Abstract
Although overweight/obesity is a major risk factor for the development of type 2 diabetes mellitus, there is increasing evidence that overweight or obese patients with type 2 diabetes mellitus experience lower mortality compared with patients of normal weight. This paradoxical finding, known as the “obesity paradox,” occurs in other chronic diseases, and in type 2 diabetes mellitus is particularly perplexing given that lifestyle intervention with one goal being weight reduction is an important feature of the management of this condition. We summarize in this review the findings from clinical and epidemiologic studies that have investigated the association between overweight and obesity (usually assessed using body mass index [BMI]) and mortality in type 2 diabetes mellitus and discuss potential causes of the obesity paradox. We conclude that most studies show evidence of an obesity paradox, but important conflicting findings still exist. We also evaluate if potential bias might explain the obesity paradox in diabetes, including, for example, the presence of confounding factors, measurement error due to use of BMI as an index of obesity, and reverse causation.
Keywords: Bias; Body mass index; Diabetes mellitus, type 2; Epidemiology; Mortality; Obesity; Survival
INTRODUCTION
The global prevalence of overweight and obesity has increased dramatically over the past several decades. The World Health Organization estimated that in 2014 more than 1.9 billion adults worldwide were overweight (39%), defined as a body mass index (BMI) of 25.0 to 29.9 kg/m2, and 600 million adults were obese (13%) (BMI ≥30 kg/m2) [1]. Overweight and obesity are established risk factors for the development of metabolic diseases such as diabetes, hypertension, dyslipidemia, cardiovascular disease, and certain cancers [2]. Nevertheless, the association between overweight/obesity and mortality remains an area of interest, importance, and debate. Some previous research has shown a U- or J-shaped association between BMI and mortality, with higher mortality in underweight or obese subjects [3,4]. On the contrary, reviews of other research have demonstrated that adults with a higher BMI have lower mortality compared with leaner individuals in population-based samples with various clinical conditions [5,6]. The lower mortality seen in overweight and obese persons presents a paradox given that greater adiposity is associated with higher risk of multiple chronic conditions associated with a shorter lifespan, and, in particular, type 2 diabetes mellitus.
Obesity is closely linked with the etiology of type 2 diabetes mellitus. Previous research has shown that the relative risk for overweight (25.0≤BMI≤29.9 kg/m2) adults to develop type 2 diabetes mellitus is 4.6-fold for women and 3.5-fold for men compared with their normal weight (18.5≤BMI≤24.9 kg/m2) same sex peers [7]. A Japanese study demonstrated that an increase in BMI of 1 kg/m2 (corresponding to a body weight gain of 2.4 to 2.9 kg) may increase the risk of diabetes by 25% [8]. In addition, weight reduction achieved through a hypocaloric diet or bariatric surgery increases the probability of remission from type 2 diabetes mellitus [9,10]. Furthermore, a lifestyle intervention has been shown to prevent the development of type 2 diabetes mellitus in overweight persons at high risk for this outcome, with weight loss being the dominant predictor of reduced diabetes risk [11]. However, as for the association between BMI and longevity, the predominant evidence from longitudinal observational studies implies that overweight or obese individuals with diabetes have lower mortality compared with normal weight individuals [6]. We reviewed the literature for research on the obesity paradox in type 2 diabetes mellitus to determine how consistently this has been observed and to discuss potential causal and non-causal explanations for this phenomenon.
CLINICAL STUDIES SUPPORTING THE OBESITY PARADOX IN DIABETES
We identified 17 studies investigating the association between obesity defined by BMI and mortality in diabetes that included more than 1,000 subjects who were followed for more than 4 years (Table 1). Eleven studies showed that being overweight or obesity was associated with lower mortality rate. Four studies reported an inverse relationship between BMI and mortality rate [12,13,14,15]. Among men with diabetes from Veterans Affairs medical centers, normal weight subjects had a higher mortality than those with class II obesity (BMI ≥35 kg/m2) [12]. Tseng [13] showed BMI was inversely associated with all cause and cancer or diabetes complication mortality in Taiwanese patients with type 2 diabetes mellitus. Zoppini et al. [16] found that the relationship between body weight and mortality varied by age. In older type 2 diabetes mellitus patients (age ≥65 years), the highest BMI quartile (≥29.9 kg/m2) was associated with lowest all-cause mortality. By contrast, among those <65 years, the highest BMI quartile (≥30.9 kg/m2) had a significantly higher mortality compared to patients in the lowest BMI quartile (≤25.4 kg/m2).
