Abstract
Aluminum (Al) is the most abundant metal in the Earth’s crust and is not an essential element for plant growth. In contrast, nitrogen (N) is the most important mineral element for plant growth, but this non-metal is often present at low levels in soils, and plants are often N deficient. Aluminum toxicity is dominant in acid soils, and so plants growing in acid soils have to overcome both Al toxicity and N limitation. Because of low N-use efficiency, large amounts of N fertilizers are applied to crop fields to achieve high yields, leading to soil acidification and potential Al toxicity. Aluminum lowers plant N uptake and N-use efficiency because Al inhibits root growth. Although numerous studies have investigated the interactions between Al and N, a complete review of these studies was lacking. This review describes: (1) the link between plant Al tolerance and ammonium/nitrate (NH4+/NO3-) preference; (2) the effects of NH4+/NO3- and pH on Al toxicity; (3) the effects of Al on soil N transformations; and (4) the effects of Al on NH4+/NO3- uptake and assimilation by plants. Acid soils are characterized chemically by a relatively high ratio of NH4+ to NO3- and high concentrations of toxic Al. Aluminum-tolerant plants generally prefer NH4+ as an N source, while Al-sensitive plants prefer NO3-. Compared with NO3-, NH4+ increases the solubilization of toxic Al into soil solutions, but NH4+ generally alleviates Al phytotoxicity under solution culture because the protons from NH4+ compete with Al3+ for adsorption sites on the root surface. Plant NO3- uptake and nitrate reductase activity are both inhibited by Al, while plant NH4+ uptake is inhibited to a smaller degree than NO3-. Together, the results of numerous studies indicate that there is a synergistic interaction between plant Al tolerance and NH4+ nutrition. This has important implications for the adaptation of plants to acid soils that are dominated chemically by toxic Al as well as NH4+. Finally, we discuss how this knowledge can be used to increase plant Al tolerance and N-use efficiency in acid soils.
Keywords: aluminum, nitrogen, ammonium, nitrate, interaction, plant, acid soil
Introduction
Acid soils cover approximately 30% of the ice-free land and up to 70% of potentially arable soils worldwide (von Uexküll and Mutert, 1995). Acid soils occur mainly in humid tropical and temperate areas (von Uexküll and Mutert, 1995), where water and heat are generally abundant for plant growth, implying that acid soils have huge productive potential. However, plant productivity in acid soils is limited primarily by aluminum (Al) toxicity accompanied by deficiencies of some nutrients (Zhao et al., 2014). The improvement of crop productivity in acid soils depends on the dual enhancement of plant Al tolerance and nutrient-use efficiency.
Nitrogen (N) is the most abundant mineral nutrient required by plants. Soil N availability greatly affects the growth and development of crops worldwide (Gutiérrez, 2012). Nitrogen deficiency is a widespread problem for plants grown in terrestrial ecosystems (Vitousek and Howarth, 1991), and it is also a major factor limiting plant growth in acid soils (Fageria and Baligar, 2001). Large amounts of N fertilizers are used in agriculture to grow crops that feed an increasing global population every year. Erisman et al. (2008) estimated that N fertilizer has supported around 4 billion people born since 1908, accounting for approximately 27% of the world’s population over the past century. At the same time, excess N fertilization is causing environmental problems such as water eutrophication, greenhouse gas emissions, nitrate (NO3-) loss, acid rain, and soil acidification due to low N-use efficiency (Ju et al., 2009). High yields and high nutrient-use efficiency are essential for contemporary agriculture. Therefore, there is an urgent need to increase plant N-use efficiency by understanding the responses to N (Kant et al., 2011).
Aluminum is the most abundant metal in the Earth’s crust. It is not an essential element for plants, and excess Al is toxic to most plants. The primary symptom of Al phytotoxicity is the inhibition of root elongation, which can occur after exposure to Al3+ at concentrations as low as μM levels within 1 h (Matsumoto, 2000; Kochian et al., 2005; Ma, 2007). This inhibition can be caused by reductions in cell elongation and cell division, which are attributed to Al interference with the cell wall, plasma membrane, the cytoskeleton, oxidative stress, signal transduction pathways, cytoplasm calcium homeostasis, magnesium uptake, and auxin polar transport (Ma, 2007). Plants have two strategies to detoxify Al (Ma, 2007). One is to exclude Al from the root tips (exclusion mechanism) and the other is to tolerate Al that enters the plant body (internal tolerance mechanism). Roots are the main organ for plants to take up nutrients from the growth medium, so Al toxicity inevitably affects the ability of plants to acquire nutrients from acid soils. On one hand, the inhibitory effects of Al on root growth can reduce the amounts of nutrients taken up by plants because of the small root volume. On the other hand, Al may directly affect the transport and metabolism of nutrients within plants. Interactions between Al and many nutrients often occur within soils and plants (Zhao et al., 2014). Most reports have focused on the effects of various externally added nutrients on Al phytotoxicity (Zhao et al., 2014), but the effects of Al on the uptake of these nutrients by plants and their corresponding mechanisms have received relatively little attention.
Aluminum is beneficial and even potentially essential for some plant species (Bojórquez-Quintal et al., 2017), because of the Al-induced stimulation of nutrient uptake (Watanabe and Osaki, 2002). Aluminum supply was shown to stimulate N uptake by several plant species adapted to acid soils (Osaki et al., 1997), and Al treatments increased shoot N contents in wheat and rye (Dinev and Stancheva, 1993). In contrast, Al reduced root N uptake and its upward translocation to shoots in sorghum and corn (Gomes et al., 1985; Pintro et al., 1996). Aluminum promoted the growth of plants supplied with ammonium (NH4+) but inhibited that of plants supplied with NO3- (Zhao et al., 2014). Nitrogen is a metabolic element involved in the synthesis of amino acids and proteins within plants. Knowledge about Al–N interactions may supply new information to explain instances where Al benefits plant growth.
Several reviews have focused on the interactions between Al and phosphorus (Chen et al., 2012), calcium (Rengel and Zhang, 2003; Meriño-Gergichevich et al., 2010), magnesium (Bose et al., 2011; Chen and Ma, 2013), boron, and silicon (Hodson and Evans, 1995; Horst et al., 2010). Aluminum is a metal and a toxic element to many plants, while N is a non-metal and is an essential element for all plants. More than 100 papers have reported on Al–N interactions so far, highlighting the importance of this topic. Despite the large amount of literature on Al–N interactions, there has been no systematic review of this topic so far. Here, we provide a detailed description and analysis of studies on the interactions between Al and N, including the link between plant Al tolerance and NH4+/NO3- preference, the effects of NH4+/NO3- and pH on Al toxicity, the effects of Al on soil N transformations, and the effects of Al on NH4+/NO3- uptake and assimilation. We also propose a strategy for improving plant Al tolerance and N-use efficiency in acid soils.
Link Between Plant Al Tolerance and Inorganic N Preference
Acid soils are characterized by poor nitrification and high levels of soluble Al, while neutral to calcareous soils show high nitrification and lower levels of Al toxicity (Zhao et al., 2014; Che et al., 2015). The two main inorganic N sources available for plant growth are NH4+ and NO3-. Therefore, on the basis of the environment driving evolution, plants originating from acid soils are Al tolerant and prefer NH4+ to NO3-, while those originating from neutral to calcareous soils are Al sensitive and prefer NO3- to NH4+ (Gigon and Rorison, 1972; Foy and Fleming, 1978; Rorison, 1985; Falkengren-Grerup, 1995; Marschner, 1995; Maathuis, 2009; Zhao et al., 2013b) (Table 1). For instance, the growth of lowbush blueberry, which is adapted to strongly acid soils, was shown to be greatly promoted by NH4+ but strongly inhibited by NO3- (Townsend, 1966; Townsend and Blatt, 1966). Wheat and barley are Al-sensitive and prefer NO3- (Malhi et al., 1988; Cramer and Lewis, 1993; Famoso et al., 2010), while tea and rice are Al-tolerant and prefer NH4+ (Ruan et al., 2007; Famoso et al., 2010; Zhao et al., 2013b). The activity of NO3- reductase could not be detected in some calcifuge species, suggesting that they have a restricted ability to utilize NO3- (Havill et al., 1974). Rice (Oryza sativa) has two subspecies, indica and japonica. Indica rice cultivars generally prefer NO3-, while japonica cultivars prefer NH4+ (Zhao et al., 2013b; Hu et al., 2015). Correspondingly, indica rice cultivars are generally Al sensitive, while japonica cultivars are Al tolerant (Zhao et al., 2013b). Among different rice cultivars, Al tolerance is closely related to NH4+ and NO3- preference (Zhao et al., 2013b).
Table 1.
Aluminum tolerance and NH4+/NO3- preference of plant species.
| Taxon | Al tolerance | NH4+/NO3- preference | Reference |
|---|---|---|---|
| Vaccinium angustifolium | Tolerant | NH4+ | Townsend, 1966; Townsend and Blatt, 1966 |
| Deschampsia flexuosa | Tolerant | NH4+ | Rorison, 1985 |
| Oxalis acetosella, Carex pilulifera, Festuca gigantea, Poa nemoralis, Deschampsia flexuosa, Stellaria holostea, Rumex acetosella | Tolerant | NH4+ | Falkengren-Grerup, 1995 |
| Camellia sinensis | Tolerant | NH4+ | Ruan et al., 2007 |
| Oryza sativa subsp. japonica | Tolerant | NH4+ | Zhao et al., 2013b |
| Holcus lanatus, Bromus erectus | Sensitive | NO3- | Rorison, 1985 |
| Hordeum vulgare | Sensitive | NO3- | Malhi et al., 1988 |
| Triticum aestivum | Sensitive | NO3- | Cramer and Lewis, 1993; Famoso et al., 2010 |
| Urtica dioica, Ficaria verna, Melandrium rubrum, Aegopodium podagraria, Geum urbanum, Bromus benekenii, Sanguisorba minor, Melica ciliata, Silene rupestris, Viscaria vulgaris, Plantago lanceolata | Sensitive | NO3- | Falkengren-Grerup, 1995 |
| Oryza sativa subsp. indica | Sensitive | NO3- | Zhao et al., 2013b |
The above analyses collectively suggest that Al-tolerant plant species and genotypes utilize NH4+ more efficiently than NO3- (Table 1). This knowledge is helpful for the selection of crop genotypes with both high Al tolerance and N-use efficiency via breeding or genetic modification. The selection of such genotypes should reduce the amount of N fertilizer required and improve plant growth in acid soils. However, the molecular mechanism underlying the link between plant Al tolerance and inorganic N preference is unclear. The two characteristics of grain protein content and acidity tolerance were found to be positively correlated among different wheat lines (Mesdag et al., 1970). In addition, a quantitative trait locus genetic analysis revealed that loci associated with Al tolerance and NH4+ utilization were located in similar regions of rice genome (Ogawa et al., 2014). An important goal for future research is to uncover the mechanism of the link between plant Al tolerance and inorganic N preference at the molecular and genetic levels.
Effects of NH4+, NO3-, and pH on Al Tolerance
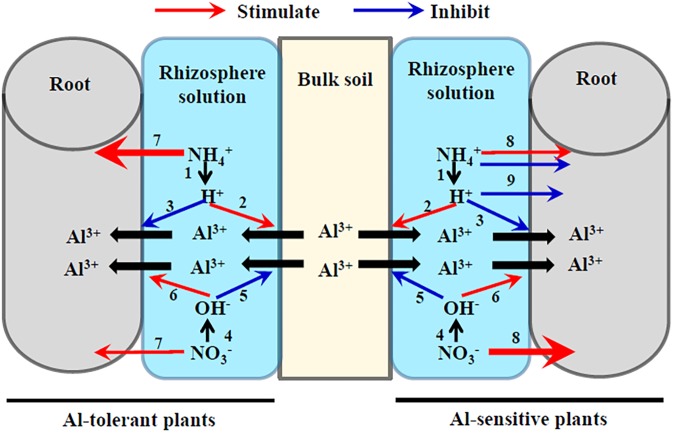
In recent decades, various anthropogenic activities have greatly accelerated soil acidification in Chinese crop fields (Guo et al., 2010; Liang et al., 2013). Among these activities is the excess use of NH4+ fertilizer (Barak et al., 1997; Fang et al., 2014). Atmospheric NH4+ deposition is also an important factor resulting in soil acidification (van Breemen et al., 1982). Nitrification is the mechanism by which NH4+ acidifies soils. During the nitrification of NH4+ to NO3-, H+ are released into soils, which increase the concentration of soluble Al (van Breemen et al., 1982; Mulder et al., 1989; Mulder and Stein, 1994; Che et al., 2015) (Figure 1). Thus, NH4+ facilitates the occurrence of Al toxicity much more than NO3- does. However, increased soluble Al content in soils caused by low pH does not always increase Al phytotoxicity, because lower pH can result in the desorption of Al from plant roots into the rhizosphere solution (Figure 1).
