egg phenotype, facilitating detection of cuckoo eggs [30–33]. Such a process would require either that host and cuckoo chicks were present in the nest together (which occurs in a minority of nests) or that hosts remember their own chick morphology from previous broods.
Some combination of innate true recognition and more flexible mechanisms in gerygones’ chick rejection would be consistent with our understanding of egg rejection mechanisms. Egg rejecter species show variation within and between populations in the form and extent of egg rejection behaviour [24,34], and individual hosts' reactions towards foreign eggs may also vary with conditions or experience [35]. The existence of both consistent and flexible patterns of egg rejection behaviour implies that both innate and learning mechanisms can be involved [34], and many host species appear to combine one or more variants of the true recognition process with proximate context-dependent factors when making rejection decisions [24]. Both egg rejection and chick rejection thus seem to be complex processes, using considerable mechanistic variation within and between species.
(b). Recognition-free discrimination
Gerygones were more than twice as likely to reject a cuckoo chick if an adult cuckoo had visited the nest during the egg-laying period than if the nest was parasitized artificially, indicating that the opportunity to observe or interact with a cuckoo at the nest strongly influenced rejection behaviour, as has also been observed in another bronze-cuckoo host [11]. Moreover, our results showed the strongest effect of exposure to adult cuckoos in interaction with chick phenotype (table 1), suggesting that this cue on its own is not enough to prompt rejection and must be coupled with cues from the chicks themselves. This indicates that the combination of this contextual cue with one or more phenotypic cues may allow gerygones to substantially reduce the risk of mistakenly rejecting their own young, particularly given the accurate host–cuckoo nestling mimicry in this system [36–39].
Notably, if hosts use the presence of adult cuckoos as a cue to reject nestling cuckoos, the cue is ‘recognition-free’ with respect to chick phenotype, but does require the recognition of adult cuckoos. Based on behavioural responses, large-billed gerygones readily distinguish between adult cuckoos near their nests, which elicit mobbing, and predators or harmless species, which do not (F. Jacomb et al. 2015, unpublished data) [40]. Although it is unknown whether mobbing ever succeeds in preventing a cuckoo from laying, our results indicate that the recognition of adult cuckoos has an important role in gerygones' antiparasite defence, by increasing the accuracy of chick rejection decisions. Accordingly, our study provides support for strategy facilitation [41], in which adaptations at one stage of the evolutionary arms race (in this case, the egg-laying stage) promote the evolution of defences at another stage (the nestling stage).
We found no evidence that large-billed gerygones use two other candidate recognition-free cues: hatch order or discordancy. A simple ‘reject the odd one out’ rule is useful only when there are multiple chicks in the nest, and only one of these is a cuckoo, a condition that is rarely met in large-billed gerygones owing to the shorter incubation period of cuckoo nestlings and the small clutch size of gerygones. A strategy of ‘reject the first hatched chick’ would, in theory, be relatively effective for gerygones in ridding themselves of cuckoos, particularly if enacted only when adult cuckoos have been seen at the nest. However, the occurrence of multiple parasitism in this system (approx. 30% of all parasitized nests receive multiple cuckoos eggs [17,42]) reduces the benefit of such a rule of thumb substantially, because often another cuckoo will simply hatch to take the place of the rejected one.
(c). Implications for cuckoo–host coevolution and diversification in little bronze-cuckoos
Our results provide the first experimental demonstration that host defences can select for the evolution of nestling mimicry in a brood parasite. Previous work revealed that the nestlings of three bronze-cuckoo species are near perfect visual mimics of the host chicks they exploit [15]. Moreover, one host, the superb fairy-wren Malurus cyaneus, was less likely to reject cuckoo chicks of a species that specializes on fairy-wrens (Horsfield's bronze-cuckoo C. basalis) than a cuckoo species that uses fairy-wrens rarely (the shining bronze-cuckoo Chalcites lucidus) [9]. However, only recognition-free cues for chick discrimination were identified in this system, so it was unclear whether host rejection selected for mimicry of host young [11]. Furthermore, some forms of chick mimicry might arise for reasons other than host rejection [43], such as to exploit biases in host–parent communication and extract the optimal resources from host parents [44]. While it remains possible that the visual mimicry of little bronze-cuckoos also increases host provisioning rates, it seems likely that it has been primarily driven by gerygones' chick rejection behaviour.