Table 1. Clinical studies about the association of BMI with mortality in diabetes (>1,000 subjects & follow-up >4 years).
| Study | Study type | Population/location | No. of study size | Endpoint | Follow-up, yr | BMI with best outcome, kg/m2 | Findings |
|---|---|---|---|---|---|---|---|
| Kokkinos et al. (2012) [12] | Prospective | United States, men | 4,156 | Total mortality | 7.5 | ≥25 | Inverse association |
| Tseng (2013) [13] | Prospective | Taiwan | 89,056 | Total mortality, cancer or non-cancer mortality | 12 | 25–29.9 | Inverse association |
| Jackson et al. (2014) [14] | Prospective | United States | 2,035 | Total mortality | 9 | 31.03–54.92 | Inverse association |
| Thomas et al. (2014) [15] | Retrospective | United Kingdom | 47,509 | Total mortality | 5 | ≥30 | Inverse association |
| Zoppini et al. (2003) [16] | Retrospective | Italy | 3,398 | Total mortality, CV mortality, cancer mortality | 10 | Old age ≥29.9, Young age <30.9 | Age ≥65 years, inverse association; age <65 years, direct association |
| Mulnier et al. (2006) [17] | Case-control | United Kingdom | 28,725 | Total mortality | 7 | 25–29 | U-shaped association |
| Logue et al. (2013) [18] | Retrospective | United Kingdom | 106,640 | Total mortality, CV mortality | 5 | 25–30 | U-shaped association |
| Zhao et al. (2014) [19] | Prospective | United States | 34,832 | Total mortality | 8.7 | 30–34.9 | U-shaped association |
| Lee et al. (2017) [20] | Prospective | Korea | 905,877 | Total mortality | 10.5 | New diagnosed DM 25–29.4, prevalent DM 26.5–29.4 | U-shaped association |
| Carnethon et al. (2012) [21] | Patient data meta-analysis | United States | 2,625 | Total mortality, CV or non-CV mortality | 27,125 PY | ≥25 | NW associated with higher mortality than OW or OB |
| Costanzo et al. (2015) [22] | Prospective | United Kingdom | 10,568 | Total mortality | 10.6 | 25–29.9 | OW associated with lower mortality risk, but OB was not |
| Chaturvedi et al. (1995) [23] | Prospective | Europeans, East Asians, Native Americans | 2,960 | Total mortality | 13 | No association | |
| Church et al. (2004) [24] | Prospective | United States | 2,196 | Total mortality | 14.6 | No association | |
| Sluik et al. (2011) [26] | Prospective | Europe | 5,435 | Total mortality, CV mortality | 9.3 | No association | |
| Bozorgmanesh et al. (2014) [25] | Prospective | Iran | 1,322 | Total mortality | 9.1 | No association | |
| Eeg-Olofsson et al. (2009) [27] | Prospective | Sweden | 13,087 | Total mortality | 5.6 | <30 | Direct association |
| Tobias et al. (2014) [28] | Prospective | United States | 11,427 | Total mortality, CV mortality | 15.8 | 22.5–24.9 | Direct association (never smoked) |
| Badrick et al. (2017) [29] | Prospective | United Kingdom | 10,464 | Total mortality | 8.7 | 22.5–24.9 | Increased mortality risk from BMI >25 kg/m2 (never smoked) |
BMI, body mass index; CV, cardiovascular; DM, diabetes mellitus; PY, person-years; NW, normal weight; OW, overweight; OB, obese.
Four studies showed a U-shaped association between mortality and BMI [17,18,19,20]. Mulnier et al. [17] reported those with a BMI 15 to 19 or ≥30 kg/m2 had an increased risk of mortality compared with a BMI 20 to 24 kg/m2 in a cohort of British patients with diabetes but no significant mortality risk difference was seen in those with a BMI 25 to 29 kg/m2 compared to 20 to 24 kg/m2. When Logue et al. [18] retrospectively examined subjects within a year of diagnosis of type 2 diabetes mellitus in Scotland, patients with normal weight (20 to <25 kg/m2) or obese (≥35 kg/m2) exhibited higher mortality compared with the overweight group. The U-shaped association between BMI and mortality was also seen among black and white patients with type 2 diabetes mellitus, with obese patients generally at lowest risk of death compared to both normal weight and overweight patients [19]. In the largest study to date that contained nearly 900,000 participants with previously diagnosed or newly diagnosed diabetes based on fasting glucose measurement, Lee et al. [20] also observed a U-shaped association between BMI and mortality in Korean diabetes patients, with lowest mortality between 25.0 to 30.9 kg/m2 depending on sex and new or previously diagnosed diabetes status. Carnethon et al. [21] reported a 2-fold higher all-cause mortality rate in normal weight subjects with new-onset diabetes compared with overweight or obese adults in an analysis of pooled data from five longitudinal cohort studies. Costanzo et al. [22] also showed that the optimal BMI for survival in a British cohort of patients with type 2 diabetes mellitus followed for a median of 10.6 years was 25.0 to 29.9 kg/m2 compared to normal weight or obese BMI categories.
