Abstract
Medical progress, the improvement of general living conditions, and an increase in life expectancy have led to an increase in the general prevalence of oncologic disease. More importantly, more and more patients survive cancer or live with the disease for long periods of time. While the battle for survivorship is continuously being fought, improving patients' quality of life has come to the fore. Psychosocial issues may modulate the course of the disease, but mainly have a deep impact on patients' physical and mental wellbeing. Psycho-oncology has risen as a relatively new interdisciplinary field with the aim of addressing these issues and providing support for patients confronting numerous challenges throughout the different stages of the disease. In this article, we provide an overview of the current knowledge of body-mind interactions in cancer and an outline of the broad spectrum of psycho-oncologic care, with a special focus on the treatment of pain, fatigue, sexual issues, and fear of progression.
Keywords: body-mind interaction, cancer, psychosocial intervention, psychotherapy, quality of life
Abstract
El progreso de la medicina, la mejora de las condiciones de vida en general y el aumento en la expectativa de vida han llevado a un incremento en la prevalencia de la enfermedad oncológica. Más importante aún es que, más y más pacientes sobreviven al cáncer o viven con la enfermedad por largos períodos de tiempo. Mientras que la batalla por la supervivencia se libra continuamente, la mejora en la calidad de vida de los pacientes ha pasado a primer plano. Los aspectos psicosociales pueden modular el curso de la enfermedad, pero tienen principalmente un profundo impacto en el bienestar físico y mental de los pacientes. La psico-oncología ha surgido hace poco como un campo interdisciplinario con el objetivo de abordar estos temas y dar soporte a los pacientes para que enfrenten numerosos desafíos a través de las diferentes etapas de la enfermedad. En este artículo se entrega una visión general del conocimiento actual de las interacciones cuerpo-mente en cáncer y un perfil del amplio espectro de los cuidados psico-oncológicos, con un foco especial en el tratamiento del dolor, la fatiga, temas sexuales y el temor al avance de la enfermedad.
Abstract
Le progrès médical, l'amélioration des conditions générales de vie et l'allongement de l'espérance de vie ont augmenté la prévalence générale des maladies cancéreuses. Plus important encore, de plus en plus de patients survivent au cancer ou vivent avec la maladie pendant longtemps. Le combat pour la survie ne s'arrêtant pas, l'amélioration de la qualité de vie des patients passe au premier plan. Des problèmes psychosociaux peuvent moduler le cours de la maladie, mais ils ont surtout un profond impact sur le bien-être physique et mental des patients. La psycho-oncologie émerge comme un domaine interdisciplinaire relativement nouveau, dont le but est de résoudre ces problèmes et d'apporter un soutien aux patients confrontés à de nombreuses difficultés au cours des différents stades de la maladie. Nous donnons dans cet article une vue d'ensemble des connaissances actuelles des interactions corps-esprit dans le cancer et un aperçu du large spectre des soins psycho-oncologiques, en insistant en particulier sur le traitement de la douleur, de la fatigue, des problèmes sexuels et la peur de la progression de la maladie.
Introduction
Cancer, and also its medical treatments, leads to a broad variety of physical and psychosocial problems. These range from physical pain, fatigue, and loss of autonomous life to anxiety, depression, and strain on personal relationships and have a deep impact on quality of life. As a result, the demand for psychosocial interventions to treat and support patients with cancer disease and cancer survivors has dramatically increased over the last decades. Other historical developments have also facilitated the birth of psycho-oncology as a relatively new interdisciplinary discipline. These include the following elements: the destigmatization of cancer and of mental disease, changes in the relationship between health care provider and patient, a change in focus from increasing survival and life expectancy to improving quality of life and development of palliative care.
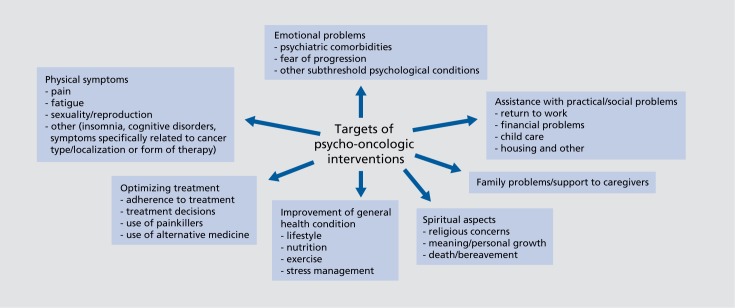
Since the 1970s, psycho-oncology has developed into a firmly established part of oncological care. Systematic research has exploded, national societies have been created in most (developed) countries and joined forces in the IPOS (International Psycho-Oncology Society 1984), guidelines have been developed, and much effort has been made to implement and coordinate supportive care networks. Also, in line with the broadening concept of health-related quality of life, the range of psychooncologic intervention has been continuously expanding (Figure 1)
Figure 1. Targets of psychooncologic interventions.
Body-mind interaction in psycho-oncology
Cancer disease is to be regarded as a complex multifactorial process, with psychosocial factors contributing to cancer genesis, as well as progression; conversely, the disease leads to many psychosocial challenges and changes.
Whereas it is well-established that health behavior and socioeconomic status have a significant influence on cancer development and prognosis,1, 2 the role of stress and personality factors is less clear. The major stress pathways of the hypothalamic-hypopituitary axis and adrenal hormone release are thought to potentially influence cancer-relevant processes like DNA damage and repair, apoptosis, migration and invasion, and angiogenesis, as well as immune processes. In animal models of disease, stress has been shown to have substantial effects on tumor genesis and progression through different pathways in a variety of experimental designs.3-7 However, the clinical relevance of such findings is yet unclear. Retrospective studies show a statistically relevant correlation between major life events and cancer incidence,8,9 but recall bias may distort the results in a retrospective design. Prospective designs have so far shown no, or only a very small, correlation between depression, personality traits, life events, and cancer development.10
There is some evidence that psychological distress, especially depression, leads to poorer prognosis in cancer disease.11,12 However, the causal nature of this correlation is not proven. Also, it is unclear if depression-related processes have a direct impact on cancer progression, or if confounding, interdependent factors like health behavior mediate this effect.
