Abstract
In numerous social species, males direct aggression towards female group members during intergroup fights, and this behaviour is commonly thought to function as mate guarding, even though males often target non-receptive females. In studying intergroup fights in a wild population of vervet monkeys, we found that male intragroup aggression was primarily directed towards individuals who had either just finished exhibiting, or were currently attempting to instigate intergroup aggression. Targeted females were less likely to instigate intergroup aggression in the future, indicating that male intragroup aggression functioned as coercion (when directed towards those who were currently trying to instigate a fight) and punishment (when directed towards those who had recently fought). These manipulative tactics effectively prevented intergroup encounters from escalating into fights and often de-escalated ongoing conflicts. Males who were likely sires were those most likely to use punishment/coercion, particularly when they were wounded, and, therefore, less able to protect vulnerable offspring should a risky intergroup fight erupt. This work, along with our previous finding that females use punishment and rewards to recruit males into participating in intergroup fights, highlights the inherent conflict of interest that exists between the sexes, as well as the role that social incentives can play in resolving this conflict. Furthermore, unlike other studies which have found punishment to be used asymmetrically between partners, these works represent a novel example of reciprocal punishment in a non-human animal.
Keywords: herding, mate defence, reciprocal punishment, offspring protection, social incentive
1. Introduction
Social groups are typically a heterogeneous assemblage of individuals, who each obtain different benefits, and experience different costs, from living and interacting with their fellow group members. As a result, conflicts of interest can arise between group members, including between siblings, parents and offspring, dominants and subordinates, individuals with varying degrees of kinship, or males and females [1]. Ultimately, the conflict of interest between the sexes stems from the asymmetry in their reproductive investment, with females typically investing more in each reproductive event. As a result, female fitness is usually thought to be limited by the resources required to produce and raise offspring, whereas male fitness is primarily limited by access to receptive females [2]. Therefore, males and females tend to have different intrinsic interests, experience different selective pressures and evolve different fitness-maximizing strategies [2–5].
Because of their different intrinsic interests, males and females in social species are expected to have different strategies for participating in intergroup fights. Females typically fight to obtain resources such as territory, food, water and shelter [6–8], while males tend to fight to defend mates [9–11], or, when they are able to assess paternity, to protect their offspring [12–14]. Regardless of an individual's motivation for fighting, winning an intergroup fight usually results in the defence of the contested area and the resources therein (e.g. food, water or shelter), at least in the short term. Because all group members have access to these resources, cooperative intergroup aggression often produces a public good [15,16]. For some group members, however, the costs associated with participating in, or even experiencing an intergroup fight, may outweigh the potential benefits associated with winning. For example, the risk of injury or death is a significant cost associated with intergroup fights [17–21] and can prohibit members of the smaller sex from fighting [11,22,23], or make the parents of vulnerable infants more averse to escalating intergroup encounters into fights than their group mates [13,14,24]. Consequently, group members may often disagree on when to fight, versus when to flee. Owing to such conflicts of interests, natural selection could favour the evolution of manipulative tactics that effectively influence the behaviour of group members.
Both theoretical models of n-player cooperation (i.e. cooperation in a group setting) and public goods experiments on humans in the laboratory often conclude that social incentives such as rewards, punishment or reputation effects can influence the behaviour of group members [15,25–30]. Thus, social incentives could theoretically be used to resolve conflicts of interest that arise between group members in the context of intergroup fights. Although there is evidence that non-human animals use social incentives in dyadic cooperation [31–34], we know relatively little about the role that social incentives play in the production of public goods [35]. However, there is recent evidence that social incentives, including the punishment of defectors and rewarding of cooperators, are used by female vervet monkeys (Chlorocebus aethiops pygerythrus) to increase the participation of male group members during intergroup fights when high-quality food resources are at stake [36]. Male vervet monkeys in the same population have also been observed to both aggress, and groom, their fellow group members during intergroup fights, and the goal of this study was to determine the function of these intragroup social interactions.
Male intragroup aggression during intergroup fights (hereafter ‘male aggression’) has always been interpreted as ‘herding’ behaviour, and is thought to serve a mate defence function, by preventing female group members from fraternizing with, or copulating with extra-group males [22,37–40]. Male intragroup grooming during intergroup fights (hereafter ‘male grooming’) could function to decrease anxiety during this stressful situation [41,42]. Alternatively, male aggression and grooming may instead function as social incentives, just as female aggression and grooming do. However, instead of promoting higher levels of participation, we hypothesize that male vervet monkeys use these social interactions to inhibit the aggressive participation of their fellow group members when an escalated intergroup fight would be costly. This may be the case when males are likely to have sired infants, or when they are using the intergroup encounter as an opportunity to assess dispersal opportunities (i.e. ‘prospect’).
Vervet monkeys live in multi-male multi-female groups, and although males are approximately 1.5 times larger than females, dimorphism is moderate enough that both sexes participate aggressively during intergroup fights [14,43,44]. Infants who are less than a year old are those most likely to suffer fatal injuries during intergroup fights [14,18], and both mothers and males who are likely to have sired infants appear to be sensitive to this risk [14,44]. However, females with infants benefit if their group wins access to valuable food resources that are limiting to female fitness; therefore, they often avoid the front line and leave the fighting to other group members to mitigate the risk posed to infants [44]. Conversely, males who are likely to have sired infants tend to sit vigilant at the front line and only participate reactively/defensively if the opposing group's members are highly aggressive [14]. Thus, males who are likely sires may benefit from preventing intergroup encounters from escalating, or from de-escalating ongoing conflicts. Additionally, non-escalated intergroup encounters also give males the chance to mingle and affiliate with members of the opposing group, assessing whether they might be able to successfully disperse [14,45].