CLINICAL STUDIES AGAINST THE OBESITY PARADOX IN DIABETES
The majority of published research provides evidence favoring the existence of an obesity paradox in type 2 diabetes mellitus. The authors of a number of studies, though, conclude that their results do not support this finding or can be explained by other factors. Chaturvedi and Fuller [23] examined mortality in a population of persons with diabetes from Europe, East Asia, and Native North Americans, but found no clear association between BMI and mortality risk. Although this study included 2,960 diabetic participants, the analysis was subdivided into three groups based on the population sampled, and different BMI cutpoints were used to define excess adiposity. The reduction in power due to such stratification may have resulted in the null findings regarding the association between BMI and mortality. Church et al. [24] observed a higher mortality risk among diabetic obese men (≥30 kg/m2) that disappeared after adjustment for fitness. Bozorgmanesh et al. [25] report that there is no obesity paradox in their population of 1,322 new-onset diabetic patients after adjustment for waist and hip circumference, but Fig. 2D of Bozorgmanesh et al. [25] showing mortality risk by BMI suggests a nadir in the overweight range, which may not have reached significance due to the relatively smaller sample size of this population. Although Sluik et al. [26] conclude that they observed no associations between BMI and mortality in their cohort of European diabetic participants followed for a median of 9.3 years, the results presented in Table 2 of Sluik et al. [26] show a significantly lower mortality risk in men with BMI 25.0 kg/m2 or greater in models adjusted for waist/height ratio, supporting the existence of the obesity paradox. We conclude that these “negative” studies do not convincingly refute the existence of the obesity paradox in type 2 diabetes mellitus.
In addition, two studies observed a direct relationship between BMI and mortality in subjects with diabetes. Eeg-Olofsson et al. [27] reported that the relative risk of total mortality for a 5-unit increase in BMI was 27% in a cohort of 13,087 diabetic participants followed for 6 years. But in this population, there was no significant difference in mortality risk comparing overweight (BMI 25.0 to 29.9 kg/m2) to normal weight participants [27]. In the Nurses' Health Study and Health Professionals Follow-up Study, Tobias et al. [28] found that a J-shaped association between BMI and mortality among all type 2 diabetes mellitus patients and among those who had ever smoked and a direct linear relationship among those who had never smoked suggesting that effect modification by smoking status acts in this association. Furthermore, there was no evidence of lower mortality among diabetes patients who were overweight or obese compared to those with normal weight in this study of health professional populations, whose weight and height were assessed shortly before diagnosis, thereby limiting potential bias due to reverse causation [28]. A prospective study of 10,464 individuals with newly diagnosed diabetes in the United Kingdom and approximately three controls per diabetic case showed no evidence of the obesity paradox in never smokers, but did see a lower mortality risk in ever smokers with BMI between 25.0 to 34.9 kg/m2 [29]. Lastly, a large prospective Swedish study examined mortality in diabetic patients compared to controls within BMI strata, but did not compare mortality within the diabetic population only by degree of adiposity [30]. Therefore, the results of this paper do not directly pertain to this overview that has focused on the role of obesity as a predictor of mortality in diabetes only.
POSSIBLE EXPLANATIONS FOR THE OBESITY PARADOX IN DIABETES
Associations observed in epidemiologic and clinical research may arise due to causation but also for many other reasons. We explore further potential mechanisms that explain the obesity paradox in diabetes by focusing on limitations in study design, the validity of BMI as an indicator of adiposity, and the value of BMI to reflect other important features of body composition.
Limitations of previous epidemiological studies
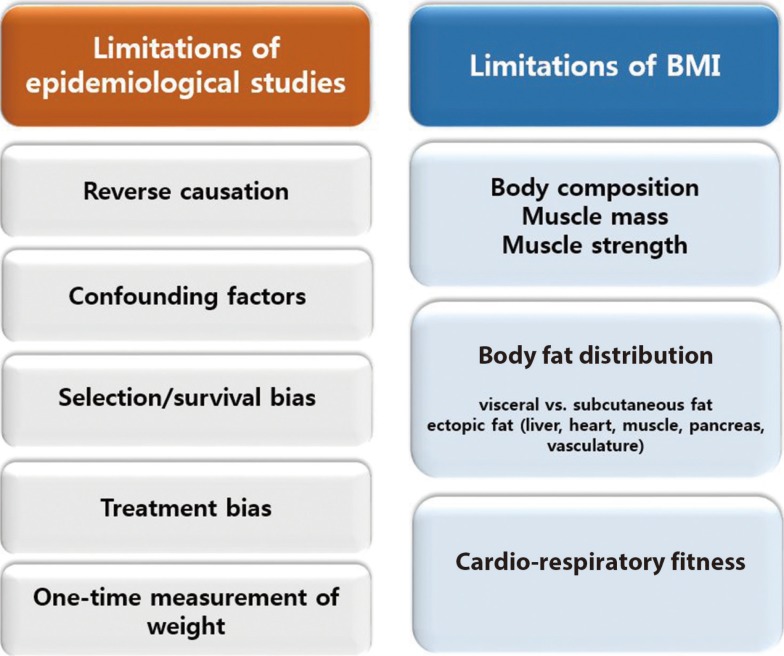
Discordant findings in observational research can often be attributed to population differences, study design issues, and measures taken to reduce and/or control for bias. Specific issues pertinent to the understanding of the obesity paradox are shown in Fig. 1 and discussed further below.