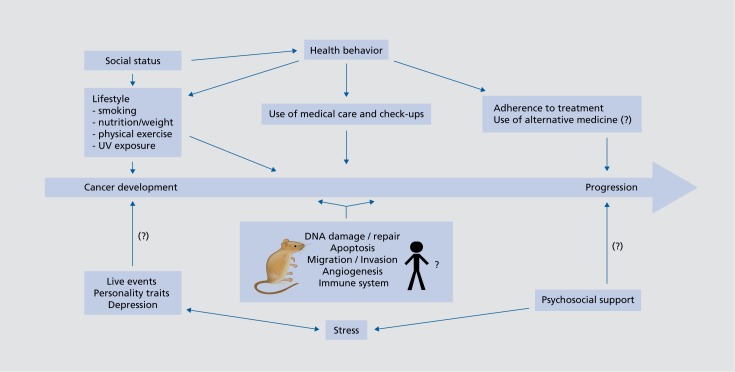
There has been much debate over the question of whether psychosocial interventions could therefore improve survival in cancer patients.13-15 Several studies have reported benefits in survival in psychosocial intervention groups,16-18 including a recent study on nonmetastatic breast cancer, in which parallel studies of the same cohort also showed reduction in Cortisol levels as well as altered leukocyte expression, including downregulation of proinflammatory and metastasis-related genes and upregulation of type I interferon response genes.19-21 In summary, evidence is still mixed and not sufficient to prove a significant effect of psychosocial interventions on cancer progression or survival. However, evidence is slowly converging, and it seems altogether likely that stress can adversely—and social support, positively—affect the course of cancer. For a model of psychosocial factors in cancer development and progression, see Figure 2 In terms of the yet inconsistent evidence and small effect sizes, these interrelations should not be overemphasized. Laypersons, as well as health care workers, tend to overestimate the role of psychological factors in cancer development. Historically, concepts like the “cancer personality” have had detrimental effects leading, for example, to dysfunctional self-attribution of guilt in cancer patients.22 Also, pressuring cancer patients into psychotherapy for the sake of life prolongation is not justified on the basis of the prevailing data.
Figure 2. Psychosocial factors in cancer development and progression. UV, ultraviolet light .
In the clinical setting, psychological consequences of cancer disease are currently more relevant. The psychological burden, as well as the direct physiological effects of cancer disease and treatment, affects body and brain, leading to changes in the neuroendocrine and immune system with wide-ranging consequences on mental health and behavior.23
Assessment of psychosocial problems and of need for intervention
Not all patients want, need, or profit from psychooncological interventions. However, the need for support is not always properly recognized, even by specialized caretakers, and thus often not met. Oncologic patients should therefore routinely undergo psychooncologic screening to assess their degree of distress throughout different stages of the disease. The term distress has been chosen in this context because it is more acceptable and less stigmatizing to patients than the terms “psychiatric,” “psychosocial,” or “emotional.”24 There are several evaluated screening instruments available, among them the easy to use distress thermometer and a short list screening for practical, physical, emotional and family problems, and spiritual and religious concerns recommended by the National Comprehensive Cancer Network (NCCN) guidelines.25 A comprehensive review of different short screening tools has been published.26 The screening should be accompanied by psychoeducational measures, including informing the patient about the range of psychooncological services and the assessment of the patient's desire for psychosocial support. Pathological results should be followed by a more thorough clinical assessment of mental problems and by offers or procurement of specific treatment. Several studies have shown that screening for distress is feasible and leads to improved outcomes when patients accept and utilize referrals to appropriate psychosocial services.27 However, this view is not entirely uncontested. Even though screening is recommended by most guidelines, a systematic review has also shown that evidence is not sufficient to prove a positive effect on distress symptoms.28
Main focuses of intervention
Management of pain and fatigue
Among the most common causes for physical distress in cancer patients is pain.29 The pain symptoms result mostly directly from the invasive growth of the tumor, but can also result from therapeutic interventions (operations, chemotherapy, radiation), immobility, or have cancer-independent causes. Guidelines of medical treatment are based on the World Health Organization (WHO) cancer pain ladder.30 Additionally, psychological treatment should be provided. Evidence is convincing that emotional distress, depression, anxiety, uncertainty, and hopelessness interact with pain. Psychological and cognitive behavioral treatments can reduce pain severity and interfere with function, as indicated in multiple meta-analyses and high-quality randomized controlled trials (RCTs). Cognitive behavioral therapy, psychoeducation, hypnosis, relaxation techniques, yoga, and exercise have been proven effective in different stages of the disease.31-34 These therapies aim at improving coping and acceptance of pain, self-efficacy, reframing of catastrophic thought, modulation of activities, and shifting the focus of attention. Educational interventions also support patients in the use of treatment options and communication with health care providers.
