Abstract
Purpose
In this study, we aimed to evaluate lymphocyte-activation gene-3 (LAG-3) expression and its prognostic value in neoadjuvant-treated triple-negative breast cancer (TNBC).
Methods
LAG-3, programmed death-1 (PD-1), programmed death ligand-1 (PD-L1), and CD8+ tumor-infiltrating lymphocyte (TILs) levels were examined using immunohistochemistry in 148 preand 114 post-neoadjuvant chemotherapy (NACT) specimens of human TNBC tissue. Correlations between expression levels and clinicopathological features were analyzed. Prognostic values for combined detection in TNBC following NACT were evaluated.
Results
In pre-NACT specimens, LAG-3 expression showed a significant association with pathological complete response (pCR, p=0.038) and was correlated with PD-1 (p<0.001) and PD-L1 (p=0.008). In post-NACT specimens, high expression of LAG-3 showed significant effects on nodal status (p=0.023) and PD-1 (p<0.001). The expression of immune markers on TILs significantly increased following NACT. Multivariate analysis indicated that only nodal status (odds ratio [OR], 0.226; 95% confidence interval [CI], 0.079–0.644; p=0.005) and high quantities of CD8+TILs (OR, 3.186; 95% CI, 1.314–7.721; p=0.010) are independent predictors of pCR. Nodal status (hazard ratio [HR], 2.666; 95% CI, 1.271–5.594; p=0.010), CD8+TILs (HR, 0.313; 95% CI, 0.139–0.705; p=0.005), and the LAG-3-high/PD-L1-high group (HR, 2.829; 95% CI, 1.050–7.623; p=0.040) provided prognostic values for patients with TNBC following NACT.
Conclusion
CD8+TILs were sensitive predictive markers in response to NACT. High expression of LAG-3 in residual tissues, especially in combination with PD-L1, was associated with poor prognosis.
Keywords: Lymphocyte-activation gene-3 protein, Neoadjuvant therapy, Programmed death-1, Programmed death ligand-1, Triple negative breast neoplasms
INTRODUCTION
Triple-negative breast cancer (TNBC) is an aggressive cancer phenotype characterized by the lack of expression of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2). It accounts for 15% to 20% of all breast cancer subtypes [1]. Chemotherapy is the only therapeutic method used for TNBC because of the lack of effective endocrine and targeted therapies.
Systemic neoadjuvant therapy is the treatment of choice for patients with locally advanced breast cancer. Pathologically complete response (pCR) is an indicator of good prognosis after neoadjuvant therapy. Previous clinical trials evaluating the efficacy of neoadjuvant chemotherapy (NACT) revealed that patients with TNBC are more sensitive to chemotherapy and show higher rates of pCR than patients with other breast cancer subtypes [2]. Therefore, identifying novel biological markers for the prediction of pCR rates or the survival of non-pCR patients is required for patients with TNBC.
Previous studies have shown that the immune microenvironment plays a key role in cancer progression and response to therapies. Tumor-infiltrating lymphocytes (TILs) have prognostic and predictive values in both adjuvant chemotherapy and NACT in breast cancer, especially in triple-negative and HER2-overexpressing breast cancers [3,4]. CD8+TILs, a subtype of TILs, are a good prognostic factor for TNBC and are related to pCR after NACT [5].
Immune checkpoints play vital roles in tumor immune escape. Cancer immunotherapy, regarded as one of the most important scientific breakthroughs, can reverse tumor immune escape by suppressing immune checkpoints. Programmed death-1 (PD-1) is a well-known immunological checkpoint and the PD-1/programmed death ligand-1 (PD-L1) axis can mediate a key immune modulatory pathway. It has recently been shown that immune checkpoint inhibitors of PD-1/PD-L1 are associated with chemotherapy response in melanoma and lung cancer and may stimulate TILs [6,7]. However, less than 25% of patients benefit from anti-PD-1/PD-L1 therapy and this treatment has not been evaluated in breast cancer. Therefore, studies exploring the role and relationship of immune checkpoints and TILs in TNBC are required to highlight the urgent need for novel immunotherapy strategies and validated immune biomarkers for guiding patient selection.
Lymphocyte-activation gene-3 (LAG-3), designated as CD223, is another important checkpoint of the immunoglobulin superfamily [8]. LAG-3 plays a negative regulatory role on T cell proliferation and activation and may exert synergistic effects with PD-1/PD-L1 to mediate immune exhaustion. Co-expression of LAG-3 and PD-1 on tolerized TILs indicates their possible contribution to tumor-mediated immune suppression [9]. Recent studies have shown the co-expression of LAG-3 and PD-1 in 15% of patients with TNBC [10]; however, the relationship between these immune checkpoints and their predictive effects remains unclear. Therefore, studies investigating the correlation of LAG-3, PD1/PD-L1, and CD8+TIL expression in TNBC with prognosis are needed to reveal the immunosuppressive effects of LAG-3 and evaluate its efficacy in patients with TNBC.