Fig. 1. The limitations of epidemiological studies and body mass index (BMI).
Reverse causation
Reverse causation refers to the outcome causing a change in the exposure instead of the other way around. If the onset of diabetes resulted in a change in body weight such that more severe disease resulted in weight loss, then this would result in an association between lower body weight/BMI and a higher rate of complications or mortality. Much of the research on the obesity paradox in diabetes measured body composition well beyond the onset of diabetes and therefore may be susceptible to reverse causation bias. A notable exception is the study of two populations of health professionals, which included measurements of body weight and height from before diabetes diagnosis in incident cases [28], finding no evidence of the obesity paradox. To mitigate reverse causation by chronic disease induced weight loss, analyses should consider exclusion of patients with major illnesses (cancer, cardiovascular disease, pulmonary disease) at baseline or who died early during follow-up.
Confounding factors
These are factors that are associated with the exposure (BMI) and the outcome (mortality), such as smoking and occult or preexisting chronic disease that lead to both lower BMI and higher mortality risk [9]. Preston and Stokes noted the absence of the obesity paradox among nonsmoking participants of National Health and Nutrition Examination Survey (NHANES) III and NHANES 1999 to 2004 with dysglycemia (glycosylated hemoglobin ≥5.7%) or prior diagnosis of diabetes, and concluded that higher mortality in normal diabetes participants can be explained by the strong inverse correlation between obesity and smoking [31]. Other factors that attenuated the obesity paradox after adjustment include fitness and waist and hip measurements reflecting body composition [24,25].
Selection/survival bias
Patients who develop type 2 diabetes mellitus in the absence of overweight or obesity may have more severe disease or higher mortality risk or both due to genetic susceptibility or the presence of other risk factors for adverse outcomes and a higher mortality rate independent of body composition [32,33]. Thinner diabetes patients in the United Kingdom with BMI <25 kg/m2 were more likely to be treated with insulin than their heavier counterparts [17,27]. Insulin use has been associated with higher risk of adverse outcomes in patients with diabetes, such as, for example, hospitalizations, foot ulcer, and mortality [34,35], and may therefore reflect greater diabetes severity. Lajous et al. [36] examined the outcomes of incident diabetes and mortality in a cohort of 88,373 French women followed for a mean of 16.7 years, and noted lower mortality associated with an overweight or obese BMI in those with diabetes, but higher mortality with the same BMI elevation in women without diabetes. The authors concluded through an analysis of casual diagrams that the apparent better survival with greater BMI among the diabetic women could be explained by selection of persons with diabetes only for assessment of the effect of obesity on mortality since risk of diabetes is affected by the exposure (obesity). They advised against assessing the effects of adiposity on mortality in persons with a disease (diabetes) for which excess adiposity is a risk factor. Badrick et al. [29], however, noted the obesity paradox among not only diabetic participants who smoked, but also non-diabetic participants, and concluded that the selection factors (bias) do not explain this phenomenon. Greater BMI was associated with better survival among older (≥65 years) patients with diabetes in two studies [16,37], suggesting that selective survival may account for the obesity paradox if patients with unhealthy obesity do not survive into old age, while those with a more healthy obesity phenotype do, and comprise a biased comparison group for evaluation of mortality differences with normal weight diabetic patients [38].
Treatment bias
Anti-diabetic medication and behavioral therapy (i.e., diet and physical activity changes) may result in weight loss. We speculate that obese patients may be diagnosed earlier with diabetes resulting in earlier initiation of treatment and potentially few diabetic and cardiovascular complications, leading to improved survival.
