Abstract
Group living is widespread among animal species, and comes with a number of costs and benefits associated with foraging, predator avoidance and reproduction. It is largely unknown, however, whether individuals sacrifice exposure to their own preferred or optimal environmental conditions so they can remain part of a social group. Here, we demonstrate that individual three-spine sticklebacks vary in the degree to which they forego exposure to their preferred ambient temperature so they can associate with a group of conspecifics. Individual fish varied widely in preferred temperature when tested in isolation. When the same individuals were presented with a choice of a warm or cold thermal regime in the presence of a social group in one of the environments, fish spent more time with the group if it was close to their own individually preferred temperature. When a group was in a relatively cool environment, focal individuals that were more social deviated most strongly from their preferred temperature to associate with the group. Standard and maximum metabolic rate were not related to temperature preference or thermal compromise. However, individuals with a higher standard metabolic rate were less social, and so energetic demand may indirectly influence the environmental costs experienced by group members. The reduced tendency to engage with a social group when there is a large difference between the group temperature and the individual's preferred temperature suggests a role for temperature in group formation and cohesion that is mediated by individual physiology and behaviour. Together, these data highlight exposure to non-preferred temperatures as a potential cost of group membership that probably has important but to date unrecognized implications for metabolic demand, energy allocation, locomotor performance and overall group functioning.
Keywords: social behaviour, trade-offs, ecophysiology, collective behaviour, teleost fishes, metabolism
1. Introduction
Group living is widespread across animal taxa and confers a range of advantages for predator avoidance [1,2], foraging [3], reproductive success [4,5] and locomotor efficiency [6–8]. To derive these benefits, however, group members must cope with costs of group living, including increased competition for resources [9], disease transfer [10] and increased visibility to predators [11]. Furthermore, although individuals within groups often adjust their behaviour towards a collective common-ground [12], individuals within species vary considerably in their behavioural and physiological phenotype [13,14]. This suggests that group members also vary in the degree of physiological and behavioural compromise they must make to align with the group as a whole.
An additional cost of group membership is that individuals sacrifice exposure to their own preferred environmental conditions so that they can be part of a group [15]. An example of such a compromise, particularly for ectotherms, is the potential for an individual group member to deviate from its own preferred temperature to remain with group mates. Temperature has an effect on a range of physiological processes, including minimum and maximum aerobic metabolic rates [16–18], growth and digestive capacity [19] and locomotor ability [20,21]. Within species, individuals can show wide variation in the temperature that they prefer to experience [22]. Studies have shown that individual preferred temperatures in some fish species tend to fall within optimum individual temperature ranges for growth [23]. In a group scenario, however, animals show relatively synchronous behaviour and individuals occupy a similar spatial location with a given set of environmental parameters. As a result, individuals within a group will be exposed to similar temperatures, regardless of individual preferences. Some individuals may, therefore, face a constant trade-off between the benefits of being in a group and experiencing temperatures that may cause them to incur a physiological cost.
The degree to which an individual is willing to depart from its preferred environmental conditions to associate with a group may be affected by its intrinsic sociability, defined as the tendency to associate with conspecifics for non-aggressive interactions [24]. Individuals within species vary in their sociability, and more social individuals may be more likely to sacrifice exposure to their own preferred temperature to remain with a group. There is also evidence that individuals with an intrinsically higher energetic demand (i.e. those with a higher standard metabolic rate (SMR), the base level of metabolism required for an ectotherm to sustain life) are less social [25]. It is, therefore, possible that SMR could have direct or indirect effects on thermal compromises via effects on sociability. Similarly, maximum aerobic metabolic rate (MMR) is directly related to aerobic scope (AS; equal to MMR – SMR), locomotor ability and potentially the ability to recover from burst-type anaerobic activity [26–28]. In many ectothermic species, MMR and AS are sensitive to acute and chronic shifts in temperature and so may influence thermal preferences [16]. In addition, aerobic capacity can positively correlate with competitive ability [29], and so animals with a higher MMR may be more social if they are able to overcome potential costs of grouping by out-competing other group members for resources.
We studied these issues in the three-spine stickleback Gasterosteus aculeatus, a shoaling fish species [30,31] that is frequently used as a model for studying collective behaviour [32–34]. Water temperatures in this species' natural habitat can show wide temporal and spatial variation, in some cases spanning a 15°C range on daily and annual bases [35]. This makes it an ideal species for studying general behavioural responses to thermal heterogeneity which remain relevant to the animal's natural ecology. Specifically, we aimed to address the following questions: (i) do individuals differ in their preferred temperature?; (ii) do individuals vary in the extent to which they will deviate from their own preferred temperature to associate with conspecifics? and (iii) does the willingness to deviate from a preferred thermal regime depend on interactions among sociability, temperature preference and metabolic traits? Our results provide insight into the relative costs and benefits of sociability and the extent to which environmental temperature can shape interactions between individual animals and their social environment.
2. Methods
(a). Study animals
The sticklebacks used in this study were the second generation progeny of individuals collected in January 2014 from the River Endrick catchment (56°03′ N, 4°22′ W). All fish were generated using in vitro fertilization from two parents. We used a total of 49 haphazardly sampled focal individuals for temperature preference and behavioural experiments, comprising five fish from each of 10 families (four fish in the case of one family). In addition to focal fish, five siblings from each of these 10 families were used to act as stimulus shoals in shoaling trials. When generating families, each male or female parent was used only once.
Approximately six to eight months after hatching (23 February 2016), juvenile focal fish from each family were tagged with one of five colours of visible implant elastomer (Northwest Marine Technology Inc., Shaw Island, USA) on either the right or left side of the dorsal fin. Individuals from each family were then moved to five separate tanks such that each tank contained one individual from each family (10 fish per tank with the exception of one tank that had nine fish). Additionally, the non-focal siblings were held in separate tanks per family.
Focal fish were measured for body mass and length at this point (mean initial mass (m) = 425 ± 126 mg; mean initial total length (TL) = 335 ± 29 mm; measurements are presented ± standard deviation). All focal fish were weighed and measured again approximately six months later (m = 807 ± 118 mg; TL = 387 ± 30 mm). Fish were kept at a constant photoperiod of 12 L : 12 D cycle throughout the study. Holding tanks were kept at 12°C within a recirculating aquarium system with biological, mechanical and ultraviolet filtration that was maintained with regular input of dechlorinated tap water. Fish were fed twice a day with frozen bloodworms.
