Abstract
Depression is associated with elevated kynurenine levels, a tryptophan metabolite generated under stress and inflammatory conditions. Agudelo et al. (2014) now reveal how PGC-1α1 overexpression in muscle mimics anti-depressant effects of exercise by promoting kynurenine aminotransferase expression, likely preventing kynurenine from crossing the blood brain barrier to disrupt neural plasticity.
Exercise has many health benefits for body and mind. Physical activity improves cognition and reduces mood disorders (Lawlor and Hopker, 2001). Research into underlying mechanisms has focused on central changes in neurotransmission, neurogenesis, growth factors, and blood flow. Less attention has been given to peripheral factors that may affect brain function during exercise. In particular, activation of skeletal muscle may play an important role. This has become particularly relevant with the identification of muscle fiber contractile and metabolic genes, which can be activated by exercise, pharmacological agents, and the overexpression of selected transcription factors. Specifically, peroxisome proliferator-activated receptor-gamma co-activator (PGC)-1α1 regulates fatty acid oxidation, gluconeogenesis, muscle fiber composition, and mitochondrial biogenesis. Exercise increases expression of PGC-1α1 in muscle and liver in rodents and humans (Arany, 2008). PGC-1α1 (and PPARα/δ) is controlled by the AMP-activated protein kinase (AMPK), a master metabolic regulator important for glucose homeostasis, appetite, and exercise physiology (Smith et al., 2013). AMPK activation enhances running endurance in sedentary mice (Narkar et al., 2008). Interestingly, brain plasticity and memory function is enhanced by administration of AMPK and PPARδ agonists in wild-type (WT) mice, but not in skeletal muscle-specific AMPK mutant mice (Kobilo et al., 2011, 2014). These studies indicate that skeletal muscle secretes factors that influence the brain and behavior in response to physiological demands. Agudelo et al. (2014) now reveal a metabolic pathway linking skeletal muscle and the brain to regulate depression.
Agudelo and colleagues exposed mice that overexpress PGC-1α1 in muscle (mck-PGC-1α1) to a 5-week-long chronic mild unpredictable stress (CMS) protocol, which causes a depression-like phenotype in rodents. They found that mck-PGC-1α1 mice are resistant to stress, as evidenced by a lack of change in behavioral assays relevant to depression, such as the forced swim and sucrose preference tests. Concurrently, expression of hippocampal synaptic plasticity related genes, such as calcium-calmodulin-dependent protein kinase (CamkII), activity-regulated cytoskeleton-associated protein (ARC), and brain-derived neurotrophic factor (BDNF), was unaffected by CMS in transgenic as compared to WT mice. In addition, mck-PGC-1α1 mice are protected from pro-inflammatory changes that can trigger depressive disorders. Specifically, cytokines typically induced by CMS such as IL-6, TNFα, and IL-1β were unchanged in mck-PGC-1α1 mouse hippocampus, while IL-10, an anti-inflammatory cytokine, was significantly increased. Furthermore, the CMS-driven increase in hypothalamic corticotrophin-releasing hormones was attenuated in mck-PGC-1α1 mice. The resilience of mck-PGC-1α1 mice to depression may be due in part to amelioration of stress-induced neuro-inflammation.