While pain has been the focus of health care from early on, cancer-related fatigue is less often addressed and recognized by health care providers even though it is at least as common and as significant a cause of distress and reduced function in patients throughout different stages of the disease.35,36 In a palliative care setting, 84% of the patients reported fatigue, and even among longterm survivors, 17% to 56% complain about fatigue as one of the major symptoms impairing their quality of life.37 The pathophysiology of fatigue in cancer is not fully understood, and causes are likely to be multifactorial. Proinflammatory cytokines directly related to the cancer disease, as well as concurrent problems like anemia, electrolyte disturbances, weight loss, metabolic disorders, infection, or effects of chemotherapy and radiotherapy, as well as the use of sedative drugs, are possible physical causes.38,39 Depression, anxiety, insomnia, or pain can also cause or contribute to fatigue.40 Practical guidelines and algorithms for treatment can be found in the NCCN guidelines and the consensus group recommendations of the European Association for Palliative Care (EAPC).41 Symptomatic treatment includes educational intervention, energy expenditure planning, and physical exercise. Pharmacological treatment with stimulants is also available, but somewhat controversial.42 Many clinical trials have shown that aerobic training and in some instances resistance or strength training will reduce fatigue.43-45 Energy expenditure interventions aim at regulating patients' activity levels according to their individual possibility and needs. They include planning of daily activities, eg, taking care of the most important things when their energy levels are highest, the prioritizing of activities, and delegation. In parallel, energy levels can be restored by ensuring rest, reducing stress, learning relaxation techniques, and participating in enjoyable activities. Psychotherapeutic interventions may also aim at reducing negative or catastrophizing thought, creating acceptance strategies, and redirecting attentional processes.41
Sexuality and reproduction in cancer disease
Cancer may affect sexuality in many direct and indirect ways. Cancer itself (especially testicular, prostate, penile, bladder, or gynecological tumors), as well as operative treatment, may harm physical structures necessary to sexual function. Disease-related hormonal changes or hormonal changes induced by chemotherapeutic, hormonal, or operative treatment interfere with sexual function. Changes in body image and self-esteem, cancer-related fatigue, pain or emotional problems, or strains on the relationship with sexual partners also play an important role.46,47 Sexual dysfunction has been shown to have a significant negative effect on quality of life.
Coping strategies vary significantly. For some patients, sexuality seems to become less important in the face of a life-threatening disease, whereas for others it gains in significance by emphasizing remaining pleasure, liveliness, and emotional bonding.
Patients can be offered support by medical treatment when available (hormonal therapy, erection aids, reconstructive surgery, etc), as well as educational and counseling interventions. Sometimes sexual or couple therapy is warranted. Multimodal treatment programs have been developed with some success, but evidence is still relatively scarce.48
Reproductive function can be impaired by some forms of cancer, but mostly it is the chemo- and radiotherapies that endanger fertility. Whereas protection of fertility is relatively easily achieved for men by freezing sperm, for women, it represents more of a challenge. Ovarian transposition before pelvic radiation and the cry opreserva Lion of fertilized ova are well-established methods, but may be helpful only in certain settings. Other, less well-evaluated possibilities are the cryopreservation of unfertilized ova or of ovary tissue. However, all these methods are quite invasive. Decisions concerning fertility preservation often have to be made under the pressure of time, during a difficult period, and while dealing with the diagnosis and other treatment decisions, which already represent a high emotional burden.
Patients need support when dealing with these decisions, with reproduction-related fears, or with the loss of reproductive function when it may not be preserved. Would-be parents are also often concerned by the possible heritability of the cancer disease, genetic damage by chemo- or radiotherapy, or possible effects of the pregnancy on recurrence risk (eg, in hormonereceptor-positive breast carcinoma). Thorough information and collaboration with reproductive medicine is required in such cases.49
Treatment of psychiatric comorbidities and fear of recurrence
Psychiatric comorbidities are common in cancer patients, especially depressive episodes, anxiety disorders, and adjustment disorders. Point prevalences are about If % for depression and dysthymia, 10.2% for anxiety disorders, and 12.5% for adjustment disorders.50 Psychiatric comorbidities lead to a significant reduction in quality of life in cancer patients and are also associated with poorer prognosis.51 Treatment of depressive episodes in cancer patients does not differ significantly from treatment in noncancer patients and care has been specified in several guidelines.50,52,53 However, due to the overlap of cancer symptoms and depression, special care is needed in the diagnostic process. Side effects and interactions of antidepressant medication also need to be taken into special consideration (eg, the interactions between some antidepressants and tamoxifen). There is ample evidence that psychotherapeutic interventions improve depressive symptoms in cancer patients throughout different stages of the disease.54,55 Classical therapeutic approaches like cognitive behavioral therapy have been investigated and, more recently, special treatment programs have been developed. In psychotherapeutic treatment, the specific treatment setting, the stage of disease, physical distress symptoms, and the existential threat have to be taken into account. Similar principles, though less thoroughly investigated, apply to anxiety disorders, where psychotherapeutic and psychopharmacological treatment follows roughly the guidelines for treatment in noncancer patients.56,57
