Abstract
Purpose
Although microinvasive carcinoma is distinct from ductal carcinoma in situ (DCIS), the clinical significance of microinvasion in DCIS remains elusive. The purpose of this study is to evaluate the clinicopathological features and clinical outcomes of microinvasive carcinoma compared with pure DCIS.
Methods
We assessed 613 cases of DCIS and microinvasive carcinoma that were consecutively resected from 2003 to 2014 and analyzed clinicopathological variables, expression of standard biomarkers such as the estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), p53, and Ki-67, and tumor recurrence.
Results
Among the 613 cases, 136 (22.2%) were classified as microinvasive carcinoma. Microinvasive carcinoma was significantly associated with DCIS with a large extent, high nuclear grade, necrosis, and comedotype architectural pattern. ER and PR expressions were dominantly observed in pure DCIS, whereas positive HER2 status, p53 overexpression, and high Ki-67 proliferation indices were more frequently observed in microinvasive carcinoma. Lymph node metastasis was found in only four cases of microinvasive carcinoma with multifocal microinvasion. In the multivariate analysis, DCIS with a large extent, comedo-type architectural pattern, and negative ER status were found to be independent predictors of microinvasion. During follow-up, 12 patients had ipsilateral breast recurrence, and no differences in recurrence rates were observed between patients with DCIS and those with microinvasive carcinoma. The triple-negative subtype was the only factor that was associated with tumor recurrence.
Conclusion
Microinvasive carcinomas are distinct from DCIS in terms of clinicopathological features and biomarker expressions but are similar to DCIS in terms of clinical outcomes. Our results suggest that microinvasive carcinoma can be treated and followed up as pure DCIS.
Keywords: Breast neoplasms, Noninfiltrating intraductal carcinoma, Recurrence, Triple negative breast neoplasms
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer in women in the United States and the second cause of cancerrelated death among women [1]. Microinvasive carcinoma, previously a subcategory of ductal carcinoma in situ (DCIS), is relatively rare and accounts for less than 1% of all breast cancers [2]. Since Lagios et al. [3] introduced the term “microinvasion” in 1982, several other terms have been used to describe microinvasive carcinoma [4,5,6]. However, the lack of a standardized definition for microinvasive carcinoma resulted in confusion regarding the entity until 1997, when the 5th edition of the American Joint Committee on Cancer (AJCC) staging manual was issued. Currently, the AJCC staging manual defines microinvasive carcinoma as “the extension of cancer cells beyond the basement membrane into adjacent tissue with no focus more than 0.1 cm in greatest dimension,” and it formally includes microinvasive carcinoma in the T staging system, where this disease is categorized as T1mi [7].
Various studies have reported clinical and pathological characteristics of microinvasive carcinoma. Microinvasion is usually present in high-grade, comedo-type DCIS and is less likely to be found in other types of DCIS or in lobular carcinoma in situ [8]. Patients with microinvasive carcinoma can present with axillary metastasis, and the incidence of lymph node metastasis ranges from 0% to 20% [9,10,11]. Thus, sentinel node biopsies or other axillary node examination methods are considered for patients with microinvasive carcinoma. However, the clinical outcome of microinvasive carcinoma remains unknown. While some studies have suggested that the clinical behavior of microinvasive carcinoma is similar to that of DCIS [12,13], others have shown that clinical outcomes are less favorable in patients with microinvasive carcinoma than in those with DCIS [10,14]. Thus, no consensus has been achieved with respect to whether microinvasive carcinoma should be treated as a stage 0 DCIS lesion or as a small, invasive carcinoma [15,16].
This study was conducted to evaluate the clinicopathological features of microinvasive carcinoma compared with pure DCIS and to identify predictive factors of microinvasion. Furthermore, we compared the clinical outcomes of patients with pure DCIS or microinvasive carcinoma and found predictive factors for recurrence in DCIS and microinvasive carcinoma.
METHODS
Samples
We assessed 613 cases of DCIS with or without microinvasion that were consecutively resected from 2003 to December 2014 at Seoul National University Bundang Hospital. At our institution, sampling for histologic examinations includes all sections of any grossly apparent lesions and margins. However, for large lesions in mastectomy specimens, sampling is performed from the whole section of the largest tumor slice and from representative sections with 0.5-cm intervals. Clinicopathological data were obtained by reviewing medical records and hematoxylin and eosin-stained sections. The following clinicopathological variables were determined: extent of DCIS, type of surgery, safety margin, axillary staging method, lymph nodal status, features of DCIS (nuclear grade, necrosis, and architectural pattern), microinvasion, and recurrence. For cases with microinvasive carcinoma, the number of microinvasive foci was also recorded. All cases were reviewed by two pathologists (M.K. and S.Y.P.). This study was approved by the Institutional Review Board, and informed consent was waived off (IRB number: B-1701/377-304).
Evaluation of basic biomarkers
Immunohistochemistry (IHC) for basic biomarkers, including the estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), p53, and Ki-67, was performed at the time of diagnosis. For cases with missing data, immunohistochemical staining was carried out using representative tissue sections and the BenchMark XT autostainer (Ventana Medical Systems, Tucson, USA) with the UltraView detection kit (Ventana Medical Systems). The following antibodies were used: ER (1:100, clone SP1; Labvision, Fremont, USA), PR (1:70, PgR 636; Dako, Carpinteria, USA), HER2 (ready to use, 4B5; Ventana Medical Systems), p53 (1:600, D07; Dako), and Ki-67 (1:250, MIB-1; Dako).