The aim of this study was to determine the relative importance of the mate defence and social incentives functions of male intragroup aggression and/or grooming. To achieve this, we examine seasonality in the occurrence of these behaviours, who was targeted and the impact these social interactions had on their subsequent behaviour, and which males exhibited these behaviours (table 1). We first assess whether male intragroup aggression in this species functions as mate defence, as is purported in the literature. If this is the case, male aggression should be exhibited primarily during the mating season when females could be receptive and primarily target female group members who are affiliating or mating with extra-group males; targets should subsequently cease fraternizing with extra-group males (table 1). We then test the alternative hypothesis, which is that male intragroup aggression and/or grooming function as social incentives, and are used to de-escalate intergroup fights. If this is the case, these behaviours should primarily occur in the summer season as this is the time of year that valuable food resources are available such that conflicts of interest between the sexes are most pronounced, as well as when intergroup encounters last the longest and have calm periods which provide the best prospecting opportunities to males (table 1) [14,44]. Male aggression should be directed towards group members who are trying to instigate intergroup aggression (i.e. to coerce them into behaving less aggressively), or individuals who have recently exhibited intergroup aggression (i.e. as a punishment for fighting) [46,47], and targets should be less likely to actively participate in the intergroup fight afterwards. Conversely, male grooming should be directed towards individuals who do not participate aggressively in the intergroup fight, and targets should continue to refrain from participating actively afterwards. If males de-escalate intergroup fights to increase prospecting opportunities, male aggression and/or grooming should be exhibited by males who are attempting to affiliate with members of the opposing group during the intergroup encounter. If males de-escalate intergroup fights to protect offspring, male aggression and/or grooming should be exhibited by males who are likely to have sired offspring, particularly if they themselves are wounded, and, therefore, less able to effectively defend vulnerable infants should the need arise.
Table 1.
Hypotheses and predictions for determining the function of male intragroup social interactions during intergroup fights in vervet monkeys.
| hypothesis | predictions | |
|---|---|---|
| mate defence | when | mating season when females are potentially receptive |
| target identity/behaviour | females who affiliate or mate with extra-group males | |
| target behaviour after | less likely to fraternize | |
| social incentives | when | summer season when conflicts of interest most likely to arise |
| target identity/behaviour | aggression: any group member who instigates/participates in intergroup aggression | |
| grooming: any group member who does not instigate/participate in intergroup aggression | ||
| target behaviour after | does not instigate/participate in intergroup aggression | |
| actor identity | likely sires (especially if wounded) or prospecting males |
We also compare male intragroup aggression and grooming to the observed patterns of punishment and rewards observed in females [36], to better understand how the conflict of interest among the sexes arises in this species, and how it is resolved. Thus, we examine the temporal co-occurrence of intragroup aggression and grooming in males and females.
2. Material and methods
(a) Subjects and study site
Data were collected on four groups of vervet monkeys at the Mawana Game Reserve (28°00′ S, 31°12′ E), South Africa, between January 2012 and February 2014. All animals were individually recognized, and each group contained one to seven adult males, five to 14 adult females, zero to seven subadults and 11–48 juveniles at any given time. There were 22 adult male and 36 adult female study animals in total. The adult members of the frequently encountered neighbouring groups were also recognized, but we did not collect detailed behavioural data on these groups. All data collection protocols were approved by the appropriate local authority, the Ezemvelo KZN Wildlife Board.
(b). Behavioural data
More than 11 000 h of behavioural data were collected, with researchers spending one to two full days with each group, each week. We calculated the proportion of matings that each male obtained in a given mating season, and classified males as likely sires if they had obtained greater than 20% of the matings in their group the previous mating season [14]. We classified males as being wounded if a visible wound had appeared within the last two weeks, as this was the average amount of time it took for wounds to heal (T.J.M. Arseneau-Robar 2012-2014, personal observation). Males were most likely to be wounded during the mating season (71% of n = 52 cases).
Intergroup fights in our study population are characterized by episodes of intergroup aggression with pauses of 3–4 min in between [36]. Because only a few group members were typically active at any given time, it was usually possible to record individual participation during intergroup encounters on an all-occurrence basis, with observers noting the time of all participation events, the participants and their behaviour [48]. However, when a larger proportion of the group was active, we only recorded the participation of adults and subadults. Participation could be non-aggressive, aggressive or affiliative (for further details on the classification of these behaviours, see the electronic supplementary material). Non-aggressive behaviours could be used to solicit support before initiating an act of intergroup aggression (hereafter, an ‘aggressive episode’). For example, individuals attempting to instigate intergroup aggression typically began to approach the opposing group while making intense contact calls (hereafter, an ‘instigating episode’).
Males engaged in intragroup social interactions when the opposing group was nearby but no fights had yet broken out, as well as during escalated fights. For each act of male intragroup aggression and grooming we recorded the identity of the actor, as well as the targets. We then determined if the targets of male intragroup aggression and grooming were currently affiliating with extra-group males, attempting to instigate intergroup aggression, or had participated in the most recent aggressive episode (in the present intergroup fight). In some cases, it was not possible to confirm if the targeted individual was instigating intergroup aggression (i.e. if they were the one who made intense contact calls), or if they were only present at the front line. In such cases, targets were conservatively scored as not attempting to instigate intergroup aggression. We also determined if the target(s) subsequently attempted to instigate intergroup aggression or participated in the next aggressive episode (in the present intergroup fight). A video of male intragroup aggression, which occurred during a playback experiment, has been uploaded to Dryad (http://dx.doi.org/10.5061/dryad.9p5hm) [49].
(c) Statistical analyses
We used generalized linear mixed models (GLMMs) to examine the seasonal variability in the occurrence of male intragroup aggression and grooming, and to test the impact that male characteristics had on their propensity to exhibit intragroup aggression (see the electronic supplementary material, for additional detail). We also used a GLMM to investigate the seasonal co-occurrence of male and female social incentives. The overall significance of each GLMM was assessed by comparing the final model to the null model (model including intercept and random effects only) using a likelihood ratio test.
Binomial tests were used to determine if certain group members were the targets of male aggression more often than would be expected. The expected values for different age/sex classes were the average availability of each class within the study groups. To assess whether male aggression functioned ‘solely’ as mate defence (i.e. was directed towards females who affiliated/mated with extra-group males), we use a slightly conservative expected proportion of 0.95. Lastly, to assess whether male aggression functioned as a social incentive (i.e. targeted individuals who instigated/participated in intergroup aggression), we used the average proportion of group members that were typically active in a given act of intergroup aggression. We note that this is a conservative expected value as intergroup conflicts in this population were characterized by discrete acts of intergroup aggression with pauses in between where no one was behaving aggressively. Thus, at any given time, the proportion of the group that was participating aggressively in the intergroup conflict was often lower than the expected proportion that we use.
We used Wilcoxon signed-rank tests to examine the impact that male intragroup aggression had on the behaviour of target females. This included comparing the proportion of cases in which each female was targeted that she had either been attempting to instigate intergroup aggression or had participated in the most recent aggressive episode, to the proportion of cases in which she participated in either an instigating episode or an aggressive episode following male aggression. It also included comparing the likelihood that targeted females participated aggressively following male aggression to their baseline likelihood of participating in two consecutive aggressive/instigating episodes (see the electronic supplementary material for additional detail). Note that targeted juveniles are not included in these analyses.