Fear of recurrence (also fear of progression) represents a distinct entity in cancer treatment. It is defined as “fear, worry, or concern about cancer returning or progressing,” and is one of the most commonly cited concerns of cancer survivors. It does not correspond to any defined disorder in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM 5) or the tenth revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) and in first line must be understood as an adaptive reaction rather than an unrealistic or neurotic fear. However, high levels of fear of recurrence can lead to significant emotional distress. Classical evaluation instruments for anxiety disorders are not effective in fear of recurrence, but specific questionnaires have been developed and validated.58 Prevalence of fear of recurrence seems to vary and may be as high as 70% with patients reporting a lack of support in this area.59 Several therapeutic concepts have been developed to address progression anxiety. A group therapy based on cognitive behavioral principles has been shown to decrease fear of progression in a (partly) RCT in a rehabilitation inpatient setting and has also shown—with some modifications—some promising effects in a pilot phase for an outpatient setting. Key elements are self-observation, exposure-based techniques, refraining techniques, and implementation of behavioral changes.60,61
Another program, which builds on Leventhal's Self-Regulation Model of Illness, uses psychoeducation, cognitive restructuring, and behavioral modification in an individual therapeutic setting and has recently shown significant improvements in a RCT in survivors of breast, prostate, and colorectal cancer.62 Other pilot studies have shown promising results or are underway.63-65
New developments
Meaning and mindfulness
Cancer may disrupt a patient's feeling of purpose and poses a challenge to the premorbid coherent self and world concept. Many patients report existential or spiritual distress. There is now growing attention on the important role of the sense of meaning in improving psychological well-being and reducing psychological distress among cancer patients.66 Patients who experience more meaning in life have higher psychological well-being, a more successful adjustment, better quality of life, and less psychological distress after the cancer diagnosis than patients who experience little life meaning.67,68 Specific meaning-oriented psychotherapeutic interventions targeted at different stages of the disease have been developed and trialed over the last 10 to 15 years with encouraging results.69,70 Meaning-Centered Psychotherapy, for example, has shown positive effects on cancer survivors, as well as in advanced disease, with improvements not only in meaning-related aspects, but also in general distress and depression scores.71,72 More studies, adapted to the palliative setting, are underway.73 Likewise the program Managing Cancer and Living Meaningfully (CALM) has shown significant results in a phase 2 study, with improvements in spiritual wellbeing, as well as anxiety and depression, and it is now being tested further.74-76
Mindfulness is defined as the skill of bringing one's attention to whatever is happening in the present moment in an accepting, nonjudgmental way. Derived from Far Eastern meditation practice, it has grown increasingly popular in Western psychotherapy over the last three decades, being used as such or as a component in the treatment of a broad variety of psychiatric disorders, as well as chronic disease. Mindfulness-based techniques, mainly Mindfulness-Based Stress Reduction (MBSR) and Mindfulness-Based Cognitive Therapy (MBCT), have been trialed in oncologic settings and seem to improve psychosocial aspects of quality of life, distress symptoms, anxiety, fatigue, or insomnia.77-79 Positive effects on immunological functions have also been shown.80 In a recent meta-analysis for mindfulness-based interventions in breast cancer, 10 RCTs were analyzed, including 1709 participants. Significant positive effects were confirmed on health-related quality of life, fatigue, stress, anxiety, sleep, and depression. However, most of the effects were not traceable at 6 or 12 months' follow-up, and the average effects were shown to be below the minimal clinically important threshold; thus, the clinical relevance remains somewhat unclear.81
Acceptance and commitment therapy (ACT) developed by Steven Hayes combines the two aspects of mindfulness and meaning (mainly under the aspect of purpose), and has already been proven effective in a range of mental and physical conditions, including depression, anxiety disorders, and chronic pain, is now also being increasingly analyzed in the oncological setting.82,83 ACT is based on the assumption that pain, grief, anxiety, and illness are inevitable features of human life, with the therapeutic goal of helping individuals adapt to these types of challenges by developing greater psychological flexibility and a committed pursuit of valued life areas. So far, there are only a few studies, including studies with the aim of supporting family members of cancer patients, which have provided pilot data showing potential positive clinical effects.84- 86 The underlying framework of ACT seems especially suited to the individualistic nature of cancer adjustment, so more research in this area is warranted.
e-Health
The increasing dissemination and exponential technical development of information and communication technologies offer a broad range of possible applications in psycho-oncologic care. Internet services are used for education and the transfer of knowledge; online platforms offer support groups and networking possibilities; computer programs or smart phone applications aid self-monitoring or training planning; electronic communication and telemedical devices support communication with health care providers; and self-management training or (partly) digital psychotherapeutic programs help coping with distress symptoms and psychosocial aspects.87 The proof of effectiveness for most of these interventions is still limited and many problems are yet to be solved, starting with issues of false or biased information through Internet, data protection issues, or barriers to usage.88-94
However, since psychosocial care is time consuming and resource intensive, meaning that feasibility is often limited, e-health services are likely to become an essential complement to traditional care.
Other points to consider
Other relatively new fields of interest and research are the specific needs of refugees and immigrants, as well as developing age and gender specificities.
In addition, the importance of returning to work has been more appreciated lately and rehabilitation efforts are being assessed.95
Conclusion
Research in psycho-oncology essentially contributes to our understanding of body-mind interaction and has been challenging a more or less mechanical view of cancer disease. The psycho-oncological therapeutic approach has become a crucial tool in the care of cancer patients, improving not only physical and psychiatric symptoms, but also quality of life. The standard management of psychiatric diseases is not sufficient for this special field, and psychotherapy has to be adapted to life-threatening conditions, as well as arising existential questions and spiritual needs. Upcoming therapy programs are encouraging, but need further evaluation and differentiation.
