Abstract
Purpose
This study aimed to identify risk factors that have significant interaction with radiation exposure to the heart, and thus to determine candidates for heart-sparing radiotherapy (RT) among women with left breast cancer.
Methods
We identified 4,333 patients who received adjuvant RT following breast-conserving surgery for ductal carcinoma in situ or invasive breast cancer from 1996 to 2010. Incidence rates of cardiovascular disease were compared between left-sided and right-sided RT, and stratified by age and risk factors such as body mass index (BMI), smoking, hyperlipidemia, hypertension, diabetes, administration of anthracycline, and trastuzumab.
Results
In all patients, the cumulative incidence of cardiovascular disease was greater in patients treated with left-sided RT than in those treated with right-sided RT, but the difference was not significant (p=0.428). Smoking (hazard ratio [HR], 5.991; 95% confidence interval [CI], 2.109–17.022; p=0.002) and hyperlipidemia (HR, 5.567; 95% CI, 3.235–9.580; p<0.001) were the most powerful risk factors for cardiovascular disease. There was no significant factor that further increased the risk of cardiovascular disease after left breast RT compared to right breast RT.
Conclusion
Although hyperlipidemia and smoking are risk factors for cardiovascular disease, they have not been proven to increase the risk of RT-related cardiovascular disease in Korean women.
Keywords: Breast neoplasms, Cardiotoxicity, Radiotherapy
INTRODUCTION
Breast cancer is the most common cancer in women, and its incidence is rising [1,2]. Radiotherapy (RT) is an essential treatment to improve local control and survival in patients who undergo breast-conserving surgery for breast cancer [3,4,5]. As approximately 90% of patients with localized breast cancer are alive at 5 years after treatment, late RT toxicity is an important consideration. For more than 20 years, a number of studies have suggested a possible association between left breast RT and cardiac morbidity or mortality [6,7,8]. A large population-based case control study in Sweden and Denmark confirmed that women who received radiation for left breast cancer had higher rates of major coronary events and the risk of these events showed a linear relationship with mean heart dose [9,10].
Nevertheless, controversy still exists regarding cardiac toxicities and RT for left breast cancer, as RT technique and chemotherapy regimens have changed over time. One study indicated that modern conformal RT does not increase cardiac toxicities in patients with left breast cancer [11]. Other studies also reported that patients treated with left breast RT after the 1990s do not have a higher risk of cardiovascular disease [12,13,14]. On the other hand, the results of several studies suggest that the use of modern anthracycline-based chemotherapy regimens enhances RT-associated cardiac disease in patients with left breast cancer [15,16]. Such conflicting conclusions are likely the result of the heterogeneity in cardiac risk among patients included in these studies. Certain subsets of patients with genetic or lifestyle risk factors or those treated with cardiotoxic agents might be more vulnerable to cardiac irradiation than others who have fewer risk factors [17].
Efforts to reduce cardiac radiation doses in patients with breast cancer have been widely implemented to reduce subsequent cardiovascular disease [18]. Various techniques such as deep inspiration breath-hold, prone position, or intensity modulating RT [19,20] are used, but these methods require considerable time and human resources. To justify the trade-off, it is necessary to select the patients who will most benefit from heart-sparing RT among those with left breast cancer.
Therefore, this study aimed to identify the risk factors that exacerbate the risk of RT-associated cardiac toxicities, and consequently to determine candidates for heart-sparing RT among women with left breast cancer.