After reviewing the pathological reports and immunohistochemically stained slides for the basic biomarkers, ER and PR were regarded as positive if the tumor showed at least 10% positive nuclear staining. HER2 expression was scored according to the 2013 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines. p53 was considered positive if the tumor showed 10% or more positive staining, and a Ki-67 proliferation index was considered to be high if 20% or more of the tumor cells showed positive staining.
Of the 136 cases of microinvasive carcinoma, slides of 86 cases were available for review of basic biomarker analyses. Among the 86 cases, 67 (77.9%) had microinvasive foci in the immunohistochemically stained slides, and the biomarker status was analyzed in the microinvasive foci. In these cases, the biomarker status was same in DCIS and microinvasive foci. When microinvasive foci were not present in the immunohistochemically stained slides, DCIS results were recorded. When slides were not available, biomarker statuses were recorded according to pathological reports.
Tissue microarray construction and determination of HER2 status
We used tissue microarray (TMA) to determine the HER2 status of HER2 IHC 2+ cases. To exclude sampling errors from TMA platform evaluations, the section that was the most representative of the tumor was selected in each case. One tissue column with a diameter of 4.0 mm was taken from the representative tumor area, which included microinvasive foci for microinvasive carcinoma, and was arranged in TMA blocks using a trephine apparatus (Superbiochips Laboratories, Seoul, Korea).
HER2 fluorescence in situ hybridization was performed using TMAs to determine the presence/absence of HER2 amplification. Briefly, 4-µm-thick deparaffinized TMA sections were incubated in a pretreatment solution (Abbott Molecular, Downers Grove, USA) at 80℃ for 30 minutes, then in a protease solution (Abbott Molecular) for 20 minutes at 37℃. Probes were diluted in tDen-Hyb-2 hybridization buffer (InSitus Biotechnologies, Albuquerque, USA). Co-denaturation of the probes and DNA from the tissue sections was achieved by incubating them for 5 minutes in 73℃ using HYBrite™ (Abbott Molecular), followed by a 16-hour hybridization at 37℃. Posthybridization washes were performed according to the manufacturer protocols. Slides were mounted using 4′,6-diamidino-2-phenylindole, and at least 20 tumor cell nuclei were counted in each case under a fluorescence microscope.
According to the 2013 ASCO/CAP guidelines, a HER2 copy number of 6.0 or higher per cell or a HER2:CEP17 ratio of 2 or higher was considered to represent HER2 amplification. HER2/CEP17 ratios of <2 and HER2 copy numbers of 4–6 signals per cell were classified as equivocal, and HER2 copy numbers of <4 signals per cell and HER2/CEP17 ratios of <2 were considered nonamplified. In this study, HER2-equivocal cases were regarded as HER2-nonamplified for statistical analyses.
Breast cancer subtypes
Breast cancer subtypes were categorized according to the 2011 St. Gallen Expert Consensus, in a similar manner to a previous study [17]: luminal A (ER+ and/or PR+, HER2–, Ki-67 <14%), luminal B (ER+ and/or PR+, HER2–, Ki-67 ≥14%; ER+ and/or PR+, HER2+), HER2+ (ER–, PR–, HER2+), and triple-negative (ER–, PR–, HER2–).
Statistical analysis
Statistical analysis was performed using SPSS version 15.0 (SPSS Inc., Chicago, USA). Categorical variables were analyzed by Pearson chi-square or Fisher exact tests. Continuous data were analyzed by independent sample t-test or the Mann-Whitney U-test. Univariate and multivariate binary logistic regression analyses were performed to identify independent predictive factors of microinvasion. Backward stepwise model selection was used to construct the multivariate logistic regression model, and odds ratios and their 95% confidence intervals (CIs) were calculated for each factor. Recurrence-free survival was defined as the time from the date of surgery to the date of recurrence. Survival curves were drawn using the Kaplan-Meier method and were compared using the log-rank test. The Cox proportional hazards regression model was also used for univariate survival analysis, and hazard ratios (HRs) and its 95% CIs were calculated for the variables. p-values less than 0.05 were considered statistically significant, and all reported p-values were two sided.
RESULTS
Clinicopathological tumor characteristics and their association with microinvasion
This study included 613 cases of DCIS with or without microinvasion, of which 136 (22.2%) were microinvasive carcinoma. The mean extent of DCIS was 3.2±2.3 cm. Axillary staging was performed for 300 cases (48.9%), of which 12 underwent axillary node dissections and 288 underwent sentinel node biopsies. The other baseline characteristics are summarized in Table 1.