FIGURE 1.
Schematic diagram of possible effects of NH4+ and NO3- on the adsorption and desorption of Al on the root–soil interface. NH4+ acidifies rhizosphere solution (1), which stimulates the desorption of Al from bulk soils into rhizosphere solution (2) but inhibits the adsorption of Al from rhizosphere solutions to plant roots (3) both because of the competition between Al3+ and H+. In contrast, NO3- alkalizes rhizosphere solution (4), which inhibits the desorption of Al from soils into rhizosphere solution (5) but stimulates the adsorption of Al from rhizosphere solutions to plant roots (6) because NO3--increased negative electrical charge of root surface. Al-tolerant plant species prefer NH4+ to NO3- (7), while Al-sensitive plant species prefer NO3- to NH4+ (8). Excess NH4+ and H+ are both toxic to the growth of Al-sensitive plant species (9). Consequently, NH4+ alleviates Al toxicity to Al-tolerant plant species while aggravates Al toxicity to Al-sensitive plant species compared with NO3-.
Early studies showed that changes in root zone pH due to ion uptake imbalances were related to Al tolerance in triticale, wheat, and rye under certain solution and soil conditions (Mugwira and Patel, 1977). The plant growth medium can be acidified due to NH4+ uptake by plant roots and the nitrification of NH4+ to NO3-. Alternatively, the growth medium can be alkalinized due to the uptake of NO3- by plant roots. Because Al toxicity occurs in acid soils, one could speculate that the preferential utilization of NO3- relative to NH4+ can enhance plant Al tolerance through increasing the pH of the growth medium via NO3- uptake. The Al tolerance of some wheat varieties was attributable to their abilities to preferentially utilize NO3- relative to NH4+ through rhizosphere alkalization (Foy et al., 1965, 1967; Foy and Fleming, 1978, 1982; Fleming, 1983; Taylor and Foy, 1985a,b,c). The results of subsequent studies, however, indicated that genotypic differences in wheat Al tolerance were not caused by differences in rhizosphere pH induced by the differential uptake of NH4+ and NO3- (Taylor, 1988a,b; Miyasaka et al., 1989). Instead, the differences in the uptake of NH4+ and NO3- among different wheat genotypes were suggested to be the result of, rather than the cause of, differences in A1 tolerance among genotypes (Taylor, 1988a,b; Miyasaka et al., 1989). Another research demonstrated that the decrease in the growth medium pH under Al stress was greater for an Al-tolerant wheat genotype than an Al-sensitive one (Ikeda and Yamanishi, 1999). Therefore, genotypic differences in the relative Al tolerance of wheat could not be explained by root-induced pH changes due to the uptake of NH4+ and NO3-.
Three reports on rice plants drew different conclusions. In two studies, an Al-tolerant rice genotype had a stronger ability than an Al-sensitive genotype to increase nutrient solution pH through efficient NO3- uptake and metabolism (Ganesan et al., 1993; Justino et al., 2006). However, another study (van Hai et al., 1989) obtained the opposite result, in that an Al-resistant genotype took up more NH4+ and acidified the nutrient solution to a greater degree than did an Al-sensitive one. In barley, Al tolerance of different cultivars was not related to the root-induced pH change by the uptake of inorganic N sources from the growth medium (Wagatsuma and Yamasaku, 1985). Similarly, differences in pH changes in the growth medium were not related to differences in A1 tolerance between two sorghum genotypes (Galvez and Clark, 1991). In fact, the NO3- uptake rate was found to be higher in an Al-sensitive sorghum genotype than in an Al-tolerant one (Cambraia et al., 1989). Genotypic differences in the Al tolerance of soybean plants were not associated with the difference in NH4+ uptake vs. NO3- uptake and root-induced pH changes (Klotz and Horst, 1988b). Changes in the medium pH were also not related to Al tolerance in triticale (Antunes and Antonieta Nunes, 1997). These analyses further demonstrated that genotypic differences in the Al tolerance of diverse plant species cannot be explained only by root-induced pH changes due to NH4+ and NO3- uptake.
Since low pH increases the concentrations of soluble Al in soils, the alkalization of the rhizosphere was proposed to be an important mechanism of plant Al tolerance (Matsumoto, 2000; Kochian et al., 2004; Ma, 2007). However, several studies demonstrated that H+ could alleviate Al toxicity because H+ competed with Al3+ for adsorption to the root surface (Kinraide et al., 1992; Godbold et al., 1995; Zhao et al., 2009; Zhao et al., 2014). A supply of H+ also alleviated Al toxicity in bacteria (Kinraide and Sweeney, 2003) and yeast (Zhao et al., 2017). These results implied that Al toxicity is much lower at low pH than at high pH under a certain acid pH range (pH < 5.0) because of the H+ alleviation of Al phytotoxicity. The uptake of NH4+ and NO3- decreases and increases the pH of the medium, respectively. Many reports have indicated that NH4+ supply can enhance plant Al tolerance, while NO3- supply aggravates Al toxicity (Table 2). In some studies, Al was found to stimulate the growth of some grasses (Rorison, 1985), tropical trees (Watanabe et al., 1998), Lespedeza bicolor (Chen et al., 2010), and rice (Zhao et al., 2013b) when supplied with NH4+, but not when supplied with NO3-. The stimulatory effects of Al on plant growth may be related to the effects of Al to alleviate H+ toxicity (Kinraide et al., 1992). Thus, NH4+ alleviates Al toxicity, and Al enhances NH4+ utilization.
Table 2.
Summary of NH4+ effects on plant Al tolerance relative to NO3-: (+) enhancement, (-) decrease, and (0) no change.
| Taxon | Effects | Reference |
|---|---|---|
| Holcus lanatus | + | McCain and Davies, 1983 |
| Deschampsia flexuosa, Holcus lanatus, Bromus erectus | + | Rorison, 1985 |
| Spruce and beech | + | Van Praag et al., 1985a |
| Glycine max | + | Klotz and Horst, 1988a,b |
| Secale cereal, Lupinus luteus | + | Grauer and Horst, 1990 |
| Pinus rigida | + | Cumming, 1990a; Cumming and Weinstein, 1990a; Schier and McQuattie, 1999a |
| Triticosecale | + | Antunes and Antonieta Nunes, 1997; Domingues, 2010 |
| Melastoma malabathricum, Acacia mangium, Melaleuca cajuputi | + | Watanabe et al., 1998 |
| Oryza sativa | + | Zhao et al., 2009, 2013b; Wang et al., 2015 |
| Lespedeza bicolor | + | Chen et al., 2010 |
| Sorghum bicolor | + or -b | Tan et al., 1992 |
| Sorghum bicolor | 0 | Keltjens, 1987 |
| Picea abies | 0 | Godbold et al., 1988 |
| Mucuna pruriens | 0 | Hairiah et al., 1994 |
| Triticum aestivum | - | Fleming, 1983; Taylor and Foy, 1985a,b,c |
aStudy was conducted using sand culture irrigated with nutrient solutions. Studies not marked by superscript letter were conducted using hydroponic systems. bEffect was dependent on plant genotypes.
It is now accepted that the NH4+-induced rhizosphere acidification is the primary mechanism underlying the NH4+ enhancement of Al tolerance in plants (Zhao et al., 2009; Wang et al., 2015) (Figure 1). Relative to NO3-, NH4+ uptake by rice roots reduces the pH of the nutrient solution. Lower pH further decreases the number of Al-binding functional groups and enhances the positive electrical potential of the root surface (Wang et al., 2015; Liu et al., 2016). Consequently, NH4+-fed roots adsorb less Al than do NO3--fed roots, thereby alleviating Al toxicity. The ability of NH4+ to alleviate Al toxicity was also observed under constant pH conditions (Rorison, 1985; Klotz and Horst, 1988a,b; Grauer and Horst, 1990), indicating that factors other than pH may be involved. It is possible that intermediate products of N metabolism such as nitric oxide (NO) play a role in the alleviation of Al toxicity by NH4+ (Zhao and Shen, 2013).
Several studies found that NH4+ aggravated Al toxicity, relative to NO3- (Table 2), which may reflect differences in plants’ sensitivity to NH4+. Some studies on the aggravating effects of NH4+ on Al toxicity used wheat as the experimental material (Fleming, 1983; Taylor and Foy, 1985a,b,c). Wheat plants prefer NO3- to NH4+ and are sensitive to both Al and NH4+ (Table 1). If wheat plants are supplied only with NH4+, then NH4+ toxicity may occur and may be more serious than Al toxicity. Thus, NH4+ may aggravate rather than alleviate Al toxicity in wheat plants. Some sorghum genotypes showed lower Al toxicity and some showed higher Al toxicity with NH4+ relative to NO3- N (Tan et al., 1992). Because an Al-sensitive sorghum genotype was more NH4+-sensitive than an Al-tolerant one, NH4+ toxicity probably masked Al toxicity in sorghum (Keltjens, 1987). Consequently, it is difficult to observe the NH4+ alleviation of Al toxicity in NH4+-sensitive plant species (Keltjens, 1987). Thus, plants grown in acid soils may suffer from Al toxicity accompanied by NH4+ toxicity due to poor soil nitrification.
Most studies on the effects of NH4+ and NO3- on Al tolerance have been conducted using hydroponic experiments (Table 2), which might not reflect the real effects of NH4+ and NO3- on Al tolerance. In soils, lower root rhizosphere pH will result in greater solubilization of Al ions from the soil into the rhizosphere solution, potentially increasing Al toxicity to plants. However, under nutrient solution culture, lower rhizosphere pH will only affect Al speciation (Keltjens and van Loenen, 1989). Lower pH due to NH4+ uptake by plants increases the solubilization of Al3+ from bulk soils into the rhizosphere solution (Figure 1). Nevertheless, for plant roots, more H+ in the rhizosphere solution can decrease Al3+ adsorption by roots through cation competition and increasing the positive electrical potential of the root surface. Thus, whether Al toxicity is exacerbated or alleviated by NH4+ or NO3- may depend on the relative dominance of the effects of pH on Al desorption from soils into the rhizosphere solution and Al adsorption from the rhizosphere solution into the roots. Further studies on this topic should be conducted on soil-grown plants.
Effects of Al on N Transformations in Soils
Although the effects of nitrification on soil pH and Al solubility are well known, less is known about the effects of Al on soil N transformations such as nitrification and ammonification. The nitrification rate is lower in acid soils than in neutral to calcareous soils (Che et al., 2015), although the reasons for this are still unclear. It is generally considered that low pH inhibits the activity of nitrifying microbes. Higher levels of soluble Al are often concomitant with lower soil pH. Soil N transformations are controlled by microbes. Most microbes are very sensitive to Al (Piña and Cervantes, 1996), while fungi are relatively more tolerant than bacteria to Al and acids (Zhao et al., 2013a, 2017). Low pH does not always result in high concentrations of active Al in soils, because Al ions can form complexes with various organic and inorganic ligands. Future research should explore the role of Al in regulating soil N transformations and in N cycle as a whole.