METHODS
Data sources and patient selection
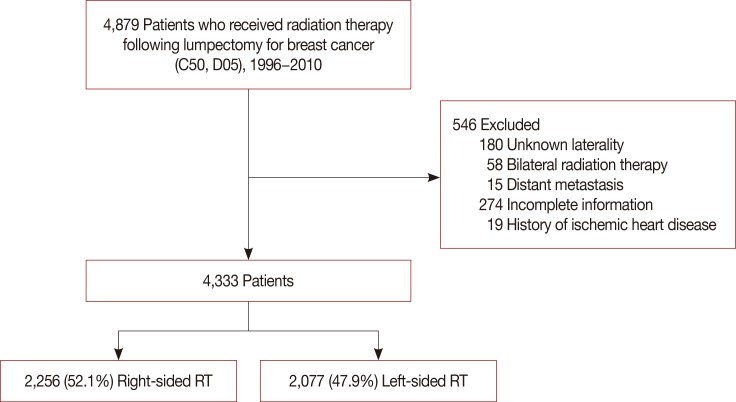
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2017-12-117), and waived the requirement for obtaining informed consent. Data were extracted from the Clinical Data Warehouse Darwin-C of Samsung Medical Center. We identified 4,879 patients treated with adjuvant RT following breast-conserving surgery for in situ or invasive breast cancer from 1996 to 2010. Patients with bilateral tumors, metastatic disease, unknown laterality, incomplete RT, lack of information, or history of ischemic heart disease prior to RT were excluded from analysis, leaving a total of 4,333 patients (Figure 1). Cardiovascular disease included acute myocardial infarction (International Classification of Diseases, 10th Revision [ICD-10] code I21), angina (ICD-10 code I20), and other ischemic heart disease (ICD-10 codes I24-25). Patients with metabolic syndrome were identified by ICD-10 codes; hyperlipidemia was classified by ICD-10 code E78, hypertension by I10, and diabetes by E10, E11, or E14. Body mass index (BMI, kg/m2) was calculated as weight (kg) divided by height (m) squared.
Figure 1. Patient selection process.
RT=radiotherapy.
Treatment
During the study period, patients were treated with whole breast irradiation following breast-conserving surgery. Supraclavicular irradiation and/or axillary irradiation were applied in cases of N2 disease or high risk N1 disease according to our institutional policy [21,22]. Internal mammary lymph node (IMN) irradiation was applied in cases with IMN involvement. The dose to the whole breast was 50.0–50.4 Gy in 25–28 fractions using 2 tangential photon beams with an electron beam boost to the tumor bed. Two-dimensional simulation and planning was performed until 2005, with three-dimensional conformal RT with computed tomography (CT) simulation performed after 2006. Adjuvant chemotherapy was administered to 2,606 patients (60.1%), and anthracycline and trastuzumab were utilized in 1,992 (46.0%) and 206 (4.8%) of analyzed patients, respectively.
Statistical analysis
The distributions of categorical variables between the patients who received right-sided RT and those who received left-sided RT were compared using chi-square tests. The baseline date was defined as the last day of adjuvant RT for breast cancer. Time to disease was defined as the time between the last day of RT for breast cancer and the date of diagnosis. To estimate the cumulative incidence of subsequent cardiac disease after exposure, accounting for the follow-up period, hazard plots were generated using the Kaplan-Meier method. Cumulative incidence curves according to risk factors were compared with the log-rank test. The Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) to control covariates and differences in follow-up periods. All analyses were conducted using SPSS Statistics version 20 (IBM Corp., Armonk, USA).
RESULTS
Patient characteristics
Patient characteristics and tumor characteristics of all patients are described in Table 1. Among all patients, 733 (16.9%) were under 40 years, 1,900 (43.8%) were 40–49 years, 1,199 (27.7%) were 50–59 years, and 501 (11.6%) were 60 years or older. BMI was available in 3,894 (89.9%), and obese women (BMI ≥25) were 1,099 (25.4%) of all patients. Smoking history was available in 3,477 (80.2%) patients and 92 (2.1%) of all patients were smokers. Diagnoses of hyperlipidemia, hypertension, and diabetes were identified in 335 (7.7%), 254 (5.9%), and 201 (4.6%) patients, respectively. Of all patients, 2,256 patients were treated with right-sided RT and 2,077 patients were treated with left-sided RT. Characteristics of the patients and tumors did not differ between the patients who received left breast RT and those who received right breast RT (Table 1).
Table 1. Characteristics of patients by tumor laterality.