In this study, our focus was demonstrating that true nestling recognition can evolve, contrary to the predictions of theory based on an imprinting model of chick rejection [8]. Mis-imprinting constraints are not the only explanation, however, for the apparent scarcity of chick rejection across hosts of brood parasites. Effective rejection of cuckoo eggs can prevent the evolution of cuckoo chick rejection by making the cuckoo nestling a ‘rare enemy’, such that the benefits of discriminating against it are outweighed by the costs of recognition errors [7,45,46]. Curiously, large-billed gerygones do not reject foreign eggs even though little bronze-cuckoo eggs look very different from their own. This is surprising given that hosts suffer fewer costs of parasitism by implementing defences early in the breeding cycle rather than later. Indeed, three non-mutually exclusive explanations for this are that (i) egg rejection is constrained by poor visibility inside the nest, because dark-coloured bronze-cuckoo eggs are cryptic inside dark host nests [17,26], (ii) egg rejection is constrained by bill morphology, because cuckoo eggs are too large or thick-shelled to be ejected and methods of egg rejection that remove or abandon whole clutches are too costly [47–49], and (iii) hosts may benefit by delaying rejection of the parasite until the chick stage when there is a risk of multiple parasitism, because allowing the cuckoo egg to remain in the nest reduces the probability that a host egg will be removed during subsequent parasitism events (the egg dilution hypothesis [17,50]).
Different subspecies of the little bronze-cuckoo exploit different hosts, and cuckoo mimicry of host nestlings can extend even down to the level of subspecies [51]. For example, C. m. minutillus mimics the dark skin and white down of nestling large-billed gerygones [15], whereas C. m. barnardi mimics the pink skin and yellow down of the offspring of white-throated gerygones Gerygone albogularis [19,43]. In addition, the little bronze-cuckoo occupies a wider distribution and has more subspecies than any other Chalcites cuckoos (10 described subspecies, compared to just one to four variants of other bronze-cuckoos) [52]. Although the rejection behaviour of other little bronze-cuckoo hosts remains to be studied, it is plausible that the observed variation in little bronze-cuckoo chicks has evolved in response to true recognition and chick rejection by their hosts, ultimately reinforcing reproductive isolation among cuckoo populations that exploit different host species [53]. Thus, unlike recognition-free mechanisms of chick rejection, true recognition of cuckoo chicks may have significant consequences for the coevolutionary trajectory of their parasites, by driving host-specific genetic diversification in parasite populations.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Virginia Abernathy, Frances Jacomb, Golo Maurer and Brian Venables for assistance in the field. We thank Hwan-Jin Yoon for advice on statistical analysis. We also kindly thank Cairns Regional Council for access to the Botanic Gardens.
Ethics
The aim of this study was to explore the mechanism of chick rejection. While chick rejection by hosts leads to the death of the rejected chick, this experiment did not increase the frequency of chick death, because in a parasitized nest either the cuckoo chick or the host chicks always die under natural conditions. Our experimental manipulations may have influenced whether it was the cuckoo or the host chicks that died, but overall the experiments did not cause mortality in more nests than would happen naturally. All experiments were conducted under approval of the Australian National University Animal Experimentation Ethics Committee Protocol number A2016/16.
Data accessibility
All data supporting this article are available from Dryad (http://dx.doi.org/10.5061/dryad.vv76g) [54].
Authors' contribution
H.-J.N. performed experiments and analyses and drafted the manuscript. H.-J.N., R.G. and N.E.L. conceived the study, discussed results, analyses and implications, and revised the manuscript.
Competing interests
We have no competing interests.
Funding
This work was supported by an Australian Research Council Discovery Grant to N.E.L.