In a paper published almost 100 years ago (Denison, 1922), Al salts stimulated ammonifying microbes but adversely affected nitrifying bacteria. However, more recent reports showed that Al did not affect the nitrification potential and abundance of ammonia-oxidizing amoA gene of archaea and bacteria (Kasuga et al., 2010; Lin et al., 2017). Bacterial growth was shown to gradually decrease as the pH decreased from 6.5 to 4.0 (Rousk et al., 2010), while soil exchangeable Al linearly increased as the pH decreased from 5.4 to 3.7 (Aciego Pietri and Brookes, 2008). In addition, the OTU richness and Shannon’s diversity index of both ammonia-oxidizing archaea and bacteria showed significantly negative correlation with soil pH ranging from 3.77 to 8.46 (Hu et al., 2013). Therefore, microbial growth was found to be limited at soil pHs lower than 5.4 when Al became soluble, but was limited by low pH rather than Al toxicity at pHs ranging from 6.5 to 5.4. These analyses suggested that the inhibition of soil nitrification that transformed NH4+ to NO3- was due to acid stress rather than Al toxicity, when soil pH decreased from 6.5 to 5.4. There are several soil N transformation processes such as nitrification, denitrification, and ammonification, and different types of microbes control the different pathways of transformations. To clarify the effects of Al on soil N transformation, further studies should evaluate N transformation-related microbial populations and Al solubility under controlled conditions with variable soil pH and NH4+/NO3- supply.
Effects of Al on NO3- Uptake by Plant Roots
Approximately 30 published studies have focused on the effects of Al toxicity on NO3- uptake, and most of them found that Al inhibited NO3- uptake (Table 3). Jerzykiewicz (2001) observed that an extremely high concentration of Al (5 mM) even resulted in NO3- efflux from cucumber roots. The mechanism by which Al inhibits NO3- uptake is still unclear, but some possible mechanisms have been proposed. In one study, a high Al concentration resulted in large amounts of Al entering the symplast of soybean roots, leading to symplastic Al concentrations that were high enough to inhibit NO3- transport across the membrane (Lazof et al., 1994). Thus, one proposed mechanism by which Al inhibits NO3- uptake is that intracellular Al may bind to NO3- transporters, NO3- metabolic enzymes, and other components of systems related to NO3- uptake. Plant NO3- transport involves at least three systems; the constitutive high-affinity transport system (cHATS), the inducible high-affinity transport system (iHATS), and the constitutive low-affinity transport system (cLATS) (Crawford and Glass, 1998; Miller et al., 2007). The constitutive systems function without NO3- pretreatment, but the inducible system is stimulated by external NO3-. The cHATS has low values of both Km (6–20 μM) and Vmax (0.3–0.82 μmol g-1h-1), while the iHATS is characterized by higher Km (20–100 μM) and Vmax (3–8 μmol g-1h-1) values and is induced by exposure to NO3- for hours to days. The cLATS functions at NO3- concentrations above 250 μM and does not become saturated even when NO3- concentrations are as high as 50 mM. Durieux et al. (1993) reported that Al exerted stronger effects on the inducible system than on the constitutive systems. Their results also suggested that high concentrations of Al inhibited the activity of NO3- transporters in the inducible system rather than affected the number of NO3- transporters (Durieux et al., 1993). Pretreatment with Al had little effect on NO3- uptake by plants (Jarvis and Hatch, 1986; Durieux et al., 1993), and NO3- transport quickly recovered when Al was removed from the external growth medium (Durieux et al., 1993). These results suggested that Al directly interacts with NO3- transporters but that this interaction is reversible, leading to the inhibition of NO3- uptake by Al.
Table 3.
Summary of effects of aluminum on NO3- uptake: (-) inhibition, (+) stimulation, and (0) no change.
| Taxon | Al (μM) | NO3- (mM) | Al duration | Effects | Reference |
|---|---|---|---|---|---|
| Triticum aestivum | 111 | 3.5 | 29 days | - | Fleming, 1983 |
| Trifolium repens | 25–100 | 0.7 | 21 days | - | Jarvis and Hatch, 1986 |
| Sorghum bicolor | 55–370 | 0.1–14 | 15 h–36 days | - | Keltjens, 1987, 1988; Keltjens and van Ulden, 1987; Cambraia et al., 1989; Galvez and Clark, 1991 |
| Pinus rigida | 200 | 2–4 | 42 days | - | Cumming, 1990a |
| Picea abies | 37–1483 | 1 | 14 days | - | Peuke and Tischner, 1991 |
| Zea mays | 5–166 | 0.2–0.6 | 1.5 h–7 days | - | Durieux et al., 1993, 1995; Calba and Jaillard, 1997; Purcino et al., 2003 |
| Glycine max | 80 | 0.3 | 30 m–2 h | - | Lazof et al., 1994 |
| Triticosecale | 185, 370 | 1.6–12 | 4–7 days | - | Antunes and Antonieta Nunes, 1997; Domingues, 2010 |
| Musa spp. | 78.5 | 1.8 | 40 days | - | Rufyikiri et al., 2001 |
| Lotus japonicus | 102–104 | 0.15 | 24 h | - | Pal’ove-Balang and Mistrík, 2007 |
| Lotus corniculatus | 103 | 0.15 | 72 h | - | Pal’ove-Balang and Zelinova, 2013 |
| Oryza sativa | 50 | 2.86 | 24–96 h | - | Zhou et al., 2016 |
| Broadleaf trees | 600 | 3.5 | 3 h | - | Burnham et al., 2017b |
| Camellia sinensis | 400 | 3.6 | 24 h | 0 | Morita et al., 1998 |
| Glycine max | 56 | 1.4 | 14 h | + | Klotz and Horst, 1988b |
| Oryza sativa | 0–1111 | 0.36 | 65 days | + (<185 μM Al) or - (>185 μM Al) | van Hai et al., 1989 |
| Hordeum vulgare | 102 | 0.37 | 5 min | + | Nichol et al., 1993 |
| Glycine max | 0–45 | 0.3 | 72 h | + (<10 μM Al) or - (>10 μM Al) | Rufty et al., 1995 |
| Cucumis sativus | 500, 103, 5 × 103 | 1 | 1–6 h | + (0.5 mM Al exposure for 3 h) or - (1 mM or 5 mM Al exposure for 6 h) | Jerzykiewicz, 2001 |
| Quercus serrata | 103 | 2.8 | 3–14 days | + | Tomioka et al., 2007 |
aStudy was conducted using sand culture irrigated with nutrient solutions. bStudy was conducted using soil culture. Studies not marked by superscript letters were conducted using hydroponic systems.
The inhibition of root elongation is the main symptom of Al phytotoxicity. Root elongation was inhibited much more than NO3- uptake in the presence of high Al concentrations in soybean (Rufty et al., 1995). The Al-inhibition of NO3- uptake was found to be similar across different Al-tolerant soybean genotypes and different root regions (Lazof et al., 1994). The root apex is the primary target of Al toxicity to plants (Ryan et al., 1993). However, NO3- uptake rates by corn root tips only accounted for a low percentage of NO3- taken up by the total root system, and N in root tips was mainly derived from N adsorbed through other root regions (Lazof et al., 1992). The mechanism by which Al inhibits root elongation was suggested to differ from the mechanism of Al inhibition of NO3- uptake in maize (Durieux et al., 1995). The results of these studies indicated that the mechanism of Al inhibition of NO3- uptake might differ from the mechanism(s) of plant Al sensitivity and Al-inhibited root elongation, at least in maize and soybean. This should be further tested using more plant species.
The effects of Al on NO3- uptake may depend on Al concentrations, Al exposure time, plant species, and plant genotype. Aluminum does not always affect NO3- uptake, for example, in Al-tolerant tea trees (Morita et al., 1998) (Table 3). A stimulatory effect of Al on root NO3- uptake has been observed in studies where Al was supplied at low concentrations (van Hai et al., 1989; Rufty et al., 1995; Jerzykiewicz, 2001), or for a short-term (Nichol et al., 1993; Jerzykiewicz, 2001), and/or in studies on wild plant species that prefer Al (Tomioka et al., 2007) (Table 3). Similar to the observed stimulatory effects of Al on NO3- uptake, N uptake and partitioning were found to be enhanced by lower Al concentrations (20–200 μM Al) but inhibited by high Al concentrations (1000 μM Al) in defoliated grasses (Thornton, 1998). In wheat, N uptake by root tips was inhibited by Al in an Al-sensitive genotype, but stimulated in an Al-tolerant genotype (Ikeda and Yamanishi, 1999). These results suggested that low Al accumulation in plants could stimulate NO3- uptake.
Several possible mechanisms were suggested to be responsible for the stimulation of NO3- uptake by low concentrations of Al (Rufty et al., 1995; Jerzykiewicz, 2001) (Figure 2). First, the increase in the positive electrical potential of the cell surface by Al3+ could facilitate the access of negatively charged NO3- to the root cell surface. Second, Al-induced H+ extrusion under acid stress could increase NO3- transport across the membrane via H+/NO3- co-transport. Finally, NO3- efflux from cells could be diminished by the binding of extracellular Al to the cell membrane if Al impairs the structural integrity of plasma membranes and alters their permeability (Cakmak and Horst, 1991). However, direct and specific evidence for each of these mechanisms is still lacking.
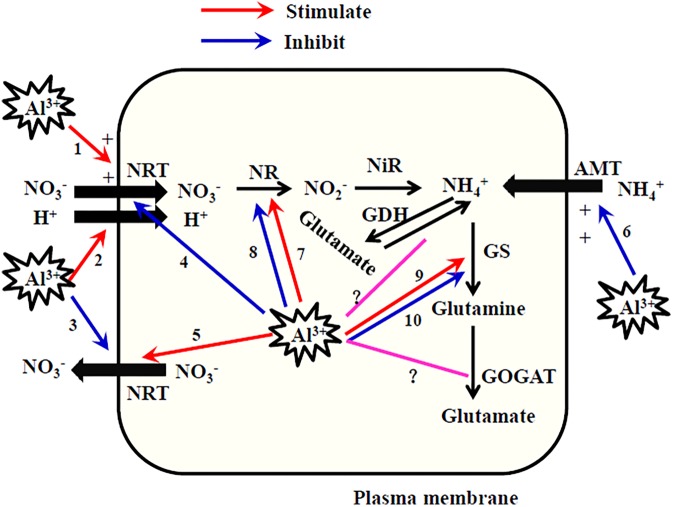
FIGURE 2.
Schematic diagram of possible effects of Al on uptake and assimilation of NH4+ and NO3- by plants. NRT, nitrate transporter; AMT, ammonium transporter; NR, nitrate reductase; NiR, nitrite reductase; GS, glutamine synthetase; GOGAT, glutamate synthase; GDH, Glutamate dehydrogenase. When plant roots accumulate low concentrations of Al in the apoplastic space, root NO3- uptake is stimulated by apoplastic Al because of Al3+-increased positive electrical charge of cell surface (1), enhanced H+-NO3- cotransport (2), and diminished NO3- efflux (3). When plant roots accumulate large amounts of Al that enters the symplasm of roots, intracellular Al inhibits NO3- uptake as Al binds to NO3- transporter (4) and induces enhanced efflux of NO3- (5). Al3+-increased positive electrical charge of cell surface results in the Al inhibition of NH4+ uptake (6). Low concentrations of Al stimulates NRA (7) because of Al-stimulated NO3- uptake by the three ways (1, 2, and 3), while high concentrations of Al inhibits NRA (8) because of Al-inhibited NO3- uptake by the two ways (4 and 5). Al stimulates GS activity (9) due to the binding of Al with GS while inhibits that (10) due to the inhibition of NH4+ uptake (6). The effects of Al on GOGAT and GDH are still uncertain (?).
Rufty et al. (1995) compared experimental conditions including the Al concentration, medium pH, and calcium concentration among several papers reporting different effects of Al on NO3- uptake. This comparative analysis suggested that pH and calcium levels, rather than Al concentrations, explained the differences in results among studies (Rufty et al., 1995). Under acid stress and low calcium levels, Al ameliorated acid stress to roots, thereby enhancing NO3- influx into cells (Rufty et al., 1995). Further studies using carefully designed experiments should explore how pH and calcium affect the ability of Al to alter NO3- uptake.
Based on the analyses summarized above, we present a schematic diagram to explain the mechanisms of the effects of Al on NO3- uptake (Figure 2). When plant roots accumulate low concentrations of Al in the apoplastic space of roots, extracellular Al may stimulate NO3- uptake because of an Al3+-induced increase in the positive electrical charge of the cell surface, enhanced H+-NO3- cotransport, and diminished NO3- efflux. When large amounts of Al enter the symplasm of roots, root NO3- uptake is inhibited by Al because Al binds to the NO3- transporter and enhances NO3- efflux. We emphasize that this schematic diagram is based only on the published reports. There is still no direct evidence for these proposed mechanisms. Just as the molecular basis for N uptake has been discovered in recent years, the molecular basis of both the Al-stimulation and Al-inhibition of NO3- transport can be explored in molecular studies on plant mutants defective in NO3- transport.