| Characteristic | All patients (n = 4,333) No. (%) |
Right-sided (n = 2,256) No. (%) |
Left-sided (n = 2,077) No. (%) |
p-value |
|---|---|---|---|---|
| Year of RT | 0.434 | |||
| 1996–2000 | 367 (8.5) | 202 (9.0) | 165 (7.9) | |
| 2001–2005 | 1,333 (30.8) | 683 (30.3) | 650 (31.3) | |
| 2006–2010 | 2,633 (60.8) | 1,371 (60.8) | 1,262 (60.8) | |
| Age (yr) | 0.981 | |||
| < 40 | 733 (16.9) | 379 (16.8) | 354 (17.0) | |
| 40–49 | 1,900 (43.8) | 996 (44.1) | 904 (43.5) | |
| 50–59 | 1,199 (27.7) | 622 (27.6) | 577 (27.8) | |
| ≥ 60 | 501 (11.6) | 259 (11.5) | 242 (11.7) | |
| BMI (kg/m2) | 0.567 | |||
| Unknown | 439 (10.1) | 218 (9.7) | 221 (10.6) | |
| < 25 | 2,795 (64.5) | 1,463 (64.8) | 1,332 (64.1) | |
| ≥ 25 | 1,099 (25.4) | 575 (25.5) | 524 (25.2) | |
| Smoking | 0.247 | |||
| Unknown | 856 (19.8) | 454 (20.1) | 402 (19.4) | |
| Yes | 92 (2.1) | 55 (2.4) | 37 (1.8) | |
| No | 3,385 (78.1) | 1,747 (77.4) | 1,638 (78.9) | |
| Hyperlipidemia | 0.465 | |||
| Yes | 335 (7.7) | 168 (7.4) | 167 (8.0) | |
| No | 3,998 (92.3) | 2,088 (92.6) | 1,910 (92.0) | |
| Hypertension | 0.257 | |||
| Yes | 254 (5.9) | 141 (6.3) | 113 (5.4) | |
| No | 4,079 (94.1) | 2,115 (93.8) | 1,964 (94.6) | |
| Diabetes | 0.845 | |||
| Yes | 201 (4.6) | 106 (4.7) | 95 (4.6) | |
| No | 4,132 (95.4) | 2,150 (95.3) | 1,982 (95.4) | |
| pT stage | 0.643 | |||
| Tis | 510 (11.8) | 264 (11.7) | 246 (11.8) | |
| T1 | 2,571 (59.3) | 1,327 (58.8) | 1,244 (59.9) | |
| T2 | 1,158 (26.7) | 609 (27.0) | 549 (26.4) | |
| T3 | 86 (2.0) | 51 (2.3) | 35 (1.7) | |
| T4 | 8 (0.2) | 5 (0.2) | 3 (0.1) | |
| pN stage | 0.592 | |||
| N0 | 3,041 (70.2) | 1,578 (69.9) | 1,463 (70.4) | |
| N1 | 967 (22.3) | 506 (22.4) | 461 (22.2) | |
| N2 | 244 (5.6) | 134 (5.9) | 110 (5.3) | |
| N3 | 81 (1.9) | 38 (1.7) | 43 (2.1) | |
| Chemotherapy | 0.234 | |||
| Yes | 2,606 (60.1) | 1,376 (61.0) | 1,230 (59.2) | |
| No | 1,727 (39.9) | 880 (39.0) | 847 (40.8) | |
| Anthracycline | 0.250 | |||
| Yes | 1,992 (46.0) | 1,056 (46.8) | 936 (45.1) | |
| No | 2,341 (54.0) | 1,200 (53.2) | 1,141 (54.9) | |
| Trastuzumab | 0.593 | |||
| Yes | 206 (4.8) | 111 (4.9) | 95 (4.6) | |
| No | 4,127 (95.2) | 2,145 (95.1) | 1,982 (95.4) |
RT=radiotherapy; BMI=body mass index.
Incidence of cardiovascular disease and risk factor analysis
Median follow-up was 8.1 years (range, 0.2–21.5 years). During the follow-up period, 69 patients experienced cardiovascular disease; the median time to development of disease was 5.5 years (range, 0.5–15.1 years). Among these patients, 45 developed angina and 24 developed other ischemic heart diseases; the median time to disease onset was 5.9 and 3.5 years, respectively. The cumulative incidences of cardiovascular disease were 0.8%, 2.0%, and 3.1% at 5, 10, and 15 years, respectively (Table 2). Table 3 shows the cumulative incidence of cardiovascular disease according to the risk factors and estimated HR of factors by univariate and multivariate analysis. In univariate analysis, age of 50 years or more (p<0.001), BMI ≥25 kg/m2 (p<0.001), smoking (p=0.042), hyperlipidemia (p<0.001), hypertension (p<0.001), diabetes (p<0.001), and administration of trastuzumab (p=0.010) were the significant risk factors associated with higher risk of cardiovascular disease. The significant risk factors (p<0.05) in univariate analysis and tumor laterality were included in the multivariate analysis. Multivariate analysis showed that an age of 50 or more (HR, 1.073; 95% CI, 1.048–4.099; p<0.001), smoking (HR, 5.991; 95% CI, 2.109–17.022; p=0.002), hyperlipidemia (HR, 5.567; 95% CI, 3.235–9.580; p<0.001), and trastuzumab (HR, 2.214; 95% CI, 1.040–4.711; p=0.039) were the significant risk factors for cardiovascular disease. Tumor laterality was not a significant risk factor for cardiovascular disease in either uni- or multivariate analysis (p=0.443 and p=0.428, respectively).