Table 1. Baseline clinicopathological characteristics (n=613).
| Characteristic | No. (%) |
|---|---|
| Age (yr)* | 50.2 ± 10.8 |
| Extent of DCIS (cm)* | 3.2 ± 2.3 |
| Operation method | |
| Mastectomy | 242 (39.5) |
| Breast-conserving surgery | 371 (60.5) |
| Axillary staging method | |
| Axillary node dissection | 12 (1.9) |
| Sentinel node biopsy | 288 (47.0) |
| Not done | 313 (51.1) |
| Histologic subtype | |
| DCIS | 477 (77.8) |
| Microinvasive carcinoma | 136 (22.2) |
| Nuclear grade | |
| 1 | 53 (8.6) |
| 2 | 278 (45.4) |
| 3 | 282 (46.0) |
| Necrosis | |
| Absent | 218 (35.6) |
| Present | 395 (64.4) |
| Comedo type | |
| Absent | 426 (69.5) |
| Present | 187 (30.5) |
| ER | |
| Negative | 157 (25.6) |
| Positive | 456 (74.4) |
| PR | |
| Negative | 216 (35.2) |
| Positive | 397 (64.8) |
| p53 overexpression | |
| Negative | 486 (79.3) |
| Positive | 127 (20.7) |
| Ki-67 proliferation index (%) | |
| < 20 | 477 (77.8) |
| ≥ 20 | 136 (22.2) |
| Subtypes | |
| Luminal A | 354 (57.7) |
| Luminal B | 107 (17.5) |
| HER2+ | 106 (17.3) |
| Triple-negative | 46 (7.5) |
| Radiation therapy | |
| Not received | 246 (40.1) |
| Received | 367 (59.9) |
| Hormonal therapy | |
| Not received | 358 (58.4) |
| Received | 255 (41.6) |
DCIS=ductal carcinoma in situ; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*Mean±SD.
We compared clinicopathological characteristics between microinvasive carcinoma and pure DCIS (Table 2). Microinvasive carcinoma was significantly associated with DCIS with a large extent (≥3.2 cm, dichotomized by the mean size); and thus, microinvasive carcinoma was more frequently treated by mastectomy (p<0.001). Microinvasive carcinoma was also associated with DCIS with high nuclear grade, necrosis, and comedo-type architectural pattern (all p<0.001) (Figure 1). In terms of biomarker expression, hormone receptor expression was high in pure DCIS. Positive HER2 status, high Ki-67 proliferation index, and p53 overexpression were more frequently observed in microinvasive carcinoma than in pure DCIS (all p<0.001) (Figure 1). As for breast cancer subtype, the luminal A subtype was more frequent in pure DCIS, whereas the HER2+ and triple-negative subtypes were more frequent in microinvasive carcinoma. Of the 300 patients who underwent axillary node staging operations, nodal metastases were found in only four patients with microinvasive carcinoma (p=0.017).
Table 2. Clinicopathological characteristics of microinvasive carcinoma in comparison with DCIS.
| Characteristic | DCIS No. (%) | Microinvasive carcinoma No. (%) | p-value |
|---|---|---|---|
| Age (yr) | 0.411 | ||
| < 50 | 261 (54.7) | 69 (50.7) | |
| ≥ 50 | 216 (45.3) | 67 (49.3) | |
| Extent of DCIS (cm) | < 0.001 | ||
| < 3.2 | 310 (65.0) | 52 (38.2) | |
| ≥ 3.2 | 167 (35.0) | 84 (61.8) | |
| Operation method | < 0.001 | ||
| Mastectomy | 161 (33.8) | 81 (59.6) | |
| Breast-conserving surgery | 316 (66.2) | 55 (40.4) | |
| Nuclear grade | < 0.001 | ||
| 1 & 2 | 300 (63.0) | 32 (30.8) | |
| 3 | 177 (35.0) | 104 (69.2) | |
| Necrosis | < 0.001 | ||
| Absent | 200 (41.9) | 18 (13.2) | |
| Present | 277 (58.1) | 118 (86.8) | |
| Comedo type | < 0.001 | ||
| Absent | 375 (78.6) | 51 (37.5) | |
| Present | 102 (21.4) | 85 (62.5) | |
| ER | < 0.001 | ||
| Negative | 83 (17.4) | 74 (54.4) | |
| Positive | 394 (82.6) | 62 (45.6) | |
| PR | < 0.001 | ||
| Negative | 128 (26.8) | 88 (64.7) | |
| Positive | 349 (73.2) | 48 (35.3) | |
| HER2 status | < 0.001 | ||
| Negative | 369 (77.4) | 58 (42.6) | |
| Positive | 108 (22.6) | 78 (57.4) | |
| p53 overexpression | < 0.001 | ||
| Negative | 403 (84.5) | 83 (61.0) | |
| Positive | 74 (15.5) | 53 (39.0) | |
| Ki-67 proliferation index (%) | < 0.001 | ||
| < 20 | 421 (88.3) | 91 (66.9) | |
| ≥ 20 | 56 (11.7) | 45 (33.1) | |
| Subtypes | < 0.001 | ||
| Luminal A | 320 (67.1) | 34 (25.0) | |
| Luminal B | 77 (16.1) | 30 (22.1) | |
| HER2+ | 51 (10.7) | 55 (40.4) | |
| Triple-negative | 29 (6.1) | 17 (12.5) | |
| Axillary node status (n = 300) | 0.017 | ||
| N0 | 190 (100) | 106 (96.3) | |
| N1mi & N1a | 0 | 4 (3.7) |
DCIS=ductal carcinoma in situ; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
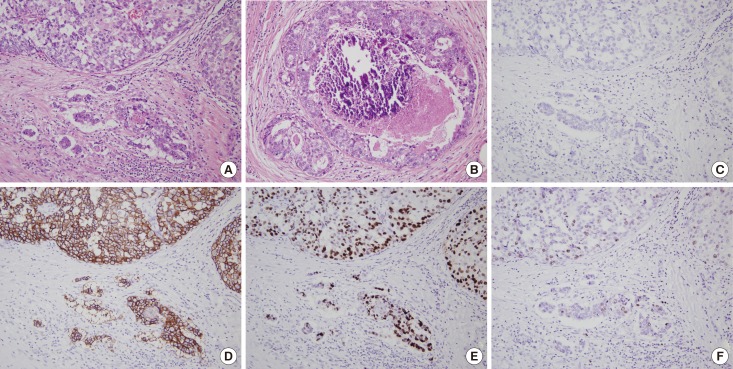
Figure 1. Histologic features and biomarker expression of microinvasive carcinoma. (A) In a representative case of microinvasive carcinoma, microinvasive foci appear as small clusters of tumor cells with inflammatory cell infiltrates in the stroma (H&E stain, ×200). (B) Surrounding ductal carcinoma in situ (DCIS) exhibits high nuclear grade and comedo-type necrosis with dystrophic calcification (H&E stain, ×200). Immunohistochemically, the tumor cells show estrogen receptor negativity (C), human epidermal growth factor receptor 2 overexpression (3+/3) (D), p53 overexpression (E), and high Ki-67 index (F) (C-F, immunohistochemistry, ×200). Immunohistochemical features of microinvasive carcinoma are identical to those of the adjacent DCIS.
