Abstract
Background
Vascular endothelial growth factor-A (VEGF-A) promotes neovascularization and is attracting considerable attention as a remarkable risk factor in patients after acute myocardial infarction (AMI). In contrast, the association between VEGF-A165b, which is the main anti-angiogenic isoform of VEGF-A, and adverse clinical outcomes after AMI remains unclear. The present study aimed to investigate the association between serum VEGF-A165b and major adverse cardiac and cerebrovascular events (MACCEs) after percutaneous coronary intervention (PCI) for AMI.
Methods
We evaluated 23 patients with AMI who underwent primary percutaneous coronary intervention. VEGF-A and VEGF-A165b levels were measured on admission (day 1) and at days 3, 7, and 30 after PCI.
Results
The levels of total VEGF-A tended to be lower, while the ratio of VEGF-A165b to total VEGF-A tended to be higher in patients with MACCEs than in those without. The patients with a high ratio of VEGF-A165b to total VEGF-A had a significantly higher risk of MACCEs using the cut-off values for MACCEs at day 30 after PCI (0.87 vs. 0.25, log-rank test, p = 0.0058).
Conclusion
The assessment of VEGF-A165b combined with VEGF-A may be a valuable screening tool for predicting MACCEs in clinical practice.
Keywords: Acute myocardial infarction, Major adverse cardiac and cerebrovascular events, VEGF-A, VEGF-A165b
1. Introduction
Vascular endothelial growth factor-A (VEGF-A) has become the focus of intense interest because of its essential role in neovascularization [1]. VEGF-A is up-regulated in hypoxia and can promote angiogenesis after acute myocardial infarction (AMI) [2]. Previous clinical studies on VEGF-A in patients with AMI have demonstrated that low levels of circulating VEGF-A were an independent risk factor for adverse clinical outcomes after AMI [3,4]. VEGF-A produces various isoforms with distinct biological activities through alternative messenger RNA splicing [5]. VEGF-A165b is the main anti-angiogenic isoform of VEGF-A [6,7].
Several reports have demonstrated the remarkable elevation of VEGF-A165b in some diseases characterized by impaired angiogenesis, vascular damage, and hypoxia such as systemic sclerosis [8], and peripheral artery disease (PAD) [[9], [10], [11]]. And it is suggested that VEGF-A165b prevents the physiological consequences of the pro-angiogenic behavior of VEGF-A by several VEGF receptor signals [14]. Recent studies demonstrated VEGF-A165b associated with infarct size in patients with AMI [17] and dysregulated VEGF-A165b in senescent endothelial cells may contribute to the risk of coronary heart disease [18]. Insufficient angiogenesis can inhibit the healing process of the myocardium and endothelial cells after AMI. However, the association between VEGF-A165b and adverse clinical outcomes after AMI has not been elucidated. Therefore, we evaluated the serial changes in circulating serum VEGF-A and VEGF-A165b levels and their association with adverse clinical outcomes after percutaneous coronary intervention (PCI) in patients with AMI.
2. Methods
This study was a prospective, single-center, observational study approved by the ethics committee of the Nagoya University Graduate School of Medicine and conducted in accordance with the ethical principles of the Declaration of Helsinki.
From July 2015 to February 2017, we evaluated 66 consecutive patients with AMI who underwent PCI within 24 h of symptom onset at Nagoya University Hospital. Exclusion criteria were as follows: patients with hemodialysis (n = 4), active malignancy (n = 5), and collagen disease (n = 2) because these conditions affect the VEGF-A and VEGF-A165b levels. Finally, 23 patients for whom blood samples could be obtained at all time points (on admission [day 1] and at days 3, 7, and 30 after PCI were enrolled in this study.
AMI was diagnosed based on the third universal definition of myocardial infarction [19]. The primary endpoint was major adverse cardiac and cerebrovascular events (MACCEs), which were defined as the composite of cardiovascular death, recurrent myocardial infarction (MI), coronary revascularization, and stroke after AMI. Coronary revascularization was defined as PCI or coronary artery bypass grafting. PCI performed on a later day for the residual lesions identified during primary PCI was excluded. Stroke was defined as a newly developed neurological deficit and relevant findings on magnetic resonance imaging. The patients were divided into two groups according to the incidence of MACCEs.
