Abstract
In social mole-rats, breeding females are larger and more elongated than non-breeding female helpers. This status-related morphological divergence is thought to arise from modifications of skeletal growth following the death or removal of the previous breeder and the transition of their successors from a non-breeding to a breeding role. However, it is not clear what changes in growth are involved, whether they are stimulated by the relaxation of reproductive suppression or by changes in breeding status, or whether they are associated with fecundity increases. Here, we show that, in captive Damaraland mole-rats (Fukomys damarensis), where breeding was experimentally controlled in age-matched siblings, individuals changed in size and shape through a lengthening of the lumbar vertebrae when they began breeding. This skeletal remodelling results from changes in breeding status because (i) females removed from a group setting and placed solitarily showed no increases in growth and (ii) females dispersing from natural groups that have not yet bred do not differ in size and shape from helpers in established groups. Growth patterns consequently resemble other social vertebrates where contrasts in size and shape follow the acquisition of the breeding role. Our results also suggest that the increases in female body size provide fecundity benefits. Similar forms of socially responsive growth might be more prevalent in vertebrates than is currently recognized, but the extent to which this is the case, and the implications for the structuring of mammalian dominance hierarchies, are as yet poorly understood.
Keywords: Bathyergidae, growth plasticity, morphological skew, strategic growth, reproductive suppression
1. Introduction
In several social vertebrates where a single dominant female monopolizes reproduction in each group, subordinates that acquire a dominant breeding position display an increase in their growth rate (fish [1–3], meerkats Suricata suricatta [4], naked mole-rats Heterocephalus glaber [5]). For example, in the social mole-rats (including the naked mole-rat and the Damaraland mole-rat Fukomys damarensis), dominant breeding females are both larger and more elongated than non-breeding subordinates [6,7], and longitudinal studies of individuals show that subordinate females removed from established breeding groups and paired with novel partners increase in size and weight [5]. As body size and weight typically confer competitive advantages, increases in growth in newly dominant individuals may help to consolidate their position and to increase their fecundity, with parallels being drawn between the elongated phenotype of female breeders in mole-rats and the physogastry (enlargement of the abdomen through increasing numbers of ovarioles) observed in queens of some eusocial insect societies [6–8].
While the presence of status-related changes in growth in social mole-rats is well established, their immediate causes are still uncertain, with studies implicating different growth patterns in naked mole-rats and Damaraland mole-rats. In naked mole-rats, longitudinal X-ray sampling of captive newly created breeders revealed upregulated growth of the lumbar vertebrae [5,6] and episodic bursts of vertebral growth in successive periods of pregnancy [9]. This causes breeding females to exhibit a longer body length relative to their skull width. The same status-related morphological difference is seen in Damaraland mole-rats, but repeated measurements of wild females suggested that breeder elongation is not driven by upregulated vertebral growth [7], as it is in naked mole-rats. Instead, it appeared to originate from a decrease in the relative growth of the skull (zygomatic arch width) compared with growth towards total body length [7]. This result prompted the idea that female Damaraland mole-rats reallocate resources from growth towards reproduction as they become dominant; during this reallocation, skull growth is reduced but growth towards body length is maintained because of the inherent fitness benefits of increased body length [7]. The focus on growth reductions in skull size is particularly pertinent in this argument as the skull of mole-rats is home to their prominent buccal incisors, which are so important for soil excavation (involved in foraging and burrow maintenance), and as a result, reproductive investment in the form of elongation can be argued to trade off directly against investment into work.
As sociality arose independently in naked and Damaraland mole-rats [10], it is plausible that the attainment of elongation (which is assumed to be directly related to their sociality) occurs through alternate developmental routes. However, as the prior analysis of Damaraland mole-rats was based on human measurements of morphological traits taken with callipers and a tape measure, a formal characterization of morphological divergence at a skeletal level is currently missing. Moreover, although Young & Bennett [7] go to great lengths to remove the possibility that status-related age differences underpin skeletal divergence—because changes in morphology only occurred in females after transitioning to dominance, and because the opportunity to acquire dominance is somewhat stochastic—a contribution of age to the shape and size of breeders has not been definitively ruled out as individuals in the wild were of unknown age.
It is also unclear whether the changes in growth in female Damaraland mole-rats that acquire the breeding position in their group are stimulated by the relaxation of reproductive suppression by the previous dominant female or by the onset of reproduction itself. The degree of reproductive suppression in the social mole-rats is extreme, manifesting itself in a complete blocking of ovulation in non-breeding Damaraland mole-rats [11]. Even so, the introduction of an unrelated male results in a recrudescence of ovarian activity in non-breeders [12,13] and stimulates high levels of aggression between females that sometimes lead to the usurpation of the incumbent breeder [14] (see [15,16] for comparable results in naked mole-rats). Non-breeding females also start ovulating in the absence of breeding females [11,17], and in the wild, the reproductive readiness of non-breeders—measured by the downstream production of luteinizing hormone following injection of pituitary gonadotrophin-releasing hormone—is elevated during periods of high rainfall when the likelihood of meeting dispersing males or of dispersing oneself is higher [18]. These physiological changes in non-breeders in anticipation of reproductive opportunities share similarities with the onset of puberty in other mammals, where sex steroid secretion is a major driver of skeletal longitudinal and radial growth [19,20], and as such, the removal from reproductive suppression and resultant hormonal changes may stimulate the morphological divergence of non-breeding Damaraland mole-rats towards a more elongated phenotype. On the other hand, studies of classical rodent laboratory models highlight that many of the most pronounced changes in skeletal remodelling occur during pregnancy [21,22], and in naked mole-rats, housing females in isolation did not cause them to lengthen their vertebrae, whereas pairing them with a receptive male (with resultant pregnancy) did [6]. Based on these studies, reproduction itself may provide the necessary cue for the skeletal remodelling of Damaraland mole-rat females.
In this study, we used information from X-rays to characterize the morphological divergence of breeders and non-breeders in Damaraland mole-rats according to three principal aims: (i) to identify the skeletal changes that lead to increases in female size, (ii) to identify the precise circumstances that stimulate growth adjustment in females and (iii) to investigate whether growth adjustments are associated with increases in fecundity. To identify the skeletal differences between breeders and non-breeders, we first carried out cross-sectional comparisons of morphology in captive and wild Damaraland mole-rats. In addition, we experimentally manipulated the life-history trajectories of female siblings in captivity to track skeletal development longitudinally within age-matched individuals. By keeping some females as non-breeders within their natal group, isolating others by placing them in their own tunnel system, and pairing others with an unrelated male to initiate reproduction, we were able to determine whether removal from reproductive suppression is involved in changes in morphology, while controlling for the influence of age on development. If females placed in isolation display growth patterns analogous to newly reproductive females, this would provide strong evidence that the elongation of subordinate females in breeding groups is hindered by reproductive suppression. A similar argument extends to wild females that have dispersed and settled solitarily but have yet to reproduce, so we also compared the morphology of solitary females with breeders and in-group non-breeders from a wild population of mole-rats. Lastly, we investigated the fecundity implications of increasing body size in breeders using correlative data from litters born in captivity.