Effects of Al on NH4+ Uptake by Plant Roots
Various studies have reported that root NH4+ uptake was either inhibited, stimulated, or unaffected by Al (Table 4). However, most studies have reported inhibitory effects of Al on NH4+ uptake by plants. Nichol et al. (1993) indicated that Al treatment for 5 min suppressed the movement of cations (NH4+, Ca2+, and K+) across the plasma membrane but facilitated the movement of anions (NO3- and phosphate). Aluminum ions may bind to the cell surface and form a positively charged layer, thereby inhibiting the adsorption of positively charged cations to the cell surface but stimulating the adsorption of negatively charged anions. Thus, similar to the mechanisms responsible for the Al stimulation of NO3- uptake described above, the Al3+-induced increase in the positive electrical charge of the cell surface is responsible for the inhibition of NH4+ uptake by Al (Figure 2).
Table 4.
Summary of effects of aluminum on NH4+ uptake: (-) inhibition, (+) stimulation, and (0) no change.
| Taxon | Al (μM) | NH4+ (mM) | Al duration | Effects | Reference |
|---|---|---|---|---|---|
| Oryza sativa | 0–1111 | 0.36 | 65 days | - | van Hai et al., 1989 |
| Sorghum bicolor | 300 | 0.36–3.6 | 2–18 days | - | Galvez and Clark, 1991 |
| Hordeum vulgare | 100 | 0.03 | 5 min | - | Nichol et al., 1993 |
| Triticum aestivum | 10, 100 | 2 | 2–3 days | - | Ikeda and Yamanishi, 1999 |
| Musa spp. | 78.5 | 0.2 | 40 days | - | Rufyikiri et al., 2001 |
| Lotus japonicus | 102–104 | 0.2 | 24 h | - | Pal’ove-Balang and Mistrík, 2007 |
| Lotus corniculatus | 103 | 0.2 | 72 h | - | Pal’ove-Balang and Zelinova, 2013 |
| Zea mays | 166 | 0.2 | 7 days | - | Purcino et al., 2003 |
| Zea mays | 5–100 | 0.2–0.24 | 0.5 h–3 days | 0 | Durieux et al., 1993; Calba and Jaillard, 1997 |
| Camellia sinensis | 400 | 3.6 | 24 h | 0 | Morita et al., 1998 |
| Triticosecale | 370 | 0.2–1.6 | 4 days | 0 | Domingues, 2010 |
| Triticosecale | 185 | 0.8, 1.4 | 5–7 days | + or 0 | Antunes and Antonieta Nunes, 1997 |
| Sorghum bicolor | 55–370 | 2–4 | 96 h–36 days | + | Keltjens, 1987, 1988; Keltjens and van Ulden, 1987 |
| Glycine max | 56 | 1.4 | 14 h | + | Klotz and Horst, 1988b |
All studies used hydroponic systems.
In general, Al exerts a smaller negative effect on NH4+ uptake than on NO3- uptake. In maize roots, Al reduced the uptake of both NH4+ and NO3- but increased the uptake ratio NH4+/NO3-, indicating that NH4+ uptake was inhibited much less than NO3- uptake by Al (Purcino et al., 2003). An Al treatment reduced NO3- uptake but not NH4+ uptake in maize and triticale (Durieux et al., 1993; Calba and Jaillard, 1997; Domingues, 2010), while Al inhibited NO3- uptake but stimulated NH4+ uptake in sorghum and triticale (Keltjens and van Ulden, 1987; Antunes and Antonieta Nunes, 1997). Leaf N content was increased by A1 when NH4+ was supplied but reduced by Al when NO3- was supplied (Van Praag et al., 1985). An Al treatment reduced the NO3- concentration but increased the free NH4+ concentration in the leaves of corn plants (Souza et al., 2016).
The studies reporting that Al stimulated root NH4+ uptake generally used N sources comprising a mixture of NH4+ and NO3- (Keltjens, 1987, 1988; Keltjens and van Ulden, 1987; Antunes and Antonieta Nunes, 1997). Since Al inhibited NO3- uptake in those studies, we may infer that N deficiency caused by the inhibition of NO3- uptake might explain the stimulation of NH4+ uptake by Al. When NO3- cannot meet the N demands of plants under Al stress, plants may take up more NH4+ in place of NO3- to alleviate N deficiency.
Effects of Al on NO3- Reduction
Nitrate reductase (NR) represents the first enzymatic and rate-limiting step of NO3- assimilation in plants. It catalyzes the reduction of nitrate to nitrite and is a substrate-inducible enzyme (Tischner, 2000). A large body of research has indicated that Al inhibits NR activity (NRA) in roots, shoots, or both (Table 5). Several studies reported that Al toxicity reduced NRA much more in Al-sensitive plant genotypes than in Al-tolerant ones (Foy and Fleming, 1982; Keltjens and van Ulden, 1987; Justino et al., 2006). In wheat and sorghum, Al significantly inhibited NRA in shoots rather than roots (Foy and Fleming, 1982; Keltjens and van Ulden, 1987). In contrast, Al inhibited NRA in roots rather than shoots in red spruce (Cumming and Brown, 1994). The inhibitory effect of Al on NRA may result from Al-inhibition of NO3- uptake, as the decreased level of the substrate, NO3-, would lead to decreased NRA (Gomes et al., 1985; Keltjens and van Ulden, 1987; Keltjens, 1988; Justino et al., 2006; Pal’ove-Balang and Mistrík, 2007; Souza et al., 2016). The Al-induced decrease in NO3- content in plants was proposed to be the main mechanism by which Al inhibits NRA, so the interaction between Al and NR may be indirect. Roots generally accumulate more Al than do shoots. However, Al significantly inhibited NRA in the shoots but not in roots of wheat and sorghum (Foy and Fleming, 1982; Keltjens and van Ulden, 1987), suggesting that a direct interaction between NR and Al is unlikely. The ratio of absorbed 15NO3- to reduced ammonia-containing N remained constant with increasing Al, also suggesting an indirect effect of Al on NR (Rufty et al., 1995). However, in another study, Al inhibited the shoot NRA of sorghum, and this could not be reversed by increased NO3- concentrations (Cambraia et al., 1989). Aluminum decreased NO3- accumulation in cucumber roots and maize leaves but enhanced their NRA (Lidon et al., 1998; Jerzykiewicz, 2001).
Table 5.
Summary of effects of aluminum on nitrate reductase activity: (-) inhibition, (+) stimulation, (0) no change and (N) not studied.
| Taxon | Al (μM) | Al duration | Effects | Reference | |
|---|---|---|---|---|---|
| Root | Shoot | ||||
| Sorghum bicolor | 50–185 | 5–30 days | - | - | Cambraia et al., 1989; Cruz et al., 2011a |
| Sorghum bicolor | 55–370 | 48 h–24 days | 0 | - | Keltjens and van Ulden, 1987; Keltjens, 1988 |
| Oryza sativa | 160–500 | 5–21 days | - | - | Ganesan et al., 1993; Justino et al., 2006; Mishra and Dubey, 2011a |
| Picea rubens | 37–370 | 2–42 days | - | N | Yandow and Klein, 1986 |
| Picea rubens | 200 | 10 weeks | - | 0 | Cumming and Brown, 1994a |
| Pinus rigida | 200 | 6 weeks | - | N | Cumming, 1990a |
| Lotus japonicus | 102–104 | 24 h | - | N | Pal’ove-Balang and Mistrík, 2007 |
| Zea mays | 5 × 104–2 × 105 | 15 days | N | - | Souza et al., 2016a |
| Helianthus annuus | 100 | 15 days | N | - | Ruiz et al., 2007 |
| Hordeum vulgare | 2 × 103–6 × 103 | 6 days | N | - | Shahnawaz et al., 2017 |
| Triticum aestivum | 19–111 | 20 | 0 | - (Al-sensitive genotype) or 0 (Al-tolerant genotype) | Foy and Fleming, 1982 |
| Mucuna pruriens | 110 | 4 weeks | N | 0 | Hairiah et al., 1994 |
| Oryza sativa | 80, 160 | 15 days | + (80 μM Al) or - (160 μM Al) | + (80 μM Al) or - (160 μM Al) | Sharma and Dubey, 2005a |
| Zea mays | 100 | 15 days | - or + (dependent on genotypes and N source) | N | Mihailovic et al., 2015 |
| Glycine max | 56 | 6 h–4 days | + or - (dependent on genotype and root distance) | N | Klotz and Horst, 1988b |
| Zea mays | 103 | 20 days | N | + | Lidon et al., 1998b |
| Triticum aestivum | 30 | 3 h | + | N | Sun et al., 2014 |
| Glycine max | 50, 100 | 24 h | + | N | Wang et al., 2017 |
| Phaseolus vulgaris | 50 | 6–24 h | + | N | Wang et al., 2010 |
| Quercus serrata | 103–2.5 × 103 | 1 h–14 days | + | N | Tomioka et al., 2007, 2012 |
| Cucumis sativus | 500, 103, 5 × 103 | 24 h | + | N | Jerzykiewicz, 2001 |
| Triticum aestivum, Triticale hexaploidae, and Secale cereale | 37–370 | 20 days | N | + (Triticum aestivum, and Triticale hexaploidae); - (Secale cereale) | Dinev and Stancheva, 1993 |
| Camellia sinensis | 300 | 14 days | + | + | Hajiboland et al., 2014 |
| Picea abies | 37–741 | 2–3 months | + (<37 μM Al) or - (>37 μM Al) | + | Peuke and Tischner, 1991 |
astudy was conducted using sand culture irrigated with nutrient solutions. bStudy was conducted using vermiculite culture irrigated with nutrient solutions. Other studies not marked with superscript letters were conducted using hydroponic systems.
In some studies, Al was found to increase NRA (Table 5). At low concentrations, Al stimulated NRA in spruce (<37 μM Al; Peuke and Tischner, 1991) and rice (80 μM Al; Sharma and Dubey, 2005). Aluminum stimulated NRA in the Al-preferring species Quercus serrata (Tomioka et al., 2007, 2012) and tea (Hajiboland et al., 2014). The production of NO mediated by NR alleviated Al toxicity in red kidney bean, wheat, and soybean by alleviating oxidative stress, where Al significantly enhanced NRA in root tips (Wang et al., 2010, 2017; Sun et al., 2014). In another study, Al more strongly promoted NRA in Al-tolerant wheat than in Al-sensitive wheat (Sun et al., 2014).
The interaction between Al and NR appears to be complex, and can be positive or negative, direct or indirect. Many environmental factors are known to modulate NRA (Tischner, 2000). In various studies, the effects of A1 on NRA depended on the plant genotype (Foy and Fleming, 1982; Keltjens and van Ulden, 1987; Justino et al., 2006; Sun et al., 2014; Mihailovic et al., 2015), plant species (Dinev and Stancheva, 1993), plant part (Foy and Fleming, 1982; Keltjens and van Ulden, 1987), medium pH (Yandow and Klein, 1986), Al levels (Peuke and Tischner, 1991; Sharma and Dubey, 2005), N source and levels (Cumming, 1990; Mihailovic et al., 2015; Gupta et al., 2016), and inoculation treatments (Cumming, 1990). Although the Al–NR interaction is complex, we can conclude that NRA is generally inhibited by high Al concentrations, and stimulated by low Al concentrations (Figure 2). This overall trend is similar to the effects of Al on NO3- uptake, because NO3- is the primary factor regulating NRA.
Further research with detailed and well-designed experiments using different plant materials is necessary to clarify the details of the interaction between NR and Al. Recently, several genes encoding NR in maize (Zea mays) were found to be differently modulated at the transcriptional level by Al toxicity (Cantú et al., 2016). Molecular biology techniques could be helpful to clarify the detailed mechanisms of the interaction between Al and NR as well as NO3- uptake.