A Fisher's exact test was used to compare the likelihood that an intergroup encounter escalated into an intergroup fight if males did versus did not exhibit intragroup aggression when the two groups were nearby, but not yet fighting. The latency to escalation following male aggression was compared to the typical latency it took for intergroup fights to erupt after a female exhibited instigating behaviour using a Wilcoxon rank-sum test. A Wilcoxon signed-rank test was used to determine if intergroup fights were more likely to end after acts of male aggression that occurred during ongoing intergroup fights than would be expected. Here, we control for the latency between the onset of the intergroup fight and each act of male aggression, and determined how long intergroup fights typically continued past this point (see the electronic supplementary material, for additional detail). Because male grooming was observed very rarely, we were unable to conduct a formal statistical test on the behaviour of targets before versus after grooming. Therefore, we present only summary statistics in discussing the function of male grooming. In all analyses, α was set at 0.05, but we also discuss statistical trends (0.05 < p < 0.10). All statistical analyses were conducted in R [v. 3.0.3, 50] and we used the lme4 package [v. 1.1–4, 51] to fit the GLMM models.
3. Results
We observed more than 400 intergroup encounters and approximately half of these (n = 236) escalated into intergroup fights, which could last for up to 8 h (mean ± s.d. = 45 ± 55 min, range = 1–475 min). Male intragroup aggression during intergroup encounters was only observed 41 times during the 2 year study period. Targets could be another adult male (n = 4), one or more adult females (n = 18), a female and a juvenile (n = 12), or a juvenile (n = 7). Importantly, the four acts of male–male intragroup aggression did not occur at the front line or appear to be related to the intergroup encounter. Instead, all were cases that occurred during periods when a subordinate male was challenging the dominant male, and hence appeared to be acts of male competition that merely happened to occur during intergroup fights. Therefore, these acts of male–male aggression are not considered further (i.e. our analyses are based on n = 37 acts of male intragroup aggression). Females were significantly more likely to be targeted than would be expected given their availability (binomial test: n = 30 out of 37 cases, expected proportion = 0.24, p < 0.001, 95% confidence interval (CI) = 0.67–0.92), while juveniles were targeted as frequently as would be expected (binomial test: n = 19 out of 37 cases, expected proportion = 0.60, p = 0.32, 95% CI = 0.35–0.68), given the composition of the study groups. Male intragroup grooming was even rarer than male intragroup aggression, with only 12 cases observed during the study period; in all cases, the target was an adult female.
(a) Male intragroup aggression as mate defence
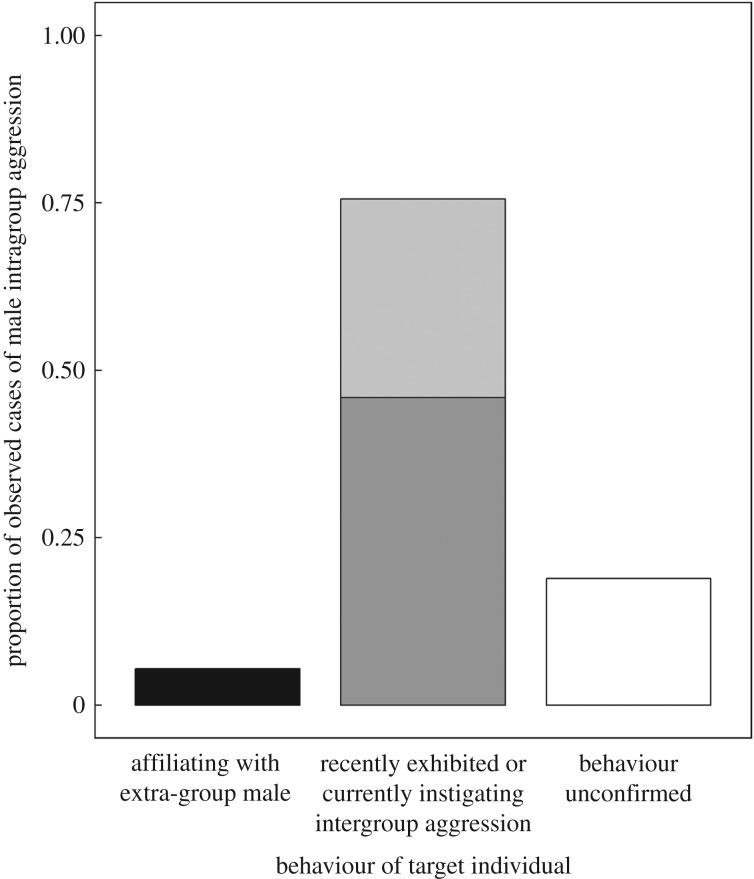
We found that male aggression was no more likely to occur during intergroup encounters that took place in the mating season than at other times of the year (GLMM: b ± s.e. = 0.12 ± 0.59, z = 0.20, p = 0.841; electronic supplementary material, table S1). Although females were often targeted, males did not exclusively direct aggression towards these potential mates (binomial test: n = 30 out of 37 cases, expected proportion = 0.95, p = 0.002, 95% CI = 0.67–0.92). Furthermore, in only two cases was male aggression directed towards female group members who behaved affiliatively with an extra-group male (figure 1). In both cases, the targeted female ceased affiliating and did not fraternize with the extra-group male for the rest of the intergroup encounter. Therefore, 5% (95% CI = 0–14%) of observations were in line with the expectations of the mate defence hypothesis (table 1). It is also notable that we observed females behaving affiliatively towards extra-group males in 7% of intergroup encounters (n = 345). Under the mate defence hypothesis, we might expect males to direct aggression towards these affiliating females in the vast majority of such occasions, yet the observed proportion of 0.08 is significantly lower than even a highly conservative expected probability of male aggression in 50% of the cases of female fraternization (binomial test: n = 2 out of 24, expected proportion = 0.5, p < 0.001, 95% CI = 0–0.21).
Figure 1.
Proportion of observed cases of male aggression during intergroup fights (n = 37) in which the targeted individual(s) were affiliating with (black) versus behaving aggressively towards (grey) members of the opposing group. Light grey signifies cases where the targeted individual had recently exhibited intergroup aggression; dark grey signifies cases where the targeted individual was currently trying to instigate intergroup aggression. The behaviour of the targeted individual was unconfirmed in n = 7 cases (white).