Blood samples were obtained from all patients on admission (day 1) and at days 3, 7, and 30 after PCI. Serum samples were stored at −80 °C. Serum VEGF-A levels were determined using an enzyme-linked immunosorbent assay (ELISA) kit (Human VEGF Quantikine ELISA Kit, DVE00, R&D Systems), and the limit of detection was 9 pg/mL (intra-assay and inter-assay coefficients of variation were 4.5% and 7.0%, respectively). VEGF-A ELISA kit does not discriminate between pro- and anti-angiogenic isoforms of VEGF-A. Serum VEGF-A165b levels were determined using the ELISA kit (Human VEGF-165b ELISA Kit, MBS720132, MyBiosource), and the limit of detection was 1 pg/mL (intra-assay and inter-assay coefficients of variation were <10% and <10%, respectively) [9]. We obtained creatine kinase (CK) levels on admission, every 3 h until peak values were reached, and 24 h after PCI. We analyzed the area under the concentration versus time curve (AUC) for CK [20]. The left ventricular ejection fraction (LVEF) was measured with echocardiography at an outpatient clinic within 6 months after PCI.
2.1. Statistical analysis
Date is presented as mean ± standard deviation, median (interquartile range [IQR]) or numbers (percentages). The differences in normally distributed values were assessed using the Student t-test, while the asymmetrically distributed values were assessed using the Mann Whitney U test. The differences in categorical variables were assessed using the Kruskal-Wallis test or chi-square test. Kaplan-Meier analysis was performed to evaluate the cumulative incidence of MACCEs, and comparisons were assessed using the log-rank test. The cut-off values for MACCEs were established using receiver operating characteristic (ROC) curve analysis. A p-value <0.05 was considered statistically significant.
3. Results
The clinical characteristics are summarized in Table 1. During the median follow-up of 187 (IQR: 73–402) days, 9 (39.1%) MACCEs occurred.
Table 1.
Clinical characteristics.
| Variables | MACCE |
p | ||
|---|---|---|---|---|
| All (n = 23) | No (n = 14) | Yes (n = 9) | ||
| Age (years) | 67.0 ± 10.8 | 64.3 ± 11.3 | 71.2 ± 9.0 | 0.14 |
| Male, n (%) | 17 (73.9) | 11 (78.5) | 6 (66.7) | 0.44 |
| Body mass index (kg/m2) | 22.9 ± 2.9 | 23.2 ± 3.0 | 22.4 ± 3.0 | 0.54 |
| Current smoking, n (%) | 7 (30.4) | 4 (28.6) | 3 (33.3) | 0.58 |
| Hypertension, n (%) | 10 (43.5) | 6 (42.9) | 4 (44.4) | 0.64 |
| Diabetes mellitus, n (%) | 7 (30.4) | 5 (35.7) | 2 (22.2) | 0.42 |
| Dyslipidemia, n (%) | 16 (69.6) | 10 (71.4) | 6 (66.7) | 0.58 |
| eGFR (mL/min/1.73 m2) | 60.9 (57.0–71.8) | 62.1 (58.8–74.1) | 59.5 (54.2–68.4) | 0.40 |
| LDL cholesterol (mg/dL) | 133 ± 28.7 | 137 ± 29.6 | 128 ± 28.1 | 0.52 |
| HDL cholesterol (mg/dL) | 50.1 ± 11.4 | 49.0 ± 10.2 | 51.9 ± 13.4 | 0.56 |
| Triglycerides (mg/dL) | 182 ± 103 | 187 ± 93.2 | 175 ± 121 | 0.78 |
| Hemoglobin A1c (%) | 5.8 (5.7–6.8) | 5.9 (5.8–6.9) | 5.8 (5.6–6.6) | 0.64 |