References
- 1.Davies NB, Brooke MdL. 1989. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J. Anim. Ecol. 58, 207–224. ( 10.2307/4995) [DOI] [Google Scholar]
- 2.Davies NB, Brooke MdL. 1989. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J. Anim. Ecol. 58, 225–236. ( 10.2307/4996) [DOI] [Google Scholar]
- 3.Feeney WE, Welbergen JA, Langmore NE. 2014. Advances in the study of coevolution between avian brood parasites and their hosts. Annu. Rev. Ecol. Evol. Syst. 45, 227–246. ( 10.1146/annurev-ecolsys-120213-091603) [DOI] [Google Scholar]
- 4.de la Colina MA, Pompilio L, Hauber ME, Reboreda JC, Mahler B. 2012. Different recognition cues reveal the decision rules used for egg rejection by hosts of a variably mimetic avian brood parasite. Anim. Cogn. 15, 881–889. ( 10.1007/s10071-012-0515-9) [DOI] [PubMed] [Google Scholar]
- 5.Hanley D, Grim T, Igic B, Samaš P, López AV, Shawkey MD, Hauber ME. 2017. Egg discrimination along a gradient of natural variation in eggshell coloration. Proc. R. Soc. B 284, 20162592 ( 10.1098/rspb.2016.2595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyllie I. 1981. The cuckoo. London, UK: Batsford. [Google Scholar]
- 7.Grim T. 2006. The evolution of nestling discrimination by hosts of parasitic birds: why is rejection so rare? Evol. Ecol. Res. 8, 785–802. [Google Scholar]
- 8.Lotem A. 1993. Learning to recognize nestlings is maladaptive for cuckoo Cuculus canorus hosts. Nature 362, 743–745. ( 10.1038/362743a0) [DOI] [Google Scholar]
- 9.Langmore NE, Hunt S, Kilner RM. 2003. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422, 157–160. ( 10.1038/nature01460) [DOI] [PubMed] [Google Scholar]
- 10.Grim T. 2007. Experimental evidence for chick discrimination without recognition in a brood parasite host. Proc. R. Soc. B 274, 373–381. ( 10.1098/rspb.2006.3731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langmore NE, Cockburn A, Russell AF, Kilner RM. 2009. Flexible cuckoo chick-rejection rules in the superb fairy-wren. Behav. Ecol. 20, 978–984. ( 10.1093/beheco/arp086) [DOI] [Google Scholar]
- 12.Anderson MG, Hauber ME. 2007. A recognition-free mechanism for reliable rejection of brood parasites. Trends Ecol. Evol. 22, 283–286. ( 10.1016/j.tree.2007.03.009) [DOI] [PubMed] [Google Scholar]
- 13.Sato NJ, Tokue K, Noske RA, Mikami OK, Ueda K. 2010. Evicting cuckoo nestlings from the nest: a new anti-parasitism behaviour. Biol. Lett. 6, 67–69. ( 10.1098/rsbl.2009.0540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tokue K, Ueda K. 2010. Mangrove Gerygones Gerygone laevigaster eject little bronze cuckoo Chalcites minutillus hatchlings from parasitized nests. Ibis 152, 835–839. ( 10.1111/j.1474-919X.2010.01056.x) [DOI] [Google Scholar]
- 15.Langmore NE, Stevens M, Maurer G, Heinsohn R, Hall ML, Peters A, Kilner RM. 2011. Visual mimicry of host nestlings by cuckoos. Proc. R. Soc. B 278, 2455–2463. ( 10.1098/rspb.2010.2391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Mársico MC, Gantchoff MG, Reboreda JC. 2012. Host-parasite coevolution beyond the nestling stage? Mimicry of host fledglings by the specialist screaming cowbird. Proc. R. Soc. B 279, 3401–3408. ( 10.1098/rspb.2012.0612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gloag R, Keller L-A, Langmore NE. 2014. Cryptic cuckoo eggs hide from competing cuckoos. Proc. R. Soc. B 281, 1–7. ( 10.1098/rspb.2014.1014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato NJ, Tanaka KD, Okahisa Y, Yamamichi M, Kuehn R, Gula R, Ueda K, Theuerkauf J. 2015. Nestling polymorphism in a cuckoo-host system. Curr. Biol. 25, R1164–R1165. ( 10.1016/j.cub.2015.11.028) [DOI] [PubMed] [Google Scholar]