(b) Male intragroup aggression as a social incentive
Male aggression was most likely to occur in the summer season (GLMM: b ± s.e. = 5.19 ± 2.12, z = 2.45, p = 0.014; electronic supplementary material, table S1), which is the time of year that high-quality fruits were abundant and females were most active in intergroup fights. In 76% of cases, male aggression targeted individuals who were behaving aggressively towards the opposing group (figure 1). This propensity to target aggressive participants appears to be highly selective, given that, on average, only 6% of group members typically participated in any given act of intergroup aggression (binomial test: n = 28 out of 37 cases, expected proportion = 0.06, p < 0.001, 95% CI = 0.62–0.89). The adult females who were targeted (n = 16 females each targeted one to seven times; total number of target events = 44) were significantly less likely to attempt to instigate aggression, or participate in the next aggressive episode after they were attacked (Wilcoxon signed-rank test: v = 105, n = 16 females, p < 0.001, r = −0.83; figure 2a). This impact on female behaviour appears biologically meaningful, as targeted females were also less likely to participate in subsequent instigating/aggressive episodes than would be expected, given their individual baseline levels (Wilcoxon signed-rank test: v = 7, n = 15 females, p = 0.003, r = −0.77; figure 2b). Thus, 76% (95% CI = 62–89%) of observations were in line with the expectations of the social incentive hypothesis (table 1).
Figure 2.
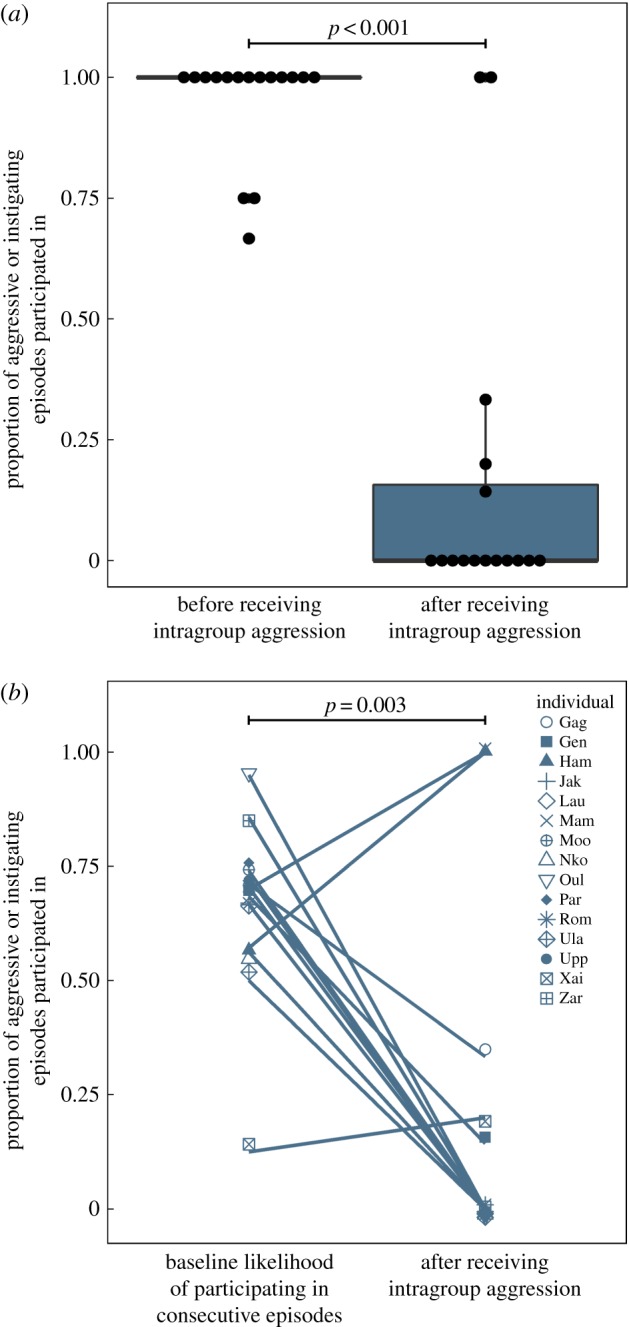
(a) The proportion of cases in which targeted females either participated in an episode of intergroup aggression, or attempted to instigate intergroup aggression, before versus after receiving intragroup aggression from a male group member (note that each dot represents one female in the population (n = 16 females)). (b) The participation of targeted females after receiving intragroup aggression versus their baseline likelihood of participating in two consecutive instigating/aggressive episodes, as determined by their typical participation in the absence of male intragroup aggression (n = 15 females).
When male aggression was exhibited prior to the onset of any intergroup aggression (i.e. the two groups were near each other, but not yet fighting), the intergroup encounter was less likely to escalate than if no male aggression had occurred (n = 314 intergroup encounters, Fisher's exact test: p = 0.005). Furthermore, in the three cases where an intergroup fight eventually erupted, this did not occur for over an hour (range: 67–73 min), which is significantly longer than it typically took intergroup fights to erupt after a female exhibited escalating behaviour (median latency: 7.5 min.; Wilcoxon rank-sum test: w = 72, n = 22, p = 0.002, r = −0.65). When male aggression was used during an escalated intergroup fight, it de-escalated the fight in half of all cases. When we control for the time into the intergroup fight that acts of male aggression occurred, there was a non-significant tendency for intergroup fights to end sooner than would be expected, given how long intergroup fights typically continued past that point in the absence of male aggression (Wilcoxon signed-rank test: v = 139, n = 19, p = 0.077, r = −0.40).
Of the 15 individual males who exhibited intragroup aggression during intergroup fights (six in group A, five in group B, three in group C and one in group D), 12 were adult males and three were subadult males who had not yet emigrated from their natal group. Among adult males, those most likely to exhibit male aggression were males who were likely to have sired offspring, particularly if they were wounded (GLMM: healthy likely sire b ± s.e. = 1.42 ± 0.65, z = 2.18, p = 0.030; wounded likely sire b ± s.e. = 2.55 ± 0.76, z = 3.35, p = 0.001; electronic supplementary material, table S2). In the majority of cases, this male was a likely sire in his current group (n = 24 out of 32 cases in which actor was an adult male), but in an additional three cases, the male may have been a likely sire in the opposing group given his residency in that group the previous mating season. The effect of being wounded appears to be additive to that of being a likely sire, because males who were wounded but were not likely sires were no more likely to exhibit male aggression than males in the reference category (males who were not wounded, likely sires or prospecting) (GLMM: b ± s.e. = 0.83 ± 1.16, z = 0.72, p = 0.473; electronic supplementary material, table S2). In fact, males who were wounded when they exhibited male aggression were always likely sires, or in one case, potentially a sire in the opposing group. Interestingly, all of the observed cases of male aggression by a wounded male occurred during the mating season. Therefore, although we did not detect a significant mating season effect in the occurrence of male aggression (electronic supplementary material, table S1), any apparent mating season effect probably arises because of the increased rates of wounding during this period.