In this study, we retrospectively evaluated the expression of LAG-3 in TNBC patients treated with NACT and investigated its correlation with clinical characteristics, patient prognosis, and response to chemotherapy. We also analyzed the relationship between LAG-3 and PD-1, PD-L1, and CD8+TILs to find TNBC patients who may benefit from the addition of immunotherapy to standard chemotherapeutic regimens.
METHODS
Patients and treatments
Breast cancer tissue samples (148 biopsy [pre-NACT] and 114 surgery [post-NACT] specimens) used in this study were obtained from Harbin Medical University Cancer Hospital. All patients were female and specimens from patients who achieved pCR were not evaluated. Specimens were from invasive ductal carcinomas. The patients were first diagnosed between January 2009 and December 2012. All patients with confirmed histological diagnosis of TNBC received no treatment before diagnosis and presented complete clinicopathological and follow-up data. pCR was defined as the absence of invasive carcinoma in breast and sampled lymph nodes (ypT0 ypN0). Disease-free survival (DFS) was defined as the interval from the date of primary surgery to the first local recurrence, distant recurrence, or death from any cause.
A total of 148 patients received pre-NACT anthracycline- and taxane-based chemotherapy regimens for a median of four cycles (range, three to six cycles) and no other anticancer treatments. After NACT, all patients underwent mastectomy or breast-conserving surgery. Subsequently, additional cycles of chemotherapy (anthracycline- and taxane-based regimens) were administered to complete six cycles at the discretion of the treating physician. Radiation was determined based on disease status before NACT.
In our analysis, patients treated with NACT were followed up for 5 to 91 months, with a median of 66.5 months. The median age of patients was 49 years (range, 27–69 years). Among these cases, 34 patients (23.0%) achieved pCR. Table 1 provides the details of the cohort.
Table 1. Expression of LAG-3 in pre-NACT specimens and the association with clinicopathologic characteristics and other variables.
| Variable | Total (n=148) No. (%) | LAG-3 | p-value | |
|---|---|---|---|---|
| High (n=33) No. (%) | Low (n=115) No. (%) | |||
| Age (yr) | 0.305 | |||
| < 45 | 39 (26.4) | 6 (18.2) | 33 (28.7) | |
| ≥ 45, < 60 | 91 (61.5) | 21 (63.6) | 70 (60.9) | |
| ≥ 60 | 18 (12.2) | 6 (18.1) | 12 (10.4) | |
| Menstruation | 0.638 | |||
| Pre | 86 (58.1) | 18 (54.5) | 68 (59.1) | |
| Post | 62 (41.9) | 15 (45.5) | 47 (40.9) | |
| Tumor size (cm) | 0.053 | |||
| ≤2 | 12 (8.1) | 6 (18.2) | 6 (5.2) | |
| > 2, ≤ 5 | 92 (62.2) | 19 (57.6) | 73 (63.5) | |
| >5 | 44 (29.7) | 8 (24.2) | 36 (31.3) | |
| Nodal status | 0.843 | |||
| Positive | 56 (37.8) | 12 (36.4) | 44 (38.3) | |
| Negative | 92 (62.2) | 21 (63.6) | 71 (61.7) | |
| NACT cycles | 0.057 | |||
| 3–4 | 96 (64.9) | 26 (78.8) | 70 (60.9) | |
| 5–6 | 52 (35.1) | 7 (21.2) | 45 (39.1) | |
| Pathological response | 0.038 | |||
| pCR | 34 (23.0) | 12 (36.4) | 22 (19.1) | |
| Non-pCR | 114 (77.0) | 21 (63.6) | 93 (80.9) | |
| PD-1 | < 0.001 | |||
| High | 40 (27.0) | 21 (63.6) | 19 (16.5) | |
| Low | 108 (73.0) | 12 (36.4) | 96 (83.5) | |
| CD8+TILs | 0.336 | |||
| High | 61 (41.2) | 16 (48.5) | 45 (39.1) | |
| Low | 87 (58.8) | 17 (51.5) | 70 (60.9) | |
| PD-L1 | 0.008 | |||
| High | 48 (32.4) | 17 (51.5) | 31 (27.0) | |
| Low | 100 (67.6) | 16 (48.5) | 84 (73.0) | |
LAG-3=lymphocyte-activation gene-3; NACT=neoadjuvant chemotherapy; pCR=pathological complete response; PD-1=programmed death-1; TILs=tumor-infiltrating lymphocytes; PD-L1=programmed death ligand-1.
Clinicopathological data and follow-up results were obtained for all patients after signing the informed consent form. This study was conducted with the approval of the Ethics Committee of the Harbin Medical University Cancer Hospital (IRB approval number: GZR2017-01).
Immunohistochemistry
Formalin-fixed and paraffin-embedded sections (4 µm) from TNBC samples were used for immunohistochemistry (IHC) staining. Briefly, these sections were deparaffinized with xylene and rehydrated with a graded ethanol series (100%, 95%, and 70%) and distilled water according to standard immunohistochemical protocols. After rehydration, heat-induced antigen retrieval was performed using citrate buffer (pH=6.0) for 1.5 minutes in a water bath. Tissue sections were then soaked in a 3% H2O2 solution for 30 minutes. Rabbit monoclonal LAG-3 (1:1,000, EPR4392; Abcam, Cambridge, USA), PD-1 (1:200, EPR4877; Abcam), PD-L1 (1:200, EPR19759; Abcam), or CD8 (1:100, ab4055; Abcam) antibody was applied at 4℃ overnight. Each section was incubated with secondary antibody at 37℃ for 20 minutes, then visualized by diaminobenzidine for 3 to 10 minutes and counterstained with hematoxylin. For the negative control samples, the primary antibody was substituted with phosphate-buffered saline. Positive control samples were developed according to the manufacturer's instructions for each antibody.