Characteristics of tumors with multifocal microinvasion
Among the 136 cases of microinvasive carcinoma, 41 had multifocal microinvasion. Clinicopathological characteristics, including age, extent of DCIS, nuclear grade, necrosis, and architectural pattern, were compared between cases with single invasion and those with multifocal invasion. We observed no significant differences in clinicopathological characteristics between microinvasive carcinoma with single and multifocal invasion, except for axillary node metastasis. Of the 110 patients who underwent axillary staging surgery, 72 patients with single microinvasions showed no lymph node metastases. Only four of the 38 patients with multifocal microinvasion showed lymph node metastases, of whom two patients were N1mi and two were N1a (p=0.013) (Figure 2). Of the four patients with axillary node metastases, three had the luminal A subtype, and the remaining one had luminal B subtype. All of the four patients had a tumor extent greater than 5.0 cm, and thus, received mastectomies. Patients with multifocal microinvasion tended to exhibit a higher Ki-67 proliferation index than those with a single microinvasion (43.9% vs. 28.4%, p=0.078).
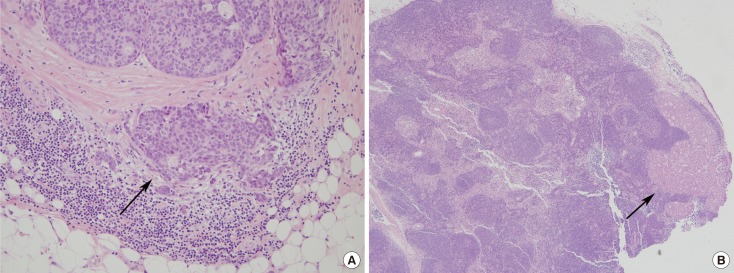
Figure 2. A representative example of node metastasis in microinvasive carcinoma. (A) Microinvasive focus is seen as a large tumor cell nest and a few small clusters accompanied by lymphoid cell infiltration in the lower portion (arrow) (H&E stain, ×200). (B) An axillary lymph node reveals macrometastasis (arrow), measuring 0.3 cm in diameter (H&E stain, ×40).
Predictors of microinvasion
In order to identify factors that independently predict microinvasion, univariate and multivariate binary logistic regression analyses were performed (Table 3). In the univariate analysis, large tumor extent (greater than 3.2 cm), high nuclear grade, necrosis, comedo type, negative ER, negative PR, positive HER2 status, p53 overexpression, and high Ki-67 proliferation index were found to be predictive factors of microinvasion (all p<0.001). Among these variables, large tumor extent, comedo-type architecture, and ER negativity were found to be independent predictors of microinvasion (all p<0.001).
Table 3. Univariate and multivariate logistic regression analyses for predictors of microinvasion.
| Variable | Univariate analysis | Multivariate analysis* | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Extent of DCIS (< 3.2 cm vs. ≥ 3.2 cm) | 2.999 (2.023–4.445) | < 0.001 | 2.605 (1.675–4.051) | < 0.001 |
| Nuclear grade (1&2 vs. 3) | 5.508 (3.556–8.533) | < 0.001 | 1.282 (0.662–2.483) | 0.461 |
| Necrosis (absent vs. present) | 4.733 (2.791–8.027) | < 0.001 | 1.366 (0.720–2.594) | 0.340 |
| Comedo type (absent vs. present) | 6.127 (4.065–9.237) | < 0.001 | 2.909 (1.809–4.678) | < 0.001 |
| ER (positive vs. negative) | 5.666 (3.752–8.556) | < 0.001 | 2.735 (1.654–4.521) | < 0.001 |
| PR (positive vs. negative) | 4.999 (3.331–7.501) | < 0.001 | 1.225 (0.595–2.520) | 0.582 |
| HER2 status (negative vs. positive) | 4.595 (3.074–6.867) | < 0.001 | 1.440 (0.833–2.489) | 0.192 |
| p53 overexpression (negative vs. positive) | 3.478 (2.275–5.317) | < 0.001 | 1.603 (0.953–2.696) | 0.075 |
| Ki-67 index ( < 20% vs. ≥ 20%) | 3.718 (2.363–5.849) | < 0.001 | 1.673 (0.979–2.859) | 0.060 |
| Triple-negative subtype (absent vs. present) | 2.207 (1.173–4.152) | 0.014 | 1.924 (0.711–5.210) | 0.198 |
CI=confidence interval; DCIS=ductal carcinoma in situ; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*Backward stepwise selection method was performed.