Although males who were prospecting were not significantly more likely to exhibit male aggression than males in the reference category (GLMM: b ± s.e. = 2.06 ± 1.20, z = 1.72, p = 0.085; electronic supplementary material, table S2), some cases of male aggression may have served a conflict de-escalating function for prospecting males, as we found a non-significant trend. In particular, de-escalating intergroup fights to protect prospecting opportunities may be an important strategy for subadult males in this population. Although we observed subadult males attempting to prospect in 6% of intergroup encounters (n = 345), prospecting was only successful (i.e. being tolerated by the neighbouring group for more than 5 min) in 3% (n = 12 intergroup encounters). Subadult males appear to make the most of these rare opportunities as, in all cases in which the actor was a subadult male (n = 5), they exhibited male aggression when the intergroup encounter presented a particularly good prospecting opportunity (i.e. they were tolerated by the neighbouring group for >30 min).
(c). Male intragroup grooming
We detected no seasonal variability in the occurrence of male grooming during intergroup fights (electronic supplementary material, table S1). Only three males were observed to exhibit this behaviour and the majority of cases were from a single individual (n = 9 out of 12 cases). There was no clear pattern in the identity of the individuals exhibiting male grooming (n = 7 cases by likely sires, n = 5 by unlikely sires), or the behaviour of the individuals targeted either before (n = 6 participated in/instigated intergroup aggression) or after being groomed (n = 3 participated in/instigated intergroup aggression).
(d) Comparing male and female social incentives
Just as female social incentives were more likely to be used in the summer season when fruits were most abundant [36], males were also more likely to exhibit male aggression in the summer season (electronic supplementary material, table S1). As a result, there was a significant positive relationship between the monthly occurrence of male and female social incentives (GLMM: b ± s.e. = 1.680 ± 0.631, z = 2.662, p = 0.008). Of the 26 separate intergroup encounters in which male aggression was observed, females also employed social incentives in eight (∼30%).
4. Discussion
The goal of this study was to investigate the function of male intragroup aggression and grooming during intergroup encounters in vervet monkeys. Our findings suggest that male aggression can function as both mate defence and as a social incentive in this species, although the latter is far more common. Male aggression appears to coerce individuals who were currently trying to escalate intergroup encounters into decreasing their level of activity, as they were less likely to attempt to instigate intergroup aggression afterwards or participate aggressively if other group members escalated an intergroup fight. When directed towards individuals who had recently exhibited intergroup aggression, male aggression functioned as punishment, effectively decreasing the likelihood that targeted individuals instigated or participated in intergroup aggression in the future [46,47]. This observed decrease is biologically meaningful, as females were less likely to participate after being coerced/punished than would be expected, given individual baseline levels. In many cases, these manipulative tactics de-escalated the entire intergroup interaction.
Males who were likely sires were those most likely to use punishment and coercion, particularly when they were wounded, suggesting that likely sires try to de-escalate the situation when an escalated fight would pose a risk to offspring. The wounds that males suffered were often severe and, as a result, wounded males may have been unable to defend an infant should the need arise. Subadult males may also use manipulative tactics when an escalated intergroup fight would disrupt a valuable prospecting opportunity. In addition to providing males the chance to assess the composition (i.e. number of potential mates and number of potential rival males) of the neighbouring group, calm periods during intergroup encounters also allowed males to mingle with the members of the opposing group. The extent to which females tolerate prospectors, or even affiliate with them, and the amount of aggression prospectors receive from males in the opposing group, probably allow prospecting males to gauge how easily they might integrate into the group if they did disperse [45]. Furthermore, prospecting during intergroup encounters may be less risky than prospecting alone because subadult males often receive support from their group members if attacked.
These findings provide novel evidence that mate defence is not always the sole, or even predominant function of male intragroup aggression during intergroup fights, as had previously been assumed [22,37,52,53]. The punishment/coercion function that we propose may also be an important motivator of male intragroup aggression in other species where intergroup fights are perceived as costly to males. This may be the case when there is a risk of attacks on infants during intergroup fights [18,54–59], or when males use intergroup encounters to assess dispersal opportunities [10,40,43,45,60–63]. To determine if male aggression functions as mate defence or to manipulate the aggressive outgroup behaviours of the targeted group member, it is critical that future studies document the behaviour of the targeted individual(s), both before and after receiving male aggression.
We did not find any strong evidence that male intragroup grooming functioned to manipulate the behaviour of group members. When aggressive conflicts arise within social groups, the participants can engage in post-conflict affiliation, either with the individual they had the conflict with, or with other group members. Such post-conflict affiliation may decrease stress levels by decreasing the heart rates of the affiliating individuals, reconcile relationships, or console and calm the targets of aggression [41,64–66]. Grooming could similarly be used to relieve stress in the intergroup conflict context. In contrast to our current finding, our previous work on the same study population indicated that females use grooming to reward males who have recently participated in an intergroup fight [36]. If social incentives evolved by hijacking a pre-existing stress response, the lack of a male reward system may arise because the necessary associations are difficult to learn [47]. In the female reward system, females groom males who have recently participated in the intergroup fight; as such, grooming takes place shortly following active participation in intergroup aggression. Conversely, if a male reward system did evolve, we would expect males to groom (i.e. reward) females who were not actively involved in the intergroup fight. Thus, females would have to learn to associate male grooming with their inactivity. This association is probably more difficult to make.
Because male and female vervet monkeys experience very different costs and benefits from participating in intergroup fights [14,44,67], they probably disagree on when to fight versus when to avoid engaging in intergroup aggression. This conflict of interest is most pronounced during the summer season, when females have much to gain from winning access to high-quality food resources, and are, therefore, more likely to instigate intergroup fights that males perceive as costly [36,44]. Conflicts of interest have severe consequences for group-level cooperation in this context; females use punishment and rewards to promote more effective group-level cooperation [36], while males use punishment and coercion to inhibit group members from fighting, which stifles group-level cooperation. The reciprocal punishment exhibited in vervet monkeys differs from the punishment that has been observed in a dyadic setting (i.e. cooperation between two partners), where only the larger partner punishes the smaller partner (i.e. punishment is asymmetric) [31–34]. Because the risk of retaliation by the target represents an additional cost to punishing, punishment is thought to be more likely to evolve when there are power asymmetries between individuals [46,47]. Given their larger size, it is not that surprising that male vervet monkeys are able to punish females. However, females face a significant risk of injury if a male retaliates when punished, and females often use coalitions to mitigate this risk and tip the balance of power in their favour [36]. Because coalitions cannot be used to undermine the power held by larger, stronger or higher-ranking individuals when cooperation takes place in a dyadic setting, it is not possible to create an asymmetry in numbers. As a result, reciprocal punishment may be less likely to evolve in a dyadic setting than in a group setting where cooperation takes place among multiple players.