Effects of Al on NH4+ Assimilation
In plants, NH4+ is mainly assimilated by the GS/GOGAT (glutamine synthetase/glutamate synthase) cycle, where GS catalyzes the reaction between NH4+ and glutamate to form glutamine. Glutamine subsequently combines with 2-oxoglutarate in a reaction catalyzed by GOGAT to form two molecules of glutamate (Masclaux-Daubresse et al., 2010). Glutamate dehydrogenase (GDH) is considered to be an alternative pathway to incorporate NH4+ into glutamate when plants are exposed to high NH4+ concentrations under stress. However, there is more evidence that GDH functions mainly in glutamate deamination (Masclaux-Daubresse et al., 2010). The presence of Al was shown to decrease the concentrations of NO3--N and asparagine but increase the concentrations of amino acid-N and glutamine in the xylem sap of sorghum plants, potentially indicating that Al interferes with the synthesis and/or interconversion of N in plants (Gomes et al., 1985).
Pécsváradi’s research group reported the activating effect of the Al(III)-tartrate 1:3 complex and the Al(III)–nitrilotriacetic acid complex on the activity of GS extracted from roots and leaves of wheat (Kertész et al., 2002; Pécsváradi et al., 2009). This activating effect was attributable to the specific binding of Al to the protein chain of GS, similar to the role of Mg in activating GS activity (Pécsváradi et al., 2009). Except for those two reports (Kertész et al., 2002; Pécsváradi et al., 2009), all of the other studies summarized here reported Al inhibition of GS activity in both roots and shoots (Table 6). However, Al either activated, suppressed, or did not affect the activities of GOGAT and GDH (Table 6). The effects of Al on the activities of N-assimilating enzymes were found to vary between Al-tolerant and Al-sensitive maize varieties and depend on the N form supplied. In maize, NH4+ facilitated the Al stimulation of N assimilation in the roots of an Al-tolerant maize genotype (Mihailovic et al., 2015). Here, we suggest that Al might stimulate GS activity by binding to it, or inhibit it by limiting NH4+ uptake (Figure 2). However, it is difficult to draw clear conclusions about the interaction between Al and NH4+ assimilation on the basis of studies published to date. Therefore, more research is required to explore the effects of Al on these enzymes involved in NH4+ assimilation.
Table 6.
Summary of effects of aluminum on the activities of glutamine synthetase (GS), glutamate synthase (GOGAT), and glutamate dehydrogenase (GDH): (-) inhibition, (+) stimulation, (0) no change, and (N) not studied.
| Taxon | Al (μM) | Al duration | Effects | Reference | |
|---|---|---|---|---|---|
| Root | Shoot | ||||
| Triticum aestivum | 10–100 | 5 days | GS: + | GS: + | Kertész et al., 2002; Pécsváradi et al., 2009 |
| Zea mays | 166 | 3–9 days | GS: -; NADH-GDH: +; GOGAT: 0 | GS: 0; NADH-GDH: -; GOGAT: 0 | Purcino et al., 2003 |
| Zea mays | 100 | 15 days | GS, NADH-GDH: (dependent on genotypes and N source) | N | Mihailovic et al., 2015 |
| Lotus japonicus | 102–104 | 24 h, 72 h | GS and GOGAT: - | N | Pal’ove-Balang and Mistrík, 2007, 2011 |
| Helianthus annuus | 100 | 15 days | N | GS and GOGAT: - | Ruiz et al., 2007 |
| Oryza sativa | 160–320 | 5–20 | GS: -; NADH-GDH: + | GS: -; NADH-GDH: + | Mishra and Dubey, 2011a |
astudy was conducted using sand culture irrigated with nutrient solutions. Other studies were conducted using hydroponic systems.
Concluding Remarks
A complex interaction between Al and N occurs in the soil–plant system. Relative to NO3-, NH4+ uptake by roots generally alleviates Al phytotoxicity under solution culture conditions, while NH4+ aggravates the solubilization of toxic Al from soils into rhizosphere solutions. Both the alleviation and aggravation effects mainly result from NH4+-induced H+ excretion due to NH4+ uptake by plant roots and/or soil nitrification.
Compared with the effects of N on Al, the effects of Al on N are much more complicated because N is involved in multiple physiological processes within plants. Many reports have demonstrated that Al toxicity inhibits NO3- uptake by plant roots because Al binds to the NO3- transporter and stimulates NO3- efflux. In some cases, such as low Al concentrations, short-term Al exposure, and Al-preferring plants, the Al stimulation of NO3- uptake is probably because of an increase in the positive electrical charge at the root-surface, enhanced H+-NO3- cotransport, and diminished NO3- efflux. The inhibitory effect of Al is generally smaller for root NH4+ uptake than for NO3- uptake. Similar to the Al inhibition of NO3- uptake, the activity of NR can be inhibited by Al treatment because of decreased internal NO3- accumulation. Low concentrations of Al can stimulate NR activity as a result of stimulating NO3- uptake. The effects of Al on the activities of GS, GOGAT, and GDH are still uncertain.
Despite the diverse interactions between Al and N in many studies as described above, it is clear that Al-tolerant plants generally prefer NH4+, while Al-sensitive plants prefer NO3-. This relationship between plant Al tolerance and NH4+/NO3- preference may be the result of ecological evolution and natural selection because acid soils are characterized by a relatively higher ratio of NH4+ to NO3- and higher concentrations of toxic Al than are neutral to calcareous soils.
Together, the results of numerous studies have suggested that the synergistic interaction between plant Al tolerance and NH4+-N nutrition may be an important strategy of plants to thrive in acid soils dominated by both toxic Al and NH4+. In addition, the Al stimulation of N uptake and assimilation can help to explain why Al stimulates plant growth in some cases.
Many studies have focused on the interactions between Al and N in plants, but the exact mechanisms underlying these interactions are still unclear. The Al–N interactions have been studied mainly at the physiological level rather than the molecular level. Physiological effects are indirectly affected by many factors and are not specific. Many genes that function in N uptake, N assimilation, and Al tolerance/toxicity have been identified (Masclaux-Daubresse et al., 2010; Ryan et al., 2011; Schroeder et al., 2013; Ma et al., 2014). The use of mutants with knocked-out or knocked-down expression of these genes could be helpful to explore the detailed mechanisms of Al–N interactions. In addition, we emphasize the importance of soil experiments for researching Al–N interactions, because the ultimate goal of understanding Al–N interactions is to improve the growth of plants in soils. Unfortunately, most studies on Al–N interactions have been conducted under solution culture conditions. As discussed above, the Al–N interactions in solutions may differ from those in soils.
How can the existing knowledge of Al–N interactions be used to improve the productivity of plants grown in acid soils? Plants need to overcome the dual limitation of Al toxicity and N deficiency in acid soils. Due to poor nitrification, acid soils have a higher NH4+ to NO3- ratio than do neutral to calcareous soils. Large-area forest decline has been linked to both NH4+ toxicity and soil acidification, and NH4+ toxicity has become an important issue in global agriculture and ecology (Britto and Kronzucker, 2002). Symptoms of NH4+ toxicity, such as leaf chlorosis, growth suppression, and even death generally appear when the external NH4+ concentrations exceed 0.1 to 0.5 mM, depending on the plant (Britto and Kronzucker, 2002). Thus, any enhancements in plant Al tolerance in acid soils should be accompanied by improvements in plant NH4+ utilization or reduced plant NH4+ sensitivity. Although NH4+ supply generally enhances plant Al tolerance, it also increases the concentrations of toxic Al in soils and leads to potentially toxic NH4+ concentrations. How can we solve this contradiction? Which type of N fertilizer should be applied in acid soils, NH4+ or NO3-? The NO3- fertilizers are much more expensive than NH4+ fertilizers. In addition, NO3- is lost to water more readily than is NH4+ because NO3- binds weakly to soil particles, which are generally negatively charged. Therefore, applying NO3- fertilizers to acid soils appears to be impractical at the moment.
Fortunately, plants originating from acid soils are generally both Al-tolerant and NH4+-preferring. Thus, one way to increase productivity from acid soils is to breed and develop genotypes that are both Al-tolerant and NH4+-preferring. This strategy may synergistically enhance plant Al tolerance and N-use efficiency, and reduce NH4+ sensitivity and NO3- loss. The improvement of N-use efficiency could reduce the amounts of N fertilizers applied to soils, thereby alleviating soil acidification and Al toxicity. Recently, an in situ 15N-labeling experiment showed that soluble soil Al inhibited the relative uptake of NO3- by six tree species, potentially increasing NO3- loss from acid soils into the surrounding water environment (Burnham et al., 2017). Thus, knowledge about Al–N interactions is important for agriculture, ecology, and the environment.
Author Contributions
XZ wrote the manuscript. RS checked and revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the National Natural Science Foundation of China, the Chinese Academy of Sciences, and the Ministry of Science and Technology of the People’s Republic of China. We thank the Editor and two reviewers for their nice and detailed comments. We also thank Jennifer Smith, Ph.D., from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Funding. This work was supported financially by the National Natural Science Foundation of China (No. 31672229), the Strategic Priority Research Program of the Chinese Academy of Sciences (Nos. XDB15030202 and XDB15030302), and the National Key Basic Research Program of China (No. 2014CB441000).