Patient outcomes
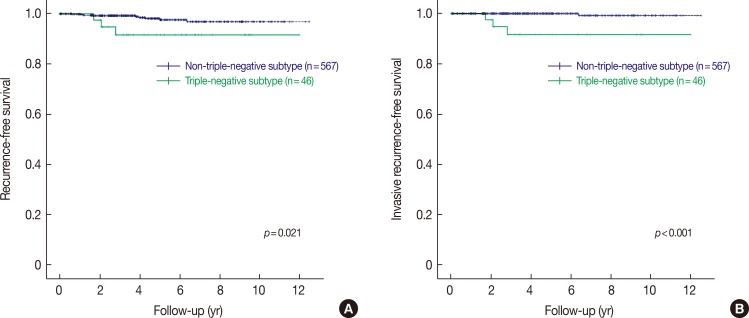
We evaluated the clinical outcomes of patients with microinvasive carcinoma and pure DCIS. Most patients were treated according to standard guidelines and were followed up regularly after surgery. The median follow-up period was 4.0 years (range, 0.1–12.4 years), during which 12 patients had ipsilateral breast cancer recurrence. The clinicopathological characteristics of these patients are presented in Table 4. Of the 12 patients, nine were originally diagnosed with pure DCIS and three with microinvasive carcinoma. The histologic type of recurrent tumors varied from pure DCIS and microinvasive carcinomas to invasive ductal carcinomas. Interestingly, three patients with triple-negative DCIS or microinvasive carcinoma had invasive carcinoma recurrences. In survival analyses, only the triple-negative subtype was found to be a prognostic factor associated with all types of ipsilateral breast recurrences (HR, 4.136, 95% CI, 1.120–15.282, p=0.033, Cox proportional hazards regression analysis; p=0.021, log-rank test) and invasive recurrences (HR, 37.188, 95% CI, 3.867–357.599, p=0.002, Cox proportional hazards regression analysis; p<0.001, log-rank test) (Table 5, Figure 3). After ipsilateral breast recurrence, one (case 83) patient subsequently developed local recurrence in the chest wall, and another patient (case 588) developed distant metastasis.
Table 4. Summary of 12 cases with ipsilateral breast recurrence.
| Case no. | Extent of DCIS (cm) | Nuclear grade | Microinvasion | Subtype | Node status | Surgery | Safety margin (cm) | Adjuvant radiation therapy | Time to recurrence (yr) | Type of recurred tumor |
|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 0.6 | 2 | - | TN | Nx | BCS | 2.5 | - | 2.05 | IDC |
| 18 | 1.5 | 2 | - | LA | Nx | BCS | 0.5 | + | 4.96 | DCIS |
| 42 | 2.0 | 3 | - | TN | Nx | BCS | 0.4 | + | 2.79 | IDC |
| 48 | 1.5 | 3 | - | HER2 | Nx | BCS | 1.5 | - | 3.98 | MIC |
| 69 | 1.6 | 3 | - | LB | Nx | BCS | 1.0 | + | 4.24 | DCIS |
| 77 | 4.0 | 2 | - | LA | Nx | BCS | < 0.1 | + | 6.32 | IDC |
| 83 | 3.5 | 3 | + | TN | N0 | BCS | 1.0 | + | 1.68 | IDC |
| 168 | 2.7 | 2 | - | LA | Nx | BCS | 0.9 | + | 3.78 | DCIS |
| 299 | 2.0 | 3 | + | LB | Nx | BCS | 0.2 | + | 1.12 | DCIS |
| 353 | 2.5 | 3 | - | HER2 | Nx | BCS | 0.5 | + | 1.65 | MIC |
| 359 | 0.8 | 2 | - | LA | Nx | BCS | 2.0 | + | 0.63 | DCIS |
| 588 | 5.0 | 3 | + | LB | N0 | MTY | 0.2 | - | 1.19 | MIC* |
DCIS=ductal carcinoma in situ; TN=triple-negative; BCS=breast-conserving surgery; IDC=invasive ductal carcinoma; LA=luminal A; HER2=human epidermal growth factor receptor 2-enriched; MIC=microinvasive carcinoma; LB=luminal B; MTY=mastectomy.
*Recurred tumor was found in the nipple after nipple-sparing mastectomy.