- 19.McGill IG, Goddard MT. 1979. The little bronze-cuckoo in New South Wales. Aust. Birds 14, 23–24. [Google Scholar]
- 20.Langmore NE, Spottiswoode CN. 2012. Visual trickery in avian brood parasites. In Host manipulation by parasites (eds DP Hughes, J Brodeur, F Thomas), pp. 95–118. Oxford, UK: Oxford University Press. [Google Scholar]
- 21.Shizuka D, Lyon BE. 2010. Coots use hatch order to learn to recognize and reject conspecific brood parasitic chicks. Nature 463, 223–226. ( 10.1038/nature08655) [DOI] [PubMed] [Google Scholar]
- 22.Davies NB. 2000. Cuckoos, cowbirds and other cheats. London, UK: T & AD Poyser. [Google Scholar]
- 23.Rothstein SI. 1974. Mechanisms of avian egg recognition: possible learned and innate factors. Auk 91, 796–807. ( 10.2307/4084731) [DOI] [Google Scholar]
- 24.Moskát C, Bán M, Székely T, Komdeur J, Lucassen RW, Van Boheemen LA, Hauber ME. 2010. Discordancy or template-based recognition? Dissecting the cognitive basis of the rejection of foreign eggs in hosts of avian brood parasites. J. Exp. Biol. 213, 1976–1983. ( 10.1242/jeb.040394) [DOI] [PubMed] [Google Scholar]
- 25.Higgins PJ. 1999. Handbook of Australian, New Zealand and Antarctic birds, vol. 4: parrots to dollarbird. Melbourne, Australia: Oxford University Press. [Google Scholar]
- 26.Langmore NE, Stevens M, Maurer G, Kilner RM. 2009. Are dark cuckoo eggs cryptic in host nests? Anim. Behav. 78, 461–468. ( 10.1016/j.anbehav.2009.06.003) [DOI] [Google Scholar]
- 27.Murtaugh PA. 2014. In defense of P values. Ecology 95, 611–617. ( 10.1890/13-0590.1) [DOI] [PubMed] [Google Scholar]
- 28.Zuur AF, Ieno EN, Elphick CS. 2010. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1, 3–14. ( 10.1111/j.2041-210X.2009.00001.x) [DOI] [Google Scholar]
- 29.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www/R-project.org/ [Google Scholar]
- 30.Øien IJ, Moksnes A, Røskaft E. 1995. Evolution of variation in egg color and marking pattern in European passerines: adaptations in a coevolutionary arms race with the cuckoo, Cuculus canorus. Behav. Ecol. 6, 166–174. ( 10.1093/beheco/6.2.166) [DOI] [Google Scholar]
- 31.Soler JJ, Pape Møller A. 1996. A comparative analysis of the evolution of variation in appearance of eggs of European passerines in relation to brood parasitism. Behav. Ecol. 7, 89–94. ( 10.10.1038/35025058) [DOI] [Google Scholar]
- 32.Stokke BG, Moksnes A, Røskaft E, Rudolfsen S, Honza M. 1999. Rejection of artificial cuckoo (Cuculus canorus) eggs in relation to variation in egg appearance among reed warblers (Acrocephalus scirpaceus). Proc. R. Soc. Lond. B 266, 1483–1488. ( 10.1098/rspb.1999.0804) [DOI] [Google Scholar]
- 33.Stokke BG, Moksnes A, Røskaft E. 2002. Obligate brood parasites as selective agents for evolution of egg appearance in passerine birds. Evolution 56, 199–205. ( 10.1111/j.0014-3820.2002.tb00861.x) [DOI] [PubMed] [Google Scholar]
- 34.Soler J, Martinez JG, Soler M, Møller AP. 1999. Genetic and geographic variation in rejection behavior of cuckoo eggs by European magpie populations: an experimental test of rejecter-gene flow. Evolution 53, 947–956. ( 10.2307/2640734) [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Yang C, Møller AP, Liang W, Lu X. 2015. Multiple mechanisms of egg recognition in a cuckoo host. Behav. Ecol. Sociobiol. 69, 1761–1767. ( 10.1007/s00265-015-1988-8) [DOI] [Google Scholar]
- 36.Reeve HK. 1989. The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407–435. ( 10.1086/284926) [DOI] [Google Scholar]