References
- Aciego Pietri J. C., Brookes P. C. (2008). Relationships between soil pH and microbial properties in a UK arable soil. Soil Biol. Biochem. 40 1856–1861. 10.1016/j.soilbio.2008.03.020 [DOI] [Google Scholar]
- Antunes A. M. G., Antonieta Nunes M. (1997). Effects of aluminum on nutrient solution pH and nitrate/ammonium uptake by triticale. J. Plant Nutr. 20 1391–1401. 10.1080/01904169709365342 [DOI] [Google Scholar]
- Barak P., Jobe B. O., Krueger A. R., Peterson L. A., Laird D. A. (1997). Effects of long-term soil acidification due to nitrogen fertilizer inputs in Wisconsin. Plant Soil 197 61–69. 10.1023/A:1004297607070 [DOI] [Google Scholar]
- Bojórquez-Quintal E., Escalante-Magaña C., Echevarría-Machado I., Martínez-Estévez M. (2017). Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 8:1767. 10.3389/fpls.2017.01767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J., Babourina O., Rengel Z. (2011). Role of magnesium in alleviation of aluminium toxicity in plants. J. Exp. Bot. 62 2251–2264. 10.1093/jxb/erq456 [DOI] [PubMed] [Google Scholar]
- Britto D. T., Kronzucker H. J. (2002). NH4+ toxicity in higher plants: a critical review. J. Plant Physiol. 159 567–584. 10.1078/0176-1617-0774 [DOI] [Google Scholar]
- Burnham M. B., Cumming J. R., Adams M. B., Peterjohn W. T. (2017). Soluble soil aluminum alters the relative uptake of mineral nitrogen forms by six mature temperate broadleaf tree species: possible implications for watershed nitrate retention. Oecologia 185 327–337. 10.1007/s00442-017-3955-8 [DOI] [PubMed] [Google Scholar]
- Cakmak I., Horst W. J. (1991). Effect of aluminum on net efflux of nitrate and potassium from root tips of soybean (Glycine max L.). J. Plant Physiol. 138 400–403. 10.1016/S0176-1617(11)80513-4 [DOI] [Google Scholar]
- Calba H., Jaillard B. (1997). Effect of aluminium on ion uptake and H+ release by maize. New Phytol. 137 607–616. 10.1046/j.1469-8137.1997.00858.x [DOI] [Google Scholar]
- Cambraia J., Pimenta J. A., Estevão M. M., Sant’Anna R. (1989). Aluminum effects on nitrate uptake and reduction in sorghum. J. Plant Nutr. 12 1435–1445. 10.1080/01904168909364048 [DOI] [Google Scholar]
- Cantú T., Vieira C. E., Piffer R. D., Luiz G. C., de Souza S. G. H. (2016). Transcriptional modulation of genes encoding nitrate reductase in maize (Zea mays) grown under aluminum toxicity. Afr. J. Biotechnol. 15 2465–2473. 10.5897/AJB2016.15585 [DOI] [Google Scholar]
- Che J., Zhao X. Q., Zhou X., Jia Z. J., Shen R. F. (2015). High pH-enhanced soil nitrification was associated with ammonia-oxidizing bacteria rather than archaea in acidic soils. Appl. Soil Ecol. 85 21–29. 10.1016/j.apsoil.2014.09.003 [DOI] [Google Scholar]
- Chen R. F., Zhang F. L., Zhang Q. M., Sun Q. B., Dong X. Y., Shen R. F. (2012). Aluminium–phosphorus interactions in plants growing on acid soils: does phosphorus always alleviate aluminium toxicity? J. Sci. Food Agric. 92 995–1000. 10.1002/jsfa.4566 [DOI] [PubMed] [Google Scholar]
- Chen Z. C., Ma J. F. (2013). Magnesium transporters and their role in Al tolerance in plants. Plant Soil 368 51–56. 10.1007/s11104-012-1433-y [DOI] [Google Scholar]
- Chen Z. C., Zhao X. Q., Shen R. F. (2010). The alleviating effect of ammonium on aluminum toxicity in Lespedeza bicolor results in decreased aluminum-induced malate secretion from roots compared with nitrate. Plant Soil 337 389–398. 10.1007/s11104-010-0535-7 [DOI] [Google Scholar]
- Cramer M. D., Lewis O. A. M. (1993). The influence of nitrate and ammonium nutrition on the growth of wheat (Triticum aestivum) and maize (Zea mays) Plants. Ann. Bot. 72 359–365. 10.1006/anbo.1993.1119 [DOI] [Google Scholar]
- Crawford N. M., Glass A. D. M. (1998). Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 3 389–395. 10.1016/S1360-1385(98)01311-9 [DOI] [Google Scholar]
- Cruz F. J. R., Lobato A. K. S., Costa R. C. L., Lopes M. J. S., Neves H. K. B., Neto C. F. O., et al. (2011). Aluminum negative impact on nitrate reductase activity, nitrogen compounds and morphological parameters in sorghum plants. Aust. J. Crop Sci. 5 641–645. [Google Scholar]
- Cumming J. R. (1990). Nitrogen source effects on Al toxicity in nonmycorrhizal and mycorrhizal pitch pine (Pinus rigida) seedlings. II. Nitrate reduction and NO3- uptake. Can. J. Bot. 68 2653–2659. 10.1139/b90-335 [DOI] [Google Scholar]
- Cumming J. R., Brown S. M. (1994). Effects of elevated nitrate and aluminum on the growth and nutrition of red spruce (Picea rubens) seedlings. Tree Physiol. 14 589–599. 10.1093/treephys/14.6.589 [DOI] [PubMed] [Google Scholar]
- Cumming J. R., Weinstein L. H. (1990). Nitrogen source effects Al toxicity in nonmycorrhizal and mycorrhizal pitch pine (Pinus rigida) seedlings. I. Growth and nutrition. Can. J. Bot. 68 2644–2652. 10.1139/b90-334 [DOI] [Google Scholar]
- Denison I. A. (1922). The nature of certain aluminum salts in the soil and their influence on ammonification and nitrification. Soil Sci. 13 81–106. [Google Scholar]
- Dinev N., Stancheva I. (1993). Changes in nitrate reductase activity, plastid pigment content, and plant mineral composition of wheat, rye, and triticale grown in the presence of aluminum. J. Plant Nutr. 16 2397–2409. 10.1080/01904169309364696 [DOI] [Google Scholar]
- Domingues A. M. (2010). Nitrogen nutrition of young triticale plants grown under aluminium stress. Rev. Ciênc. Agrár. 33 40–52. [Google Scholar]
- Durieux R. P., Bartlett R. J., Magdoff F. R. (1995). Separate mechanisms of aluminum toxicity for nitrate uptake and root elongation. Plant Soil 172 229–234. 10.1007/BF00011325 [DOI] [Google Scholar]
- Durieux R. P., Jackson W. A., Kamprath E. J., Moll R. H. (1993). Inhibition of nitrate uptake by aluminum in maize. Plant Soil 151 97–104. 10.1007/BF00010790 [DOI] [Google Scholar]
- Erisman J. W., Sutton M. A., Galloway J., Klimont Z., Winiwarter W. (2008). How a century of ammonia synthesis changed the world. Nat. Geosci. 1 636–639. 10.1038/ngeo325 [DOI] [Google Scholar]
- Fageria N. K., Baligar V. C. (2001). Improving nutrient use efficiency of annual crops in Brazilian acid soils for sustainable crop production. Commun. Soil Sci. Plant Anal. 32 1303–1319. 10.1081/CSS-100104114 [DOI] [Google Scholar]
- Falkengren-Grerup U. (1995). Interspecies differences in the preference of ammonium and nitrate in vascular plants. Oecologia 102 305–311. 10.1007/BF00329797 [DOI] [PubMed] [Google Scholar]
- Famoso A. N., Clark R. T., Shaff J. E., Craft E., McCouch S. R., Kochian L. V. (2010). Development of a novel aluminum tolerance phenotyping platform used for comparisons of cereal aluminum tolerance and investigations into rice aluminum tolerance mechanisms. Plant Physiol. 153 1678–1691. 10.1104/pp.110.156794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X. M., Chen F. S., Hu X. F., Yuan P. C., Li J., Chen X. (2014). Aluminum and nutrient interplay across an age-chronosequence of tea plantations within a hilly red soil farm of subtropical China. Soil Sci. Plant Nutr. 60 448–459. 10.1080/00380768.2014.912950 [DOI] [Google Scholar]
- Fleming A. L. (1983). Ammonium uptake by wheat varieties differing in Al tolerance. Agron. J. 75 726–730. 10.2134/agronj1983.00021962007500050003x [DOI] [Google Scholar]
- Foy C. D., Burns G. R., Brown J. C., Fleming A. L. (1965). Differential aluminum tolerance of two wheat varieties associated with plant induced pH changes around their roots. Soil Sci. Soc. Am. Proc. 29 64–67. 10.2136/sssaj1965.03615995002900010019x [DOI] [Google Scholar]
- Foy C. D., Fleming A. L. (1978). “The physiology of plant tolerance to excess available aluminum and manganese in acid soils,” in Crop Tolerance to Suboptimal Land Conditions, ed. Jung G. A. (Madison, WI: American Society of Agronomy special publication; ), 301–328. [Google Scholar]
- Foy C. D., Fleming A. L. (1982). Aluminum tolerances of two wheat genotypes related to nitrate reductase activities. J. Plant Nutr. 5 1313–1333. 10.1080/01904168209363064 [DOI] [Google Scholar]
- Foy C. D., Fleming A. L., Burns G. R., Armiger W. H. (1967). Characterization of differential aluminum tolerance among varieties of wheat and barley. Soil Sci. Soc. Am. Proc. 31 513–521. 10.2136/sssaj1967.03615995003100040027x [DOI] [Google Scholar]
- Galvez L., Clark R. B. (1991). Nitrate and ammonium uptake and solution pH changes for Al-tolerant and Al-sensitive sorghum (Sorghum bicolor) genotypes grown with and without aluminium. Plant Soil 134 179–188. 10.1007/BF00010730 [DOI] [Google Scholar]
- Ganesan K., Sankaranarayanan C., Balakumar T. (1993). Physiological basis of differential aluminum tolerance in rice genotypes. Commun. Soil Sci. Plant Anal. 24 2179–2191. 10.1080/00103629309368947 [DOI] [Google Scholar]
- Gigon A., Rorison I. H. (1972). The response of some ecologically distinct plant species to nitrate- and to ammonium-nitrogen. J. Ecol. 60 93–102. 10.2307/2258043 [DOI] [Google Scholar]
- Godbold D. L., Dictus K., Hüttermann A. (1988). Influence of aluminium and nitrate on root growth and mineral nutrition of Norway spruce (Picea abies) seedlings. Can. J. For. Res. 18 1167–1171. 10.1139/x88-179 [DOI] [Google Scholar]
- Godbold D. L., Jentschke G., Marschner P. (1995). Solution pH modifies the response of Norway spruce seedlings to aluminium. Plant Soil 171 175–178. 10.1007/BF00009583 [DOI] [Google Scholar]
- Gomes M. M. S., Cambraia J., Sant’anna R., Estevão M. M. (1985). Aluminum effects on uptake and translocation of nitrogen in sorghum (Sorghum bicolor, L. Moench). J. Plant Nutr. 8 457–465. 10.1080/01904168509363360 [DOI] [Google Scholar]
- Grauer U. E., Horst W. J. (1990). Effect of pH and nitrogen source on aluminium tolerance of rye (Secale cereale L.) and yellow lupin (Lupinus luteus L.). Plant Soil 127 13–21. 10.1007/BF00010832 [DOI] [Google Scholar]
- Guo J. H., Liu X. J., Zhang Y., Shen J. L., Han W. X., Zhang W. F., et al. (2010). Significant acidification in major Chinese croplands. Science 327 1008–1010. 10.1126/science.1182570 [DOI] [PubMed] [Google Scholar]
- Gupta P., Sarengthem J., Dhamgaye S., Gadre R. (2016). Differential effect of aluminium on enzymes of nitrogen assimilation in excised bean leaf segments. Adv. Biol. Chem. 6 106–113. 10.4236/abc.2016.63009 [DOI] [Google Scholar]
- Gutiérrez R. A. (2012). Systems biology for enhanced plant nitrogen nutrition. Science 336 1673–1675. 10.1126/science.1217620 [DOI] [PubMed] [Google Scholar]
- Hairiah K., Stulen I., Noordwijk M., Kuiper P. J. C. (1994). Al avoidance and Al tolerance of Mucuna pruriens var. utilis: effects of a heterogeneous root environment and the nitrogen form in the root environment. Plant Soil 167 67–72. 10.1007/BF01587600 [DOI] [Google Scholar]
- Hajiboland R., Bahrami-Rad S., Bastani S. (2014). Aluminum alleviates boron-deficiency induced growth impairment in tea plants. Biol. Plant. 58 717–724. 10.1007/s10535-014-0425-6 [DOI] [Google Scholar]
- Havill D. C., Lee J. A., Stewart G. R. (1974). Nitrate utilisation by species from acidic and calcareous soils. New Phytol. 73 1221–1231. 10.1111/j.1469-8137.1974.tb02151.x [DOI] [Google Scholar]
- Hodson M. J., Evans D. E. (1995). Aluminium/silicon interactions in higher plants. J. Exp. Bot. 46 161–171. 10.1093/jxb/46.2.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst W. J., Wang Y., Eticha D. (2010). The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann. Bot. 106 185–197. 10.1093/aob/mcq053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Wang W., Ou S., Tang J., Li H., Che R., et al. (2015). Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 47 834–838. 10.1038/ng.3337 [DOI] [PubMed] [Google Scholar]