Social life is rife with conflicts of interest, and these conflicts probably have consequences for group-level cooperation in a number of cooperative contexts, including intergroup conflict. In humans, a number of strategies are used to manipulate the participation of group members in primitive warfare, including punishment, coercion, ostracism, rewards and prestige [68–71]. However, we currently understand little of the strategies that other group-living animals use to resolve the conflicts of interest that arise during n-player cooperative activities. In vervet monkeys, the observed intra- and inter-individual variability in participation indicates that males and females experience very different costs and benefits from participating in intergroup fights [14,44,67]. These differences probably create selective pressure for the evolution of manipulative tactics. In this study, as well as in our previous work, we demonstrate that both male and female vervet monkeys use social incentives to resolve these conflicts of interest [36]. We have also tried to examine the real-world conditions that have promoted the evolution of these manipulative tactics by examining the social and ecological conditions in which both male and female social incentives are used [36]. We thereby hope to provide important and novel insight into the role that social incentives can play in the evolution and maintenance of group-level cooperation in non-human animals.
Supplementary Material
Acknowledgements
We thank two referees for their constructive feedback on the manuscript. We are grateful to K. and E. van der Walt for permission to conduct the study at the Mawana Game Reserve and A. Driescher for support in the field. We thank E. van de Waal, C. Borgeaud, S. Mercier, and all the other students and research assistants who helped with data collection.
Ethics
All data collection protocols were approved by the Ezemvelo KZN Wildlife Board in South Africa.
Data accessibility
The datasets supporting this article have been deposited in Dryad, as has a video of male intragroup aggression (which took place during a playback experiment) (http://dx.doi.org/10.5061/dryad.9p5hm) [49].
Authors' contributions
T.J.M.A.-R., C.v.S. and E.P.W. conceived the study. T.J.M.A.-R., E.M. and A.L.T. collected the data. T.J.M.A.-R., E.M. and A.L.T. analysed the data. All the authors wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
The study was supported by Swiss National Science Foundation (Sinergia grant CRS133_133040), Claraz-Stiftung and University of Zurich Forschungskredit.
References
- 1.Huntingford FA, Turner AK. 1987. Animal conflict, p. 448 New York, NY: Chapman and Hall. [Google Scholar]
- 2.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 136–179. Chicago, IL: Aldine Publishing Company. [Google Scholar]
- 3.Smuts BB, Smuts RW. 1993. Male aggression and sexual coercion of females in nonhuman primates and other mammals: evidence and theoretical implications. Adv. Study Behav. 22, 1–63. ( 10.1016/S0065-3454(08)60404-0) [DOI] [Google Scholar]
- 4.Muller MN, Wrangham R. 2009. Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females, p. 496 Cambridge, MA: Harvard University Press. [Google Scholar]
- 5.Fashing PJ. 2001. Male and female strategies during intergroup encounters in guerezas (Colobus guereza): evidence for resource defense mediated through males and a comparison with other primates. Behav. Ecol. Sociobiol. 50, 219–230. ( 10.1007/s002650100358) [DOI] [Google Scholar]
- 6.Boydston EE, Morelli TL, Holekamp KE. 2001. Sex differences in territorial behavior exhibited by the spotted hyena (Hyaenidae, Crocuta crocuta). Ethology 107, 369–385. ( 10.1046/j.1439-0310.2001.00672.x) [DOI] [Google Scholar]
- 7.Nunn CL, Deaner RO. 2004. Patterns of participation and free riding in territorial conflicts among ringtailed lemurs (Lemur catta). Behav. Ecol. Sociobiol. 57, 50–61. ( 10.1007/s00265-004-0830-5) [DOI] [Google Scholar]
- 8.Zhao Q, Tan CL. 2010. Inter-unit contests within a provisioned troop of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in the Qinling Mountains, China. Am. J. Primatol. 73, 262–269. ( 10.1002/ajp.20892) [DOI] [PubMed] [Google Scholar]
- 9.Kitchen DM, Cheney DL, Seyfarth RM. 2004. Factors mediating inter-group encounters in savannah baboons (Papio cynocephalus ursinus). Behaviour 141, 197–218. ( 10.1163/156853904322890816) [DOI] [Google Scholar]
- 10.Majolo B, Ventura R, Koyama NF. 2005. Sex, rank and age differences in the Japanese macaque (Macaca fuscata yakui) participation in inter-group encounters. Ethology 111, 455–468. ( 10.1111/j.1439-0310.2005.01087.x) [DOI] [Google Scholar]
- 11.Koch F, Signer J, Kappeler PM, Fichtel C. 2016. Intergroup encounters in Verreaux's sifakas (Propithecus verreauxi): who fights and why? Behav. Ecol. Sociobiol. 70, 797–808. ( 10.1007/s00265-016-2105-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grinnell J, Packer C, Pusey AE. 1995. Cooperation in male lions: kinship, reciprocity or mutualism? Anim. Behav. 49, 95–105. ( 10.1016/0003-3472(95)80157-X) [DOI] [Google Scholar]
- 13.Wich SA, Assink PR, Sterck EHM. 2004. Thomas langurs (Presbytis thomasi) discriminate between calls of young solitary versus older group-living males: a factor in avoiding infanticide? Behaviour 141, 41–51. ( 10.2307/4536111) [DOI] [Google Scholar]
- 14.Arseneau TJM, Taucher A, Van Schaik CP, Willems EP. 2015. Male monkeys fight in between-group conflicts as protective parents and reluctant recruits. Anim. Behav. 110, 39–50. ( 10.1016/j.anbehav.2015.09.006) [DOI] [Google Scholar]
- 15.Olson M. 1965. The logic of collective action: public goods and the theory of groups. Cambridge, MA: Harvard University Press. [Google Scholar]