Pathological evaluation of LAG-3, PD1, CD8+TILs, and PD-L1
Five randomly selected high-power fields (400×) were averaged in each case to calculate the percentage of positive cells. LAG-3, PD-1, and CD8+TILs were evaluated in tumor stroma. The high and low cutoff value for the first two indices was 5%, as described in prior studies [11,12]. CD8+TILs were evaluated using the method described in a previous study [13]. PD-L1 staining was evaluated in both tumor and stromal cells using the histo-score (H-score) system [14]. H-score can assess the extent of immunoreactivity based on staining intensity (1+, 2+, and 3+) and percentage of staining in invasive tumor cells. For PD-L1, a cutoff H-score ranging from 0 to 99 indicates low expression, whereas an H-score of 100 to 300 indicates high expression.
All IHC results were independently reviewed by two experienced pathologists (Ling Q and Yang L) who were blinded to the clinical data. When inconsistencies were greater than 5%, re-evaluation was conducted to reach a consensus.
Statistical analysis
The chi-square test was used to evaluate the association of LAG-3 expression with clinicopathologic features and biomarkers as variables. Fisher exact test was performed only when necessary. A logistic regression model was used to investigate the relationships between correlative factors and pCR. Paired t-tests were applied to compare differences in the four markers in biopsy and surgery specimens. Survival data were estimated using the Kaplan-Meier method and analyzed with a log-rank test. Univariate and multivariate analyses for DFS were performed using the Cox proportional hazard regression model. Hazard ratios and their 95% confidence intervals (CIs) were recorded for each marker. All statistical analyses were performed using SPSS version 20.0 (IBM, Armonk, USA). All tests were two-sided and a p-value < 0.05 was considered statistically significant.
RESULTS
Levels of LAG-3, PD-1, CD8+TILs, and PD-L1 in pre- or post-NACT specimens are associated with clinicopathological characteristics
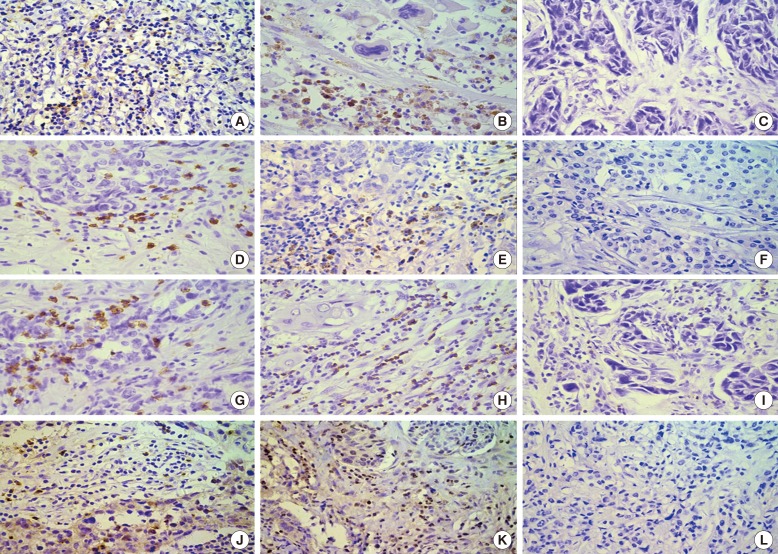
LAG-3, PD-1, PD-L1, and CD8+TILs in cell membranes were analyzed by IHC staining (Figure 1). LAG-3, PD-1, and CD8+TILs were detected in tumor stroma and PD-L1 was expressed in both tumor and stromal cells.
Figure 1. Expressions of lymphocyte-activation gene-3 (LAG-3), programmed death-1 (PD-1), CD8+ tumor-infiltrating lymphocytes (TILs), and programmed death ligand-1 (PD-L1) in triple-negative breast cancer (TNBC) as observed during immunohistochemistry (×400). High expression of LAG-3 in pre-neoadjuvant chemotherapy (NACT) (A) and in post-NACT (B) specimens; low expression of LAG-3 in TNBC (C); high expression of PD-1 in pre-NACT (D) and in post-NACT (E) specimens; low expression of PD-1 in TNBC (F); high expression of CD8+TILs in pre-NACT (G) and in post-NACT (H) specimens; low expression of CD8+TILs TNBC (I); high expression of PD-L1 in pre-NACT (J) and in post-NACT (K) specimens; low expression of PD-L1 TNBC (L).