One-time measurement of weight
Weight measurement only once at baseline may not reflect a person's weight history. In a recent report that included the Nurses' Health Study I and II and the Health Professionals Follow-Up Study, when BMI was defined using a single baseline measurement, a significant inverse association between overweight and mortality was observed [39]. However, this paradoxical association was reversed in analyses using instead the maximum BMI over 16 years of weight history. Having a history of being overweight or obese was linked to an increase in all-cause mortality risk. Thus, this study implies that maximum BMI reflects weight history better for mortality prediction and its use may reduce reverse causation bias associated with a single BMI assessment.
Limitations of BMI as an obesity index
Although BMI is a simple, convenient, and noninvasive surrogate measure of body fat, it is a measure of excess weight per height rather than a direct measure of fat mass. In addition, age, sex, ethnicity, and muscle mass can affect the relationship between BMI and body fat. To our knowledge, there is no demonstration of an “obesity paradox” based on a direct measurement of body fat. Until such demonstration is made, whether the obesity paradox equals the BMI paradox or the adiposity paradox will remain unknown.
Body composition/muscle mass/muscle strength
BMI fails to distinguish between fat and muscle mass, so that very muscular persons may have a high BMI and be misclassified as overweight or obese. Because low muscle mass has been independently associated with mortality [40], it is possible that higher mortality in normal weight subjects may associated with low muscle mass and not low adiposity [41]. On the other hand, many obese individuals have not only increased fat mass but also increased muscle mass [42,43]. Thus, relatively higher muscle mass may contribute to better survival outcomes in persons classified as obese. In addition, recent research has demonstrated a role for muscle function as well by demonstrating that greater muscle strength predicts lower risk of mortality [44,45,46]. Hamasaki et al. [46] reported that greater handgrip strength was significantly associated with lower mortality in Japanese men with type 2 diabetes mellitus.
Body fat distribution
BMI does not capture information on body fat distribution. Previous research suggests that the fat distribution pattern has a greater influence on cardiometabolic risk than BMI [47,48]. Whereas visceral adiposity plays an important role in development of obesity-associated metabolic disorders, others have proposed that peripheral fat depots may protect against cardiovascular disease [49]. Visceral fat accumulation is greatest in younger adults, and thus would be expected to increase risk of cardiometabolic conditions for many decades [50,51]. Although best assessed using imaging, satisfactory surrogate measures of visceral fat exist such as waist circumference, waist/hip ratio, and waist/height ratio, that are all positively associated with mortality [26]. BMI, however, is not able to capture differences in body fat distribution and therefore may misrepresent some diabetic patients as “normal” when in fact excess visceral adiposity is present. In addition, ectopic fat undetectable by measurement of BMI may occur in multiple other anatomic locations including liver, heart, pancreas, and muscle, and is also linked in these locations to adverse cardiometabolic outcomes [52].
Cardiorespiratory fitness
The Aerobics Center Longitudinal Study showed no significant association between BMI and mortality after adjustment for fitness in males with diabetes, and furthermore observed that fitness predicted mortality independent of BMI [24]. McAuley et al. [53] confirmed these findings in male veterans with type 2 diabetes mellitus. Thus, the level of fitness may affect survival of obese individuals, and potentially may confound research on the association between BMI and mortality in persons with diabetes.
CONCLUSIONS
The obesity paradox in type 2 diabetes mellitus has undergone extensive scrutiny by many investigators of whom many have concluded that high BMI does not cause lower mortality. Some of the theories proposed to explain the obesity paradox as due to bias as opposed to a causal benefit of high BMI on lowering mortality are not universally supported by the data identified in this review. For example, if the obesity paradox is simply the result of bias due to selection (conditioning) of patients with diabetes, why then was it observed in a non-diabetic control population of ever smokers [29,36]? Furthermore, why was the obesity paradox not evident in two well conducted studies that selected diabetic participants [27,28]? We believe that the obesity paradox most likely represents a non-causal association between higher BMI and mortality in diabetes, but await a comprehensive explanation