2. Methods
(a). Study species and X-ray methodology
Our study used information from X-rays taken on both captive and wild Damaraland mole-rats. The wild study population of mole-rats is located around the Kuruman River Reserve in the Northern Cape of South Africa (S 26.98706° E 21.81229°), with individuals from this population founding a captive study system based at the same location in February 2013. The details of the captive and wild populations are presented in full in the electronic supplementary material (see also [23]). The X-ray data from captive animals were collected between November 2015 and July 2017, and from wild animals between February 2015 and June 2017.
X-rays were taken using the Gierth TR 90/20 battery-operated generator unit with portable Leonardo DR Mini plate (OR Technology, Rostock, Germany) under protocols approved by the University of Pretoria ethics committee. For each X-ray, mole-rats were immobilized under isoflurane anaesthesia and gently positioned in a dorsoventral position with straightened spine and splayed limbs. Nine skeletal traits were measured from each X-ray (figure 1) using ImageJ software. As in studies that have examined the morphology of naked mole-rats, the length of a lumbar vertebra (L5 in our case) served as an index of lumbar length [5,9,24]. Similarly, the log ratio of the L5 vertebra length to zygomatic arch width served as a metric of elongation (‘elongation factor’ below). Hereafter, we refer to zygomatic arch width as skull width. All animals were weighed after anaesthesia. All statistical tests were performed in R v. 3.2.3 [25], and standard model assumptions (normal errors and homogeneity of residual variance) were checked throughout.
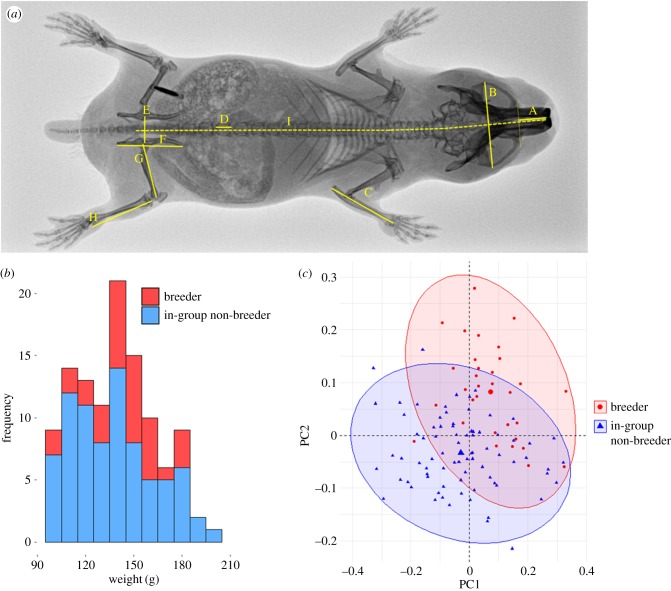
Figure 1.
(a) Dorsoventral X-ray annotated with the nine morphological traits used in multivariate analysis. A, rostrum length; B, skull width; C, ulna; D, L5 vertebra length; E, pelvic girdle width; F, pelvis length; G, femur length; H, tibia length; I, skeletal body length. (b) Weight distribution of captive breeding and non-breeding in-group females in multivariate analysis. (c) PC1 and PC2 separate captive females according to their social status. (Online version in colour.)
(b). Morphological divergence
To quantify the morphological divergence of breeders and non-breeders, we analysed a cross-sectional dataset of X-rays taken in captivity. The cross-sectional analysis from captive females was restricted to individuals larger than 100 g, as the smallest reproductive female in the captive population was 100 g. This resulted in data from 32 breeders and 79 in-group non-breeders spanning 40 different groups, where group refers either to the group of original capture or group of birth. All nine skeletal traits were included in a standard principal component analysis (PCA), and a multivariate analysis of variance (MANOVA) was performed on the resulting principal components to test for broad morphological differences between the two classes of female. Follow-up univariate ANOVAs on each of the first five principal components were used to determine on which of the principal components reproductive status was exerting its influence.
To examine whether the bivariate scaling relationships of morphological traits differed with breeding status, we fitted a series of linear models of the form loge(trait1) ∼ loge(trait2) * status, where trait2 represents either the skull width or skeletal body length, two metrics of size. In these models, a significant interaction term denotes significantly different slope between breeders and in-group non-breeders. When the slopes did not differ, we tested for a difference in intercept by removing the interaction term from each model: loge(trait1) ∼ loge(trait2) + status.
(c). Skeletal changes
To further identify the skeletal changes that lead to increases in female size and to determine the circumstances leading to growth adjustment, we manipulated the life-history trajectory of 30 natal sisters born in captivity by altering their social status, and tracked their development longitudinally (originally 32 females, but two died shortly after pairing and were excluded throughout). These females came from 14 litters and were of known age, which also removed any possible confounding effect of age that could have been present in the cross-sectional analysis of captive mole-rats. Females were randomly allocated to one of three treatments: remaining in their natal group as a non-breeder, placed in a new artificial tunnel system as a solitary non-breeder, or paired with an unfamiliar male in a new tunnel system to become a breeder. X-rays were taken on these females at two-month intervals from the point of treatment initiation for 12 months (except in a few cases where we deemed that females were too heavily pregnant to be anaesthetized and X-rayed). Of the females that survived to eight months (n = 28), the mean age at manipulation was 526.8 ± 34.18, 536.45 ± 30.91 and 526.9 ± 36.78 days for in-group non-breeders, solitary non-breeders and breeders, respectively. Of the 11 females that were paired with a male, only two had failed to produce a litter by six months of age and only one individual by 10 months. The median time to the first parturition after pairing was 101.5 days. All females in the ‘breeder’ treatment were included in all analyses.
General linear models were used to investigate skeletal growth trajectories across the three social treatments. The within-individual change in L5 lumbar vertebra length, skull width and the elongation factor were each fitted as a response, with treatment and the initial trait value specified as fixed covariates. Models were fitted for each response variable at every two-month sampling interval to determine the point at which skeletal morphological divergence occurred. Pairwise comparisons of significant treatment effects were assessed with Tukey's multiple comparisons (multcomp package [26]), and similarities/differences between breeders and non-breeders were used to assess whether growth adjustment is a result of breeding itself (in which case breeder morphology would differ from both classes of non-breeding female) or rather due to the relaxation of reproductive suppression (in which case breeder morphology would only differ from females remaining in their natal group).