In pre-NACT specimens, 33 patients (22.3%) showed high expression of LAG-3 on TILs and 115 (77.7%) patients showed low expression. A total of 40 patients (27.0%) had high expression of PD-1 on TILs and 108 patients (73.0%) had low expression. High quantities of CD8+TILs were observed in 61 patients (41.2%). A total of 48 samples (32.4%) were stained positive for PD-L1 on tumor cells and/or TILs. Table 1 presents LAG-3 and other marker expression levels on TILs and their correlations with clinicopathologic characteristics. High expression of LAG-3 showed significant effects on pCR (p=0.038) and a correlation was observed between high expression of LAG-3 and PD-1 (p<0.001) and PD-L1 (p=0.008).
Table 2 summarizes the clinicopathological characteristics and marker expression in 114 patients with post-NACT samples. High expression of LAG-3, PD-1, and PD-L1 was seen in 33.3%, 28.9%, and 37.7% of samples, respectively. A high number of CD8+TILs was seen in 50.9% of samples. The expression of these markers was higher in post-NACT specimens than in pre-NACT specimens. The pre- and post-NACT tumor specimens were matched and the changes in expression level for each marker before and after NACT are shown in Figure 2. Expression of LAG-3, PD-1, and CD8+ on TILs showed significant changes after NACT (p<0.05), whereas the expression of PD-L1 on TILs and tumor cells did not change (p=0.073). Expression of LAG-3 on TILs showed significant differences with nodal status (p=0.023) and PD-1 (p<0.001). Meanwhile, no significant differences were observed between age, menstruation, tumor size, histological grade, Ki-67, and PD-L1 expression and the number of CD8+TILs (p>0.05).
Table 2. Expression of LAG-3 in post-NACT specimens and the association with clinicopathologic characteristics and other variables.
| Variable | Total (n=114) No. (%) | LAG-3 | p-value | |
|---|---|---|---|---|
| High (n=38) No. (%) | Low (n=76) No. (%) | |||
| Age (yr) | 0.140 | |||
| < 45 | 30 (26.3) | 10 (26.3) | 20 (26.3) | |
| ≥ 45, < 60 | 72 (63.2) | 21 (55.3) | 51 (67.1) | |
| ≥ 60 | 12 (10.5) | 7 (18.4) | 5 (6.6) | |
| Menstruation | 0.421 | |||
| Pre | 66 (57.9) | 24 (63.2) | 42 (55.3) | |
| Post | 48 (42.1) | 14 (36.8) | 34 (44.7) | |
| Tumor size (cm) | 0.959 | |||
| ≤2 | 46 (40.4) | 16 (42.1) | 30 (39.5) | |
| > 2, ≤ 5 | 50 (43.9) | 16 (42.1) | 34 (44.7) | |
| >5 | 18 (15.8) | 6 (15.8) | 12 (15.8) | |
| Histological grade | 0.227 | |||
| I, II | 48 (42.1) | 13 (34.2) | 35 (46.1) | |
| III | 66 (57.9) | 25 (65.8) | 41 (53.9) | |
| Nodal status | 0.023 | |||
| Positive | 64 (56.1) | 27 (71.1) | 37 (48.7) | |
| Negative | 50 (43.9) | 11 (28.9) | 39 (51.3) | |
| Ki-67 (%) | 0.330 | |||
| ≤ 14 | 24 (21.1) | 6 (15.8) | 18 (23.7) | |
| > 15 | 90 (78.9) | 32 (84.2) | 58 (76.3) | |
| PD-1 | < 0.001 | |||
| High | 33 (28.9) | 23 (60.5) | 10 (13.2) | |
| Low | 81 (71.1) | 15 (39.5) | 66 (86.8) | |
| CD8+TILs | 0.289 | |||
| High | 58 (50.9) | 22 (57.9) | 36 (47.4) | |
| Low | 56 (49.1) | 16 (42.1) | 40 (52.6) | |
| PD-L1 | 0.056 | |||
| High | 43 (37.7) | 19 (50.0) | 24 (31.6) | |
| Low | 71 (62.3) | 19 (50.0) | 52 (68.4) | |
LAG-3=lymphocyte-activation gene-3; NACT=neoadjuvant chemotherapy; PD-1=programmed death-1; TILs=tumor-infiltrating lymphocytes; PD-L1=programmed death ligand-1.
Figure 2. One-to-one correspondence of immune markers between biopsy (pre-neoadjuvant chemotherapy [NACT]) and resected specimens (post-NACT) for all cases. Changes of lymphocyte-activation gene-3 (LAG-3) (A), programmed death-1 (PD-1) (B), CD8+ tumor-infiltrating lymphocytes (TILs) (C), and programmed death ligand-1 (PD-L1) (D) before and after NACT.
CD8+TILs in pre-NACT specimens are independent prognostic factors for pCR
To identify the possible factors that affect pCR, we used univariate and multivariate logistic analyses, as shown in Table 3. Univariate analyses were performed on the following variables: age; menstruation; tumor size; nodal status; NACT cycles; and levels of LAG-3, PD-1, CD8+TILs, and PD-L1. The results revealed that nodal status; NACT cycles; and levels of LAG-3, CD8+TILs, and PD-L1 were predictors of pCR after NACT. On the other hand, only nodal status and expression of CD8 on TILs showed significance (p=0.005 and p=0.010, respectively) in the prediction of pCR in multivariate analysis.