Table 5. Univariate analysis for recurrence-free survival.
| Variable | All types of recurrence | Invasive recurrence | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Operation method (mastectomy vs. breast-conserving surgery) | 6.726 (0.868–52.118) | 0.068 | 44.466 (0.011–187,240.294) | 0.373 |
| Extent of DCIS (< 3.2 cm vs. ≥ 3.2 cm) | 0.527 (0.143–1.948) | 0.337 | 1.555 (0.219–11.037) | 0.659 |
| Microinvasion (absent vs. present) | 0.981 (0.264–3.645) | 0.977 | 0.962 (0.099–9.376) | 0.973 |
| Radiation therapy (not received vs. received) | 2.897 (0.634–13.234) | 0.170 | 42.777 (0.009–194,514.159) | 0.382 |
| Hormone therapy (not received vs. received) | 1.097 (0.346–3.475) | 0.876 | 0.515 (0.053–5.021) | 0.568 |
| Safety margin (≤ 0.1 cm vs. > 0.1 cm) | 1.416 (0.183–10.972) | 0.739 | 5.368 (0.558–51.611) | 0.146 |
| Comedo type (absent vs. present) | 1.119 (0.337–3.718) | 0.855 | 2.248 (0.316–16.006) | 0.418 |
| ER (positive vs. negative) | 2.049 (0.650–6.461) | 0.221 | 8.549 (0.886–82.455) | 0.063 |
| HER2 status (negative vs. positive) | 1.174 (0.354–3.901) | 0.793 | 0.028 (0.000–227.055) | 0.437 |
| Ki-67 proliferation index ( < 20% vs. ≥ 20%) | 2.924 (0.879–9.728) | 0.080 | 1.983 (0.206–19.094) | 0.554 |
| Triple-negative subtype (absent vs. present) | 4.136 (1.120–15.282) | 0.033 | 37.188 (3.867–357.599) | 0.002 |
CI=confidence interval; DCIS=ductal carcinoma in situ; ER=estrogen receptor; HER2=human epidermal growth factor receptor 2.
Figure 3. Kaplan-Meier survival curves for recurrence-free survival stratified by subtypes. Tumors of triple-negative subtype show decreased recurrence-free survival compared to those with non-triple-negative subtype for all types of recurrence including ductal carcinoma in situ, microinvasive carcinoma and invasive carcinoma (A), and invasive recurrence (B).
DISCUSSION
In this study, we observed that microinvasive carcinomas were frequently accompanied by DCIS with large extent, high nuclear grade, necrosis, and comedo-type architectural pattern. As for biomarker expression, microinvasive carcinoma frequently showed hormone receptor negativity, HER2 positivity, p53 overexpression, and high Ki-67 proliferation index.
Unlike invasive breast cancer, the immunohistochemical assessment of biomarker expression is seldom performed for DCIS, either for classification or management. However, to identify features that are characteristic of microinvasive carcinoma, besides those of pure DCIS, we evaluated and compared biomarker expression in both of these groups. Previous studies have reported no significant differences in ER and PR expressions between microinvasive carcinoma and pure DCIS [13,18]. In our study, ER and PR expressions were dominant in pure DCIS compared with microinvasive carcinoma. Lari and Kuerer [19] found that ER/PR expression was frequently associated with low-grade DCIS and that the expression of these markers was associated with a lower rate of local recurrence. In our study, the conspicuous hormone receptor expression in pure DCIS can be explained by the fact that unlike microinvasive carcinoma, the majority of pure DCIS lesions had lower nuclear grades and non-comedo types. These characteristics are known to be associated with ER positivity.
Moreover, we observed a significantly higher rate of HER2 positivity in microinvasive carcinoma compared with pure DCIS. Previous studies have reported varying results in terms of HER2 expression in microinvasive carcinoma. A recent study by Wang et al. [13] described similar rates of HER2 overexpression in DCIS and microinvasive carcinoma. In contrast, Margalit et al. [16] reported a significantly higher rate of HER2 overexpression in microinvasive carcinoma than in both invasive carcinomas and DCIS. This finding is in agreement with our study results because we observed a significantly higher rate of HER2 positivity in microinvasive carcinoma than in pure DCIS. Traditionally, HER2 amplification and/or overexpression are more frequently associated with DCIS than with invasive carcinoma [15,20]. In some studies, HER2 overexpression in DCIS has been suggested as a predictor of rapid progression to invasive carcinoma [21,22]. Another study showed no significant association between HER2 expression with DCIS and disease recurrence [23]. Our study also demonstrated that HER2 status was not associated with ipsilateral breast recurrence in DCIS and microinvasive carcinoma. Thus, although positive HER2 status was associated with microinvasion in our study, its role in invasive progression remains unclear.
Although axillary staging is performed for patients with microinvasive carcinoma, lymph node metastasis is a rare event for such patients. Similar to the results from a previous study [18], we observed axillary nodal metastases in only four (3.7%) of 110 patients with microinvasive carcinoma who underwent axillary staging surgery. Of note, all of these patients had multifocal microinvasion. A recent study that compared the risk of nodal involvement in single vs. multifocal microinvasive carcinoma concluded that there was no correlation between nodal metastasis and multifocality [24]. However, similar to our results, Kapoor et al. [25], in their study of 45 patients with microinvasive carcinoma, reported a trend toward lymph node metastasis in patients with multifocal microinvasion compared with those with unifocal disease.