To control for a possible artefact of captivity on morphological patterns, we also examined X-rays from wild mole-rats. X-rays from wild animals only included females heavier than 101 g, the weight of the smallest breeder that was captured for X-ray sampling. This produced a dataset of 56 females captured between February 2016 and June 2017, including 21 breeders, 12 in-group non-breeders and 23 solitary non-breeders. The presence of an unperforated vagina confirmed that these solitary females had not previously engaged in sexual activities, and previous trapping records indicated that many of these solitary non-breeding females had been solitary for at least 2 years (and in a few cases 4 years). The relative elongation (log L5 vertebra/log skull width) of the three classes of female was fitted in a linear mixed effects model with normal errors, with group identity set as a random effect to control for possible morphological similarities within groups. Pairwise comparisons were treated as above.
(d). Body length and fitness
To investigate whether increases in body length in breeders are associated with increases in fecundity, we used an extensive dataset of birth events in captivity. The total body length of female breeders is measured during routine sampling in the laboratory, which made it possible to investigate whether body length was related to three measures of fecundity among a large cohort of breeders: litter size at birth, the total mass of pups at birth (total neonate mass) and individual pup mass at birth. Information was only included from females whose total body length was measured within 90 days of the birth of a litter to ensure that it reflects size around parturition. Further, to remove any uncertainty around the birth date, litters were only used if checks of the nest-box indicated that the litter must have been born on the day of the check or the day preceding it. The total dataset included 186 litters born to 58 mothers that produced 587 pups. Pups were measured to the nearest gram on an electronic balance. The three traits were fitted to linear mixed effects models, where total neonate mass and individual pup mass were fitted to a normal error structure, and litter size was fitted to a Poisson error structure. For total neonate mass and litter size, a single model was fitted in each case that included breeder body length and whether it was the females’ first reproductive episode (primiparity) as fixed terms, and maternal identity as a random term. For individual pup mass, the model contained breeder body length, primiparity and litter size as fixed terms and maternal identity and litter identity as random terms; including litter size allows us to test whether longer mothers produced relatively larger pups; partial residuals of body length were extracted using the remef package [27] before being plotted. The significance of fixed terms was assessed by likelihood ratio tests.
To check that any morphological pattern in the results could not be driven by a bias in the X-ray annotation by the lead author, a random subset of 150 X-ray images that formed part of the study were annotated blind by a second person unrelated to the study. The correlation between the measurements across skeletal traits was consistently high (r > 0.93, except for pelvis length, where r = 0.497), and so it was highly improbable that measurer bias affected the results.
3. Results
(a). Morphological divergence
In captive Damaraland mole-rats, breeding females are both larger and more elongated than subordinate non-breeders residing in groups, after controlling for the weight of individuals (figure 1b; breeder mean = 143.47 g ± 3.56 versus in-group non-breeder mean 137.91 g ± 2.78; Welch's t-test, t70.1 = −1.23, p = 0.22): PCA revealed a morphometric separation of breeders and in-group non-breeders according to both size and shape (figure 1c; trait loadings for the first five PCs, which together explain 91.8% of the variance, are in electronic supplementary material, table S1). There was considerable overlap in the morphological space occupied by either class. PC1 revealed positive loading for all traits and is indicative of general size, breeders being generally larger than in-group females. PC2 separated breeders and in-group non-breeders by shape, and reflects the relatively longer lumbar vertebra, longer skeletal body length, wider pelvis and shorter femurs of breeders. The MANOVA performed on PCs 1–5 revealed a significant effect of social status (F5,104 = 26.48, p < 0.001), with follow-up univariate ANOVAs on each PC 1–5 suggesting the status effect is most prominent in the first three components (PC1: F1 = 11.04, p < 0.001; PC2: F1 = 57.1, p < 0.001; PC3: F1 = 7.43, p = 0.007; PC4: F1 = 2.80, p = 0.097; PC5: F1 = 2.87, p = 0.093).
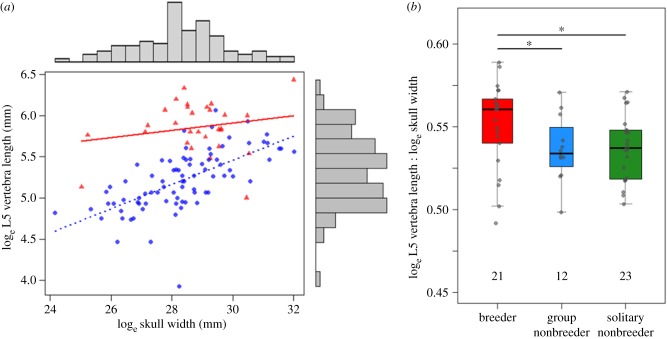
Breeders in captivity were more elongated than subordinate non-breeders, as shown by their relatively longer lumbar vertebrae for a given size (skull width, figure 2a; or skeletal body length, electronic supplementary material, table S2), as well as being longer overall (total body length: breeder mean = 18.51 ± 0.14 cm, in-group non-breeder mean 17.72 ± 0.11 cm, Welch's t-test, t64.3 = −4.36, p < 0.001). Breeders and in-group non-breeders also showed different bivariate scaling relationships in several other skeletal traits (all bivariate relationships in electronic supplementary material, table S2), most notable being the wider pelvic girdle of breeders.
Figure 2.
(a) The bivariate relationship between the lumbar vertebra length and skull width in captive female mole-rats indicates that breeders (triangles) are relatively more elongated than in-group non-breeders (circles). (b) Breeding females are also more elongated than both in-group females and solitary females in the wild, the latter having dispersed and settled in isolation. Numbers within the plot refer to sample size. (Online version in colour.)