of the remaining loose ends. We believe that the findings of obesity paradox should not change current clinical advice regarding the importance of weight reduction in patients with type 2 diabetes mellitus who are overweight or obese. We agree with Lajous et al. [54] who conclude that we should not recommend that patients with chronic diseases gain weight. A systematic review of randomized controlled trials of weight loss interventions for adults with obesity concluded that these interventions may reduce premature all-cause mortality in adults with obesity [55]. We believe that the literature also provides good support for recommending weight loss to overweight and obese patients who have type 2 diabetes mellitus. The Look AHEAD Study conducted an intensive lifestyle intervention in 5,145 overweight or obese patients with type 2 diabetes mellitus to assess whether this would reduce cardiovascular morbidity and mortality [56]. The intervention group succeeded in losing 8.6% of body weight by year 1 and 6.0% by the end of the study. Although this randomized controlled trial did not demonstrate a reduction in cardiovascular morbidity or mortality, no increase in either of these outcomes occurred, which might have been expected if excess adiposity conferred a survival benefit consistent with the obesity paradox. This clinical trial therefore has a 2-fold benefit as it argues against the obesity paradox and provides reassurance to clinicians that overweight and obese patients with diabetes may safely attempt to reduce weight.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.World Health Organization. Obesity and overweight: fact sheet N°311. Geneva: World Health Organization; 2015. [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Troiano RP, Frongillo EA, Jr, Sobal J, Levitsky DA. The relationship between body weight and mortality: a quantitative analysis of combined information from existing studies. Int J Obes Relat Metab Disord. 1996;20:63–75. [PubMed] [Google Scholar]
- 4.Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, Samet JM. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 5.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 6.Carnethon MR, Rasmussen-Torvik LJ, Palaniappan L. The obesity paradox in diabetes. Curr Cardiol Rep. 2014;16:446. doi: 10.1007/s11886-013-0446-3. [DOI] [PubMed] [Google Scholar]
- 7.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 8.Nagaya T, Yoshida H, Takahashi H, Kawai M. Increases in body mass index, even within non-obese levels, raise the risk for type 2 diabetes mellitus: a follow-up study in a Japanese population. Diabet Med. 2005;22:1107–1111. doi: 10.1111/j.1464-5491.2005.01602.x. [DOI] [PubMed] [Google Scholar]
- 9.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 10.Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, Rodrigues AM, Rehackova L, Adamson AJ, Sniehotta FF, Mathers JC, Ross HM, McIlvenna Y, Stefanetti R, Trenell M, Welsh P, Kean S, Ford I, McConnachie A, Sattar N, Taylor R. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541–551. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- 11.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokkinos P, Myers J, Faselis C, Doumas M, Kheirbek R, Nylen E. BMI-mortality paradox and fitness in African American and Caucasian men with type 2 diabetes. Diabetes Care. 2012;35:1021–1027. doi: 10.2337/dc11-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tseng CH. Obesity paradox: differential effects on cancer and noncancer mortality in patients with type 2 diabetes mellitus. Atherosclerosis. 2013;226:186–192. doi: 10.1016/j.atherosclerosis.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Jackson CL, Yeh HC, Szklo M, Hu FB, Wang NY, Dray-Spira R, Brancati FL. Body-mass index and all-cause mortality in US adults with and without diabetes. J Gen Intern Med. 2014;29:25–33. doi: 10.1007/s11606-013-2553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas G, Khunti K, Curcin V, Molokhia M, Millett C, Majeed A, Paul S. Obesity paradox in people newly diagnosed with type 2 diabetes with and without prior cardiovascular disease. Diabetes Obes Metab. 2014;16:317–325. doi: 10.1111/dom.12217. [DOI] [PubMed] [Google Scholar]
- 16.Zoppini G, Verlato G, Leuzinger C, Zamboni C, Brun E, Bonora E, Muggeo M. Body mass index and the risk of mortality in type II diabetic patients from Verona. Int J Obes Relat Metab Disord. 2003;27:281–285. doi: 10.1038/sj.ijo.802199. [DOI] [PubMed] [Google Scholar]
- 17.Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med. 2006;23:516–521. doi: 10.1111/j.1464-5491.2006.01838.x. [DOI] [PubMed] [Google Scholar]