Acknowledgments
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Gallo V., Mackenbach JP., Ezzati M., et al. Social inequalities and mortality in Europe - results from a large multi-national cohort. PLoS One. e39013. doi:10.1371/journal.pone.0039013. 2012;7(7) doi: 10.1371/journal.pone.0039013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalton SO., Steding-Jessen M., Engholm G., Schuz J., Olsen JH. Social inequality and incidence of and survival from lung cancer in a population-based study in Denmark, 1994-2003. Eur J Cancer. 2008;44(14):1989–1995. doi: 10.1016/j.ejca.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Sloan EK., Priceman SJ., Cox BF., et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(18):7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thaker PH., Han LY., Kamat AA., et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 5.Hassan S., Karpova Y., Baiz D., et al. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest. 2013;123(2):874–886. doi: 10.1172/JCI63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eng JW., Kokolus KM., Reed CB., Hylander BL., Ma WW., Repasky EA. A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother. 2014;63(11):1115–1128. doi: 10.1007/s00262-014-1617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saul AN., Oberyszyn TM., Daugherty C., et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97(23):1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruk J. Self-reported psychological stress and the risk of breast cancer: a case-control study. Stress. 2012;15(2):162–171. doi: 10.3109/10253890.2011.606340. [DOI] [PubMed] [Google Scholar]
- 9.Lillberg K., Verkasalo PK., Kaprio J., et al. Stressful life events and risk of breast cancer in 10 808 women: a cohort study. Am J Epidemiol. 2003;157(5):415–423. doi: 10.1093/aje/kwg002. [DOI] [PubMed] [Google Scholar]
- 10.Schoemaker MJ., Jones ME., Wright LB., et al. Psychological stress, adverse life events and breast cancer incidence: a cohort investigation in 106 000 women in the United Kingdom. Breast Cancer Res. 2016;18(1):72. doi: 10.1186/s13058-016-0733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskelinen M., Korhonen R., Selander T., Ollonen P., Beck Depression inventory as a predictor of long-term outcome among patients admitted to the Breast Cancer Diagnosis Unit: a 25-year cohort study in Finland. Anticancer Res. 2017;37(2):819–824. doi: 10.21873/anticanres.11383. [DOI] [PubMed] [Google Scholar]
- 12.Satin JR., Linden W., Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115(22):5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel D., Bloom JR., Kraemer HC., Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2(8668):888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- 14.Kissane DW. Letting go of the hope that psychotherapy prolongs cancer survival. J Clin Oncol. 2007;25(36):5689–5690. doi: 10.1200/JCO.2007.13.9451. [DOI] [PubMed] [Google Scholar]
- 15.Spiegel D. Minding the body: psychotherapy and cancer survival. Br J Health Psychol. 2014;19(3):465–485. doi: 10.1111/bjhp.12061. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin PJ., Leszcz M., Ennis M., et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345(24):1719–1726. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 17.Kissane DW., Grabsch B., Clarke DM., et al. Supportive-expressive group therapy for women with metastatic breast cancer: survival and psychosocial outcome from a randomized controlled trial. Psychooncology. 2007;16(4):277–286. doi: 10.1002/pon.1185. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel D., Butler LD., Giese-Davis J., et al. Effects of supportive-expressive group therapy on survival of patients with metastatic breast cancer: a randomized prospective trial. Cancer. 2007;110(5):1130–1138. doi: 10.1002/cncr.22890. [DOI] [PubMed] [Google Scholar]
- 19.Stagl JM., Lechner SC., Carver CS., et al. A randomized controlled trial of cognitive-behavioral stress management in breast cancer: survival and recurrence at 1 1-year follow-up. Breast Cancer Res Treat. 2015;154(2):319–328. doi: 10.1007/s10549-015-3626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips KM., Antoni MH., Carver CS., et al. Stress management skills and reductions in serum Cortisol across the year after surgery for nonmetastatic breast cancer. Cogn Therapy Res. 2011;35(6):595–600. [Google Scholar]
- 21.Antoni MH., Lutgendorf SK., Blomberg B., et al. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71(4):366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz R. Psychosocial factors in carcinogenesis: on the problem of the so-called cancer-prone personality [in German]. Psychother Psychosom Med Psychol. 1993;43(1):1–9. [PubMed] [Google Scholar]
- 23.Miller AH., Ancoli-lsrael S., Bower JE., Capuron L., Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Distress management. 2010. Available at: https://www.nccn. org/store/login/login.aspx ReturnURL=https://www. nccn.org/professionals/physician_gls/pdf/distress.pdf. accessed September 20, 2017 [Google Scholar]
- 25.Kayser K., Acquati C., Tran TV. No patients left behind: a systematic review of the cultural equivalence of distress screening instruments. J Psychosoc Oncol. 2012;30(6):679–693. doi: 10.1080/07347332.2012.721489. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2010;8(4):487–494. doi: 10.6004/jnccn.2010.0035. [DOI] [PubMed] [Google Scholar]
- 27.Bultz BD., Waller A., Cullum J., et al. Implementing routine screening for distress, the sixth vital sign, for patients with head and neck and neurologic cancers. J Natl Compr Canc Netw. 20131;11(10):1249–1261. doi: 10.6004/jnccn.2013.0147. [DOI] [PubMed] [Google Scholar]