(b). Individual temperature preference
Fish were scored individually for temperature preference using a shuttlebox tank (Loligo Systems, Tjele, Denmark) consisting of two 30 cm diameter circular tanks joined by an 8 cm long connecting section. The tank was filled with water to a depth of 5 cm, and both of the two sub-chambers had an inlet and an outlet, connected by tubing to two separate external buffer tanks. The temperatures in each buffer tank could be increased or decreased independently, and water fed to each side of the shuttlebox tank to alter the temperature of that side. External heating and cooling units connected to the buffer tanks gave a maximum possible temperature range of 4°C–24°C. The inflows and outflows of the shuttlebox tank were arranged such that water flowed clockwise around one section and anti-clockwise around the other, minimizing mixing between the chambers. Water entering the shuttlebox tank passed over a temperature probe which was connected to external temperature sensors and a data acquisition module (DAQ-M, Loligo Systems, Denmark). These were in turn connected to a computer running ShuttleSoft software (Loligo Systems, Denmark), which could, therefore, control the temperature in each side of the shuttlebox tank independently. The computer was also connected to an infrared-sensitive camera (uEye, Imaging Development Systems GmbH, Obersulm, Germany), 1 m above the tank and looking down, which allowed the software to track a fish placed in the tank by contrast. The tank was lit from below by two infrared spotlights to increase the contrast of the fish. Two LED lamps provided faint illumination to the shuttlebox tank, which was kept behind black curtains for the duration of trials to minimize disturbance to the fish.
Fish were transferred between holding tanks and experimental tanks in a bucket of water. Fish were first left in the tank for 16 h overnight (from 17.00 to 09.00) with the software set in ‘static’ mode, during which each side was kept at a constant temperature, here 12.5°C and 15.5°C ± 0.2°C. After this point, the system was set to the ‘dynamic’ mode. During this time, fish were able to explore the two sides for 8 h, between 09.00 and 17.00. In dynamic mode, the software maintained a set difference in temperature between the two sections (here 3°C), but altered the actual temperatures based on the location of the fish within the tank. If the fish were in the cooler section, the temperature of both chambers was decreased at a rate of 2°C h−1, maintaining the set differential between them. Should the fish move to the warmer chamber, both sides increased in temperature at this same rate. The fish was, therefore, able to behaviourally regulate the temperature that it experienced. Data were logged once per second, including fish position and the temperatures in each side of the shuttlebox. Preferred temperatures are reported as the modal temperature experienced by the fish during the full 8 h of its time in dynamic mode (note that the modal temperature is robust to the duration of the time period used to determine preferred temperature).
(c). Social behaviour and thermal compromise
Fish were then scored for social behaviour in varying thermal environments over five successive trials, each of which ran for 30 min using the same shuttlebox tank. For these trials, two transparent 12 cm diameter PVC cylinders were placed in the centre of each shuttlebox chamber to allow the placement of a physically isolated shoal of five sticklebacks. Small holes were drilled into the sides of these cylinders. An empty cylinder acted as a control when required. Fish making up the stimulus shoals were full kin of focal fish, and reared in the same tank until tagging. During each trial, the proportion of time the fish spent in either chamber of the shuttlebox was quantified.
Trials investigating changes in spatial usage of the tank in response to the presence of a group were performed with static temperatures which did not vary based on the location of the focal fish. Each trial consisted of a different treatment condition as follows: in the first trial, one shuttlebox chamber was kept at 12.5°C and the other at 15.5°C, with neither side containing a shoal. In trials 2 and 3, a stimulus shoal was placed in the cylinder on either the warm or cool section for 30 min, then moved to the opposite section for another 30 min. Since the shoal was physically moved from one side of the shuttlebox to the other between trials 2 and 3, the order in which these trials were carried out was varied among individuals in a family such that half of individuals began with the shoal on the warm side, then had it moved to the cool side, and half the other way round. All fish in the stimulus shoal were netted simultaneously and transferred between sides of the tank as quickly as possible to minimize stress from disturbance.
Space use within each trial was quantified using the ratio of the time (s) spent on the cool side to time spent on the warm side. The degree to which fish changed behaviour from when they were alone based on the presence of a shoal was calculated as the change in space use (as a ratio of time) between a trial with no shoal present and the trials with a shoal present on either the cool side or warm side. These figures were then changed to a percentage, with 50% meaning no preference for either side, 100% being all time spent on the warmer side and 0% being all time spent on the cooler side.
Finally, in trials 4 and 5, both sides of the tank were set to 14°C (the average temperature across the shuttlebox chambers in the static choice trials), and the trials repeated again, once with the shoal in each section. As in trials 2 and 3, the order of shoal placement on each side was varied between individuals. These trials allowed quantification of sociability without a temperature differential. Individual sociability score was a unitless value, equivalent to the mean percentage of time spent in the same chamber as the stimulus shoal across trials 4 and 5.