- Hu H. W., Zhang L. M., Dai Y., Di H. J., He J. Z. (2013). pH-dependent distribution of soil ammonia oxidizers across a large geographical scale as revealed by high-throughput pyrosequencing. J. Soil Sediment 13 1439–1449. 10.1007/s11368-013-0726-y [DOI] [Google Scholar]
- Ikeda M., Yamanishi T. (1999). Accumulation of nitrogen supplied as ammonium in the root tips of aluminum-stressed wheat cultivars differing in aluminum sensitivity. J. Fac. Agric. Kyushu Univ. 44 33–38. [Google Scholar]
- Jarvis S. C., Hatch D. J. (1986). The effects of low concentrations of aluminium on the growth and uptake of nitrate-N by white clover. Plant Soil 95 43–55. 10.1007/BF02378851 [DOI] [Google Scholar]
- Jerzykiewicz J. (2001). Aluminium effect on nitrate assimilation in cucumber (Cucumis sativus L.) roots. Acta Physiol. Plant. 23 213–219. 10.1007/s11738-001-0011-3 [DOI] [Google Scholar]
- Ju X. T., Xing G. X., Chen X. P., Zhang S. L., Zhang L. J., Liu X. J., et al. (2009). Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. U.S.A. 106 3041–3046. 10.1073/pnas.0813417106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justino G. C., Cambraia J., Oliva M. A., Oliveira J. A. (2006). Uptake and reduction of nitrate in two rice cultivars in the presence of aluminum. Pesqui. Agropec. Bras. 41 1285–1290. 10.1590/S0100-204X2006000800011 [DOI] [Google Scholar]
- Kant S., Bi Y. M., Rothstein S. J. (2011). Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 62 1499–1509. 10.1093/jxb/erq297 [DOI] [PubMed] [Google Scholar]
- Kasuga I., Nakagaki H., Kurisu F., Furumai H. (2010). Abundance and diversity of ammonia-oxidizing archaea and bacteria on biological activated carbon in a pilot-scale drinking water treatment plant with different treatment processes. Water Sci. Technol. 61 3070–3077. 10.2166/wst.2010.204 [DOI] [PubMed] [Google Scholar]
- Keltjens W. G. (1987). Nitrogen source and aluminum toxicity of two sorghum genotypes differing in aluminum susceptibility. J. Plant Nutr. 10 841–856. 10.1080/01904168709363614 [DOI] [Google Scholar]
- Keltjens W. G. (1988). Short-term effects of Al on nutrient uptake, H+ efflux, root respiration and nitrate reductase activity of two sorghum genotypes differing in Al-susceptibility. Commun. Soil Sci. Plant Anal. 19 1155–1163. 10.1080/00103628809368002 [DOI] [Google Scholar]
- Keltjens W. G., van Loenen E. (1989). Effects of aluminium and mineral nutrition on growth and chemical composition of hydroponically grown seedlings of five different forest tree species. Plant Soil 119 39–50. 10.1007/BF02370267 [DOI] [Google Scholar]
- Keltjens W. G., van Ulden P. S. R. (1987). Effects of Al on nitrogen (NH4+ and NO3-) uptake, nitrate reductase activity and proton release in two sorghum cultivars differing in Al tolerance. Plant Soil 104 227–234. 10.1007/BF02372536 [DOI] [Google Scholar]
- Kertész S., Fábián A., Zsoldos F., Vashegyi Á., Labádi I., Bona L., et al. (2002). Changes in glutamate synthetase activity in presence of aluminium complexes. Acta Biol. Szeged. 45 103–104. [Google Scholar]
- Kinraide T. B., Ryan P. R., Kochian L. V. (1992). Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiol. 99 1461–1468. 10.1104/pp.99.4.1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Sweeney B. K. (2003). Proton alleviation of growth inhibition by toxic metals (Al, La, Cu) in rhizobia. Soil Biol. Biochem. 35 199–205. 10.1016/S0038-0717(02)00246-8 [DOI] [Google Scholar]
- Klotz F., Horst W. J. (1988a). Effect of ammonium- and nitrate-nitrogen nutrition on aluminium tolerance of soybean (Glycine max L.). Plant Soil 111 59–65. 10.1007/BF02182037 [DOI] [Google Scholar]
- Klotz F., Horst W. J. (1988b). Genotypic differences in aluminium tolerance of soybean (Glycine max L.) as affected by ammonium and nitrate-nitrogen nutrition. J. Plant Physiol. 132 702–707. 10.1016/S0176-1617(88)80232-3 [DOI] [Google Scholar]
- Kochian L. V., Hoekenga O. A., Piñeros M. A. (2004). How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 55 459–493. 10.1146/annurev.arplant.55.031903.141655 [DOI] [PubMed] [Google Scholar]
- Kochian L. V., Piñeros M. A., Hoekenga O. A. (2005). The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274 175–195. 10.1007/s11104-004-1158-7 [DOI] [Google Scholar]
- Lazof D. B., Rincon M., Rufty T. W., MacKown C. T., Carter T. E. (1994). Aluminum accumulation and associated effects on 15NO3- influx in roots of two soybean genotypes differing in A1 tolerance. Plant Soil 164 291–297. 10.1007/BF00010081 [DOI] [Google Scholar]
- Lazof D. B., Rufty T. W., Redinbaugh M. G. (1992). Localization of nitrate absorption and translocation within morphological regions of the corn root. Plant Physiol. 100 1251–1258. 10.1104/pp.100.3.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L. Z., Zhao X. Q., Yi X. Y., Chen Z. C., Dong X. Y., Chen R. F., et al. (2013). Excessive application of nitrogen and phosphorus fertilizers induces soil acidification and phosphorus enrichment during vegetable production in Yangtze River Delta, China. Soil Use Manage. 29 161–168. 10.1111/sum.12035 [DOI] [Google Scholar]
- Lidon F. C., Ramalho J. C., Barreiro M. G. (1998). Aluminium toxicity modulates nitrate to ammonia reduction. Photosynthetica 35 213–222. 10.1023/A:1006906722469 [DOI] [Google Scholar]
- Lin J., Zhong Y., Fan H., Song C., Yu C., Gao Y., et al. (2017). Chemical treatment of contaminated sediment for phosphorus control and subsequent effects on ammonia-oxidizing and ammonia-denitrifying microorganisms and on submerged macrophyte revegetation. Environ. Sci. Pollut. Res. 24 1007–1018. 10.1007/s11356-016-7828-1 [DOI] [PubMed] [Google Scholar]
- Liu Z., Wang H., Xu R. (2016). The effects of root surface charge and nitrogen forms on the adsorption of aluminum ions by the roots of rice with different aluminum tolerances. Plant Soil 408 43–53. 10.1007/s11104-016-2909-y [DOI] [Google Scholar]
- Ma J. F. (2007). Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int. Rev. Cytol. 264 225–252. 10.1016/S0074-7696(07)64005-4 [DOI] [PubMed] [Google Scholar]
- Ma J. F., Chen Z. C., Shen R. F. (2014). Molecular mechanisms of Al tolerance in gramineous plants. Plant Soil 381 1–12. 10.1007/s11104-014-2073-1 [DOI] [Google Scholar]
- Maathuis F. J. M. (2009). Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 12 250–258. 10.1016/j.pbi.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Malhi S. S., Nyborg M., Caldwell C. D., Hoyt P. B., Leitch R. H. (1988). Effect of ammonium and nitrate on growth and yield of barley on acid soils. Commun. Soil Sci. Plant Anal. 19 1049–1063. 10.1080/00103628809367994 [DOI] [Google Scholar]
- Marschner H. (1995). Mineral Nutrition of Higher Plants. London: Academic Press. [Google Scholar]
- Masclaux-Daubresse C., Daniel-Vedele F., Dechorgnat J., Chardon F., Gaufichon L., Suzuki A. (2010). Nitrogen uptake, assimilation and remobilisation in plants: challenges for sustainable and productive agriculture. Ann. Bot. 105 1141–1157. 10.1093/aob/mcq028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H. (2000). Cell biology of aluminum toxicity and tolerance in higher plants. Int. Rev. Cytol. 200 1–46. 10.1016/S0074-7696(00)00001-2 [DOI] [PubMed] [Google Scholar]
- McCain S., Davies M. S. (1983). The influence of background solution on root responses to aluminium in Holcus lanatus L. Plant Soil 73 425–430. 10.1007/BF0218432 [DOI] [Google Scholar]
- Meriño-Gergichevich C., Alberdi M., Ivanov A. G., Reyes-Díaz M. (2010). Al3+-Ca2+ interaction in plants growing in acid soils: Al-phytotoxicity response to calcareous amendments. J. Soil Sci. Plant Nutr. 10 217–243. [Google Scholar]
- Mesdag J., Slootmaker L. A. J., Post J. (1970). Linkage between tolerance to high soil acidity and genetically high protein content in the kernel of wheat, Triticum aestivum L. and its possible use in breeding. Euphytica 19 163–174. 10.1007/BF01902940 [DOI] [Google Scholar]
- Mihailovic N., Vucinic Z., Sukalovic V. H. (2015). Ammonium enables aluminum-induced stimulation of nitrogen assimilation in roots of Al-tolerant maize genotypes. J. Plant Nutr. 38 371–383. 10.1080/01904167.2014.934471 [DOI] [Google Scholar]
- Miller A. J., Fan X., Orsel M., Smith S. J., Wells D. M. (2007). Nitrate transport and signalling. J. Exp. Bot. 58 2297–2306. 10.1093/jxb/erm066 [DOI] [PubMed] [Google Scholar]
- Mishra P., Dubey R. S. (2011). Nickel and Al-excess inhibit nitrate reductase but upregulate activities of aminating glutamate dehydrogenase and aminotransferases in growing rice seedlings. Plant Growth Regul. 64 251–261. 10.1007/s10725-011-9566-1 [DOI] [Google Scholar]
- Miyasaka S. C., Kochian L. V., Shaff J. E., Foy C. D. (1989). Mechanisms of aluminum tolerance in wheat. An investigation of genotypic differences in rhizosphere pH, K+, and H+ transport and root cell membrane potentials. Plant Physiol. 91 1188–1196. 10.1104/pp.91.3.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita A., Ohta M., Yoneyama T. (1998). Uptake, transport and assimilation of 15N-nitrate and 15N-ammonium in tea (Camellia sinensis L.) plants. Soil Sci. Plant Nutr. 44 647–654. 10.1080/00380768.1998.10414488 [DOI] [Google Scholar]
- Mugwira L. M., Patel S. U. (1977). Root zone pH changes and ion uptake imbalances by triticale, wheat and rye. Agron. J. 69 719–722. 10.2134/agronj1977.00021962006900040047x [DOI] [Google Scholar]
- Mulder J., Stein A. (1994). The solubility of aluminum in acidic forest soils: long-term changes due to acid deposition. Geochim. Cosmochim. Acta 58 85–94. 10.1016/0016-7037(94)90448-0 [DOI] [Google Scholar]
- Mulder J., van Breemen N., Eijck H. C. (1989). Depletion of soil aluminium by acid deposition and implications for acid neutralization. Nature 337 247–249. 10.1038/337247a0 [DOI] [Google Scholar]
- Nichol B. E., Oliveira L. A., Glass A. D. M., Siddiqi M. Y. (1993). The effects of aluminum on the influx of calcium, potassium, ammonium, nitrate, and phosphate in an aluminum-sensitive cultivar of barley (Hordeum vulgare L.). Plant Physiol. 101 1263–1266. 10.1104/pp.101.4.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Selvaraj M. G., Fernando A. J., Lorieux M., Ishitani M., McCouch S., et al. (2014). N- and P-mediated seminal root elongation response in rice seedlings. Plant Soil 375 303–315. 10.1007/s11104-013-1955-y [DOI] [Google Scholar]
- Osaki M., Watanabe T., Tadano T. (1997). Beneficial effect of aluminum on growth of plants adapted to low pH soils. Soil Sci. Plant Nutr. 43 551–563. 10.1080/00380768.1997.10414782 [DOI] [Google Scholar]
- Pal’ove-Balang P., Mistrík I. (2007). Impact of low pH and aluminium on nitrogen uptake and metabolism in roots of Lotus japonicus. Biologia 62 715–719. 10.2478/s11756-007-0133-1 [DOI] [Google Scholar]
- Pal’ove-Balang P., Mistrík I. (2011). Effect of aluminium on nitrogen assimilation in roots of Lotus japonicus. Plant Biosyst. 145 527–531. 10.1080/11263504.2011.575608 [DOI] [Google Scholar]
- Pal’ove-Balang P., Zelinova A. M. V. (2013). Nitrogen uptake and free amino-acid accumulation in roots of Lotus corniculatus cultivars under Al-stress. Agric. Trop. Subtrop. 46 5–9. 10.2478/ats-2013-0001 [DOI] [Google Scholar]
- Pécsváradi A., Nagy Z., Varga A., Vashegyi Á., Labádi I., Galbács G., et al. (2009). Chloroplastic glutamine synthetase is activated by direct binding of aluminium. Physiol. Plant. 135 43–50. 10.1111/j.1399-3054.2008.01167.x [DOI] [PubMed] [Google Scholar]