- 28.Meijer A., Roseman M., Delisle VC., et al. Effects of screening for psychological distress on patient outcomes in cancer: a systematic review. J Psychosom Res. doi:10.1016/j.jpsychores.2013.01.012. 2013;75(1):1–17. doi: 10.1016/j.jpsychores.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Beuken-van Everdingen MH., Hochstenbach LM., Joosten EA., Tjan-Heijnen VC., Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. e9. doi:10.1016/j.jpainsymman.2015.12.340. 2016;51(6):1070–1090. doi: 10.1016/j.jpainsymman.2015.12.340. [DOI] [PubMed] [Google Scholar]
- 30.WHO guidelines, cancer palliative care. Available at: http://www. who.int/cancer/palliative/painladder/en/. Accessed September 20 2017. [Google Scholar]
- 31.Sheinfeld Gorin S., Krebs P., Badr H., et al. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol. 2012;30(5):539–547. doi: 10.1200/JCO.2011.37.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johannsen M., Farver I., Beck N., et al. The efficacy of psychosocial intervention for pain in breast cancer patients and survivors: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;138(3):675–690. doi: 10.1007/s10549-013-2503-4. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery GH., Schnur JB., Kravits K. Hypnosis for cancer care: over 200 years young. CA Cancer J Clin. 2013;63(1):31–44. doi: 10.3322/caac.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tatrow K., Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med. 2006;29:17–27. doi: 10.1007/s10865-005-9036-1. [DOI] [PubMed] [Google Scholar]
- 35.Vogelzang NJ., Breitbart W., Cella D., et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol. 1997;34(3 suppl 2):4–12. [PubMed] [Google Scholar]
- 36.Williams LA., Bohac C., Hunter S., Cella D. Patient and health care provider perceptions of cancer-related fatigue and pain. Support Care Cancer. 2016;24(10):4357–4363. doi: 10.1007/s00520-016-3275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence DP., Kupelnick B., Miller K., Devine D., Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;32:40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 38.Wood LJ., Nail LM., Gilster A., Winters KA., Elsea CR. Cancer chemotherapy-related symptoms: evidence to suggest a role for proinflammatory cytokines. Oncol Nurs Forum. 2006;33(3):535–542. doi: 10.1188/06.ONF.535-542. [DOI] [PubMed] [Google Scholar]
- 39.Saligan LN., Olson K., Filler K., et al. Multinational Association of Supportive Care in Cancer Fatigue Study Group-Biomarker Working Group. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer. 2015;23(8):2461–2478. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabi A., Falcicchio C., Giannarelli D., Maggi G., Cognetti F., Pugliese P. The course of cancer related fatigue up to ten years in early breast cancer patients: what impact in clinical practice? Breast. 2017;34:44–52. doi: 10.1016/j.breast.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Radbruch L., Strasser F., Elsner F., et al. Research Steering Committee of the European Association for Palliative Care (EAPC). Fatigue in palliative care patients - an EAPC approach. Palliat Med. 2008;22(1):13–32. doi: 10.1177/0269216307085183. [DOI] [PubMed] [Google Scholar]
- 42.Qu D., Zhang Z., Yu X., Zhao J., Qiu F., Huang J. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2016;25(6):970–979. doi: 10.1111/ecc.12397. [DOI] [PubMed] [Google Scholar]
- 43.Dimeo FC. Effects of exercise on cancer-related fatigue. Cancer. 2001;92(6 suppl):1689–1693. doi: 10.1002/1097-0142(20010915)92:6+<1689::aid-cncr1498>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 44.Schmitz KH., Holtzman J., Courneya KS., Masse LC., Duval S., Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005l;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 45.Dittus KL., Gramling RE., Ades PA. Exercise interventions for individuals with advanced cancer: a systematic review. Prev Med. 2017;104:124–132. doi: 10.1016/j.ypmed.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Male DA., Fergus KD., Cullen K. Sexual identity after breast cancer: sexuality, body image, and relationship repercussions. Curr Opin Support Palliat Care. 2016;10(1):66–74. doi: 10.1097/SPC.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 47.Katz A., Dizon DS. Sexuality after cancer: a model for male survivors. J Sex Med. 2016;13(1):70–78. doi: 10.1016/j.jsxm.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Krychman ML., Katz A. Breast cancer and sexuality: multi-modal treatment options. J Sex Med. 2012;9(1):5–13; quiz 14-5.. doi: 10.1111/j.1743-6109.2011.02566.x. [DOI] [PubMed] [Google Scholar]
- 49.Wollenschein M., Rohde A., van der Ven K. Weibliche Fertilitat und Krebserkrankung. In: Mehnert A, Koch U, eds. Handbuch Pychoonkologie. Gottingen, Germany: Hogrefe Verlag. 2016:635–645. [Google Scholar]
- 50.Arbeitsgemeinschaft der wissenschaftlichen medizinischen Fachgesellschaften (AWMF). Guideline (S3-Leitlinie): “Psychoonkologische Diagnostic Beratung und Behandlung von erwachsenen Krebspatienten”. 31.01.2014. Available at: http://www.awmf.org/leitlinien/detail/ll/032051OL.html. Accessed September 1. 2017 [Google Scholar]
- 51.Sotelo JL., Musselman D., Nemeroff C. The biology of depression in cancer and the relationship between depression and cancer progression. Int Rev Psychiatry. 2014;26(1):16–30. doi: 10.3109/09540261.2013.875891. [DOI] [PubMed] [Google Scholar]
- 52.National Cancer Institute (NCI). Depression. Available at: https://www. cancer.gov/about-cancer/coping/feel ings/depression-hp-pdq/#section/_1. Accessed September 20. 2017 [Google Scholar]