To explore mechanisms underlying muscle-brain communication, Agudelo et al. (2014) conducted gene-expression analysis, which showed elevated levels of kynurenine amino transferases (KAT) 1, 3, and 4 in the muscle of mck-PGC-1α1 mice. Kynurenine (KYN) is a metabolite of tryptophan converted into kynurenic acid (KYNA) by KAT enzymes. The KYN pathway is considered important for depression (Schwarcz et al., 2012). In fact, the expression level of several rate-limiting enzymes in the KYN pathway was increased by CMS in WT (Figure 1A) but not in mck-PGC-1α1 mice (Figure 1B). Moreover, plasma KYN levels were unchanged, while plasma KYNA levels were higher in the transgenic mice. However, these alterations of the KYN pathway did not affect total plasma or brain tryptophan and serotonin levels or its metabolites, suggesting that tryptophan metabolism changes specifically within muscle. The authors also showed that administration of exogenous KYN reduced sucrose consumption in WT but not in mck-PGC-1α1 mice, providing further evidence that the transgenic mice are resistant to depression. Furthermore, KYN treatment in WT mice resulted in brain gene-expression patterns similar to that of the pro-inflammatory, KYN pathway, with reduced expression of synaptic plasticity genes, including immediate early gene ARC and AMPA receptor subunit GluA2. Consistently, mice with skeletal muscle-specific PGC-1α1 deletion had reduced KAT expression levels in muscle and displayed depressive behavior (anhedonia) following KYN administration.
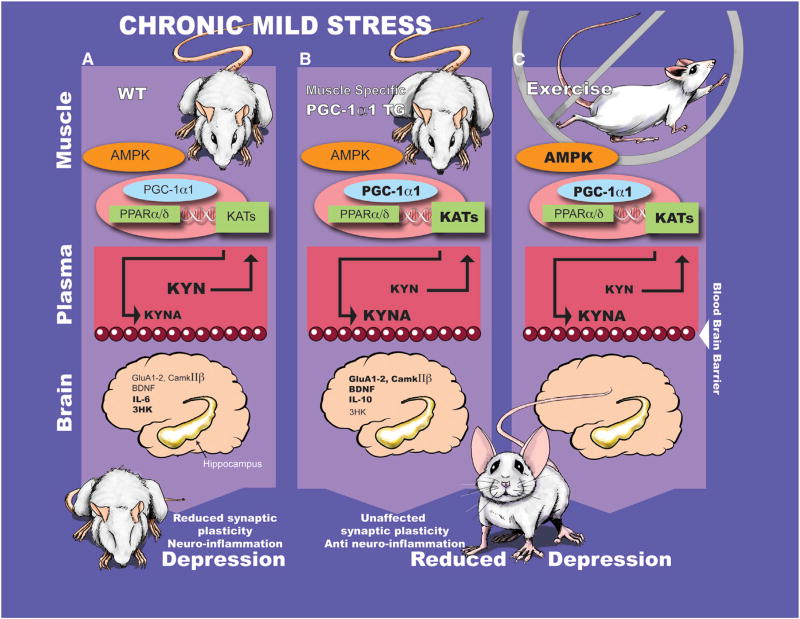
Figure 1.
Schematic of the Role of Muscle-Specific (mck)-PGC-1α1 Overexpression in a Mouse Model of Depression Caused by Chronic Mild Stress
(A) Plasma kynurenine (KYN) levels are elevated by chronic mild stress (top) in wild-type (WT) mice. KYN crosses the blood-brain barrier (middle panel) and reduces neuronal plasticity by increasing neuro-inflammation (bottom)—and, in turn, causes depression-like behaviors.
(B) Activation of the skeletal muscle AMPK - PGC-1α1 pathway influences secretion of proteins involved in muscle adaptation. Agudelo et al. show that mck-PGC-1α1 mice are protected from exhibiting depressive-like behavior induced by chronic mild stress through upregulation of the PPARα/δ-KAT-Kynurenine pathway.
(C) The discovery of a novel paracrine mechanism mediating communication between skeletal muscle and brain may provide insight into the beneficial effects of exercise on cognition and mood. Future studies may lead to identification of key molecules for brain function, induced by muscle PGC-1α1 overexpression or AMPK activation during exercise.