- 16.Willems EP, Arseneau TJM, Schleuning X, van Schaik CP. 2015. Communal range defence in primates as a public goods dilemma. Phil. Trans. R. Soc. B 370 ( 10.1098/rstb.2015.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cant MA, Otali E, Mwanguhya F. 2002. Fighting and mating between groups in a cooperatively breeding mammal, the banded mongoose. Ethology 108, 541–555. ( 10.1046/j.1439-0310.2002.00795.x) [DOI] [Google Scholar]
- 18.Cheney DL, Seyfarth RM. 1987. The influence of intergroup competition on the survival and reproduction of female vervet monkeys. Behav. Ecol. Sociobiol. 21, 375–386. ( 10.1007/BF00299932) [DOI] [Google Scholar]
- 19.Hölldobler B, Lumsden CJ. 1980. Territorial strategies in ants. Science 210, 732–739. ( 10.1126/science.210.4471.732) [DOI] [PubMed] [Google Scholar]
- 20.Mech LD. 1994. Buffer zones of territories of gray wolves as regions of intraspecific strife. J. Mammal. 75, 199–202. ( 10.2307/1382251) [DOI] [Google Scholar]
- 21.Mosser A, Packer C. 2009. Group territoriality and the benefits of sociality in the African lion, Panthera leo. Anim. Behav. 78, 359–370. ( 10.1016/j.anbehav.2009.04.024) [DOI] [Google Scholar]
- 22.Cheney D.L. 1987. Interactions and relationships between groups. In Primate societies (eds Smuts BB, Cheney DL, Seyfarth RM, Wrangham R, Struhsaker TT), pp. 267–281. Chicago, IL: University of Chicago Press. [Google Scholar]
- 23.Willems EP, Hellriegel B, van Schaik CP. 2013. The collective action problem in primate territory economics. Proc. R. Soc. B 280, 20130081 ( 10.1098/rspb.2013.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitchen DM. 2004. Alpha male black howler monkey responses to loud calls: effect of numeric odds, male companion behaviour and reproductive investment. Anim. Behav. 67, 125–139. ( 10.1016/j.anbehav.2003.03.007) [DOI] [Google Scholar]
- 25.Hardin G. 1968. The tragedy of the commons. Science 162, 1243–1248. ( 10.1126/science.162.3859.1243) [DOI] [PubMed] [Google Scholar]
- 26.Nunn CL, Lewis RJ. 2001. Cooperation and collective action. In Economics in nature (eds Noe R, van Hooff JARAM, Hammerstein P), pp. 42–66. Cambridge, UK, Cambridge University Press. [Google Scholar]
- 27.Fehr E, Gächter S. 2000. Cooperation and punishment in public goods experiments. Am. Econ. Rev. 90, 980–994. ( 10.1257/aer.90.4.980) [DOI] [Google Scholar]
- 28.Sefton M, Shupp R, Walker JM. 2007. The effect of rewards and sanctions in provision of public goods. Econ. Inq. 45, 671–690. ( 10.1111/j.1465-7295.2007.00051.x) [DOI] [Google Scholar]
- 29.Balliet D, Mulder LB, Van Lange PAM. 2011. Reward, punishment, and cooperation: a meta-analysis. Psychol. Bull. 137, 594–615. ( 10.1037/a0023489) [DOI] [PubMed] [Google Scholar]
- 30.Milinski M, Semmann D, Krambeck H-J. 2002. Reputation helps solve the ‘tragedy of the commons’. Nature 415, 424–426. ( 10.1038/415424a) [DOI] [PubMed] [Google Scholar]
- 31.Bshary R, Grutter AS. 2005. Punishment and partner switching cause cooperative behaviour in a cleaning mutualism. Biol. Lett. 1, 396–399. ( 10.1098/rsbl.2005.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raihani NJ, Grutter AS, Bshary R. 2010. Punishers benefit from third-party punishment in fish. Science 327, 171 ( 10.1126/science.1183068) [DOI] [PubMed] [Google Scholar]
- 33.Raihani NJ, Grutter AS, Bshary R. 2012. Female cleaner fish cooperate more with unfamiliar males. Proc. R. Soc. B 279, 2479–2486. ( 10.1098/rspb.2012.0063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raihani NJ, Pinto AI, Grutter AS, Wismer S, Bshary R. 2012. Male cleaner wrasses adjust punishment of female partners according to the stakes. Proc. R. Soc. B 279, 365–370. ( 10.1098/rspb.2011.0690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bshary A, Bshary R. 2010. Self-serving punishment of a common enemy creates a public good in reef fishes. Curr. Biol. 20, 2032–2035. ( 10.1016/j.cub.2010.10.027) [DOI] [PubMed] [Google Scholar]
- 36.Arseneau-Robar TJM, Taucher AL, Müller E, van Schaik C, Bshary R, Willems EP. 2016. Female monkeys use both the carrot and the stick to promote male participation in intergroup fights. Proc. R. Soc. B 283, 20161817 ( 10.1098/rspb.2016.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kummer H. 1968. Social organization of hamadryas baboons, p. 189 Chicago, IL: University of Chicago Press. [Google Scholar]
- 38.Feist JD, McCullough DR. 1976. Behavior patterns and communication in feral horses. Zeitschrift für Tierpsychologie 41, 337–371. ( 10.1111/j.1439-0310.1976.tb00947.x) [DOI] [PubMed] [Google Scholar]
- 39.Sicotte P. 1993. Inter-group encounters and female transfer in mountain gorillas: Influence of group composition on male behavior. Am. J. Primatol. 30, 21–36. ( 10.1002/ajp.1350300103) [DOI] [PubMed] [Google Scholar]
- 40.Cheney DL, Seyfarth RM. 1977. Behaviour of adult and immature male baboons during inter-group encounters. Nature 269, 404–406. ( 10.1038/269404a0) [DOI] [Google Scholar]
- 41.Aureli F, Preston SD, de Waal FBM. 1999. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J. Comp. Psychol. 113, 59–65. ( 10.1037/0735-7036.113.1.59) [DOI] [PubMed] [Google Scholar]
- 42.Radford AN, Majolo B, Aureli F. 2016. Within-group behavioural consequences of between-group conflict: a prospective review. Proc. R. Soc. B 283, 20161567 ( 10.1098/rspb.2016.1567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheney DL. 1981. Intergroup encounters among free-ranging vervet monkeys. Folia Primatol. 35, 124–146. ( 10.1159/000155970) [DOI] [PubMed] [Google Scholar]
- 44.Arseneau-Robar TJM, Taucher AL, Schnider AB, van Schaik CP, Willems EP. 2017. Intra- and interindividual differences in the costs and benefits of intergroup aggression in female vervet monkeys. Anim. Behav. 123, 129–137. ( 10.1016/j.anbehav.2016.10.034) [DOI] [Google Scholar]