- 53.Li M., Kennedy EB., Byrne N., et al. Management of depression in patients with cancer: a clinical practice guideline. J Oncol Pract. 2016;12(8):747–756. doi: 10.1200/JOP.2016.011072. [DOI] [PubMed] [Google Scholar]
- 54.Osborn RL., Demoncada AC., Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analyses. Int J Psychiatry Med. 2006;36(1):13–34. doi: 10.2190/EUFN-RV1K-Y3TR-FK0L. [DOI] [PubMed] [Google Scholar]
- 55.Akechi T., Okuyama T., Onishi J., Morita T., Furukawa TA. Psychotherapy for depression among incurable cancer patients. Cochrane Database Syst Rev. 2008;16(2):CD005537. doi: 10.1002/14651858.CD005537.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rayner L., Price A., Hotopf M., Higginson IJ. The development of evidence-based European guidelines on the management of depression in palliative cancer care. Eur J Cancer. 2011;47:702–712. doi: 10.1016/j.ejca.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 57.Howell D., Keshavarz H., Esplen MJ., et al. on behalf of the Cancer Journey Advisory Group of the Canadian Partnership Against Cancer. A pan Canadian practice guideline: screening, assessment and care of psychosocial distress (depression, anxiety) in adults with cancer. Canadian Partnership Against Cancer (Cancer Journey Advisory Group) and the Canadian Association of Psychosocial Oncology. 2015. Available at: https://www.capo. ca/wp-content/uploads/2010/10/Distress_guideline_CAPO_201 50731 1.pdf. Accessed September 20, 2017 [Google Scholar]
- 58.Thewes B., Butow P., Zachariae R., Christensen S., Simard S., Gotay C. Fear of cancer recurrence: a systematic literature review of self-report measures. Psychooncology. 2012;21(6):571–587. doi: 10.1002/pon.2070. [DOI] [PubMed] [Google Scholar]
- 59.Simard S., Thewes B., Humphris G., et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv. 2013;7(3):300–322. doi: 10.1007/s11764-013-0272-z. [DOI] [PubMed] [Google Scholar]
- 60.Herschbach P., Book K., Dinkel A., et al. Evaluation of two group therapies to reduce fear of progression in cancer patients. Support Care Cancer. 2010;18(4):471–479. doi: 10.1007/s00520-009-0696-1. [DOI] [PubMed] [Google Scholar]
- 61.Rudolph B., Wunsch A., Herschbach P., Dinkel A. Cognitive-behavioral group therapy addressing fear of progression in cancer out-patients [in German]. Psychother Psychosom Med Psychol. 2018;68(1):38–43. doi: 10.1055/s-0043-107774. [DOI] [PubMed] [Google Scholar]
- 62.van de Wal M., Thewes B., Gielissen M., Speckens A., Prins J. Efficacy of blended cognitive behavior therapy for high fear of recurrence in breast, prostate, and colorectal cancer survivors: the SWORD study, a randomized controlled trial. J Clin Oncol. 2017;35(19):2173–2183. doi: 10.1200/JCO.2016.70.5301. [DOI] [PubMed] [Google Scholar]
- 63.Lebel S., Maheu C., Lefebvre M., et al. Addressing fear of cancer recurrence among women with cancer: a feasibility and preliminary outcome study. J Cancer Surviv. 2014;8(3):485–496. doi: 10.1007/s11764-014-0357-3. [DOI] [PubMed] [Google Scholar]
- 64.Smith A., Thewes B., Turner J., et al. Pilot of a theoretically grounded psychologist-delivered intervention for fear of cancer recurrence (Conquer Fear). Psychooncology. 2015;24(8):967–970. doi: 10.1002/pon.3775. [DOI] [PubMed] [Google Scholar]
- 65.Butow PN., Bell ML., Smith AB., et al. Conquer Fear Authorship Group. Conquer fear: protocol of a randomised controlled trial of a psychological intervention to reduce fear of cancer recurrence. BMC Cancer.doi:10.1 186/1471-2407-13-201. 2013;13:201. doi: 10.1186/1471-2407-13-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.LeMay K., Wilson KG. Treatment of existential distress in life threatening illness: a review of manualized interventions. Clin Psychol Rev. 2008;28(3):472–493. doi: 10.1016/j.cpr.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Park CL., Edmondson D., Fenster JR., Blank TO. Meaning making and psychological adjustment following cancer: the mediating roles of growth, life meaning, and restored just-world beliefs. J Consult Clin Psychol. 2008; 76(5):863–875. doi: 10.1037/a0013348. [DOI] [PubMed] [Google Scholar]
- 68.Jaarsma TA., Pool G., Ranchor AV., Sanderman R. The concept and measurement of meaning in life in Dutch cancer patients. Psychooncology. 2007;16(3):241–248. doi: 10.1002/pon.1056. [DOI] [PubMed] [Google Scholar]
- 69.Fitchett G., Emanuel L., Handzo G., Boyken L., Wilkie DJ. Care of the human spirit and the role of dignity therapy: a systematic review of dignity therapy research. BMC Palliat Care. 2015;14:8. doi: 10.1186/s12904-015-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henry M., Cohen SR., Lee V., et al. The Meaning-Making intervention (MMi) appears to increase meaning in life in advanced ovarian cancer: a randomized controlled pilot study. Psychooncology. 2010;19(12):1340–1347. doi: 10.1002/pon.1764. [DOI] [PubMed] [Google Scholar]
- 71.van der Spek N., Vos J., van Uden-Kraan CF., et al. Efficacy of meaningcentered group psychotherapy for cancer survivors: a randomized controlled trial. Psychol Med. 2017;47(11):1990–2001. doi: 10.1017/S0033291717000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Breitbart W., Rosenfeld B., Pessin H., et al. Meaning-centered group psychotherapy: an effective intervention for improving psychological well-being in patients with advanced cancer. J Clin Oncol. 2015;33(7):749–754. doi: 10.1200/JCO.2014.57.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenfeld B., Saracino R., Tobias K., et al. Adapting meaning-centered psychotherapy for the palliative care setting: results of a pilot study. Palliat Med. 2017;31(2):140–146. doi: 10.1177/0269216316651570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lo C., Hales S., Jung J., et al. Managing Cancer and Living Meaningfully (CALM): phase 2 trial of a brief individual psychotherapy for patients with advanced cancer. Palliat Med. 2014;28(3):234–242. doi: 10.1177/0269216313507757. [DOI] [PubMed] [Google Scholar]