Table 2. Subsequent incidence of cardiovascular disease.
| Cardiovascular disease category (ICD-10 code) | Events (no.) | Median time (yr) | Cumulative incidence (%) | ||
|---|---|---|---|---|---|
| 5 yr | 10 yr | 15 yr | |||
| Angina (I20) | 45 | 5.9 | 0.5 | 1.4 | 2.0 |
| Other ischemic heart disease (I24-25) | 24 | 3.5 | 0.4 | 0.7 | 1.2 |
| All cardiovascular disease | 69 | 5.5 | 0.8 | 2.0 | 3.1 |
ICD-10=International Classification of Diseases, 10th Revision.
Table 3. Five-, 10-, and 15-year cumulative incidence according to risk factors and hazard ratio in multivariate analysis.
| Characteristic | 5 yr (%) | 10 yr (%) | 15 yr (%) | p-value* | HR (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Age at diagnosis (yr) | < 0.001† | |||||
| < 50 | 0.4 | 0.8 | 1.7 | < 0.001 | 1 | < 0.001 |
| ≥ 50 | 1.5 | 4.3 | 5.7 | 1.073 (1.048–4.099) | ||
| Laterality | 0.443 | 0.428 | ||||
| Right | 0.6 | 1.9 | 2.8 | 1 | ||
| Left | 1.0 | 2.3 | 3.5 | 1.212 (0.753–1.950) | ||
| Year of radiotherapy | 0.491 | |||||
| 1996–2000 | 0.9 | 2.3 | 2.3 | |||
| 2001–2005 | 0.6 | 1.6 | 1.6 | |||
| 2006–2010 | 0.9 | 2.2 | 3.5 | |||
| BMI (kg/m2) | < 0.001† | 1.005 (0.962–1.051) | 0.816 | |||
| < 25 | 0.6 | 1.6 | 2.2 | < 0.001 | 1 | 0.151 |
| ≥ 25 | 1.5 | 3.5 | 5.8 | 1.764 (0.607–5.128) | ||
| Smoking | 0.042 | 0.002 | ||||
| No | 0.8 | 2.2 | 3.4 | 1 | ||
| Yes | 3.3 | 6.2 | 6.2 | 5.991 (2.109–17.022) | ||
| Hyperlipidemia | < 0.001 | < 0.001 | ||||
| No | 0.4 | 1.2 | 1.7 | 1 | ||
| Yes | 5.2 | 10.6 | 15.3 | 5.567 (3.235–9.580) | ||
| Hypertension | < 0.001 | 0.228 | ||||
| No | 0.7 | 1.7 | 2.4 | 1 | ||
| Yes | 3.0 | 7.5 | 12.1 | 1.460 (0.789–2.703) | ||
| Diabetes | < 0.001 | 0.068 | ||||
| No | 0.7 | 1.6 | 2.2 | 1 | ||
| Yes | 3.7 | 8.8 | 16.0 | 1.786 (0.957–3.331) | ||
| Anthracycline | 0.079 | |||||
| No | 0.7 | 1.3 | 2.5 | |||
| Yes | 0.9 | 3.0 | 3.7 | |||
| Trastuzumab | 0.010 | 0.039 | ||||
| No | 0.8 | 1.8 | 2.9 | 1 | ||
| Yes | 1.0 | 4.7 | 6.7 | 2.214 (1.040–4.711) |
HR=hazard ratio; CI=confidence interval; BMI=body mass index.
*p-value obtained from univariate Cox regression analysis; †p-value obtained from univariate Cox regression analysis calculated as continuous variables.