(d). Estimation of metabolic rates
One week after all temperature preference and social trials had taken place, metabolic rates of the focal fish were estimated using intermittent stopped-flow respirometry [36,37]. Each day at approximately 14.00, eight fish that had been fasted for 36 h were subjected to exhaustive exercise by manually chasing the fish in a circular tank (50 cm diameter) with a water depth of 10 cm [27,38]. All fish were exhausted, defined as being non-responsive to additional stimuli and would not correct themselves if turned upside down, within 3 min of chasing. Fish were immediately transferred to individual cylindrical 50 ml glass respirometers; transfer time was always less than 20 s. For all measurements, water oxygen content was quantified once every 2 s using a Firesting 4-channel oxygen meter and associated sensors (PyroScience GmbH, Aachen, Germany). Rates of oxygen uptake were then calculated in 3 min intervals during a 20 min closed phase in the respirometers, and the maximum rate of oxygen uptake measured during this time by measuring the slope of oxygen decline in each chamber and accounting for the chamber water volume (and associated tubing), minus the volume of the fish (assuming 1 g of fish approximates 1 ml). The maximum rate during this time was taken as MMR (in mg O2 h−1). The fish were then left undisturbed overnight. Respirometers were located within a water bath kept at 12°C. Every 10 min an automated flush pump would switch on or off. When off, respirometers were sealed and the decrease in oxygen content could be analysed to indicate rate of oxygen uptake. When open, respirometers would be flushed with aerated water. Oxygen content within chambers was always above 75% air saturation. Fish were removed from respirometers at around 10.00 the following day. Once fish were removed, chambers were re-sealed and left to run empty for at least 1 h to control for background bacterial oxygen consumption (chambers were cleaned daily with bleach and bacterial oxygen consumption was always less than 10% of the oxygen uptake by fish). Whole animal SMR (mg O2 h−1) was estimated by first calculating rates of oxygen uptake from slopes as described for MMR, then determining the lowest 10th percentile of measurements taken throughout the measurement period, excluding the first 5 h of confinement in the chambers. Absolute AS was calculated as the difference between MMR and SMR. Owing to a technical issue with the respirometry set-up, some data did not record correctly. Therefore, the actual sample sizes were 25 for SMR and AS, and 41 for MMR. These samples recorded normally and can be considered to be representative of the larger population of fish in the study.
(e). Data and statistical analyses
All data are available in the Mendeley Data Repository (http://dx.doi.org/10.17632/34npwr97vn.1). Analyses were performed in R [39] using linear mixed-effects models (LME) using the package ‘lme4’ [40]. The first used tank spatial usage as a response variable (an individual's mean position in a specific trial, ranging from 100% cool side to 100% warm side.) Explanatory variables were preferred temperature (of that individual), location of the shoal (three levels: warm side, cold side and no shoal), sociability (of that individual at an intermediate temperature), log body mass, log MMR and the interaction between sociability and shoal location.
Two additional LMEs were created to explore relationships between metabolic rate and sociability. These models each had sociability as a response variable, and log body mass as an explanatory variable. One model had log MMR as an explanatory variable, while the other had both log SMR and log AS as explanatory variables. These models were created separately to account for the difference in sample sizes between SMR/AS and MMR. For all models and tests, p < 0.05 was used as the significance threshold; non-significant model terms were systematically removed in a backwards-step model selection process based on Akaike information criterion scores [41]. Model assumptions were verified by examining residuals compared to the fitted values. It was found that families varied in metabolic rate, therefore, family was included as a random effect in all models. Additionally, individual ID was included in the model of spatial tank usage to account for the effect of repeated measures on the same individual. Finally, Julian date was added as a fixed effect to control for any systematic changes in mean thermal preference across individuals through time.
ANOVA was performed on models to obtain F-values. Model r2 values were computed using the MuMIn 1.9.13 package for R [42]. This included marginal r2
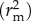 and conditional r2
and conditional r2
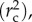 which indicate the variance explained by fixed factors and by both fixed and random factors, respectively [43]. Full details of model terms and output can be found in the electronic supplementary material, tables S1-S3.
which indicate the variance explained by fixed factors and by both fixed and random factors, respectively [43]. Full details of model terms and output can be found in the electronic supplementary material, tables S1-S3.
Tests for correlations between variables were performed using Pearson's product-moment correlation coefficients (r), and tests for differences among families in preferred temperature and sociability were performed using Friedman rank sum tests (Q).
3. Results
(a). Individual temperature preference
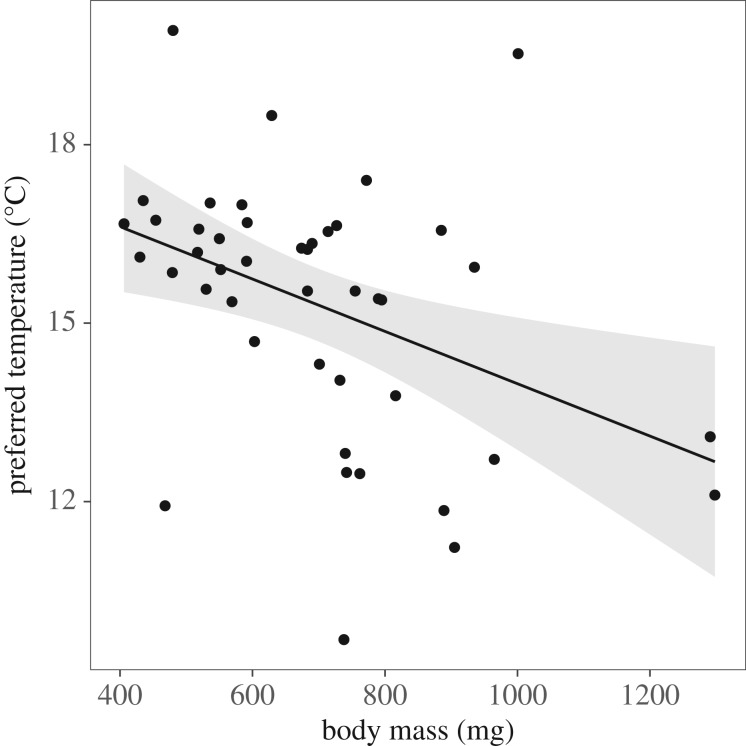
Individuals showed a wide range of preferred temperatures, ranging from 9.68°C to 19.82°C. Individuals with a lower body mass preferred higher temperatures (figure 1; Pearson correlation coefficient: r = −0.40, p = 0.006). Even after correction for body mass using residuals of the relationship between preferred temperature and mass, there remained a 3.92°C range of preferred temperatures among individuals (12.67°C–16.59°C). Subsequent statistical models do not use these corrected values, but instead use raw temperature preferences with body mass included as a covariate. There was no effect of SMR, MMR or AS on preferred temperature among individuals. Families did not differ in preferred temperature (Friedman test: Q4 = 6.54, p = 0.161).
Figure 1.
Smaller fish had significantly higher preferred temperatures (Pearson correlation; r = −0.41, p = 0.005). Each point represents one individual fish. Shaded area is 95% confidence interval (CI) around regression line; y = −0.004x + 18.38.