- 75.Scheffold K., Philipp R., Engelmann D., et al. Efficacy of a brief manualized intervention Managing Cancer and Living Meaningfully (CALM) adapted to German cancer care settings: study protocol for a randomized controlled trial. BMC Cancer. 2015;15:592. doi: 10.1186/s12885-015-1589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lo C., Hales S., Chiu A., et al. Managing Cancer and Living Meaningfully (CALM): randomised feasibility trial in patients with advanced cancer. BMJ Support Palliat Care. 2016 Jan 19. Epub ahead of print. pii:bmjspcare-201 5-000866. doi:10.1136/bmjspcare-2015000866. doi: 10.1136/bmjspcare-2015-000866. [DOI] [PubMed] [Google Scholar]
- 77.Ledesma D., Kumano H. Mindfulness-based stress reduction and cancer: a meta-analysis. Psychooncology. 2009;18(6):571–579. doi: 10.1002/pon.1400. [DOI] [PubMed] [Google Scholar]
- 78.Musial F., Bussing A., Heusser P., Choi KE., Ostermann T. Mindfulness-based stress reduction for integrative cancer care: a summary of evidence. Forsch Komplementmed. 2011;18(4):192–202. doi: 10.1159/000330714. [DOI] [PubMed] [Google Scholar]
- 79.Biegler KA., Chaoul MA., Cohen L. Cancer, cognitive impairment, and meditation. Acta Oncol. 2009;48(1):18–26. doi: 10.1080/02841860802415535. [DOI] [PubMed] [Google Scholar]
- 80.Kenne Sarenmalm E., Martensson LB., Andersson BA., Karlsson P., Bergh I. Mindfulness and its efficacy for psychological and biological responses in women with breast cancer. Cancer Med. 2017;6(5):1108–1122. doi: 10.1002/cam4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haller H., Winkler MM., Klose P., Dobos G., Kummel S., Cramer H. Mindfulness-based interventions for women with breast cancer: an updated systematic review and meta-analysis. Acta Oncol. 2017;56(12):1665–1676. doi: 10.1080/0284186X.2017.1342862. [DOI] [PubMed] [Google Scholar]
- 82.Arch JJ., Mitchell JL. An Acceptance and Commitment Therapy (ACT) group intervention for cancer survivors experiencing anxiety at re-entry. Psychooncology. 2016;25(5):610–615. doi: 10.1002/pon.3890. [DOI] [PubMed] [Google Scholar]
- 83.Low J., Serfaty M., Davis S., et al. Acceptance and commitment therapy for adults with advanced cancer (CanACT): study protocol for a feasibility randomised controlled trial. Trials. 2016;17:77. doi: 10.1186/s13063-016-1169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dindo L., Van Liew JR., Arch JJ. Acceptance and commitment therapy: a transdiagnostic behavioral intervention for mental health and medical conditions. Neurotherapeutics. 2017;14(3):546–553. doi: 10.1007/s13311-017-0521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patterson P., McDonald FE., Ciarrochi J., et al. A study protocol for Truce: a pragmatic controlled trial of a seven-week acceptance and commitment therapy program for young people who have a parent with cancer. BMC Psychol. 2015;3:31. doi: 10.1186/s40359-015-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hulbert-Williams NJ., Storey L., Wilson KG. Psychological interventions for patients with cancer: psychological flexibility and the potential utility of Acceptance and Commitment Therapy. Eur J Cancer Care (Engl). 2015;24(1):15–27. doi: 10.1111/ecc.12223. [DOI] [PubMed] [Google Scholar]
- 87.Schulz H., Fink J., Watzke B. E-Health-angebote in der onkologie. In: Mehnert A, Koch U, eds. Handbuch Pychoonkologie. Gottingen, Germany: Hogrefe Verlag. 2016:635–645. [Google Scholar]
- 88.Galiano-Castillo N., Cantarero-Villanueva I., Fernandez-Lao C., et al. Telehealth system: a randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer. 2016;122(20):3166–3174. doi: 10.1002/cncr.30172. [DOI] [PubMed] [Google Scholar]
- 89.Williams AR., Williams DD., Williams PD., et al. The development and application of an oncology Therapy-Related Symptom Checklist for Adults (TRSC) and Children (TRSC-C) and e-health applications. Biomed Eng Online. 2015;14(suppl 2):S1–xx. doi: 10.1186/1475-925X-14-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hong Y., Peña-Purcell NC., Ory MG. Outcomes of online support and resources for cancer survivors: a systematic literature review. Patient Educ Couns. 2012;86(3):288–296. doi: 10.1016/j.pec.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 91.Beatty L., Koczwara B., Wade T. Evaluating the efficacy of a self-guided Web-based CBT intervention for reducing cancer-distress: a randomised controlled trial. Support Care Cancer. 2016;24(3):1043–1051. doi: 10.1007/s00520-015-2867-6. [DOI] [PubMed] [Google Scholar]
- 92.Myall M., May CR., Grimmett C., et al. RESTORE: an exploratory trial of a web-based intervention to enhance self-management of cancer-related fatigue: findings from a qualitative process evaluation. BMC Med Inform DecisMak. 2015;15:94. doi: 10.1186/s12911-015-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mikolasek M., Berg J., Witt CM., Barth J. Effectiveness of mindfulness- and relaxation-based eHealth Interventions for patients with medical conditions: a systematic review and synthesis, Int J Behav Med. 2018;25(1):1–16. doi: 10.1007/s12529-017-9679-7. [DOI] [PubMed] [Google Scholar]
- 94.Post KE., Flanagan J. Web-based survivorship interventions for women with breast cancer: an integrative review. Eur J Oncol Nurs. 2016;25:90–99. doi: 10.1016/j.ejon.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 95.Abstracts of the 2017 World Congress of Psycho-oncology, 14-18 August 2017 Berlin, Germany. Psycho-Oncology. 2017;26(suppl 3):3–167. doi: 10.1002/pon.4476. [DOI] [PubMed] [Google Scholar]