Table 3. Univariate and multivariate logistic analysis for predicting pathological complete response.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age (yr) | ||||||
| Overall | - | - | 0.542 | - | - | - |
| < 45 vs. ≥ 45, < 60 | 0.533 | 0.152–1.866 | 0.325 | - | - | - |
| < 45 vs. ≥ 60 | 0.556 | 0.185–1.666 | 0.294 | - | - | - |
| Menstruation | ||||||
| Pre vs. post | 0.963 | 0.442–2.095 | 0.923 | - | - | - |
| Tumor size (cm) | ||||||
| Overall | - | - | 0.222 | - | - | - |
| ≤ 2 vs. > 2, ≤ 5 | 3.083 | 0.703–13.518 | 0.135 | - | - | - |
| ≤ 2 vs. > 5 | 2.145 | 0.805–5.713 | 0.127 | - | - | - |
| Nodal status | ||||||
| Positive vs. negative | 0.274 | 0.105–0.714 | 0.008 | 0.226 | 0.079–0.644 | 0.005 |
| NACT cycles | ||||||
| 3–4 vs. 5–6 | 0.398 | 0.160–0.990 | 0.047 | 0.399 | 0.150–1.061 | 0.066 |
| LAG-3 | ||||||
| High vs. low | 2.416 | 1.035–5.640 | 0.041 | 2.169 | 0.837–5.578 | 0.112 |
| PD-1 | ||||||
| High vs. low | 0.636 | 0.252–1.604 | 0.338 | - | - | - |
| CD8+TILs | ||||||
| High vs. low | 2.988 | 1.354–6.594 | 0.007 | 3.186 | 1.314–7.721 | 0.010 |
| PD-L1 | ||||||
| High vs. low | 2.278 | 1.036–5.007 | 0.041 | 1.336 | 0.541–3.301 | 0.530 |
OR=odds ratio; CI=confidence interval; NACT=neoadjuvant chemotherapy; LAG-3=lymphocyte-activation gene-3; PD-1=programmed death-1; TILs=tumor-infiltrating lymphocytes; PD-L1=programmed death ligand-1.
Post-NACT expression levels of LAG-3 and other markers are associated with patient prognosis
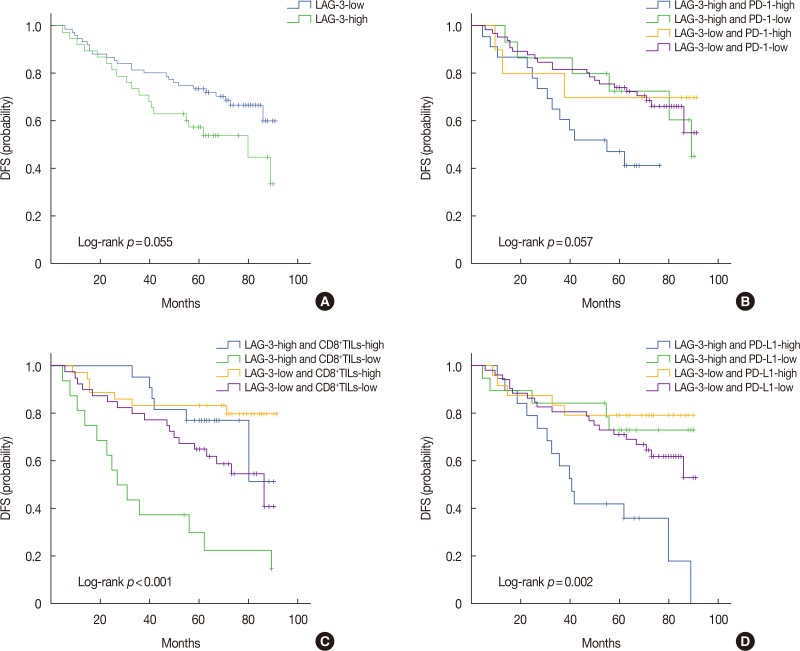
Median DFS was 33 months (range, 5–89 months). Kaplan-Meier analysis revealed no significant difference (p=0.055) in the DFS of post-NACT patients with high and low expression of LAG-3 (Figure 3A).
Figure 3. Expression of lymphocyte-activation gene-3 (LAG-3) and combined prognostic value of LAG-3 and other markers (programmed death-1 [PD-1], CD8+ tumor-infiltrating lymphocytes [TILs], and programmed death ligand-1 [PD-L1]) in triple-negative breast cancer. Kaplan-Meier curves showing estimated disease-free survival (DFS) for the LAG-3 expression (A), combined effects of LAG-3 and PD-1 (B), combined effects of LAG-3 and CD8+TILs (C), and combined effects of LAG-3 and PD-L1 (D).