(b). The effect of behavioural and metabolic traits on thermal compromise
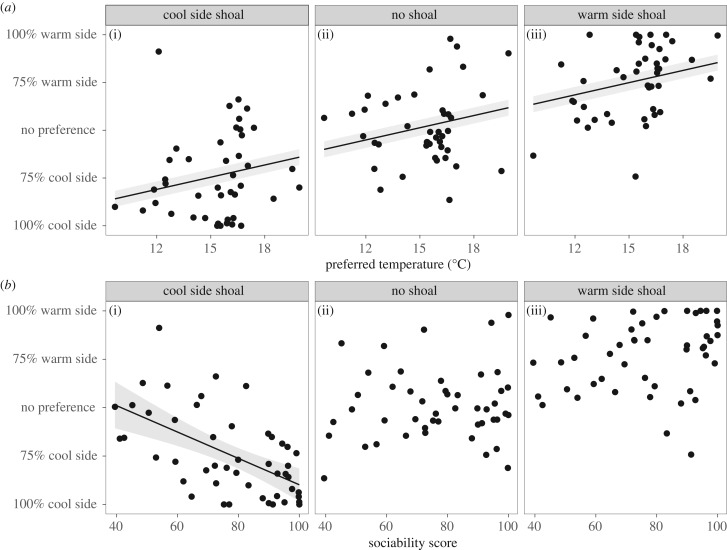
When a shoal of conspecifics was added to either side of the tank, fish spent more time on that side of the tank (figure 2; LME: F2,68 = 81.66, p < 0.001; electronic supplementary material, table S1). However, the degree to which fish changed their space use in the presence of conspecifics—and therefore, the temperature they experienced—differed greatly among individuals (figure 3). This difference was modulated by their preferred temperature: fish with a preference for warmer temperatures when alone spent more time in the warmer environment, regardless of the location of the shoal (figure 3; LME: F1,68 = 7.90, p = 0.009).
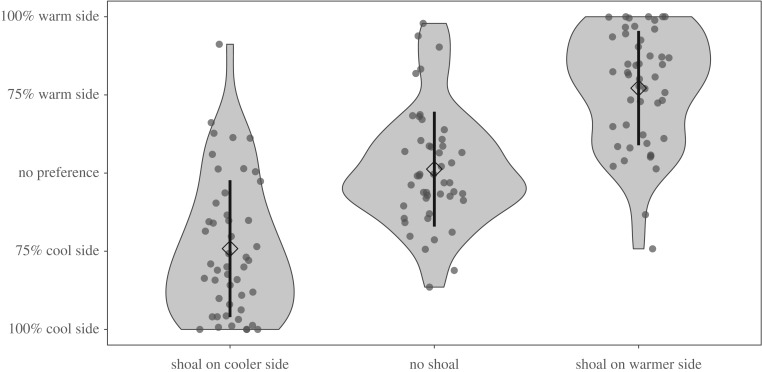
Figure 2.
Compared to their position when no shoal was present, animals spent significantly more time on whichever side the shoal was located (LME; F2,68 = 81.66, p < 0.001). This violin plot shows where, on average, fish spent time in the two-chambered tank under three experimental shoal positions. Each point represents one individual fish. Diamonds represents the mean ± s.d. The width of each violin represents observation density at that y-value.
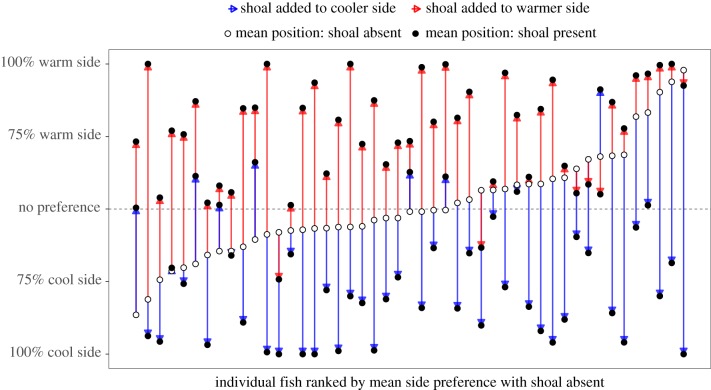
Figure 3.
Individual fish vary greatly in the degree to which they associate with conspecifics at different temperatures. White points show the fish's tank usage with no shoal, and black points show tank usage when a shoal is added. Black points connected by a red arrow to the white point represent the shift in the fish's tank usage when a shoal is added to the warmer side. Conversely, black points connected by a blue arrow to the white point represent the shift in the fish's tank usage when a shoal is added to the cooler side. Most fish tended to shift towards the shoal on either side.
The tendency of fish to change their space use upon addition of a shoal was also dependent on sociability. When the stimulus shoal was in the cool environment, the fish that were more social showed a greater tendency to move towards the shoal (figure 4b(i); LME: t1,68 =−4.74, p < 0.001). When the shoal was on the warm side, however, there was no effect of sociability on space use (figure 4b(iii); LME: t1,68 = 0.06, p < 0.956). Sociability did not affect space use when the shoal was not present (figure 4b(ii); Pearson correlation coefficient: r = 0.08, p = 0.581), nor did it have any effect on preferred temperature (Pearson correlation coefficient: r = 0.08, p = 0.622). Families did not differ in sociability (Friedman test: Q4 = 4.80, p = 0.308).
Figure 4.
(a) While individuals changed location based on the position of conspecifics, individuals with a higher temperature preference always spend more time on the warmer side (LME; F1,27 = 7.90, p = 0.009). Regression line shows the same slope for all three panels with a different intercept for each level of the ‘shoal location’ variable as part of a LME model. (b) More social fish spent more time with the shoal when it was on the cool side (LME; t2,67 = −4.74, p < 0.001), but not when the shoal was on the warm side. Lines represent significant trends based on LMEs described in the text. Equations for lines in (a) are: y = 4.26x − 113.04 for the shoal on the cooler side; y = 4.26x − 61.26 for no shoal; and y = 4.26x − 14.02 for the shoal on the warmer side. The equation for the line in (b) is y = −1.36x + 56.57. Shaded area is 95% CI around each regression line. Refer to the electronic supplementary material, table S1 for further statistical analysis.