Several studies have investigated factors that may indicate the presence of invasive cancer in patients who were initially diagnosed with DCIS [26,27,28,29]. Nevertheless, there exist only a limited number of studies on independent predictors of microinvasive carcinoma. In a study of 174 patients with microinvasive carcinoma, Orzalesi et al. [18] found neither specific parameters nor specific cancer subtypes that were associated with microinvasive carcinoma. However, Tunon-de-Lara et al. [29] reported that high nuclear grade of DCIS was associated with a greater risk of microinvasion in resection specimens. Using a multivariate analysis, we found that a large extent, comedo-type architectural pattern, and negative ER status of DCIS were independent predictors of microinvasion, suggesting that when these factors are present in tumors that seem to be pure DCIS, a thorough review may be necessary to determine the hidden foci of microinvasion.
During follow-up, 12 patients presented with ipsilateral breast recurrence; however, there was no difference in recurrence rates between pure DCIS and microinvasive carcinoma. Similar results have been reported by Wang et al. [13], with 5-year overall survival rates of 99.0% and 99.2% in patients with microinvasive carcinoma and DCIS, respectively. As in a previous study that reported that patients with triple-negative DCIS had a higher risk of developing invasive breast cancer [17], the only factor found to be associated with tumor recurrence, either all types of recurrence or invasive recurrence, was the triple-negative subtype. Recently, Wu et al. [30] reported that patients with triple-negative breast carcinoma in situ (BCIS) had decreased breast cancer-specific and overall survivals compared with patients with hormone receptor-positive/HER2-negative BCIS, which suggests that tumor subtype has a significant effect on the clinical outcomes of patients with BCIS as well.
As a strong point, the current study included a comparative analysis of microinvasive carcinoma and pure DCIS across a large cohort of 613 patients. Nevertheless, some limitations should be noted. Being a retrospective study, the treatment decisions were affected by physician recommendations and patient preferences and as such were not randomized. In addition, unmeasured selection bias may exist due to the loss of patients to follow-up despite a diligent search of electronic medical records.
In conclusion, microinvasive carcinoma is different from pure DCIS with respect to pathological features and biomarker expressions but is similar to DCIS with respect to clinical outcomes. The triple-negative subtype is the only factor associated with tumor recurrence. These results suggest that microinvasive carcinomas can be treated and followed up as pure DCIS, although axillary staging surgery is necessary. This study also indicates that patients with triple-negative DCIS or microinvasive carcinoma need close follow-up because such cancers are associated with tumor recurrence, especially invasive recurrence.
Footnotes
This study was supported by a grant from the National Research Foundation of Korea (NRF)'s Basic Science Research Program funded by the Ministry of Science, ICT and Future Planning (Grant number: NRF-2018R1A2B6005559) and a grant from Seoul National University Bundang Hospital (Grant number: 02-2017-004) to So Yeon Park.
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Silver SA, Tavassoli FA. Mammary ductal carcinoma in situ with microinvasion. Cancer. 1998;82:2382–2390. doi: 10.1002/(sici)1097-0142(19980615)82:12<2382::aid-cncr12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Lagios MD, Westdahl PR, Margolin FR, Rose MR. Duct carcinoma in situ: relationship of extent of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer. 1982;50:1309–1314. doi: 10.1002/1097-0142(19821001)50:7<1309::aid-cncr2820500716>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein MJ, Waisman JR, Gamagami P, Gierson ED, Colburn WJ, Rosser RJ, et al. Intraductal carcinoma of the breast (208 cases): clinical factors influencing treatment choice. Cancer. 1990;66:102–108. doi: 10.1002/1097-0142(19900701)66:1<102::aid-cncr2820660119>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Royal College of Pathologists Working Group. Pathology reporting in breast cancer screening. J Clin Pathol. 1991;44:710–725. doi: 10.1136/jcp.44.9.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosner D, Lane WW, Penetrante R. Ductal carcinoma in situ with microinvasion: a curable entity using surgery alone without need for adjuvant therapy. Cancer. 1991;67:1498–1503. doi: 10.1002/1097-0142(19910315)67:6<1498::aid-cncr2820670606>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast cancer: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 8.Perry N, Broeders M, de Wolf C. European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis. 4th ed. Luxembourg: Office for Official Publications of the European Communities; 2006. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi S, Vezzosi V. Microinvasive carcinoma of the breast. Pathol Oncol Res. 2008;14:105–111. doi: 10.1007/s12253-008-9054-8. [DOI] [PubMed] [Google Scholar]
- 10.de Mascarel I, MacGrogan G, Mathoulin-Pélissier S, Soubeyran I, Picot V, Coindre JM. Breast ductal carcinoma in situ with microinvasion: a definition supported by a long-term study of 1,248 serially sectioned ductal carcinomas. Cancer. 2002;94:2134–2142. doi: 10.1002/cncr.10451. [DOI] [PubMed] [Google Scholar]