- Peuke A. D., Tischner R. (1991). Nitrate uptake and reduction of aseptically cultivated spruce seedlings, Picea abies (L.) Karst. J. Exp. Bot. 239 723–728. 10.1093/jxb/42.6.723 [DOI] [Google Scholar]
- Piña R. G., Cervantes C. (1996). Microbial interactions with aluminium. Biometals 9 311–316. 10.1007/BF00817932 [DOI] [PubMed] [Google Scholar]
- Pintro J., Barloy J., Fallavier P. (1996). Aluminum effects on the growth and mineral composition of corn plants cultivated in nutrient solution at low aluminum activity. J. Plant Nutr. 19 729–741. 10.1080/01904169609365156 [DOI] [Google Scholar]
- Purcino A. A. C., Alves V. M. C., Parentoni S. N., Belele C. L., Loguercio L. L. (2003). Aluminum effects on nitrogen uptake and nitrogen assimilating enzymes in maize genotypes with contrasting tolerance to aluminum toxicity. J. Plant Nutr. 26 31–61. 10.1081/PLN-120016496 [DOI] [Google Scholar]
- Rengel Z., Zhang W. H. (2003). Role of dynamics of intracellular calcium in aluminium-toxicity syndrome. New Phytol. 159 295–314. 10.1046/j.1469-8137.2003.00821.x [DOI] [PubMed] [Google Scholar]
- Rorison I. H. (1985). Nitrogen source and the tolerance of Deschampsia flexuosa, Holcus lanatus and Bromus erectus to aluminium during seedling growth. J. Ecol. 73 83–90. [Google Scholar]
- Rousk J., Brookes P. C., Bååth E. (2010). Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 42 926–934. 10.1016/j.soilbio.2010.02.009 [DOI] [Google Scholar]
- Ruan J., Gerendás J., Hardter R., Sattelmacher B. (2007). Effect of nitrogen form and root zone pH on growth and nitrogen uptake of tea (Camellia sinensis) plants. Ann. Bot. 99 301–310. 10.1093/aob/mcl258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., MacKnown C. T., Lazof D. B., Carter T. E. (1995). Effects of aluminium on nitrate uptake and assimilation. Plant Cell Environ. 18 1325–1331. 10.1111/j.1365-3040.1995.tb00192.x [DOI] [Google Scholar]
- Rufyikiri G., Dufey J. E., Nootens D., Delvaux B. (2001). Effect of aluminium on bananas (Musa spp.) cultivated in acid solutions. II. Water and nutrient uptake. Fruits 56 5–16. 10.1051/fruits:2001107 [DOI] [Google Scholar]
- Ruiz J. M., Rivero R. M., Romero L. (2007). Comparative effect of Al, Se, and Mo toxicity on NO3- assimilation in sunflower (Helianthus annuus L.) plants. J. Environ. Manage. 83 207–212. 10.1016/j.jenvman.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Ryan P. R., Ditomaso J. M., Kochian L. V. (1993). Aluminum toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J. Exp. Bot. 44 437–446. 10.1093/jxb/44.2.437 [DOI] [Google Scholar]
- Ryan P. R., Tyerman S. D., Sasaki T., Furuichi T., Yamamoto Y., Zhang W. H., et al. (2011). The identification of aluminium-resistance genes provides opportunities for enhancing crop production on acid soils. J. Exp. Bot. 62 9–20. 10.1093/jxb/erq272 [DOI] [PubMed] [Google Scholar]
- Schier G. A., McQuattie C. J. (1999). Effect of nitrogen source on aluminum toxicity in nonmycorrhizal and ectomycorrhizal pitch pine seedling. J. Plant Nutr. 22 951–965. 10.1080/01904169909365685 [DOI] [Google Scholar]
- Schroeder J. I., Delhaize E., Frommer W. B., Guerinot M. L., Harrison M. J., Herrera-Estrella L., et al. (2013). Using membrane transporters to improve crops for sustainable food production. Nature 497 60–66. 10.1038/nature11909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnawaz M. D., Chouhan R., Sanadhya D. (2017). Impact of aluminum toxicity on physiological aspects of barley (Hordeum vulgare L.) cultivars and its alleviation through ascorbic acid and salicylic acid seed priming. Int. J. Curr. Microbiol. Appl. Sci. 6 875–891. 10.20546/ijcmas.2017.605.098 [DOI] [Google Scholar]
- Sharma P., Dubey R. S. (2005). Modulation of nitrate reductase activity in rice seedlings under aluminium toxicity and water stress: role of osmolytes as enzyme protectant. J. Plant Physiol. 162 854–864. 10.1016/j.jplph.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Souza L. C., Nogueira D. C. S., Machado L. C., Costa T. C., Martins J. T. S., Mendes C. A. P., et al. (2016). Nitrogen compounds, proteins and amino acids in corn subjected to doses of aluminum. Afr. J. Agric. Res. 11 1519–1524. 10.5897/AJAR2015.10758 [DOI] [Google Scholar]
- Sun C., Lu L., Liu L., Liu W., Yu Y., Liu X., et al. (2014). Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol. 201 1240–1250. 10.1111/nph.12597 [DOI] [PubMed] [Google Scholar]
- Tan K., Keltjens W. G., Findenegg G. R. (1992). Effect of nitrogen form on aluminum toxicity in sorghum genotypes. J. Plant Nutr. 15 1383–1394. 10.1080/01904169209364405 [DOI] [Google Scholar]
- Taylor G. J. (1988a). Aluminum tolerance is independent of rhizosphere pH in Triticum aestivum L. Commun. Soil Sci. Plant Anal. 19 1217–1227. 10.1080/00103628809368007 [DOI] [Google Scholar]
- Taylor G. J. (1988b). Mechanisms of aluminum tolerance in Triticum aestivum (wheat). V. Nitrogen nutrition, plant-induced pH, and tolerance to aluminum; correlation without causality? Can. J. Bot. 66 694–699. 10.1139/cjb-66-4-694 [DOI] [Google Scholar]
- Taylor G. J., Foy C. D. (1985a). Mechanisms of aluminum tolerance in Triticum aestivum L. (wheat). I. Differential pH induced by winter cultivars in nutrient solutions. Am. J. Bot. 72 695–701. 10.2307/2443681 [DOI] [Google Scholar]
- Taylor G. J., Foy C. D. (1985b). Mechanisms of aluminum tolerance in Triticum aestivum L. (wheat). II. Differential pH induced by spring cultivars in nutrient solutions. Am. J. Bot. 72 702–706. 10.2307/2443682 [DOI] [Google Scholar]
- Taylor G. J., Foy C. D. (1985c). Mechanisms of aluminum tolerance in Triticum aestivum (wheat). IV. The role of ammonium and nitrate nutrition. Can. J. Bot. 63 2181–2186. 10.1139/b85-309 [DOI] [Google Scholar]
- Thornton B. (1998). Influence of pH and aluminum on nitrogen partitioning in defoliated grasses. Grass Forage Sci. 53 170–178. 10.1046/j.1365-2494.1998.5320170.x [DOI] [Google Scholar]
- Tischner R. (2000). Nitrate uptake and reduction in higher and lower plants. Plant Cell Environ. 23 1005–1024. 10.1046/j.1365-3040.2000.00595.x [DOI] [Google Scholar]
- Tomioka R., Takenaka C., Maeshima M., Tezuka T., Kojima M., Sakakibara H. (2012). Stimulation of root growth induced by aluminum in Quercus serrata Thunb. is related to activity of nitrate reductase and maintenance of IAA concentration in roots. Am. J. Plant Sci. 3 1619–1624. 10.4236/ajps.2012.311196 [DOI] [Google Scholar]
- Tomioka R., Uchida A., Takenaka C., Tezuka T. (2007). Effect of aluminum on nitrate reductase and photosynthetic activities in Quercus serrata seedlings. Environ. Sci. 14 157–165. [PubMed] [Google Scholar]
- Townsend L. R. (1966). Effect of nitrate and ammonium nitrogen on the growth of the lowbush blueberry. Can. J. Plant Sci. 46 209–210. 10.4141/cjps66-033 [DOI] [Google Scholar]
- Townsend L. R., Blatt C. R. (1966). Lowbush blueberry: evidence for the absence of a nitrate reducing system. Plant Soil 25 456–460. 10.1007/BF01394468 [DOI] [Google Scholar]
- van Breemen N., Burrough P. A., Velthorst E. J., van Dobben H. F., de Wit T., Ridder T. B., et al. (1982). Soil acidification from atmospheric ammonium sulphate in forest canopy throughfall. Nature 299 548–550. 10.1038/299548a0 [DOI] [Google Scholar]
- van Hai T., Nga T. T., Laudelout H. (1989). Effect of aluminium on the mineral nutrition of rice. Plant Soil 114 173–185. 10.1007/BF02220796 [DOI] [Google Scholar]
- Van Praag H. J., Weissen F., Sougnez-Remy S., Carletti G. (1985). Aluminum effects on spruce and beech seedlings. II. Statistical analysis of sand culture experiments. Plant Soil 83 339–356. 10.1007/BF02184446 [DOI] [Google Scholar]
- Vitousek P. M., Howarth R. W. (1991). Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13 87–115. 10.1007/BF00002772 [DOI] [Google Scholar]
- von Uexküll H. R., Mutert E. (1995). Global extent, development and economic impact of acid soils. Plant Soil 171 1–15. 10.1007/BF00009558 [DOI] [Google Scholar]
- Wagatsuma T., Yamasaku K. (1985). Relationship between differential aluminium tolerance and plant induced pH change of medium among barley cultivars. Soil Sci. Plant Nutr. 31 521–535. 10.1080/00380768.1985.10557461 [DOI] [Google Scholar]
- Wang H., Li Y., Hou J., Huang J., Liang W. (2017). Nitrate reductase-mediated nitric oxide production alleviates Al-induced inhibition of root elongation by regulating the ascorbate-glutathione cycle in soybean roots. Plant Soil 410 453–465. 10.1007/s11104-016-3045-4 [DOI] [Google Scholar]
- Wang H. H., Huang J. J., Bi Y. R. (2010). Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant Sci. 179 281–288. 10.1016/j.plantsci.2010.05.014 [DOI] [Google Scholar]
- Wang W., Zhao X. Q., Chen R. F., Dong X. Y., Lan P., Ma J. F., et al. (2015). Altered cell wall properties are responsible for ammonium-reduced aluminum accumulation in rice roots. Plant Cell Environ. 38 1382–1390. 10.1111/pce.12490 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Osaki M. (2002). Mechanisms of adaptation to high aluminum condition in native plant species growing in acid soils: a review. Commun. Soil Sci. Plant Anal. 33 1247–1260. 10.1081/CSS-120003885 [DOI] [Google Scholar]
- Watanabe T., Osaki M., Tadano T. (1998). Effects of nitrogen source and aluminum on growth of tropical tree seedlings adapted to low pH soils. Soil Sci. Plant Nutr. 44 655–666. 10.1080/00380768.1998.10414489 [DOI] [Google Scholar]
- Yandow T. S., Klein R. M. (1986). Nitrate reductase of primary roots of red spruce seedlings. Effects of acidity and metal ions. Plant Physiol. 81 723–725. 10.1104/pp.81.3.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. Q., Aizawa T., Schneider J., Wang C., Shen R. F., Sunairi M. (2013a). Complete mitochondrial genome of the aluminum-tolerant fungus Rhodotorula taiwanensis RS1 and comparative analysis of Basidiomycota mitochondrial genomes. Microbiologyopen 2 308–317. 10.1002/mbo3.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. Q., Bao X. M., Wang C., Xiao Z. Y., Hu Z. M., Zheng C. L., et al. (2017). Hydroxy-Al and cell-surface negativity are responsible for the enhanced sensitivity of Rhodotorula taiwanensis to aluminum by increased medium pH. Arch. Microbiol. 199 1185–1194. 10.1007/s00203-017-1387-9 [DOI] [PubMed] [Google Scholar]
- Zhao X. Q., Chen R. F., Shen R. F. (2014). Coadaptation of plants to multiple stresses in acidic soils. Soil Sci. 179 503–513. 10.1097/SS.0000000000000086 [DOI] [Google Scholar]
- Zhao X. Q., Guo S. W., Shinmachi F., Sunairi M., Noguchi A., Hasegawa I., et al. (2013b). Aluminum tolerance in rice is antagonistic with nitrate preference and synergistic with ammonium preference. Ann. Bot. 111 69–77. 10.1093/aob/mcs234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. Q., Shen R. F. (2013). Interactive regulation of nitrogen and aluminum in rice. Plant Signal. Behav. 8:e24355. 10.4161/psb.24355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. Q., Shen R. F., Sun Q. B. (2009). Ammonium under solution culture alleviates aluminum toxicity in rice and reduces aluminum accumulation in roots compared with nitrate. Plant Soil 315 107–121. 10.1007/s11104-008-9736-8 [DOI] [Google Scholar]
- Zhou X., Gu Z., Xu H., Chen L., Tao G., Yu Y., et al. (2016). The effects of exogenous ascorbic acid on the mechanism of physiological and biochemical responses to nitrate uptake in two rice cultivars (Oryza sativa L.) under aluminum stress. J. Plant Growth Regul. 35 1013–1024. 10.1007/s00344-016-9599-9 [DOI] [Google Scholar]