- 18.Logue J, Walker JJ, Leese G, Lindsay R, McKnight J, Morris A, Philip S, Wild S, Sattar N Scottish Diabetes Research Network Epidemiology Group. Association between BMI measured within a year after diagnosis of type 2 diabetes and mortality. Diabetes Care. 2013;36:887–893. doi: 10.2337/dc12-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Li W, Johnson J, Heymsfield SB, Cefalu WT, Ryan DH, Hu G. Body mass index and the risk of all-cause mortality among patients with type 2 diabetes mellitus. Circulation. 2014;130:2143–2151. doi: 10.1161/CIRCULATIONAHA.114.009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee EY, Lee YH, Yi SW, Shin SA, Yi JJ. BMI and all-cause mortality in normoglycemia, impaired fasting glucose, newly diagnosed diabetes, and prevalent diabetes: a cohort study. Diabetes Care. 2017;40:1026–1033. doi: 10.2337/dc16-1458. [DOI] [PubMed] [Google Scholar]
- 21.Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell-Jenkins B, Dyer AR. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costanzo P, Cleland JG, Pellicori P, Clark AL, Hepburn D, Kilpatrick ES, Perrone-Filardi P, Zhang J, Atkin SL. The obesity paradox in type 2 diabetes mellitus: relationship of body mass index to prognosis: a cohort study. Ann Intern Med. 2015;162:610–618. doi: 10.7326/M14-1551. [DOI] [PubMed] [Google Scholar]
- 23.Chaturvedi N, Fuller JH. Mortality risk by body weight and weight change in people with NIDDM. The WHO Multinational Study of Vascular Disease in Diabetes. Diabetes Care. 1995;18:766–774. doi: 10.2337/diacare.18.6.766. [DOI] [PubMed] [Google Scholar]
- 24.Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27:83–88. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- 25.Bozorgmanesh M, Arshi B, Sheikholeslami F, Azizi F, Hadaegh F. No obesity paradox-BMI incapable of adequately capturing the relation of obesity with all-cause mortality: an inception diabetes cohort study. Int J Endocrinol. 2014;2014:282089. doi: 10.1155/2014/282089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sluik D, Boeing H, Montonen J, Pischon T, Kaaks R, Teucher B, Tjonneland A, Halkjaer J, Berentzen TL, Overvad K, Arriola L, Ardanaz E, Bendinelli B, Grioni S, Tumino R, Sacerdote C, Mattiello A, Spijkerman AM, van der A DL, Beulens JW, van der Schouw YT, Nilsson PM, Hedblad B, Rolandsson O, Franks PW, Nothlings U. Associations between general and abdominal adiposity and mortality in individuals with diabetes mellitus. Am J Epidemiol. 2011;174:22–34. doi: 10.1093/aje/kwr048. [DOI] [PubMed] [Google Scholar]
- 27.Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Nunez L, Gudbjornsdottir S, Eliasson B. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia. 2009;52:65–73. doi: 10.1007/s00125-008-1190-x. [DOI] [PubMed] [Google Scholar]
- 28.Tobias DK, Pan A, Jackson CL, O'Reilly EJ, Ding EL, Willett WC, Manson JE, Hu FB. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233–244. doi: 10.1056/NEJMoa1304501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badrick E, Sperrin M, Buchan IE, Renehan AG. Obesity paradox and mortality in adults with and without incident type 2 diabetes: a matched population-level cohort study. BMJ Open Diabetes Res Care. 2017;5:e000369. doi: 10.1136/bmjdrc-2016-000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edqvist J, Rawshani A, Adiels M, Bjorck L, Lind M, Svensson AM, Gudbjornsdottir S, Sattar N, Rosengren A. BMI and mortality in patients with new-onset type 2 diabetes: a comparison with age- and sex-matched control subjects from the general population. Diabetes Care. 2018;41:485–493. doi: 10.2337/dc17-1309. [DOI] [PubMed] [Google Scholar]
- 31.Preston SH, Stokes A. Obesity paradox: conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiology. 2014;25:454–461. doi: 10.1097/EDE.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry JR, Voight BF, Yengo L, Amin N, Dupuis J, Ganser M, Grallert H, Navarro P, Li M, Qi L, Steinthorsdottir V, Scott RA, Almgren P, Arking DE, Aulchenko Y, Balkau B, Benediktsson R, Bergman RN, Boerwinkle E, Bonnycastle L, Burtt NP, Campbell H, Charpentier G, Collins FS, Gieger C, Green T, Hadjadj S, Hattersley AT, Herder C, Hofman A, Johnson AD, Kottgen A, Kraft P, Labrune Y, Langenberg C, Manning AK, Mohlke KL, Morris AP, Oostra B, Pankow J, Petersen AK, Pramstaller PP, Prokopenko I, Rathmann W, Rayner W, Roden M, Rudan I, Rybin D, Scott LJ, Sigurdsson G, Sladek R, Thorleifsson G, Thorsteinsdottir U, Tuomilehto J, Uitterlinden AG, Vivequin S, Weedon MN, Wright AF MAGIC; DIAGRAM Consortium; GIANT Consortium. Hu FB, Illig T, Kao L, Meigs JB, Wilson JF, Stefansson K, van Duijn C, Altschuler D, Morris AD, Boehnke M, McCarthy MI, Froguel P, Palmer CN, Wareham NJ, Groop L, Frayling TM, Cauchi S. Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases. PLoS Genet. 2012;8:e1002741. doi: 10.1371/journal.pgen.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carnethon MR. Diabetes mellitus in the absence of obesity: a risky condition. Circulation. 2014;130:2131–2132. doi: 10.1161/CIRCULATIONAHA.114.013534. [DOI] [PubMed] [Google Scholar]
- 34.Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22:1036–1042. doi: 10.2337/diacare.22.7.1036. [DOI] [PubMed] [Google Scholar]