The effect of a shoal in the cooler environment on space use by the focal individual was greater than the effect of a shoal in the warmer environment (figure 2; LME, effect of cool side shoal: t2,68 = −7.31, p < 0.001; effect of warm side shoal: t2,68 = 5.30, p < 0.001). There was also greater variance in spatial positioning of focal individuals when the shoal was on the cool side compared with the warm side (coefficients of variance: cool side shoal = 1916.32; warm side shoal = 1336.37). Smaller individuals also spent more time in the warmer environment across all shoal treatments (figure 1; LME: F1,68 = 4.26, p = 0.048), mirroring their tendency towards higher preferred temperatures when alone.
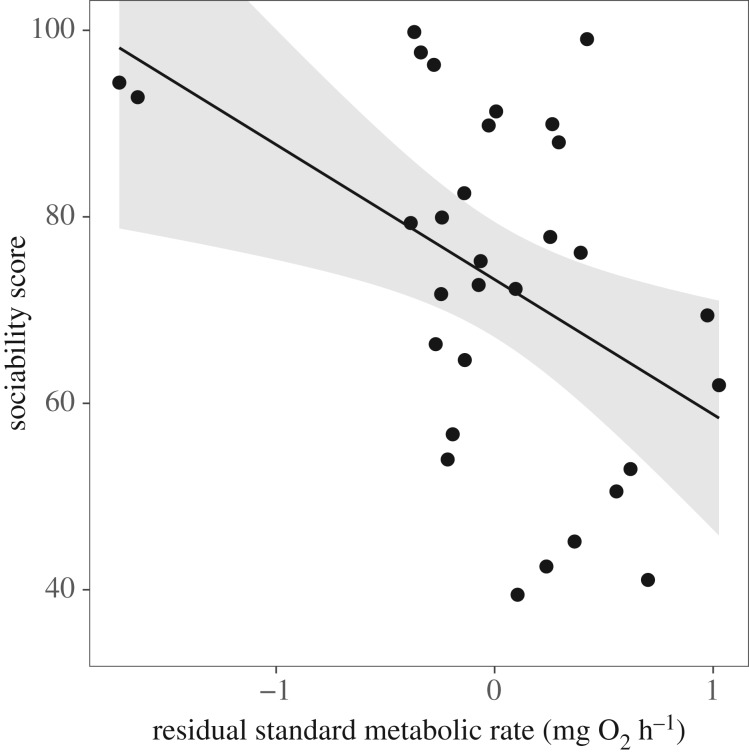
Individual fish varied in their SMR, MMR and AS after correcting for body mass. Sociability decreased with increasing SMR (figure 5; LME: F1,23 = 7.35, p = 0.012; electronic supplementary material, table S2) and increasing MMR (LME: F1,37 = 4.49, p = 0.041), while AS had no effect on sociability (LME: F116 = 0.22, p = 0.644). Animals with a higher SMR spent more time away from the shoal when the shoal was on the warmer side (Pearson correlation; r = 0.42, p = 0.019). No other links were found among preferred temperature, tank spatial usage and any of SMR, MMR or AS. Full details on these correlations can be found in the electronic supplementary material, table S3.
Figure 5.
Fish with a higher SMR were less social. Sociability score was taken as a unitless value, equivalent to the percentage of time an individual spent with the shoal in the absence of a temperature differential. SMR is shown as residual values after correcting for variation in body mass. Shaded area is 95% CI around regression line. The equation for the line is y = −14.47 + 73.27. Refer to the electronic supplementary material, table S2 for further statistical analysis.
4. Discussion
These results demonstrate that animals will compromise exposure to their individually preferred thermal regime in order to associate with conspecifics. However, preferred temperature still influenced where individuals chose to go when a group was present, and therefore the degree of thermal compromise that each individual experienced. Almost all fish shifted towards the shoal's location in either a warm or cool environment, but the magnitude of this shift depended upon individual temperature preference. Many fish had an individual preferred temperature above the warmer environment, but were still willing to make a profound thermal compromise to associate with the shoal on the cooler side.
These results not only indicate that the environment could be an important modulator of group cohesion in gregarious species, but also that exposure to non-preferred temperatures may be a compromise associated with group living that varies among groupmates. In the specific case of sticklebacks, water temperatures in this species' natural habitat can show wide temporal and spatial variation, in some cases spanning a 15°C range on daily and annual bases [35]. Furthermore, riverine systems similar to that from which the experimental fish were sourced can have microthermal gradients of up to 7°C on a scale of centimetres to metres, based on changing depth, shading and floating vegetation [44]. In the wild, sticklebacks exist in variable shoal sizes ranging from a few to dozens of individuals [30]. Depending on factors such as the degree of environmental heterogeneity and the area or volume occupied by the group, it is likely that sticklebacks experience trade-offs between social group membership and exposure to preferred temperatures. Alternatively, individuals may minimize this trade-off by grouping with individuals that prefer similar temperatures.
Exposure to a non-preferred thermal regime is likely to affect the physiology and behaviour of individual animals within social groups. The mechanistic basis for individual variation in thermal preference in ectotherms is not well understood, and the exact physiological costs of being at a non-preferred temperature in ectotherms is in need of further study. However, the available evidence suggests that exposure to temperatures that are warmer or cooler than an individual's preference will affect metabolic demand and energy budgeting among growth, activity and possibly reproduction [22]. If individuals experience varying degrees of thermal compromise while part of a group, foraging activity of the group may not be aligned to the demands of each individual. Additional work could examine how foraging and growth rates change among individuals in response to the temperature experienced by the group. For ectotherms, exposure to cooler or warmer temperatures than preferred could cause an individual to display more or less activity than that of the group, potentially increasing their conspicuousness to predators via the oddity effect [45]. Individual movement speed has been shown to be a key trait allowing individuals to direct group movements in animal collectives [32], and so changes in movement speed could influence which individuals become leaders within groups. AS can be affected by temperature in ectotherms [36], and a reduced AS could also constrain the ability to simultaneously feed and digest food while continuing to match the performance of the group [46,47]. For sticklebacks, growth can occur over a wide range of temperatures (3–29°C), with an optimum for growth occurring around 12–24°C, depending on available rations [35,48]. Finally, it is worth noting escape performance in fishes is affected by temperature [26,49], and fishes exposed to non-preferred temperatures could experience a reduced ability to avoid predators during an attack.