- 45.Teichroeb JA, Wikberg EC, Sicotte P. 2011. Dispersal in male ursine colobus monkeys (Colobus vellerosus): influence of age, rank and contact with other groups on dispersal decisions. Behaviour 148, 765–793. ( 10.1163/000579511X577157) [DOI] [Google Scholar]
- 46.Clutton-Brock TH, Parker GA. 1995. Punishment in animal societies. Nature 373, 209–216. ( 10.1038/373209a0) [DOI] [PubMed] [Google Scholar]
- 47.Raihani NJ, Thornton A, Bshary R. 2012. Punishment and cooperation in nature. Trends Ecol. Evol. 27, 288–295. ( 10.1016/j.tree.2011.12.004) [DOI] [PubMed] [Google Scholar]
- 48.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–267. ( 10.2307/4533591) [DOI] [PubMed] [Google Scholar]
- 49.Arseneau-Robar TJM, Müller E, Taucher AL, van Schaik CP, Bshary R, Willems EP. 2018. Data from: Male monkeys use punishment and coercion to de-escalate costly intergroup fights Dryad Digital Repository. ( 10.5061/dryad.9p5hm) [DOI] [PMC free article] [PubMed]
- 50.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 51.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 52.Klingel H. 1969. The social organisation and population ecology of the plains zebra (Equus quagga). Zoologica Africana 4, 249–263. ( 10.1080/00445096.1969.11447374) [DOI] [Google Scholar]
- 53.Monard A-M, Duncan P, Boy V. 1996. The proximate mechanisms of natal dispersal in female horses. Behaviour 133, 1095–1124. ( 10.1163/156853996X00611) [DOI] [Google Scholar]
- 54.Steenbeek R. 1999. Tenure related changes in wild Thomas's langurs I: between-group interactions. Behaviour 136, 595–625. ( 10.1163/156853999501487) [DOI] [Google Scholar]
- 55.Watts DP. 1989. Infanticide in mountain gorillas: new cases and a reconsideration of the evidence. Ethology 81, 1–18. ( 10.1111/j.1439-0310.1989.tb00754.x) [DOI] [Google Scholar]
- 56.Cords M, Fuller JL. 2010. Infanticide in Cercopithecus mitis stuhlmanni in the Kakamega Forest, Kenya: variation in the occurrence of an adaptive behavior. Int. J. Primatol. 31, 409–431. ( 10.1007/s10764-010-9400-z) [DOI] [Google Scholar]
- 57.Watts DP, Muller M, Amsler SJ, Mbabazi G, Mitani JC. 2006. Lethal intergroup aggression by chimpanzees in Kibale National Park, Uganda. Am. J. Primatol. 68, 161–180. ( 10.1002/ajp.20214) [DOI] [PubMed] [Google Scholar]
- 58.Shopland JM. 1982. An intergroup encounter with fatal consequences in yellow baboons (Papio cynocephalus). Am. J. Primatol. 3, 263–266. ( 10.1002/ajp.1350030123) [DOI] [PubMed] [Google Scholar]
- 59.Harris TR, Monfort SL. 2003. Behavioral and endocrine dynamics associated with infanticide in a black and white colobus monkey (Colobus guereza). Am. J. Primatol. 61, 135–142. ( 10.1002/ajp.10116) [DOI] [PubMed] [Google Scholar]
- 60.Doolan SP, Macdonald DW. 1996. Dispersal and extra-territorial prospecting by slender-tailed meerkats (Suricata suricata) in the south-western Kalahari. J. Zool. 240, 59–73. ( 10.1111/j.1469-7998.1996.tb05486.x) [DOI] [Google Scholar]
- 61.Lazaro-Perea C. 2001. Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defence and assessment of neighbours. Anim. Behav. 62, 11–21. ( 10.1016/anbe.2000.1726) [DOI] [Google Scholar]
- 62.van Noordwijk M, van Schaik C. 2001. Career moves: transfer and rank challenge decisions by male long-tailed macaques. Behaviour 138, 359–395. ( 10.1163/15685390152032505) [DOI] [Google Scholar]
- 63.Marty PR, Hodges K, Agil M, Engelhardt A. 2016. Determinants of immigration strategies in male crested macaques (Macaca nigra). Sci. Rep. 6, 32028 ( 10.1038/srep32028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Waal FBM, van Roosmalen A. 1979. Reconciliation and consolation among chimpanzees. Behav. Ecol. Sociobiol. 5, 55–66. ( 10.1007/BF00302695) [DOI] [Google Scholar]
- 65.Aureli F, Schaik CPv. 1991. Post-conflict behaviour in long-tailed macaques (Macaca fascicularis). Ethology 89, 89–100. ( 10.1111/j.1439-0310.1991.tb00296.x) [DOI] [Google Scholar]
- 66.Cheney DL, Seyfarth RM. 1989. Redirected aggression and reconciliation among vervet monkeys, Cercopithecus aethiops. Behaviour 110, 258–275. ( 10.1163/156853989X00501) [DOI] [Google Scholar]
- 67.Arseneau-Robar TJM, Müller E, Taucher AL, van Schaik CP, Willems EP. 2016. Male food defence as a by-product of intersexual cooperation in a non-human primate. Sci. Rep. 6, 35800 ( 10.1038/srep35800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glowacki L, Wrangham RW. 2013. The role of rewards in motivating participation in simple warfare. Hum. Nat. 24, 444–460. ( 10.1007/s12110-013-9178-8) [DOI] [PubMed] [Google Scholar]
- 69.Mathew S, Boyd R. 2011. Punishment sustains large-scale cooperation in prestate warfare. Proc. Natl Acad. Sci. USA 108,11 375–11 380. ( 10.1073/pnas.1105604108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyd R, Gintis H, Bowles S. 2010. Coordinated punishment of defectors sustains cooperation and can proliferate when rare. Science 328, 617–620. ( 10.1126/science.1183665) [DOI] [PubMed] [Google Scholar]
- 71.Wrangham R, Glowacki L. 2012. Intergroup aggression in chimpanzees and war in nomadic hunter-gatherers. Hum. Nat. 23, 5–29. ( 10.1007/s12110-012-9132-1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Arseneau-Robar TJM, Müller E, Taucher AL, van Schaik CP, Bshary R, Willems EP. 2018. Data from: Male monkeys use punishment and coercion to de-escalate costly intergroup fights Dryad Digital Repository. ( 10.5061/dryad.9p5hm) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been deposited in Dryad, as has a video of male intragroup aggression (which took place during a playback experiment) (http://dx.doi.org/10.5061/dryad.9p5hm) [49].