(b). Skeletal changes in breeders
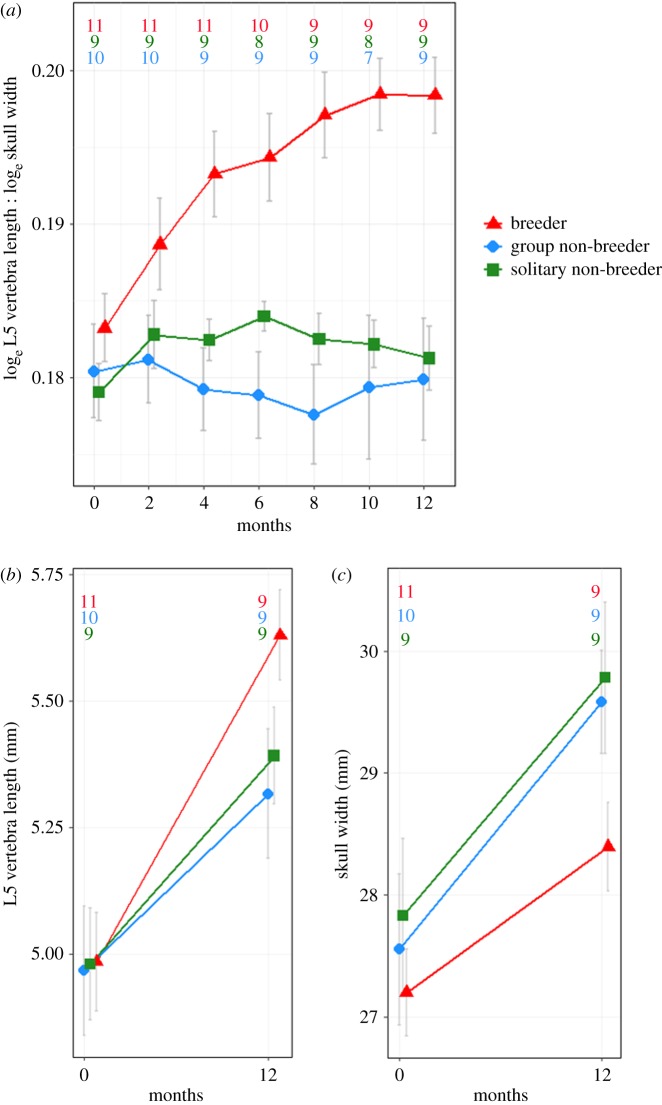
Experimental manipulation of the social status of natal sisters of known age in captive animals showed that the elongation of breeding females was caused by an increased lengthening of the skeletal vertebrae (figure 3). These increases in growth are a consequence of breeding rather than of the relaxation of reproductive suppression, because females removed from a group setting and placed solitarily displayed a similar growth pattern to non-breeding females residing in groups. Females paired with unfamiliar males to become breeders were already more elongated than the two classes of non-breeding female four months into the experiment (figure 3a; F2 = 11.31, both pairwise contrasts p < 0.001). This coincided with the point at which the L5 vertebra of breeders were significantly longer (figure 3b displays the contrasts in L5 vertebra length at 12 months; LMM contrast = 0.251 mm ± 0.05 relative to in-group breeders, p < 0.001, contrast = 0.014 mm ± 0.06, p = 0.025), an effect that persisted thereafter (electronic supplementary material, figure S1a displays contrasts at each sampling interval). The lengthening effect in breeders was also shown by other lumbar vertebrae (see electronic supplementary material, figure S2 for growth in L4 and L6 vertebrae). By contrast, there was no evidence that changes in skull width contributed significantly to the relative elongation of breeders (figure 3c; electronic supplementary material, figure S1b; p > 0.10 for all pairwise contrasts).
Figure 3.
(a) Change in the relative elongation, (b) L5 lumbar vertebra length and (c) skull width of captive females experimentally manipulated to follow different social trajectories. Numbers within the plot refer to sample sizes at each timepoint of the experiment. (Online version in colour.)
Evidence from wild mole-rats agreed with the role of reproduction in breeder elongation. Here too, breeders displayed a more elongated phenotype than female non-breeders residing in groups (figure 2b; elongation factor contrast = 0.013 ± 0.005, p = 0.027) and solitary non-breeders (contrast = 0.014 ± 0.006, p = 0.036), whereas the two classes of non-breeder did not differ from one another (contrast = −0.0001 ± 0.006, p = 0.89). Breeders (mean = 18.81 ± 0.17 cm) also exhibited a longer total body length than both solitary non-breeders (mean 17.86 ± 0.36 cm; contrast p-value < 0.001) and in-group non-breeders (mean 17.81 ± 0.27 cm; contrast p-value = 0.052).
(c). Body length and fitness
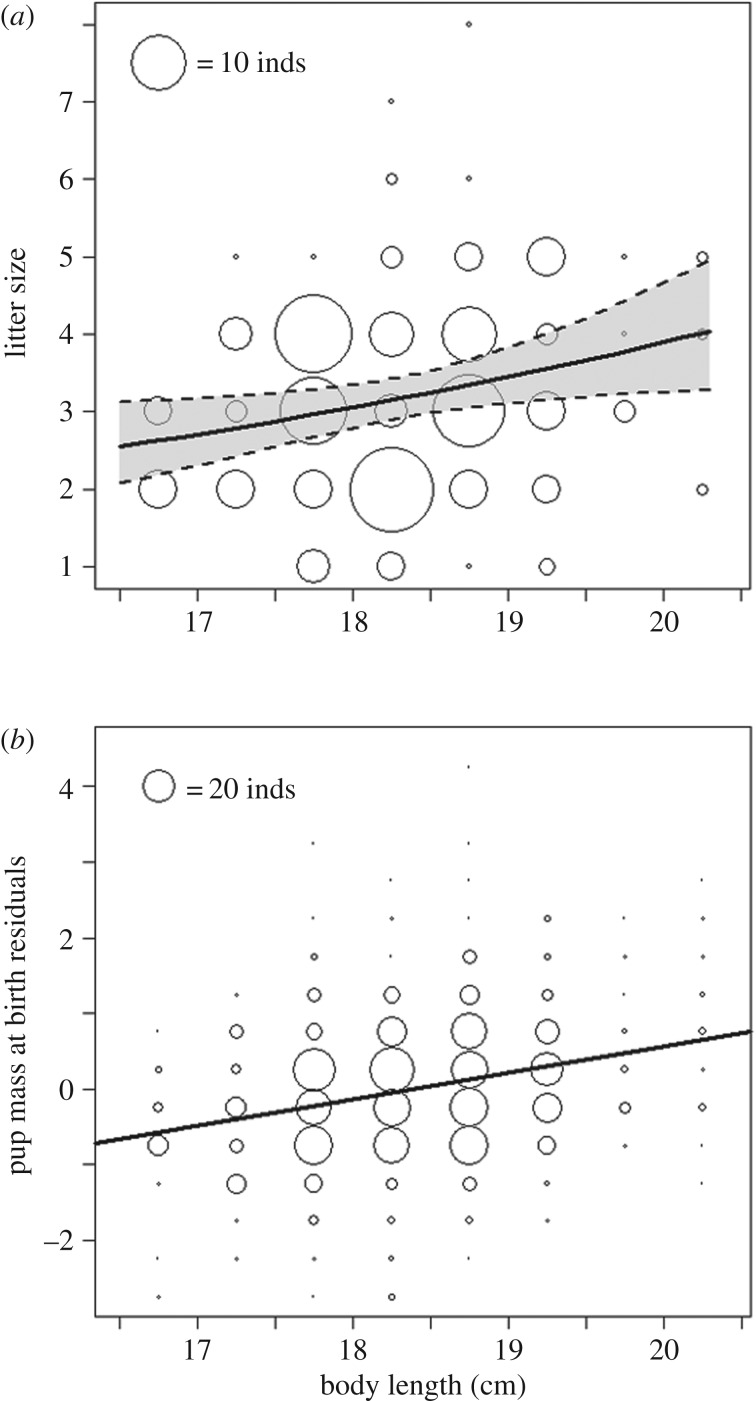
Across breeding females, increased body length is associated with higher fecundity (electronic supplementary material, table S3) as longer females have larger litters (figure 4a; GLMM: β = 0.12 ± 0.05, 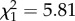 , p = 0.016). As litter size is highly correlated with total neonate mass (r = 0.92), longer females also produced litters with larger total mass (LMM: β = 3.83 ± 1.23,
, p = 0.016). As litter size is highly correlated with total neonate mass (r = 0.92), longer females also produced litters with larger total mass (LMM: β = 3.83 ± 1.23, 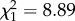 , p = 0.003). However, longer females do not trade off increasing offspring quantity for reduced quality offspring. On the contrary, controlling for litter size, longer females invest proportionally more per pup (figure 4b; LMM: β = 0.36 ± 0.17,
, p = 0.003). However, longer females do not trade off increasing offspring quantity for reduced quality offspring. On the contrary, controlling for litter size, longer females invest proportionally more per pup (figure 4b; LMM: β = 0.36 ± 0.17, 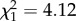 , p = 0.040), which suggests that increases in body length may facilitate increases in both offspring quality and quantity.