To further study the combined prognostic values of LAG-3 and other markers, patients were divided into four groups: LAG-3-high/PD-1-high, LAG-3-high/PD-1-low, LAG-3-low/PD-1-high, and LAG-3-low/PD-1-low. The groups were formed with CD8+TILs and PD-L1. Kaplan-Meier graphical analysis showed that LAG-3-high/PD-1-high, LAG-3-high/CD8+TILs-low, and LAG-3-high/PD-L1-high groups had the poorest prognosis. In addition, DFS differed significantly among the four groups for the combined effects of LAG-3 and CD8+TILs (p<0.001) or PD-L1 (p=0.002) (Figure 3C and 3D). No significant difference (p=0.057) (Figure 3B) was observed between LAG-3 and PD-1.
Prognostic value of combined detection of markers
To identify the possible factors that affect DFS, we used univariate and multivariate Cox proportional hazard regression analysis and the results are shown in Table 4. Based on Kaplan-Meier analysis, we incorporated LAG-3-high/PD-1-high, LAG-3-high/CD8+TILs-low, and LAG-3-high/PD-L1-high groups into the Cox risk model. Univariate analysis revealed that nodal status, Ki-67, CD8+TILs, LAG-3-high/PD-1-high, LAG-3-high/CD8+TILs-low, and LAG-3-high/PD-L1-high were predictors of DFS in post-NACT patients. Meanwhile, nodal status (p=0.010), CD8+TILs (p=0.005), and LAG-3-high/PD-L1-high (p=0.040) exhibited prognostic value in multivariate analysis.
Table 4. Prognostic value of the variables in univariate and multivariate analysis.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (yr) | ||||||
| Overall | - | - | 0.439 | - | - | - |
| < 45 vs. ≥ 45, < 60 | 1.362 | 0.375–4.954 | 0.639 | - | - | - |
| < 45 vs. ≥ 60 | 1.884 | 0.575–6.170 | 0.295 | - | - | - |
| Menstruation | ||||||
| Pre vs. post | 0.970 | 0.532–1.770 | 0.921 | - | - | - |
| Tumor size (cm) | ||||||
| Overall | - | - | 0.299 | - | - | - |
| ≤ 2 vs. > 2, ≤ 5 | 0.895 | 0.394–2.034 | 0.792 | - | - | - |
| ≤ 2 vs. > 5 | 0.571 | 0.244–1.336 | 0.196 | - | - | - |
| Histological grade | ||||||
| I, II vs. III | 1.789 | 0.947–3.378 | 0.730 | - | - | - |
| Nodal status | ||||||
| Positive vs. negative | 2.882 | 1.475–5.631 | 0.001 | 2.666 | 1.271–5.594 | 0.010 |
| Ki-67 (%) | ||||||
| ≤ 14 vs. > 15 | 1.013 | 1.002–1.024 | 0.023 | 1.007 | 0.995–1.019 | 0.249 |
| LAG-3 | ||||||
| High vs. low | 1.785 | 0.978–3.258 | 0.059 | - | - | - |
| PD-1 | ||||||
| High vs. low | 1.750 | 0.944–3.244 | 0.076 | - | - | - |
| CD8+TILs | ||||||
| High vs. low | 0.331 | 0.173–0.633 | 0.001 | 0.313 | 0.139–0.705 | 0.005 |
| PD-L1 | ||||||
| High vs. low | 1.421 | 0.781–2.586 | 0.249 | - | - | - |
| LAG-3*PD-1 | ||||||
| LAG-3-high/PD-1-high vs. non-LAG-3-high/PD-1-high | 2.450 | 1.257–4.774 | 0.008 | 1.248 | 0.464–3.353 | 0.661 |
| LAG-3*CD8+TILs | ||||||
| LAG-3-high/CD8+TILs-low vs. non-LAG-3-high/CD8+TILs-low | 3.947 | 2.038–7.644 | < 0.001 | 1.149 | 0.439–3.006 | 0.777 |
| LAG-3*PD-L1 | ||||||
| LAG-3-high/PD-L1-high vs. non-LAG-3-high/PD-L1-high | 3.198 | 1.681–6.087 | < 0.001 | 2.829 | 1.050–7.623 | 0.040 |
HR=hazard ratio; CI=confidence interval; LAG-3=lymphocyte-activation gene-3; PD-1=programmed death-1; TILs=tumor-infiltrating lymphocytes; PD-L1=programmed death ligand-1.
*Interaction.
DISCUSSION
LAG-3 controls T cell proliferation and inhibits cell cycle progression, leading to a dysfunctional state [15]. It plays a role in anticancer immunity and has been studied in many cancer types. As early as 2003, Cappello et al. [16] observed that anti-LAG-3 treatment may control breast cancer in mouse models. LAG-3 expression is significantly upregulated in CD8+TILs of patients with hepatocellular carcinoma [17]. Recent evidence suggested that co-expression of LAG-3 and PD-1 may increase tumor-mediated immunosuppression [9]. The inhibition of both LAG-3 and PD-1 pathways resulted in longer survival than PD-1 inhibition alone [18]. In addition, the expression of these two immune checkpoints is correlated with the presence of TILs [10]. These results with immune checkpoint inhibitors have renewed interest in immune checkpoints in the tumor microenvironment and their relationship with TILs and chemotherapy responses.