Sociability influenced the degree of thermal compromise individuals made to be with the group, but only when the group was located in the cooler environment. When the shoal was in the warmer environment, nearly all fish moved towards the group regardless of their own level of sociability. This may have been owing to the fact that the warmer environment was closer in temperature to the individual preferences of the majority of fish, which may have, therefore, masked the effect of sociability. The overall picture that emerges from these findings is that individual fish did not elect to move towards cooler temperatures unless a shoal was present in that location and they themselves were relatively social, or unless they already preferred to be at cooler temperatures. The reasons for this shift are unknown but, under conditions of high food availability, warmer temperatures can increase growth rate in ectotherms until the point at which their optimal thermal range for growth is exceeded [50]. This effect could also explain why smaller individuals in the present study preferred warmer temperatures. Studies have shown that individual fish may prefer temperatures which represent their own optimum temperature for growth [23]. In this study, many fish, and especially those that were smaller, had a preferred temperature above even the warmer temperature presented in the shoaling trials, therefore both environments may have presented a compromise, but differing in magnitude. This suggests that there may be relationships among size, preferred temperature and sociability which may be important for group formation and cohesion.
An individual's tendency to associate with a shoal depended on the temperature of the shoal, and those individuals that associated most with a shoal on the warm side associated least when the shoal was on the cool side. Very few individuals were observed that either readily joined, or clearly avoided the shoal at both temperatures. We therefore suggest that the observed behaviours are not just the result of individual variation in sociability, but interactions among sociability, ambient temperature and possible intrinsic factors such as body mass or metabolic rate. Further study into interactions among factors may elucidate the degree to which exposure to non-preferred temperatures may impose a cost in terms of locomotor performance (and by extension, foraging ability and predator avoidance), growth or reproduction.
Metabolic traits, as measured at a common temperature, were not directly related to the temperature preference of individuals, or the degree of thermal compromise they made. Individuals with a lower SMR, however, were more social and so individual metabolic demand may indirectly influence thermal compromises experienced by individual group members via effects on sociability. Group living can increase competition for food [9], and individuals with increased maintenance costs have previously been found to be less social, presumably to increase their own food intake [25]. Previous work has observed a negative correlation between preferred temperature and SMR among individual fishes [22], a relationship that was not observed in the current study. It is possible that the relationships among metabolic traits and temperature preference vary among species or are labile in response to environmental factors [51]. A caveat with the current findings is that SMR and MMR were measured at a single temperature, while fish in the behavioural studies would have been experiencing variable environmental temperatures. Given that SMR and MMR can be affected by temperature in ectotherms [36,52], additional work is required to determine how reaction norms for metabolic traits among individuals across temperatures align with reaction norms for sociability across temperatures.
Any effects on behaviour and physiology experienced by individuals by exposure to non-preferred temperatures could have emergent effects on how social groups are formed, their composition and their functioning as a unit. Social groups such as fish shoals are believed to form through a combination of active and passive processes [53]. Active group assortment occurs when individuals preferentially associate with conspecifics of a particular phenotype, while passive assortment occurs when individuals associate in space and time owing to mutual environmental association, perhaps based on factors such as nutritional requirements, or sensitivity to environmental stressors [54]. The current study also suggests that temperature preference of individuals may interact with sociability to affect these mechanisms of group formation. If given a choice, individuals should associate with conspecifics with a similar preferred temperature to themselves. However, associations based on temperature preference could also occur passively if individuals with similar thermal preferences tend to occupy the same spatial location. Regardless of the mechanism, if social groups consist of individuals with a similar thermal preference, this could cause clustering of individuals with traits correlated with thermal preference and possibly influence assortative mating. Sticklebacks in particular have been shown to demonstrate a degree of shoal fidelity in the wild [30]. While it is likely that familiarity plays an important role in facilitating stable group composition [55], common preferences for temperature among individuals could initially determine the conspecifics with which they associate.
In conclusion, the data here demonstrate that individuals will deviate from their preferred environmental conditions to associate with a group of conspecifics and that thermal compromise in particular is likely to be a cost experienced by individual fish within shoals. Additional work is needed to precisely quantify the costs of exposure to non-preferred temperatures in a social context and how effects on physiology and behaviour may alter the functioning of the group as a whole.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Graham Law, Ross Phillips and Alistair Kirk for assistance with fish husbandry, and Davide Thambithurai, Tommy Norin and Brooke Allan for advice with respirometry. Additional thanks go to a range of members of the Society of Experimental Biology for their comments on the first presentation of these data, and to Neil B. Metcalfe, David McKenzie, and three anonymous reviewers for comments on the manuscript.
Ethics
The procedures described in this paper comply with animal care guidelines approved within the UK and were conducted under Home Office Project 60/4461.
Data accessibility
All data are available in the Mendeley Data Repository (http://dx.doi.org/10.17632/34npwr97vn.1).
Authors' contributions
S.S.K., B.C. and B.A. conceived the study; B.C. collected the data and analysed the data with assistance from S.S.K. and B.A.; B.C. and S.S.K. drafted the manuscript; all authors contributed to further manuscript development and gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
S.S.K. is supported by Natural Environment Research Council Advanced Fellowship NE/J019100/1 and European Research Council starting grant 640004. B.A. was funded by NERC grant no. NE/K00400X/1 awarded to Neil B. Metcalfe, P. Monaghan and P. Shiels.