- 37.Wiley RH. 1994. Errors, exaggeration, and deception in animal communication. In Behavioral mechanisms in evolutionary ecology (ed. LA Real), pp. 157–189. Chicago, IL: University of Chicago Press. [Google Scholar]
- 38.Sherman PW, Reeve HK, Pfennig DW. 1997. Recognition systems. In Behavioral ecology: an evolutionary approach (eds Krebs JR, Davies NB), pp. 69–96. Oxford, UK: Blackwell Scientific. [Google Scholar]
- 39.Holen ØH, Johnstone RA. 2006. Context-dependent discrimination and the evolution of mimicry. Am. Nat. 167, 377–389. ( 10.1086/499567) [DOI] [PubMed] [Google Scholar]
- 40.Mulyani YA. 2004. Reproductive ecology of tropical mangrove-dwelling warblers: the roles of nest predation, brood parasitism and food limitation. PhD thesis, Charles Darwin University, Australia. [Google Scholar]
- 41.Kilner RM, Langmore NE. 2011. Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol. Rev. 86, 836–852. ( 10.1111/j.1469-185X.2010.00173.x) [DOI] [PubMed] [Google Scholar]
- 42.Brooker MG, Brooker L. 1989. Cuckoo hosts in Australia. Aust. Zool. Rev. 2, 1–67. [Google Scholar]
- 43.Grim T. 2005. Mimicry vs. similarity: which resemblances between brood parasites and their hosts are mimetic and which are not? Biol. J. Linn. Soc. 84, 69–78. ( 10.1111/j.1095-8312.2005.00414.x) [DOI] [Google Scholar]
- 44.Davies NB. 2011. Cuckoo adaptations: trickery and tuning. J. Zool. 284, 1–14. ( 10.1111/j.1469-7998.2011.00810.x) [DOI] [Google Scholar]
- 45.Dawkins R. 2016. The extended phenotype. Oxford, UK: Oxford University Press. [Google Scholar]
- 46.Britton NF, Planqué R, Franks NR. 2007. Evolution of defence portfolios in exploiter–victim systems. Bull. Math. Biol. 69, 957–988. ( 10.1007/s11538-006-9178-5) [DOI] [PubMed] [Google Scholar]
- 47.Antonov A, Stokke BG, Moksnes A, Røskaft E. 2009. Evidence for egg discrimination preceding failed rejection attempts in a small cuckoo host. Biol. Lett. 5, 169–171. ( 10.1098/rsbl.2008.0645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Mársico MC, Gloag R, Ursino CA, Reboreda JC. 2013. A novel method of rejection of brood parasitic eggs reduces parasitism intensity in a cowbird host. Biol. Lett. 9, 20130076 ( 10.1098/rsbl.2013.0076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rasmussen JL, Underwood TJ, Sealy SG. 2010. Functional morphology as a barrier to the evolution of grasp-ejection in hosts of the Brown-headed Cowbird (Molothrus ater). Can. J. Zool. 88, 1210–1217. ( 10.1139/Z10-099) [DOI] [Google Scholar]
- 50.Sato NJ, Mikamf OK, Ueda K. 2010. The egg dilution effect hypothesis: a condition under which parasitic nestling ejection behaviour will evolve. Ornithol. Sci. 9, 115–121. ( 10.2326/osj.9.115) [DOI] [Google Scholar]
- 51.Langmore NE, Maurer G, Adcock GJ, Kilner RM. 2008. Socially acquired host-specific mimicry and the evolution of host races in Horsfield's bronze-cuckoo Chalcites basalis. Evolution 62, 1689–1699. ( 10.1111/j.1558-5646.2008.00405.x) [DOI] [PubMed] [Google Scholar]
- 52.Payne RB. 2005. The cuckoos. Oxford, UK: Oxford University Press. [Google Scholar]
- 53.Thompson JN. 2005. The geographic mosaic of coevolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 54.Noh H-J, Gloag R, Langmore NE. 2018. Data from: True recognition of nestlings by hosts selects for mimetic cuckoo chicks Dryad Digital Repository. ( 10.5061/dryad.vv76g) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Noh H-J, Gloag R, Langmore NE. 2018. Data from: True recognition of nestlings by hosts selects for mimetic cuckoo chicks Dryad Digital Repository. ( 10.5061/dryad.vv76g) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data supporting this article are available from Dryad (http://dx.doi.org/10.5061/dryad.vv76g) [54].