Risk of cardiovascular disease in left breast cancer (versus right breast cancer)
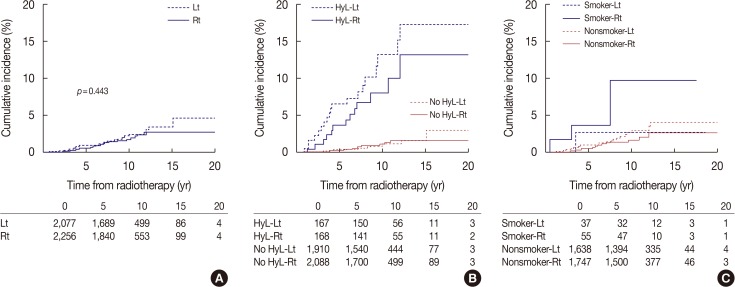
In the entire patient group, the cumulative hazard of cardiovascular disease was numerically greater in patients treated with left-sided RT than those treated with right-sided RT, but the difference was not significant (p=0.443) (Figure 2A). The cumulative incidence of cardiovascular disease was 0.6% at 5 years and 1.9% at 10 years in patients treated with right breast RT and 1.0% at 5 years and 2.3% at 10 years in patients treated with left breast RT (Table 3). Figure 2B shows the hazard plots for cardiovascular disease according to tumor laterality and hyperlipidemia. Regardless of hyperlipidemia, patients treated with left breast RT showed slightly higher incidence of cardiovascular disease than those treated with right breast RT, but the difference was not significant. Figure 2C shows the hazard plots for incidence of cardiovascular disease according to tumor laterality and smoking. Although smokers have a higher risk of cardiovascular disease, no difference was observed between left and right breast cancer in either group, the smokers or the nonsmokers. Table 4 indicates incidence rates and unadjusted HRs for cardiovascular disease in patients with left breast cancer relative to right breast cancer in various subgroups. None of the subgroups were identified to have a higher risk of cardiovascular disease in left breast cancer compared to right breast cancer.
Figure 2. Cumulative incidence of cardiovascular disease in patients who received radiotherapy for breast cancer according to laterality. Incidence of cardiovascular disease in all patients according to tumor laterality (A), hyperlipidemia and tumor laterality (B), smoking and tumor laterality (C).
Lt=left; Rt=right; HyL=hyperlipidemia.
Table 4. Hazard ratio of cardiovascular disease by logistic regression analysis: left versus right.
| Variable | Incidence rate (Lt vs. Rt) (%) | Unadjusted HR (95% CI) (Lt vs. Rt) | p-value | ||
|---|---|---|---|---|---|
| 5 yr | 10 yr | 15 yr | |||
| Age (yr) | |||||
| ≥50 | 2.0 vs. 1.1 | 4.1 vs. 3.7 | 6.3 vs. 5.1 | 1.223 (0.692–2.161) | 0.488 |
| <50 | 0.4 vs. 0.3 | 0.7 vs. 0.8 | 1.9 vs. 1.5 | 1.094 (0.454–2.637) | 0.842 |
| BMI (kg/m2) | |||||
| < 25 | 0.8 vs. 0.5 | 0.8 vs. 0.5 | 5.1 vs. 0.5 | 1.038 (0.533–2.022) | 0.913 |
| ≥ 25 | 0.8 vs. 0.4 | 1.8 vs. 1.4 | 1.8 vs. 2.5 | 1.262 (0.610–2.612) | 0.530 |
| Smoking | |||||
| Yes | 2.7 vs. 3.8 | 2.7 vs. 9.8 | 2.7 vs. 9.8 | 0.481 (0.048–4.815) | 0.534 |
| No | 1.1 vs. 0.6 | 2.7 vs. 1.7 | 6.7 vs. 2.7 | 1.385 (0.809–2.370) | 0.235 |
| Hyperlipidemia | |||||
| Yes | 6.7 vs. 3.8 | 13.1 vs. 8.1 | 17.3 vs. 13.2 | 1.531 (0.730–3.210) | 0.260 |
| No | 0.5 vs. 0.4 | 1.1 vs. 1.3 | 1.1 vs. 1.7 | 0.929 (0.485–1.778) | 0.823 |
| Hypertension | |||||
| Yes | 2.8 vs. 3.1 | 7.4 vs. 7.4 | 13.5 vs. 11.7 | 0.998 (0.380–2.618) | 0.997 |
| No | 0.9 vs. 0.5 | 1.9 vs. 1.5 | 2.6 vs. 2.2 | 1.315 (0.755–2.292) | 0.333 |
| Diabetes | |||||
| Yes | 2.2 vs. 5.0 | 10.4 vs. 7.3 | 17.4 vs. 15.7 | 0.991 (0.366–2.682) | 0.986 |
| No | 1.0 vs. 0.4 | 1.7 vs. 1.6 | 2.4 vs. 2.0 | 1.269 (0.733–2.197) | 0.394 |
| Anthracycline | |||||
| Yes | 1.4 vs. 0.5 | 3.2 vs. 2.8 | 3.8 vs. 3.5 | 1.192 (0.632–2.247) | 0.588 |
| No | 0.8 vs. 0.7 | 1.5 vs. 1.1 | 2.9 vs. 2.1 | 1.205 (0.585–2.480) | 0.613 |
| Trastuzumab | |||||
| Yes | 0 vs. 1.9 | 1.7 vs. 4.3 | 11.2 vs. 4.3 | 1.176 (0.286–4.834) | 0.822 |
| No | 1.1 vs. 0.5 | 1.9 vs. 1.7 | 3.2 vs. 2.7 | 1.197 (0.722–1.987) | 0.486 |
Lt=left; Rt=right; HR=hazard ratio; CI=confidence interval; BMI=body mass index.
DISCUSSION