References
- 1.Herbert-Read JE, et al. 2017. How predation shapes the social interaction rules of shoaling fish. Proc. R. Soc. B 284, 20171126 ( 10.1098/rspb.2017.1126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krause J. 1994. The influence of food competition and prédation risk on size-assortative shoaling in juvenile chub (Leuciscus cephalus). Ethology 96, 105–116. ( 10.1111/j.1439-0310.1994.tb00886.x) [DOI] [Google Scholar]
- 3.Herbert-Read JE, et al. 2016. Proto-cooperation: group hunting sailfish improve hunting success by alternating attacks on grouping prey. Proc. R. Soc. B 283, 20161671 ( 10.1098/rspb.2016.1671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilastro A, Benetton S, Bisazza A. 2003. Female aggregation and male competition reduce costs of sexual harassment in the mosquitofish Gambusia holbrooki. Anim. Behav. 65, 1161–1167. ( 10.1006/anbe.2003.2118) [DOI] [Google Scholar]
- 5.Bekkevold D, Hansen MM, Loeschcke V. 2002. Male reproductive competition in spawning aggregations of cod (Gadus morhua, L.). Mol. Ecol. 11, 91–102. ( 10.1046/j.0962-1083.2001.01424.x) [DOI] [PubMed] [Google Scholar]
- 6.Marras S, Killen S, Lindström J, McKenzie D, Steffensen J, Domenici P. 2015. Fish swimming in schools save energy regardless of their spatial position. Behav. Ecol. Sociobiol. 69, 219–226. ( 10.1007/s00265-014-1834-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herskin J, Steffensen JF. 1998. Energy savings in sea bass swimming in a school: measurements of tail beat frequency and oxygen consumption at different swimming speeds. J. Fish Biol. 53, 366–376. ( 10.1111/j.1095-8649.1998.tb00986.x) [DOI] [Google Scholar]
- 8.Portugal SJ, Hubel TY, Fritz J, Heese S, Trobe D, Voelkl B, Hailes S, Wilson AM, Usherwood JR. 2014. Upwash exploitation and downwash avoidance by flap phasing in ibis formation flight. Nature 505, 399–402. ( 10.1038/nature12939) [DOI] [PubMed] [Google Scholar]
- 9.Webster MM, Hart PJ. 2006. Kleptoparasitic prey competition in shoaling fish: effects of familiarity and prey distribution. Behav. Ecol. 17, 959–964. ( 10.1093/beheco/arl037) [DOI] [Google Scholar]
- 10.Han BA, Park AW, Jolles AE, Altizer S. 2015. Infectious disease transmission and behavioural allometry in wild mammals. J. Anim. Ecol. 84, 637–646. ( 10.1111/1365-2656.12336) [DOI] [PubMed] [Google Scholar]
- 11.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Ward A, Webster M. 2016. Sociality: the behaviour of group-living animals. Berlin, Germany: Springer. [Google Scholar]
- 13.Burton T, Killen SS, Armstrong JD, Metcalfe NB. 2011. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B 278, 3465–3473. ( 10.1098/rspb.2011.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Careau V, Garland T Jr. 2012. Performance, personality, and energetics: correlation, causation, and mechanism. Physiol. Biochem. Zool. 85, 543–571. ( 10.1086/666970) [DOI] [PubMed] [Google Scholar]
- 15.Borowiec BG, O'Connor CM, Goodick K, Scott GR, Balshine S. 2017. The preference for social affiliation renders fish willing to accept lower O2 levels. Physiol. Biochem. Zool. 91, 716–724. ( 10.1086/695566) [DOI] [PubMed] [Google Scholar]
- 16.Lefevre S. 2016. Are global warming and ocean acidification conspiring against marine ectotherms? A meta-analysis of the respiratory effects of elevated temperature, high CO2 and their interaction. Conserv. Physiol. 4, cow009 ( 10.1093/conphys/cow009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandblom E, Gräns A, Axelsson M, Seth H. 2014. Temperature acclimation rate of aerobic scope and feeding metabolism in fishes: implications in a thermally extreme future. Proc. R. Soc. B 281, 20141490 ( 10.1098/rspb.2014.1490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angilletta MJ, Huey RB, Frazier MR. 2010. Thermodynamic effects on organismal performance: is hotter better? Physiol. Biochem. Zool. 83, 197–206. ( 10.1086/648567) [DOI] [PubMed] [Google Scholar]
- 19.Legler ND, Johnson TB, Heath DD, Ludsin SA. 2010. Water temperature and prey size effects on the rate of digestion of larval and early juvenile fish. Trans. Am. Fish. Soc. 139, 868–875. ( 10.1577/T09-212.1) [DOI] [Google Scholar]
- 20.Claireaux G, Couturier C, Groison A-L. 2006. Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J. Exp. Biol. 209, 3420–3428. ( 10.1242/jeb.02346) [DOI] [PubMed] [Google Scholar]
- 21.Artacho P, Jouanneau I, Galliard J-FL. 2013. Interindividual variation in thermal sensitivity of maximal sprint speed, thermal behavior, and resting metabolic rate in a lizard. Physiol. Biochem. Zool. 86, 458–469. ( 10.1086/671376) [DOI] [PubMed] [Google Scholar]
- 22.Killen SS. 2014. Growth trajectory influences temperature preference in fish through an effect on metabolic rate. J. Anim. Ecol. 83, 1513–1522. ( 10.1111/1365-2656.12244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan J, Pether S, Bruce M, Walker S, Herbert N. 2014. Optimum temperatures for growth and feed conversion in cultured hapuku (Polyprion oxygeneios): is there a link to aerobic metabolic scope and final temperature preference? Aquaculture 430, 107–113. ( 10.1016/j.aquaculture.2014.03.046) [DOI] [Google Scholar]
- 24.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 25.Killen SS, Fu C, Wu Q, Wang YX, Fu SJ. 2016. The relationship between metabolic rate and sociability is altered by food-deprivation. Funct. Ecol. 30, 1358–1365. ( 10.1111/1365-2435.12634) [DOI] [Google Scholar]
- 26.Killen SS, Reid D, Marras S, Domenici P. 2015. The interplay between aerobic metabolism and antipredator performance: vigilance is related to recovery rate after exercise. Front. Physiol. 6, 111 ( 10.3389/fphys.2015.00111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norin T, Clark T. 2016. Measurement and relevance of maximum metabolic rate in fishes. J. Fish Biol. 88, 122–151. ( 10.1111/jfb.12796) [DOI] [PubMed] [Google Scholar]