, p = 0.040), which suggests that increases in body length may facilitate increases in both offspring quality and quantity.
Figure 4.
Increases in body length are associated with increases in (a) litter size and (b) individual pup mass across 186 litters born in captivity. (a) Points represent raw data scaled to according to sample size, and lines show predicted litter size ± 95 confidence intervals from linear mixed effects models. (b) Partial residuals of individual pup mass at birth, corrected for litter size and primiparity. Points represent partial residuals estimated from linear mixed effects model. Line displays the partial effect of body length on individual pup mass, but confidence intervals are not provided as the generation of a partial effect removes variation from the fitted model. The partial residuals were standardized as they do not provide meaningful values.
4. Discussion
The morphological divergence of reproductive and non-reproductive individuals has only been documented in two vertebrate societies, those of the naked mole-rat and the Damaraland mole-rat [6,7]. In this study, by altering the life-history trajectories of age-matched females in captivity, we show that the lengthening of breeding females in Damaraland mole-rats is caused by the upregulated growth of the skeletal vertebrae in breeders, causing breeders to be more elongated than non-breeders. We also show that it is the onset of breeding, rather than the removal from reproductive suppression, that acts as the driver of this skeletal remodelling. Two lines of evidence support this view. First, we found that reproductively naive females in the wild that dispersed from their natal group and settled solitarily were morphologically equivalent to female non-breeders still resident in their natal group, despite many of the former being isolated for multiple years. Second, we found that individuals in captivity only changed shape and size when they were paired with an unrelated male, whereas no such change took place in females that were housed solitarily. The increase in elongation demonstrated by breeding females presumably serves to enhance fecundity by allowing the female to be larger without gaining extra girth. It is probably also highly advantageous for a species that occupies a system of narrow subterranean tunnels where increases in girth must impose strong constraints on mobility, as, unlike in eusocial insects, reproductive female mole-rats are not bound to the nest (e.g. Ansell's mole-rat Fukomys anselli [28]). The fecundity benefits of body lengthening were confirmed by analyses of birth events in captivity which showed that longer breeders produced larger litters and invested more prenatally in each pup after the effect of litter size was statistically controlled for.
In finding that upregulated vertebral growth is central to the elongation of breeding females, the results of this study deviate from a previous result in wild Damaraland mole-rats which suggested that breeder elongation is achieved through a relative reduction in the growth of the skull compared with growth towards total body length [7]. The ad libitum feeding of individuals in captivity might also have generated different patterns of growth in captivity compared to the wild. This possibility can only be addressed with longitudinal X-rays of wild animals. Without this information, our study implicates vertebral growth as the key process involved in elongation. As such, similar developmental routes appear to underpin the status-related morphological divergence of naked and Damaraland mole-rats, albeit the extent of divergence is greater in naked mole-rats [6]. This may be an indirect result of the much larger group sizes of naked mole-rats (up to 295 individuals [29]); with increasing group size, breeder fecundity will be stronger (larger workforces can rear more offspring; mean and maximum litter sizes in the field = 11.3 ± 6.2 and 28 individuals, respectively [30]) and the reproductive potential of helpers declines (because the likelihood of inheriting the dominance position is reduced), which together would favour increased elongation in breeders and developmental arrest in non-breeders. Although naked and Damaraland mole-rats display the largest group sizes and most extreme forms of sociality in the mole-rats, other members of the Bathyergidae family exhibit comparable social features (group-living, a reproductive division of labour and delayed dispersal [31]), and one could speculate that if the social environment is important in morphological divergence then morphological divergence may also be present in other mole-rats. Alternatively, if skeletal elongation is principally a consequence of subterranean living and associated constraints on abdominal width, then even the solitary species of mole-rat may undergo vertebral lengthening across reproductive episodes (e.g. the Cape dune mole-rat Bathyergus suillus, the Cape mole-rat Georychus capensis, the silvery mole-rat Heliophobius argenteocinerus, among others); though group-living and sociality could still increase the magnitude of this effect trait. A broader examination of rank-related growth would reveal whether skeletal lengthening is a unique adaptation associated with the highly social mole-rats or a more general feature of the Bathyergidae family and their subterranean habits.
Our study indicates that the vertebral lengthening of formerly subordinate helpers is stimulated by the onset of reproductive activities. The alternative possibility that relaxation of reproductive suppression is sufficient to induce divergence towards an elongated phenotype can be ruled out, because non-breeding females removed from their natal group and housed solitarily for a year exhibited a growth trajectory equivalent to non-breeders retained in complete groups (see [6] for analogous result in naked mole-rats). Likewise, solitary females in the wild were morphologically indistinguishable from in-group non-breeders. Various endocrinological changes that take place throughout pregnancy could be implicated in this parity-driven bone growth. Sex steroids such as oestradiol and progesterone increase during pregnancy and are heavily involved in bone formation [26] (see [12] for progesterone in DMRs), often by mediating levels of growth hormone and insulin-like growth factor 1 [32]. Testosterone also promotes bone growth [33], and although commonly thought of as a male hormone, the heightened levels of testosterone measured in breeding female Damaraland mole-rats [34]—a pattern also seen in other cooperative breeders [35]—raises the possibility that testosterone is also involved in vertebral lengthening. The action of such hormones sets up a skeletal mineral reserve that is subsequently resorbed and used in offspring development and milk production [21,36,37]. Yet, despite skeletal demineralization during lactation, reproduction has been shown to lead to a net increase in total skeletal size. For example, in mice it has been shown that reproduction generates permanent increases in total body length [38], a result bearing obvious relevance to mole-rats, whilst in humans, high parity has been associated with increases in bone size [39,40]. Similar physiological mechanisms might, therefore, operate to drive the elongation and increased size of female mole-rats, whose unusual physiology could yet offer important biomedical insights concerning skeletal development [41].