Many studies have focused on LAG-3; however, little is known about the role of LAG-3 in TNBC, especially in NACT. In this work, we demonstrated LAG-3 expression pre- and post-NACT in TNBC and also described the correlations between LAG-3 and PD-1, PD-L1, and CD8+TILs.
In our study, we showed that the levels of these four markers increased at different degrees following NACT. These results are similar to those of previously published studies [10,19]. Furthermore, one-to-one correspondence of each marker between biopsy and resected specimens indicated that the immune marker expression on TILs increased significantly after NACT. Mesnage et al. [20] showed that chemotherapy may lead to the activation of immunosuppressive mechanisms. Preclinical data showed upregulation of PD-L1 in ovarian cancer cell lines under chemotherapy [21]. In addition, increased infiltration by CD8+ and CD4+ T cells and relatively stable numbers of co-inhibitory molecules were detected after NACT [22]. The effect of chemotherapy on tumor control will be weakened under an immunosuppressive microenvironment. Therefore, the addition of immunotherapy to chemotherapy may be a good choice, as NACT induces immune antigen exposure, especially for non-pCR patients. Our research provides strong evidence to support this hypothesis. At present, some case reports and preclinical studies have demonstrated the efficacy of combining chemoradiotherapy with immunotherapy [23,24,25].
A previous study has shown that in luminal B/HER2-negative breast cancer, high quantities of intratumoral CD8+TILs serve as a predictor of pCR after NACT and represent a good prognostic factor for overall survival [26]. In the present work, we also observed that expression of LAG-3 in pre-NACT specimens showed significant effects on pCR. At the same time, univariate and multivariate logistic analyses showed the possible factors that may affect pCR. In univariate analysis, multiple factors (nodal status, NACT cycles, quantity of CD8+TILs, and expression of LAG-3 and PD-L1) were predictors of pCR after NACT. On the other hand, only the nodal status and quantity of CD8+TILs were significant in predicting pCR according to multivariate analysis. Nevertheless, high expression of LAG-3 was associated with a high pCR rate. After inhibiting PD-1, increased CD8+ T cell infiltration was observed in tumors and effector and memory T cells in the peripheral blood [27]. LAG-3 inhibition may show the same result. Therefore, the addition of anti-LAG-3 therapy to NACT may increase the number of CD8+TILs, thereby increasing the pCR rate.
Recent studies have revealed that LAG-3, in combination with PD-1, plays a negative regulatory role in T cells by mediating a state of exhaustion and antitumor immune response in many tumor samples [8]. In mice, LAG-3 and PD-1 exhibit synergistic effects that can alleviate large tumor burdens, whereas only delayed tumor growth was observed in single-knockout mice [28]. In an immunotherapeutic model, LAG-3 expression is upregulated with anti-PD-L1 therapy, possibly by a compensatory measure [18]. This may explain the combinatorial blockade. We also observed a correlation between high expression levels of LAG-3, PD-1, and PD-L1 in pre-NACT specimens, whereas high expression of LAG-3 on TILs showed significant differences with nodal status and PD-1 in post-NACT specimens. However, the interactions of related regulatory pathways remain poorly understood [29].
Although pCR status suggests good prognosis, studies have shown that residual lesions have become a useful tool for NACT in breast cancer patients [30]. In the present study, survival analysis of patients following NACT demonstrated that high expression of LAG-3 in residual tissues suggests poor prognosis; however, such expression cannot be used as a prognostic indicator (p>0.05). Meanwhile, the combination of LAG-3 and CD8+TILs or PD-L1 expression showed poor prognosis for DFS. In the Kaplan-Meier analyses, LAG-3-high/PD-1-high, LAG-3-high/CD8+TILs-low, and LAG-3-high/PD-L1-high indicated the poorest prognosis. Although LAG-3-high/PD-1-high was associated with the poorest prognosis, it showed no statistical significant difference. In contrast, Bottai et al. [10] demonstrated that co-expression of PD-1 and LAG-3 on TILs in the tumor microenvironment may be associated with good prognosis in TNBC. This finding may be inconsistent with the selected population in the present study. We selected patients who underwent neoadjuvant therapy, whereas Bottai et al. [10] studied postoperative patients. This difference in findings may also be caused by tumor heterogeneity. Therefore, a more comprehensive analysis should be performed using whole-tissue sections to reduce sampling bias caused by tumor heterogeneity.
We further confirmed prognostic factors in TNBC by univariate and multivariate Cox proportional hazard regression analyses. Nodal status, CD8+TILs, and LAG-3-high/PD-L1-high showed prognostic values in multivariate analysis. This result is similar to findings in a study on non-small-cell lung cancer [19]. Thus, LAG-3, especially in combination with other immune checkpoints, is comprehensive and accurate in predicting patient survival in TNBC following NACT. The finding provides the basis for screening candidates for immune combination therapy. The combination of immunotherapy with chemotherapy may be more effective for those with poor prognosis.