The aim of the present study was to identify the factors having an interaction with cardiac irradiation and resulting in a higher risk of cardiovascular disease. However, in this study, the risk of cardiovascular disease did not increase following left breast RT compared to right breast RT in Korean women. Similarly, a recent study failed to demonstrate an increased risk of cardiovascular disease following left breast RT in Korean women, most of whom have fewer risk factors for cardiovascular disease [23]. Several other studies also reported that left breast RT did not increase the risk of acute coronary events or cardiac mortality in patients with breast cancer [11,12,13,14]. However, a numerically higher incidence of cardiovascular disease in patients with left breast cancer implies that left breast RT might enhance the risk of cardiovascular disease in certain subsets of patients.
Smoking was the strongest risk factor for cardiovascular disease, although an interaction with left breast RT was not proven in this study. Taylor et al. [24] reported that smoking was a powerful risk factor for cardiovascular disease in patients with breast cancer, while another study showed conflicting results, finding that smoking was not a risk factor for cardiovascular disease in Korean women [23]. In the present study, despite a large amount of missing data and lack of information regarding ex-smokers, smoking was the most significant risk factor with the highest HR of 5.991 for cardiovascular disease in patients with breast cancer, a finding consistent with the results of Taylor et al. and with other studies in the general population [17,24].
Hyperlipidemia was the second most powerful risk factor for cardiovascular disease. The similarity in mechanisms of RT-induced vascular disease and hyperlipidemia-associated vascular disease implies a possible synergistic effect of the two factors: coronary vessels that receive radiation during breast RT are assumed to become susceptible to thrombus accumulation provoked by cytokines from irradiated endothelial cells. Hyperlipidemia also promotes atherosclerotic change in the endothelium of coronary vessels. However, the present study failed to demonstrate an interaction between hyperlipidemia and RT-induced cardiovascular disease.
Hyperlipidemia, diabetes, hypertension, and obesity, called metabolic syndrome, are the major risk factors for cardiovascular disease, combined with old age and smoking [17]. The effect of metabolic syndrome on cardiovascular disease and cardiac mortality has been investigated in several studies. Obesity is the most frequently reported risk factor for cardiovascular disease, especially in Western studies [9]. However, in Asian women, most of whom are slim, the association between higher BMI and higher risk of cardiovascular disease seems to be not as great as that in Western women. Chang et al. [23] who investigated the effect of risk factors including high BMI, hypertension, and diabetes on cardiovascular disease in patients with breast cancer, showed that hypertension and diabetes were risk factors for cardiovascular disease, while higher BMI was not. The present study also demonstrated that hyperlipidemia was more associated with risk of cardiovascular disease than higher BMI in patients with breast cancer. Information regarding medication for hyperlipidemia could not be acquired for analysis since statin medications are usually prescribed by primary care physicians. If we had information about statin medications and disease control status, the interaction between hyperlipidemia and irradiation could be clarified.