The metabolic challenges of reproduction [42,43] can be expected to be particularly large in cooperative breeding mammals like Damaraland mole-rats because dominant females within cooperative societies often breed multiple times per year, and it is not uncommon for a female to conceive during the period that she is lactating for her current litter [8,44,45]. In this context, continued skeletal growth in mole-rats is particularly remarkable because short inter-birth intervals must necessarily reduce the opportunity to recover calcium lost during lactation. In some cooperative breeders such as the mongooses and the canids, some of the maternal burden of lactation is offset by helpers lactating for non-descendent pups (e.g. African wild dog Lycaon pictus [46]; dwarf mongoose Helogale parvula [47]; meerkat [48]), but allo-lactation is absent in mole-rats. In the absence of allo-lactation, the atypically long gestation period of Damaraland mole-rats could be important by allowing more time for pregnant females to accrue mineral reserves prior to lactation (78–92 days [31]). In meeting their calcium requirement, breeding females may also rely on the exceptionally high calcium content of their primary food source—the subterranean tubers of the gemsbok cucumber Acanthosicyos naudininianus—and a highly efficient mode of calcium uptake to drive their skeletal growth [49,50]. In fact, the calcium content of gemsbok cucumber is four to five times higher per unit mass than the sweet potato and cucumber diet given to the animals in the captive population, and this lowered calcium diet may even have led to an underestimation of the degree of vertebral lengthening documented in this study.
Overall, this study confirms the morphological divergence of breeding female Damaraland mole-rats at the skeletal level. By examining the growth trajectories of age-matched individuals, it provides the first definitive evidence that the growth patterns underlying skeletal dimorphism cannot be explained by early-life developmental divergence or by status-specific age differences like those that underpin phenotypic development in some eusocial ants and honeybees [51–53]. Instead, growth patterns resemble other cooperative breeding vertebrates (e.g. meerkats [4], naked mole-rats [5], Haplochromis burtoni cichlid [3]) and other social insects (e.g. ponerine ants [54], termites [55]) where contrasts in size and shape between breeders and non-breeders are the result of changes that occur on or around the acquisition of a dominant breeding position. Viewed more broadly, the secondary growth of mole-rat breeders provides a clear example of socially responsive growth adjustment, or what might be termed ‘strategic growth’ [1,56]. Similar forms of adaptive, socially responsive growth might be more prevalent in mammals than is currently recognized, but the extent to which this is the case, and the implications for the structuring of mammalian dominance hierarchies, are as yet poorly understood. Of the few cases documented in mammals, the emphasis has been placed on the status-related upregulation of growth [4,5], but an equally interesting perspective will be to understand the circumstances and mechanisms that cause development to be delayed or arrested in subordinate individuals in the first place. For example, in adult male orang-utans Pongo abelii, some individuals develop conspicuous, sexually selected cheek flanges soon after reaching sexual maturity, whereas others may reach sexual maturity and remain unflanged for 20 years before developing this conspicuous secondary trait [57]; the causes of this individual variation in development are largely unknown. Societies with marked reproductive skew and suppression of subordinate reproduction provide an obvious place to investigate socially responsive growth further, but as subordinate group members often enjoy a small share of reproduction in even heavily skewed societies [58,59], it seems that if rank-related divergence in size and shape is identified in other mammals, it is unlikely to be of a magnitude comparable with the social mole-rats, where subordinates never reproduce.
Supplementary Material
Acknowledgements
We are grateful to the research managers, students, volunteers and other on-site employees who have contributed to the running of the Kalahari mole-rat project since its inception; Philippe Vullioud, Rute Mendonça, Dave Gaynor and Tim Vink have all played major roles in this. Thanks to Kyle Finn and Kyle Flesness for their efforts capturing some of the wild mole-rats that formed part of this study. We are also grateful to the Kalahari Research Trust for access to the facilities, to Prof. Marta Manser for her contribution to maintaining the Kalahari Research Centre, and to the Northern Cape Department of Environment and Nature Conservation for permission to conduct research in the Northern Cape.
Ethics
All the research carried out in this study was approved by the University of Pretoria Animal Ethics Committee (permits EC006-15, EC091-15, EC087-15).
Data accessibility
Data are deposited in Dryad (http://dx.doi.org/10.5061/dryad.d783p98) [60].
Authors' contributions
J.T. conceived the study with input from T.C.-B. and M.Z. J.T. performed the statistical analyses. J.T., N.K., K.G. and M.Z. organized and/or carried out data collection. J.T. wrote the paper, with input from the other authors at later stages. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
The Kalahari Mole-rat Project is supported by a European Research Council Grant awarded to T.C.-B. (no. 294494); J.T. was funded by a Natural Environment Research Council Doctoral Training Program; parts of the fieldwork were funded by a British Ecological Society Grant awarded to Markus Zöttl (no. 5301/6343).