- 35.Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, Everson-Stewart S, Kinder L, Oliver M, Boyko EJ, Katon WJ. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 36.Lajous M, Bijon A, Fagherazzi G, Boutron-Ruault MC, Balkau B, Clavel-Chapelon F, Hernan MA. Body mass index, diabetes, and mortality in French women: explaining away a “paradox”. Epidemiology. 2014;25:10–14. doi: 10.1097/EDE.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yano Y, Kario K, Ishikawa S, Ojima T, Gotoh T, Kayaba K, Tsutsumi A, Shimada K, Nakamura Y, Kajii E JMS Cohort Study Group. Associations between diabetes, leanness, and the risk of death in the Japanese general population: the Jichi Medical School Cohort Study. Diabetes Care. 2013;36:1186–1192. doi: 10.2337/dc12-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hainer V, Aldhoon-Hainerova I. Obesity paradox does exist. Diabetes Care. 2013;36(Suppl 2):S276–S281. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu E, Ley SH, Manson JE, Willett W, Satija A, Hu FB, Stokes A. Weight history and all-cause and cause-specific mortality in three prospective cohort studies. Ann Intern Med. 2017;166:613–620. doi: 10.7326/M16-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JH, Lim S, Choi SH, Kim KM, Yoon JW, Kim KW, Lim JY, Park KS, Jang HC. Sarcopenia: an independent predictor of mortality in community-dwelling older Korean men. J Gerontol A Biol Sci Med Sci. 2014;69:1244–1252. doi: 10.1093/gerona/glu050. [DOI] [PubMed] [Google Scholar]
- 41.Batsis JA, Mackenzie TA, Emeny RT, Lopez-Jimenez F, Bartels SJ. Low lean mass with and without obesity, and mortality: results from the 1999–2004 National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci. 2017;72:1445–1451. doi: 10.1093/gerona/glx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–829. doi: 10.1016/S2213-8587(14)70034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seidell JC, Bjorntorp P, Sjostrom L, Sannerstedt R, Krotkiewski M, Kvist H. Regional distribution of muscle and fat mass in men: new insight into the risk of abdominal obesity using computed tomography. Int J Obes. 1989;13:289–303. [PubMed] [Google Scholar]
- 44.Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55:M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 45.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Jr, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R, Rahman O, Swaminathan S, Iqbal R, Gupta R, Lear SA, Oguz A, Yusoff K, Zatonska K, Chifamba J, Igumbor E, Mohan V, Anjana RM, Gu H, Li W, Yusuf S Prospective Urban Rural Epidemiology (PURE) Study investigators. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386:266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 46.Hamasaki H, Kawashima Y, Katsuyama H, Sako A, Goto A, Yanai H. Association of handgrip strength with hospitalization, cardiovascular events, and mortality in Japanese patients with type 2 diabetes. Sci Rep. 2017;7:7041. doi: 10.1038/s41598-017-07438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neeland IJ, Turer AT, Ayers CR, Berry JD, Rohatgi A, Das SR, Khera A, Vega GL, McGuire DK, Grundy SM, de Lemos JA. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol. 2015;65:2150–2151. doi: 10.1016/j.jacc.2015.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. 2008;57:1269–1275. doi: 10.2337/db07-1378. [DOI] [PubMed] [Google Scholar]
- 49.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, Adams PW. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 50.Lee CG, Fujimoto WY, Brunzell JD, Kahn SE, McNeely MJ, Leonetti DL, Boyko EJ. Intra-abdominal fat accumulation is greatest at younger ages in Japanese-American adults. Diabetes Res Clin Pract. 2010;89:58–64. doi: 10.1016/j.diabres.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hairston KG, Scherzinger A, Foy C, Hanley AJ, McCorkle O, Haffner S, Norris JM, Bryer-Ash M, Wagenknecht LE. Five-year change in visceral adipose tissue quantity in a minority cohort: the Insulin Resistance Atherosclerosis Study (IRAS) family study. Diabetes Care. 2009;32:1553–1555. doi: 10.2337/dc09-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013;169:166–176. doi: 10.1016/j.ijcard.2013.08.077. [DOI] [PubMed] [Google Scholar]
- 53.McAuley PA, Myers JN, Abella JP, Tan SY, Froelicher VF. Exercise capacity and body mass as predictors of mortality among male veterans with type 2 diabetes. Diabetes Care. 2007;30:1539–1543. doi: 10.2337/dc06-2397. [DOI] [PubMed] [Google Scholar]
- 54.Lajous M, Banack HR, Kaufman JS, Hernan MA. Should patients with chronic disease be told to gain weight? The obesity paradox and selection bias. Am J Med. 2015;128:334–336. doi: 10.1016/j.amjmed.2014.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, Sharma P, Fraser C, MacLennan G. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849. doi: 10.1136/bmj.j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Look AHEAD, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]