Age is the most frequently reported risk factor for cardiovascular disease in patients treated with RT for breast cancer. Paszat et al. [8] reported that age over 60 is a significant risk factor for death from myocardial infarction in patients with breast cancer. Darby et al. [9] showed that the older the age of women at the time of cancer diagnosis, the greater the percentage of cardiac mortality in those treated with adjuvant RT for breast cancer. The present study also indicated that the risk of cardiovascular disease increases in patients older than 50 years. We divided the patients into four age groups: <40; 40 to 49; 50 to 59; and ≥60 years, and found that the differentiation between women under 50 years and those over 50 years was the most prominent.
The risk factors for cardiac disease differ according to which disease we define as the end point of RT-related cardiac toxicities. A recent study in Denmark indicated that anthracycline-based chemotherapy further raises the risk of heart disease in left-sided breast cancer compared to right sided breast cancer [25], when heart failure was defined as an RT-induced cardiac disease. Boekel et al. [26] also reported that anthracycline-based chemotherapy increased the risk of heart failure in patients with breast cancer. The present study defined cardiovascular disease as an end point excluding heart failure, and use of anthracycline-based chemotherapy was not related to cardiovascular disease.
A limitation of our study is that cardiovascular disease diagnosed outside the institution was not included in this analysis. Owing to potential missing events, the absolute incidence of cardiovascular disease might be underestimated; however, we can at least estimate the difference between left and right breast cancer if we hypothesize that the amount of missing data between the groups is similar. Furthermore, hyperlipidemia, hypertension, and diabetes are extracted by ICD codes, which can also result in underestimated incidence of the underlying diseases. The lack of information about RT dose and field is another drawback of the current study. No difference in the incidence of cardiovascular disease among different time periods of RT in the present study, which can be a surrogate of irradiated heart dose, implies that the impact of heart dose on cardiovascular disease can be trivial. However, additional studies including detailed dosimetric analysis are warranted to clarify the relationship between heart dose and the risk of cardiovascular disease. Nevertheless, the present study is the first study highlighting the significance of hyperlipidemia on the risk of cardiovascular disease in patients with breast cancer, especially in Asian women, most of whom are not obese. Further prospective studies addressing the association between hyperlipidemia and RT-related cardiovascular disease are warranted.
In conclusion, none of the risk factors is proven to have an interaction with RT-related cardiovascular disease in patients with breast cancer. Adequate management of hyperlipidemia and smoking rather than heart-sparing RT might be more crucial for preventing cardiovascular disease, especially in Asian women.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Park EH, Min SY, Kim Z, Yoon CS, Jung KW, Nam SJ, et al. Basic facts of breast cancer in Korea in 2014: the 10-year overall survival progress. J Breast Cancer. 2017;20:1–11. doi: 10.4048/jbc.2017.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017;109:djx030. doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 4.Kang JK, Kim MS, Jang WI, Seo YS, Kim HJ, Cho CK, et al. The clinical utilization of radiation therapy in Korea between 2009 and 2013. Radiat Oncol J. 2016;34:88–95. doi: 10.3857/roj.2016.01753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim KS, Shin KH, Choi N, Lee SW. Hypofractionated whole breast irradiation: new standard in early breast cancer after breast-conserving surgery. Radiat Oncol J. 2016;34:81–87. doi: 10.3857/roj.2016.01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 7.Vallis KA, Pintilie M, Chong N, Holowaty E, Douglas PS, Kirkbride P, et al. Assessment of coronary heart disease morbidity and mortality after radiation therapy for early breast cancer. J Clin Oncol. 2002;20:1036–1042. doi: 10.1200/JCO.2002.20.4.1036. [DOI] [PubMed] [Google Scholar]