References
- 1.Heg D, Bender N, Hamilton I. 2004. Strategic growth decisions in helper cichlids. Proc. R. Lond. Soc. B 271(Suppl. 6), S505–S508. ( 10.1098/rsbl.2004.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buston PM. 2003. Size and growth modification in clownfish. Nature 424, 145–146. ( 10.1038/424145a) [DOI] [PubMed] [Google Scholar]
- 3.Hofmann HA, Benson ME, Fernald RD. 1999. Social status regulates growth rate: consequences for life-history strategies. Proc. Natl Acad. Sci. USA 96, 14 171–14 176. ( 10.1073/pnas.96.24.14171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell AF, Carlson AA, McIlrath GM, Jordan NR, Clutton-Brock T. 2004. Adaptive size modification by dominant female meerkats. Evolution 58, 1600–1607. ( 10.1111/j.0014-3820.2004.tb01739.x) [DOI] [PubMed] [Google Scholar]
- 5.Dengler-Crish CM, Catania KC. 2007. Phenotypic plasticity in female naked mole-rats after removal from reproductive suppression. J. Exp. Biol. 210, 4351–4358. ( 10.1242/jeb.009399) [DOI] [PubMed] [Google Scholar]
- 6.O'Riain MJ, Jarvis JU, Alexander R, Buffenstein R, Peeters C. 2000. Morphological castes in a vertebrate. Proc. Natl Acad. Sci. USA 97, 13 194–13 197. ( 10.1073/pnas.97.24.13194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young AJ, Bennett NC. 2010. Morphological divergence of breeders and helpers in wild Damaraland mole-rat societies. Evolution 64, 3190–3197. ( 10.1111/j.1558-5646.2010.01066.x) [DOI] [PubMed] [Google Scholar]
- 8.Jarvis J. 1991. Reproduction of naked mole-rats. In The biology of the naked mole rat (eds Sherman P, Jarvis J, Alexander R), pp. 384–425. Princeton, NJ: Princeton University Press. [Google Scholar]
- 9.Henry EC, Dengler-Crish CM, Catania KC. 2007. Growing out of a caste—reproduction and the making of the queen mole-rat. J. Exp. Biol. 210, 261–268. ( 10.1242/jeb.02631) [DOI] [PubMed] [Google Scholar]
- 10.Faulkes CG, Bennett NC, Bruford MW, O'Brien HP, Aguilar GH, Jarvis JU. 1997. Ecological constraints drive social evolution in the African mole-rats. Proc. R. Soc. Lond. B 264, 1619–1627. ( 10.1098/rspb.1997.0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molteno AJ, Bennett NC. 2000. Anovulation in non-reproductive female damaraland mole-rats (Cryptomys damarensis). J. Reprod. Fertil. 119, 35–41. ( 10.1530/jrf.0.1190035) [DOI] [PubMed] [Google Scholar]
- 12.Rickard CA, Bennett NC. 1997. Recudescence of sexual activity in a reproductively quiescent colony of the Damaraland mole-rat (Cryptmys damarensis), by the introduction of an unfamilar and genetically unrelated male: a case of incest advoidance in ‘queenless’ colonies. J. Zool. 241, 185–202. ( 10.1111/j.1469-7998.1997.tb05508.x) [DOI] [Google Scholar]
- 13.Clarke FM, Miethe GH, Bennett NC. 2001. Reproductive suppression in female Damaraland mole-rats Cryptomys damarensis: dominant control or self-restraint? Proc. R. Soc. Lond. B 268, 899–909. ( 10.1098/rspb.2000.1426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooney R, Bennett NC. 2000. Inbreeding avoidance and reproductive skew in a cooperative mammal. Proc. R. Soc. Lond. B 267, 802–806. ( 10.1098/rspb.2000.1074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faulkes CG, Abbott DH. 1993. Evidence that primer pheromones do not cause social suppression of reproduction in male and female naked mole-rats (Heterocephalus glaber). J. Reprod. Fertil. 99, 225–230. ( 10.1530/jrf.0.0990225) [DOI] [PubMed] [Google Scholar]
- 16.Clarke FM, Faulkes CG. 1997. Dominance and queen succession in captive colonies of the eusocial naked mole-rat, Heterocephalus glaber. Proc. R. Soc Lond. B 264, 993–1000. ( 10.1098/rspb.1997.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyman PC, Jackson CR, Bennett NC. 2006. Do dispersing non-reproductive female Damaraland mole-rats, Cryptomys damarensis (Rodentia: Bathyergidae) exhibit spontaneous or induced ovulation? Physiol. Behav. 87, 88–94. ( 10.1016/j.physbeh.2005.09.003) [DOI] [PubMed] [Google Scholar]
- 18.Young AJ, Oosthuizen MK, Lutermann H, Bennett NC. 2010. Physiological suppression eases in Damaraland mole-rat societies when ecological constraints on dispersal are relaxed. Horm. Behav. 57, 177–183. ( 10.1016/j.yhbeh.2009.10.011) [DOI] [PubMed] [Google Scholar]
- 19.Clarke BL, Khosla S. 2010. Female reproductive system and bone. Arch. Biochem. Biophys. 503, 118–128. ( 10.1016/j.abb.2010.07.006.Female) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syed F, Khosla S. 2005. Mechanisms of sex steroid effects on bone. Biochem. Biophys. Res. Commun. 328, 688–696. ( 10.1016/j.bbrc.2004.11.097) [DOI] [PubMed] [Google Scholar]
- 21.Bowman BM, Miller SC. 2001. Skeletal adaptations during mammalian reproduction. J. Musculoskelet. Neuronal Interact. 1, 347–355. [PubMed] [Google Scholar]
- 22.Qing H, Ardeshirpour L, Divieti Pajevic P, Dusevich V, Jähn K, Kato S, Wysolmerski J, Bonewald LF. 2012. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J. Bone Miner. Res. 27, 1018–1029. ( 10.1002/jbmr.1567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zöttl M, Vullioud P, Mendonça R, Torrents Ticó M, Gaynor D, Mitchell A, Clutton-Brock T. 2016. Differences in cooperative behavior among Damaraland mole rats are consequences of an age-related polyethism. Proc. Natl Acad. Sci. USA 113, 201607885 ( 10.1073/pnas.1607885113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dengler-Crish CM, Catania K. 2009. Cessation of reproduction-related spine elongation after multiple breeding cycles in female naked mole-rats. Anat. Rec. 292, 131–137. ( 10.1002/ar.20793.Cessation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical omputing. [Google Scholar]
- 26.Hothorn T, Bretz F, Westfall P. 2008. Simulation inference in general parametric models. Biometrical J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 27.Hohenstein S, Kliegl R. 2013. remef: remove partial effects. R Package version 1.0.6.9000.