To further understand the regulation of KAT expression, the authors analyzed the direct repeat 1 (DR1) sequence, an element located in the genomic region surrounding the KAT1, 3, and 4 genes that is recognized by PPAR α and δ. They showed that an association between PGC-1α1 and PPAR α/δ controls KAT expression in the myotube. Additionally, 8 weeks of voluntary wheel running increased skeletal muscle KAT 1, 3, and 4 and plasma KYNA levels in WT mice (Figure 1C). Consistently, a 3 week training program in humans elevated skeletal muscle KAT 1–4 expression. Altogether, these findings suggest that skeletal muscle PGC-1α1 plays an important role in regulation of muscle KAT enzyme expression, which modulates plasma KYN levels to, in turn, influence hippocampal neuronal plasticity and to alleviate stress-induced depression. This novel function of PGC-1α1 in regulation of the KYN pathway further substantiates the concept of communication between skeletal muscle and brain.
This research raises further questions. On a systemic level, elevated PGC-1α1 in muscle improves insulin metabolism, which may convey antidepressant-like effects (Oxenkrug, 2013). In addition, the role of muscle in brain function could be explored further. For example, uptake of tryptophan and its metabolites into muscles of the transgenic mice could be measured. This is important because total plasma tryptophan levels were unchanged in mck-PGC-1α1 mice after CMS, raising the possibility that higher levels of tryptophan within muscle lead to elevated efflux of KYNA. In addition, to further evaluate whether there is a causal connection between muscle PGC-1α1 and depression, it would be interesting to examine whether muscle specific deletion of PGC-1α1 or KAT abolishes the antidepressant effect of exercise. Furthermore, PGC-1α1 protein measurement in the muscles of stressed WT mice and mood disorder patients may give additional insight into depression etiology. Finally, the brains of mck-PGC-1α1 mice warrant a closer examination. Genes and proteins important for glutamatergic neurotransmission (Hashimoto, 2009) such as AMPA receptors, CamKII, and glial fibrillary acidic protein (GFAP) are upregulated in the transgenic mice at baseline, without CMS. These neural changes could modify brain structure, size, neuronal number, and function. In addition, hippocampal synaptic physiology, adult neurogenesis, and memory function (Kobilo et al., 2011) may be affected by PGC-1α1 overexpression in skeletal muscle.
Depression causes morbidity and is a burden on society (Lawlor and Hopker, 2001). The study by Agudela et al. demonstrates that skeletal muscle PGC-1α1 overexpression can counteract depression and acts as an exercise-mimetic that modulates paracrine factors. Understanding the mechanisms underlying muscle-to-brain communication may open up avenues to new therapeutic approaches. Indeed, it will be important to identify the key proteins and metabolites that are dynamically secreted by muscle during exercise to meet the brain’s physiological demands. In the future, these may lead to treatments for a variety of brain disorders, including psychiatric and cognitive conditions as well as neurodegenerative diseases.
Acknowledgments
This work was supported in part by the Intramural Research Program of the NIH, National Institute on Aging (NIA) and by the Korea Health Industry Development Institute (KHIDI, grant number: HI13C1149). We thank Kristen Alexander and Jimmy Burril for assistance with figure illustration.
References
- Agudelo LZ, Femenía T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, et al. Cell. 2014 doi: 10.1016/j.cell.2014.07.051. Published online September 25, 2014. http://dx.doi.org/10.1016/j.cell.2014.07.051. [DOI] [PubMed]
- Arany Z. Curr. Opin. Genet. Dev. 2008;18:426–434. doi: 10.1016/j.gde.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Brain Res. Rev. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Yuan C, van Praag H. Learn. Mem. 2011;18:103–107. doi: 10.1101/lm.2001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H. Learn. Mem. 2014;21:119–126. doi: 10.1101/lm.033332.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Hopker SW. BMJ. 2001;322:763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug G. Mol. Neurobiol. 2013;48:294–301. doi: 10.1007/s12035-013-8497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Nat. Rev. Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BK, Mukai K, Lally JS, Maher AC, Gurd BJ, Heigenhauser GJ, Spriet LL, Holloway GP. J. Physiol. 2013;591:1551–1561. doi: 10.1113/jphysiol.2012.245944. [DOI] [PMC free article] [PubMed] [Google Scholar]