Our results show that LAG-3 may be a viable candidate for targeted monotherapy and has significant potential, in combination with other immune factors, for the prediction of prognosis. However, our study presents some limitations. For example, our sample size is small. We must further expand the sample size to verify our results. The interaction of LAG-3 with other immune molecules must also be further validated with cytology. There are no clinical research reports investigating whether changes in LAG-3 occur in patients receiving poor anti-PD-1/PD-L1 treatment or identifying the possible signaling pathways connecting these molecules. These problems should be resolved in future studies.
In summary, we demonstrated that CD8+TILs are sensitive predictive markers in response to NACT. Moreover, tumor immunity was activated by increased immune cells after NACT. In residual tissues, high expression of LAG-3, especially in combination with low quantities of CD8+TILs or high expression of PD-L1, was associated with poor prognosis. These results will have an important bearing on future studies on immune maintenance therapy, immunotherapy combined with NACT, and evaluation of the efficacy of and prognosis of tumors for immunotherapy following NACT. Lastly, future studies should analyze the molecular mechanisms of LAG-3 and its synergistic effects with other immune checkpoints in TNBC.
Footnotes
This study was supported by grants from the National Natural Science Foundation of China (8167100710).
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 2.Caudle AS, Yu TK, Tucker SL, Bedrosian I, Litton JK, Gonzalez-Angulo AM, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012;14:R83. doi: 10.1186/bcr3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 5.Dushyanthen S, Beavis PA, Savas P, Teo ZL, Zhou C, Mansour M, et al. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 2015;13:202. doi: 10.1186/s12916-015-0431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnis ME, Andrews LP, Vignali DA. Inhibitory receptors as targets for cancer immunotherapy. Eur J Immunol. 2015;45:1892–1905. doi: 10.1002/eji.201344413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottai G, Raschioni C, Losurdo A, Di Tommaso L, Tinterri C, Torrisi R, et al. An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Res. 2016;18:121. doi: 10.1186/s13058-016-0783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res. 2015;21:3969–3976. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667–676. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song IH, Heo SH, Bang WS, Park HS, Park IA, Kim YA, et al. Predictive value of tertiary lymphoid structures assessed by high endothelial venule counts in the neoadjuvant setting of triple-negative breast cancer. Cancer Res Treat. 2017;49:399–407. doi: 10.4143/crt.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Dong P, Ren M, Song Y, Qian X, Yang Y, et al. PD-L1 expression is associated with tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and poor prognosis of patient. J Cancer. 2016;7:784–793. doi: 10.7150/jca.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waugh KA, Leach SM, Moore BL, Bruno TC, Buhrman JD, Slansky JE. Molecular profile of tumor-specific CD8+ T cell hypofunction in a transplantable murine cancer model. J Immunol. 2016;197:1477–1488. doi: 10.4049/jimmunol.1600589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cappello P, Triebel F, Iezzi M, Caorsi C, Quaglino E, Lollini PL, et al. LAG-3 enables DNA vaccination to persistently prevent mammary carcinogenesis in HER-2/neu transgenic BALB/c mice. Cancer Res. 2003;63:2518–2525. [PubMed] [Google Scholar]
- 17.Li FJ, Zhang Y, Jin GX, Yao L, Wu DQ. Expression of LAG-3 is coincident with the impaired effector function of HBV-specific CD8(+) T cell in HCC patients. Immunol Lett. 2013;150:116–122. doi: 10.1016/j.imlet.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Foy SP, Sennino B, dela Cruz T, Cote JJ, Gordon EJ, Kemp F, et al. Poxvirus-based active immunotherapy with PD-1 and LAG-3 dual immune checkpoint inhibition overcomes compensatory immune regulation, yielding complete tumor regression in mice. PLoS One. 2016;11:e0150084. doi: 10.1371/journal.pone.0150084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Yu H, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, et al. LAG-3 protein expression in non-small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol. 2017;12:814–823. doi: 10.1016/j.jtho.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Mesnage SJ, Auguste A, Genestie C, Dunant A, Pain E, Drusch F, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC) Ann Oncol. 2017;28:651–657. doi: 10.1093/annonc/mdw625. [DOI] [PubMed] [Google Scholar]
- 21.Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-kappaB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034–5045. doi: 10.1158/0008-5472.CAN-14-3098. [DOI] [PubMed] [Google Scholar]
- 22.Teng F, Meng X, Kong L, Mu D, Zhu H, Liu S, et al. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res. 2015;166:721–732.e1. doi: 10.1016/j.trsl.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1:365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son CH, Bae JH, Shin DY, Lee HR, Choi YJ, Jo WS, et al. CTLA-4 blockade enhances antitumor immunity of intratumoral injection of immature dendritic cells into irradiated tumor in a mouse colon cancer model. J Immunother. 2014;37:1–7. doi: 10.1097/CJI.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 26.Al-Saleh K, Abd El-Aziz N, Ali A, Abozeed W, Abd El-Warith A, Ibraheem A, et al. Predictive and prognostic significance of CD8(+) tumor-infiltrating lymphocytes in patients with luminal B/HER 2 negative breast cancer treated with neoadjuvant chemotherapy. Oncol Lett. 2017;14:337–344. doi: 10.3892/ol.2017.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3: potential mechanisms of action. Nat Rev Immunol. 2015;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 30.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]