- 8.Paszat LF, Mackillop WJ, Groome PA, Schulze K, Holowaty E. Mortality from myocardial infarction following postlumpectomy radiotherapy for breast cancer: a population-based study in Ontario, Canada. Int J Radiat Oncol Biol Phys. 1999;43:755–762. doi: 10.1016/s0360-3016(98)00412-x. [DOI] [PubMed] [Google Scholar]
- 9.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 10.Park K, Lee Y, Cha J, You SH, Kim S, Lee JY. Influence of different boost techniques on radiation dose to the left anterior descending coronary artery. Radiat Oncol J. 2015;33:242–249. doi: 10.3857/roj.2015.33.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merzenich H, Bartkowiak D, Schmidberger H, Schmidt M, Schwentner L, Wiegel T, et al. 3D conformal radiotherapy is not associated with the long-term cardiac mortality in breast cancer patients: a retrospective cohort study in Germany (PASSOS-Heart Study) Breast Cancer Res Treat. 2017;161:143–152. doi: 10.1007/s10549-016-4042-2. [DOI] [PubMed] [Google Scholar]
- 12.Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108:179–182. doi: 10.1038/bjc.2012.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul Wright G, Drinane JJ, Sobel HL, Chung MH. Left-sided breast irradiation does not result in increased long-term cardiac-related mortality among women treated with breast-conserving surgery. Ann Surg Oncol. 2016;23:1117–1122. doi: 10.1245/s10434-015-4949-6. [DOI] [PubMed] [Google Scholar]
- 14.Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–424. doi: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onwudiwe NC, Kwok Y, Onukwugha E, Sorkin JD, Zuckerman IH, Shaya FT, et al. Cardiovascular event-free survival after adjuvant radiation therapy in breast cancer patients stratified by cardiovascular risk. Cancer Med. 2014;3:1342–1352. doi: 10.1002/cam4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boero IJ, Paravati AJ, Triplett DP, Hwang L, Matsuno RK, Gillespie EF, et al. Modern radiation therapy and cardiac outcomes in breast cancer. Int J Radiat Oncol Biol Phys. 2016;94:700–708. doi: 10.1016/j.ijrobp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375:2349–2358. doi: 10.1056/NEJMoa1605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayan M, Wilson K, Nelson C, Gagne H, Rubin D, Heimann R. A novel schedule of accelerated partial breast radiation using intensity-modulated radiation therapy in elderly patients: survival and toxicity analysis of a prospective clinical trial. Radiat Oncol J. 2017;35:32–38. doi: 10.3857/roj.2016.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung K, Lee KC, Lee SH, Ahn SH, Lee SH, Choi J. Cardiac dose reduction with breathing adapted radiotherapy using self respiration monitoring system for left-sided breast cancer. Radiat Oncol J. 2014;32:84–94. doi: 10.3857/roj.2014.32.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HY, Chang JS, Lee IJ, Park K, Kim YB, Suh CO, et al. The deep inspiration breath hold technique using Abches reduces cardiac dose in patients undergoing left-sided breast irradiation. Radiat Oncol J. 2013;31:239–246. doi: 10.3857/roj.2013.31.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu JI, Park W, Shin KH, Lee NK, Choi DH, Huh SJ. Prophylactic supraclavicular radiotherapy after surgery in high-risk n1 breast cancer. Oncology. 2013;85:14–20. doi: 10.1159/000352002. [DOI] [PubMed] [Google Scholar]
- 22.Yu JI, Park W, Choi DH, Huh SJ, Nam SJ, Kim SW, et al. Prognostic modeling in pathologic N1 breast cancer without elective nodal irradiation after current standard systemic management. Clin Breast Cancer. 2015;15:e197–e204. doi: 10.1016/j.clbc.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Chang JS, Ko BK, Bae JW, Yu JH, Park MH, Jung Y, et al. Radiation-related heart disease after breast cancer radiation therapy in Korean women. Breast Cancer Res Treat. 2017;166:249–257. doi: 10.1007/s10549-017-4398-y. [DOI] [PubMed] [Google Scholar]
- 24.Taylor C, Correa C, Duane FK, Aznar MC, Anderson SJ, Bergh J, et al. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35:1641–1649. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehammar JC, Jensen MB, McGale P, Lorenzen EL, Taylor C, Darby SC, et al. Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977–2005. Radiother Oncol. 2017;123:299–305. doi: 10.1016/j.radonc.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boekel NB, Schaapveld M, Gietema JA, Russell NS, Poortmans P, Theuws JC, et al. Cardiovascular disease risk in a large, population-based cohort of breast cancer survivors. Int J Radiat Oncol Biol Phys. 2016;94:1061–1072. doi: 10.1016/j.ijrobp.2015.11.040. [DOI] [PubMed] [Google Scholar]