- 28.Šklíba J, Lövy M, Burda H, Šumbera R. 2016. Variability of space-use patterns in a free living eusocial rodent, Ansell's mole-rat indicates age-based rather than caste polyethism. Sci. Rep. 6, 37497 ( 10.1038/srep37497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brett R. 1991. The population structure of naked mole-rat colonies. In The biology of the naked mole Rat (eds PW Sherman, JUM Jarvis, Alexander R.), pp. 97–137. Princeton, NJ: Princeton University Press. [Google Scholar]
- 30.Sherman PW, Braude S, Jarvis JUM. 1999. Litter sizes and mammary numbers of naked mole-rats: breaking the one-half rule. J. Mammal. 80, 720–733. ( 10.2307/1383241) [DOI] [Google Scholar]
- 31.Bennett NC, Faulkes CG. 2000. The African mole-rats: ecology and eusociality. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 32.Locatelli V, Bianchi VE. 2014. Effect of GH/IGF-1 on bone metabolism and osteoporsosis. Int. J. Endocrinol. 2014, 235060 ( 10.1155/2014/235060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke B, Khosla S. 2009. Androgens and bone. Steroids 74, 296–305. ( 10.1016/j.steroids.2008.10.003.Androgens) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutermann H, Young AJ, Bennett NC. 2013. Reproductive status and testosterone among females in cooperative mole-rat societies. Gen. Comp. Endocrinol. 187, 60–65. ( 10.1016/j.ygcen.2013.03.026) [DOI] [PubMed] [Google Scholar]
- 35.Davies CS, Smyth KN, Greene LK, Walsh DA, Mitchell J, Clutton-Brock T, Drea CM. 2016. Exceptional endocrine profiles characterise the meerkat Sex, status, and reproductive patterns. Sci. Rep. 6, 1–9. ( 10.1038/srep35492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovacs CS, Kronenberg HM. 1997. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 18, 832–872. ( 10.1210/edrv.18.6.0319) [DOI] [PubMed] [Google Scholar]
- 37.Kovacs CS. 2016. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol. Rev. 96, 449–547. ( 10.1152/physrev.00027.2015) [DOI] [PubMed] [Google Scholar]
- 38.Schutz H, Donovan ER, Hayes JP. 2009. Effects of parity on pelvic size and shape dimorphism in Mus. J. Morphol. 270, 834–842. ( 10.1002/jmor.10723) [DOI] [PubMed] [Google Scholar]
- 39.Specker B, Binkley T. 2005. High parity is associated with increased bone size and strength. Osteoporos. Int. 16, 1969–1974. ( 10.1007/s00198-005-1978-1) [DOI] [PubMed] [Google Scholar]
- 40.Wiklund PK, et al. 2012. Lactation is associated with greater maternal bone size and bone strength later in life. Osteoporos. Int. 23, 1939–1945. ( 10.1007/s00198-011-1790-z) [DOI] [PubMed] [Google Scholar]
- 41.Pinto M, Jepsen KJ, Terranova CJ, Buffenstein R. 2010. Lack of sexual dimorphism in femora of the eusocial and hypogonadic naked mole-rat: a novel animal model for the study of delayed puberty on the skeletal system. Bone 46, 112–120. ( 10.1016/j.bone.2009.08.060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clutton-Brock TH, Albon S, Guinness F. 1989. Fitness costs of gestation and lactation in wild mammals. Nature 337, 260–262. ( 10.1038/337260a0) [DOI] [PubMed] [Google Scholar]
- 43.Speakman JR. 2008. The physiological costs of reproduction in small mammals. Phil. Trans. R. Soc. B 363, 375–398. ( 10.1098/rstb.2007.2145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrette MF, Monfort SL, Festa-Bianchet M, Clutton-Brock TH, Russell AF. 2012. Reproductive rate, not dominance status, affects fecal glucocorticoid levels in breeding female meerkats. Horm. Behav. 61, 463–471. ( 10.1016/j.yhbeh.2011.12.005) [DOI] [PubMed] [Google Scholar]
- 45.Russell A, Brotherton P, McIlrath G, Sharpe L, Clutton-Brock T. 2003. Breeding success in cooperative meerkats: effects of helper number and maternal state. Behav. Ecol. 14, 486–492. ( 10.1093/beheco/arg022) [DOI] [Google Scholar]
- 46.Creel S, Creel NM. 2002. The African wild dog: behaviour, ecology and evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 47.Creel S, Monfort S, Wildt D, Waser P. 1991. Spontaneous lactation is an adaptive result of pseudopregnancy. Nature 351, 660–662. ( 10.1038/351660a0) [DOI] [PubMed] [Google Scholar]
- 48.MacLeod KJ, Nielsen JF, Clutton-Brock TH. 2013. Factors predicting the frequency, likelihood and duration of allonursing in the cooperatively breeding meerkat. Anim. Behav. 86, 1059–1067. ( 10.1016/j.anbehav.2013.09.012) [DOI] [Google Scholar]
- 49.Pitcher T, Buffenstein R, Keegan JD, Moodley GP, Yahav S. 1992. Dietary calcium content, calcium balance and mode of uptake in a subterranean mammal, the damara mole-rat. J. Nutr. 122, 108–114. ( 10.1093/jn/122.1.108) [DOI] [PubMed] [Google Scholar]
- 50.Buffenstein R. 2000. Ecophysiological responses of subterranean rodents to underground habits. In Life underground: the biology of subterranean rodents (eds EA Lacey, JL Patton, GN Cameron), pp. 63–110. Chigago, IL: Chicago University Press. [Google Scholar]
- 51.Volny VP, Gordon DM. 2002. Genetic basis for queen-worker dimorphism in a social insect. Proc. Natl Acad. Sci. USA 99, 6108–6111. ( 10.1073/pnas.092066699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kucharski R, Maleszka J, Foret S, Maleszka R. 2008. Nutritional control of reproductive status in honeybees via DNA methylation. Science 319, 1827–1830. ( 10.1126/science.1153069) [DOI] [PubMed] [Google Scholar]
- 53.Schwander T, Lo N, Beekman M, Oldroyd BP, Keller L. 2010. Nature versus nurture in social insect caste differentiation. Trends Ecol. Evol. 25, 275–282. ( 10.1016/j.tree.2009.12.001) [DOI] [PubMed] [Google Scholar]
- 54.Peeters C, Ito F. 2001. Colony dispersal and the evolution of queen morphology in social hymenoptera. Annu. Rev. Entomol. 46, 601–630. ( 10.1227/01.NEU.0000038928.61329.44) [DOI] [PubMed] [Google Scholar]
- 55.Noirot C. 1990. Sexual castes and reproductive strategies in termites. In Social insects: an evolutionary approach to castes and reproduction (ed. Engels W.), pp. 5–35. Berlin, Germany: Springer. [Google Scholar]
- 56.Huchard E, English S, Bell MBV, Thavarajah N, Clutton-Brock T. 2016. Competitive growth in a cooperative mammal. Nature 533, 532–534. ( 10.1038/nature17986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Utami S, Goossens B, Bruford M, de Ruiter J, van Hooff JARA. 2002. Male bimaturism and reproductive success in male bimaturism and reproductive success in Sumatran orang-utans. Behav. Ecol. 5, 643–652. ( 10.1093/beheco/13.5.643) [DOI] [Google Scholar]
- 58.Russell AF. 2004. Mammals: comparisons and contrasts. In Ecology and evolution of cooperative breeding in birds (eds WD Koenig, Dickinson J.), pp. 210–227. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 59.Clutton-Brock T. 2016. Mammalian societies, pp. 557–604. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 60.Thorley J, Katlein N, Goddard K, Zöttl M, Clutton-Brock T. 2018. Data from: Reproduction triggers adaptive increases in body size in female mole-rats Dryad Digital Repository. ( 10.5061/dryad.d783p98) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Thorley J, Katlein N, Goddard K, Zöttl M, Clutton-Brock T. 2018. Data from: Reproduction triggers adaptive increases in body size in female mole-rats Dryad Digital Repository. ( 10.5061/dryad.d783p98) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are deposited in Dryad (http://dx.doi.org/10.5061/dryad.d783p98) [60].