- 11.Solin LJ, Fowble BL, Yeh IT, Kowalyshyn MJ, Schultz DJ, Weiss MC, et al. Microinvasive ductal carcinoma of the breast treated with breast-conserving surgery and definitive irradiation. Int J Radiat Oncol Biol Phys. 1992;23:961–968. doi: 10.1016/0360-3016(92)90900-3. [DOI] [PubMed] [Google Scholar]
- 12.Shatat L, Gloyeske N, Madan R, O'Neil M, Tawfik O, Fan F. Microinvasive breast carcinoma carries an excellent prognosis regardless of the tumor characteristics. Hum Pathol. 2013;44:2684–2689. doi: 10.1016/j.humpath.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Zhang W, Lyu S, Liu X, Zhang T, Liu S, et al. Clinicopathologic characteristics and molecular subtypes of microinvasive carcinoma of the breast. Tumour Biol. 2015;36:2241–2248. doi: 10.1007/s13277-014-2652-z. [DOI] [PubMed] [Google Scholar]
- 14.Sopik V, Sun P, Narod SA. Impact of microinvasion on breast cancer mortality in women with ductal carcinoma in situ. Breast Cancer Res Treat. 2018;167:787–795. doi: 10.1007/s10549-017-4572-2. [DOI] [PubMed] [Google Scholar]
- 15.Yu KD, Wu LM, Liu GY, Wu J, Di GH, Shen ZZ, et al. Different distribution of breast cancer subtypes in breast ductal carcinoma in situ (DCIS), DCIS with microinvasion, and DCIS with invasion component. Ann Surg Oncol. 2011;18:1342–1348. doi: 10.1245/s10434-010-1407-3. [DOI] [PubMed] [Google Scholar]
- 16.Margalit DN, Sreedhara M, Chen YH, Catalano PJ, Nguyen PL, Golshan M, et al. Microinvasive breast cancer: ER, PR, and HER-2/neu status and clinical outcomes after breast-conserving therapy or mastectomy. Ann Surg Oncol. 2013;20:811–818. doi: 10.1245/s10434-012-2640-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhou W, Jirström K, Amini RM, Fjällskog ML, Sollie T, Lindman H, et al. Molecular subtypes in ductal carcinoma in situ of the breast and their relation to prognosis: a population-based cohort study. BMC Cancer. 2013;13:512. doi: 10.1186/1471-2407-13-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orzalesi L, Casella D, Criscenti V, Gjondedaj U, Bianchi S, Vezzosi V, et al. Microinvasive breast cancer: pathological parameters, cancer subtypes distribution, and correlation with axillary lymph nodes invasion. Results of a large single-institution series. Breast Cancer. 2016;23:640–648. doi: 10.1007/s12282-015-0616-9. [DOI] [PubMed] [Google Scholar]
- 19.Lari SA, Kuerer HM. Biological markers in DCIS and risk of breast recurrence: a systematic review. J Cancer. 2011;2:232–261. doi: 10.7150/jca.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang M, Kim E, Choi Y, Lee H, Kim Y, Kim J, et al. FGFR1 is amplified during the progression of in situ to invasive breast carcinoma. Breast Cancer Res. 2012;14:R115. doi: 10.1186/bcr3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roses RE, Paulson EC, Sharma A, Schueller JE, Nisenbaum H, Weinstein S, et al. HER-2/neu overexpression as a predictor for the transition from in situ to invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1386–1389. doi: 10.1158/1055-9965.EPI-08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen XF, Yang G, Mao W, Thornton A, Liu J, Bast RC, Jr, et al. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25:6986–6996. doi: 10.1038/sj.onc.1209685. [DOI] [PubMed] [Google Scholar]
- 23.Kühn T. Ductal carcinoma in situ: clinical perspective. Breast Care (Basel) 2010;5:227–232. doi: 10.1159/000319325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsen CB, Hirsch A, Eaton A, Stempel M, Heerdt A, Van Zee KJ, et al. Extent of microinvasion in ductal carcinoma in situ is not associated with sentinel lymph node metastases. Ann Surg Oncol. 2014;21:3330–3335. doi: 10.1245/s10434-014-3920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapoor NS, Shamonki J, Sim MS, Chung CT, Giuliano AE. Impact of multifocality and lymph node metastasis on the prognosis and management of microinvasive breast cancer. Ann Surg Oncol. 2013;20:2576–2581. doi: 10.1245/s10434-013-2924-7. [DOI] [PubMed] [Google Scholar]
- 26.Yen TW, Hunt KK, Ross MI, Mirza NQ, Babiera GV, Meric-Bernstam F, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg. 2005;200:516–526. doi: 10.1016/j.jamcollsurg.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Chan MY, Lim S. Predictors of invasive breast cancer in ductal carcinoma in situ initially diagnosed by core biopsy. Asian J Surg. 2010;33:76–82. doi: 10.1016/S1015-9584(10)60013-9. [DOI] [PubMed] [Google Scholar]
- 28.Park HS, Park S, Cho J, Park JM, Kim SI, Park BW. Risk predictors of underestimation and the need for sentinel node biopsy in patients diagnosed with ductal carcinoma in situ by preoperative needle biopsy. J Surg Oncol. 2013;107:388–392. doi: 10.1002/jso.23273. [DOI] [PubMed] [Google Scholar]
- 29.Tunon-de-Lara C, Chauvet MP, Baranzelli MC, Baron M, Piquenot J, Le-Bouédec G, et al. The role of sentinel lymph node biopsy and factors associated with invasion in extensive DCIS of the breast treated by mastectomy: the Cinnamome prospective multicenter study. Ann Surg Oncol. 2015;22:3853–3860. doi: 10.1245/s10434-015-4476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Q, Li J, Sun S, Zhu S, Chen C, Wu J, et al. Breast carcinoma in situ: an observational study of tumor subtype, treatment and outcomes. Oncotarget. 2017;8:2361–2371. doi: 10.18632/oncotarget.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]