- 28.Metcalfe NB, Van Leeuwen TE, Killen SS. 2016. Does individual variation in metabolic phenotype predict fish behaviour and performance? J. Fish Biol. 88, 298–321. ( 10.1111/jfb.12699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killen SS, Mitchell MD, Rummer JL, Chivers DP, Ferrari MCO, Meekan MG, McCormick MI. 2014. Aerobic scope predicts dominance during early life in a tropical damselfish. Funct. Ecol. 28, 1367–1376. ( 10.1111/1365-2435.12296) [DOI] [Google Scholar]
- 30.Ward AJW, Botham MS, Hoare DJ, James R, Broom M, Godin J-GJ, Krause J. 2002. Association patterns and shoal fidelity in the three-spined stickleback. Proc. R. Soc. Lond. B 269, 2451–2455. ( 10.1098/rspb.2002.2169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Östlund-Nilsson S, Mayer I, Huntingford FA. 2006. Biology of the three-spined stickleback. Boca Raton, FL: CRC Press. [Google Scholar]
- 32.Jolles JW, Boogert NJ, Sridhar VH, Couzin ID, Manica A. 2017. Consistent individual differences drive collective behavior and group functioning of schooling fish. Curr. Biol. 27, 2862–2868.e2867. ( 10.1016/j.cub.2017.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faria JJ, Dyer JR, Clément RO, Couzin ID, Holt N, Ward AJ, Waters D, Krause J. 2010. A novel method for investigating the collective behaviour of fish: introducing ‘Robofish’. Behav. Ecol. Sociobiol. 64, 1211–1218. ( 10.1007/s00265-010-0988-y) [DOI] [Google Scholar]
- 34.Ward AJ, Sumpter DJ, Couzin ID, Hart PJ, Krause J. 2008. Quorum decision-making facilitates information transfer in fish shoals. Proc. Natl Acad. Sci. USA 105, 6948–6953. ( 10.1073/pnas.0710344105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen JRM, Wootton RJ. 1982. The effect of ration and temperature on the growth of the three-spined stickleback, Gasterosteus aculeatus L. J. Fish Biol. 20, 409–422. ( 10.1111/j.1095-8649.1982.tb03934.x) [DOI] [Google Scholar]
- 36.Clark TD, Sandblom E, Jutfelt F. 2013. Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J. Exp. Biol. 216, 2771–2782. ( 10.1242/jeb.084251) [DOI] [PubMed] [Google Scholar]
- 37.Svendsen MBS, Bushnell PG, Steffensen JF. 2016. Design and setup of intermittent-flow respirometry system for aquatic organisms. J. Fish Biol. 88, 26–50. ( 10.1111/jfb.12797) [DOI] [PubMed] [Google Scholar]
- 38.Killen SS, Norin T, Halsey LG. 2017. Do method and species lifestyle affect measures of maximum metabolic rate in fishes? J. Fish Biol. 90, 1037–1046. ( 10.1111/jfb.13195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.R Core Team. 2017. R: a Language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing See http://www.R-project.org/.
- 40.Bates D, et al. 2016. Package ‘lme4’. In R Package Version 11–10, See https://cran.r-project.org/web/packages/Lme4/index.html.
- 41.Sakamoto Y, Ishiguro M, Kitagawa G. 1986. Akaike information criterion statistics. Dordrecht, The Netherlands: D. Reidel Publishing Company. [Google Scholar]
- 42.Bartoń K. 2013. MuMIn: multi-model inference. R package version 1.9. 13. Vienna, Austria: The Comprehensive R Archive Network (CRAN). [Google Scholar]
- 43.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 44.Clarke E, Webb BW, Ladle M. 2009. Microthermal gradients and ecological implications in Dorset rivers. Hydrol. Processes 13, 423–438. ( 10.1002/(SICI)1099-1085(19990228)13:3%3C423::AID-HYP747%3E3.0.CO;2-#) [DOI] [Google Scholar]
- 45.Croft DP, Darden SK, Ruxton GD. 2009. Predation risk as a driving force for phenotypic assortment: a cross-population comparison. Proc. R. Soc. B 276, 1899–1904. ( 10.1098/rspb.2008.1928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Killen SS, Marras S, Steffensen JF, McKenzie DJ. 2012. Aerobic capacity influences the spatial position of individuals within fish schools. Proc. R. Soc. B 279, 357–364. ( 10.1098/rspb.2011.1006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Killen SS, Persson A, Norin T, Killen SS. 2018. Metabolic costs of feeding predictively alter the spatial distribution of individuals in fish schools. Curr. Biol. 28, 1144–1149. ( 10.1016/j.cub.2018.02.043) [DOI] [PubMed] [Google Scholar]
- 48.Lefébure R, Larsson S, Byström P. 2011. A temperature-dependent growth model for the three-spined stickleback Gasterosteus aculeatus. J. Fish Biol. 79, 1815–1827. ( 10.1111/j.1095-8649.2011.03121.x) [DOI] [PubMed] [Google Scholar]
- 49.Domenici P, Blake R. 1997. The kinematics and performance of fish fast-start swimming. J. Exp. Biol. 200, 1165–1178. [DOI] [PubMed] [Google Scholar]
- 50.Jobling M. 1995. Fish bioenergetics. London, UK: Chapman & Hall. [Google Scholar]
- 51.Killen SS, Marras S, Metcalfe NB, McKenzie DJ, Domenici P. 2013. Environmental stressors alter relationships between physiology and behaviour. Trends Ecol. Evol. 28, 651–658. ( 10.1016/j.tree.2013.05.005) [DOI] [PubMed] [Google Scholar]
- 52.Killen SS, Atkinson D, Glazier DS. 2010. The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol. Lett. 13, 184–193. ( 10.1111/j.1461-0248.2009.01415.x) [DOI] [PubMed] [Google Scholar]
- 53.Killen SS, Marras S, Nadler L, Domenici P. 2017. The role of physiological traits in assortment among and within fish shoals. Phil. Trans. R. Soc. B 372, 20160233 ( 10.1098/rstb.2016.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Croft DP, Arrowsmith BJ, Bielby J, Skinner K, White E, Couzin ID, Magurran AE, Ramnarine I, Krause J. 2003. Mechanisms underlying shoal composition in the Trinidadian guppy, Poecilia reticulata. Oikos 100, 429–438. ( 10.1034/j.1600-0706.2003.12023.x) [DOI] [Google Scholar]
- 55.Ward AJW, Hart PJB. 2003. The effects of kin and familiarity on interactions between fish. Fish Fish. 4, 348–358. ( 10.1046/j.1467-2979.2003.00135.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the Mendeley Data Repository (http://dx.doi.org/10.17632/34npwr97vn.1).