| C-reactive protein (mg/L) | 1.0 (0.7–2.5) | 1.0 (0.7–4.8) | 0.8 (0.7–3.0) | 0.56 |
| Creatine kinase (AUC) (IU/L × h) | 43,142 (29788–88,934) | 51,164 (31,922–92,034) | 40,748 (18,932–78,411) | 0.69 |
| Peripheral artery disease, n (%) | 2 (8.7) | 1 (7.1) | 1 (11.1) | 0.66 |
| Time to reperfusion (min) | 90.9 ± 31.9 | 85.6 ± 24.9 | 98.4 ± 40.4 | 0.37 |
| Culprit lesion (LAD, LCX, RCA) | 56.5%, 13.0%, 30.4% | 21.4%, 57.1%, 21.4% | 44.4%, 55.6%, 0% | 0.10 |
| Killip class (I, II, III, IV) | 70%, 4.3%, 8.7%, 17% | 79%, 0%, 0%, 21% | 56%, 11%, 22%, 11% | 0.40 |
| TIMI flow grade before PCI (0, 1, 2, 3) | 74%, 4.3%, 13%, 8.7% | 57%, 7.1%, 21%, 14% | 100%, 0%, 0%, 0% | 0.03 |
| TIMI flow grade after PCI (0, 1, 2, 3) | 0%, 4.3%, 4.3%, 91% | 0%, 0%, 7.1%, 93% | 0%, 11%, 0%, 89% | 0.39 |
| LVEF after PCI (%) | 53.3 ± 10.9 | 55.6 ± 8.3 | 49.7 ± 13.8 | 0.21 |
| Medication at discharge | ||||
| Antiplatelet agents, n (%) | 23 (100) | 14 (100) | 9 (100) | |
| ACE-I or ARB, n (%) | 21 (91.3) | 13 (92.9) | 8 (88.9) | 0.64 |
| Beta-blocker, n (%) | 14 (60.9) | 10 (71.4) | 4 (44.4) | 0.20 |
| Calcium channel blocker, n (%) | 3 (13.0) | 2 (14.3) | 1 (11.1) | 0.67 |
| Statin, n (%) | 22 (95.7) | 13 (92.9) | 9 (100) | 0.61 |
Data are indicated as means ± SD or median (interquartile range) or number (percentages). MACCE, major adverse cardiac and cerebrovascular events; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein; HDL, high-density lipoprotein; AUC, area under the concentration versus time curve; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction; LVEF, Left ventricular ejection fraction; PCI, percutaneous coronary intervention; ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker.
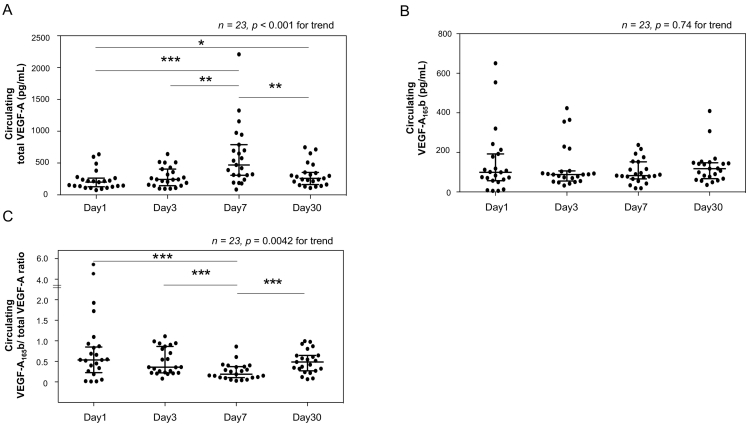
The levels of total VEGF-A increased after PCI and reached their peak at day 7, whereas the levels of VEGF-A165b maintained the similar values at each time point (Fig. 1). We also assessed the serial changes in the ratio of VEGF-A165b to total VEGF-A in order to evaluate the total VEGF-A and VEGF-A165b levels simultaneously. The ratio of VEGF-A165b to total VEGF-A was at its lowest at day 7 after PCI, and it increased again at day 30 after AMI (Fig. 1).
Fig. 1.
Serial changes in circulating total vascular endothelial growth factor-A (VEGF-A) (A), VEGF-A165b (B), and the ratio of VEGF-A165b to total VEGF-A (C) in patients after PCI. The Kruskal-Wallis test was performed to evaluate the differences at day 1, 3, 7, and 30 (shown the p values for trend). The values of median (interquartile range) were as follows:
A. Day 1: 199.6 (132.1–251.0), Day 3: 242.9 (152.0–382.7), Day 7: 470.1 (309.1–743.7), Day 30: 260.2 (185.7–348.3) pg/mL. B. Day1: 98.9 (59.8–185.4), Day 3: 88.2 (62.5–105.1), Day 7: 82.7 (69.8–134.0), Day 30 117.8 (72.1–146.2) pg/mL. C. Day 1: 0.54 (0.30–0.90), Day 3: 0.36 (0.23–0.83), Day 7: 0.18 (0.11–0.37), Day 30: 0.49 (0.29–0.64).
⁎p < 0.05, ⁎⁎p < 0.01, ⁎⁎⁎p < 0.001(Mann Whitney U test).
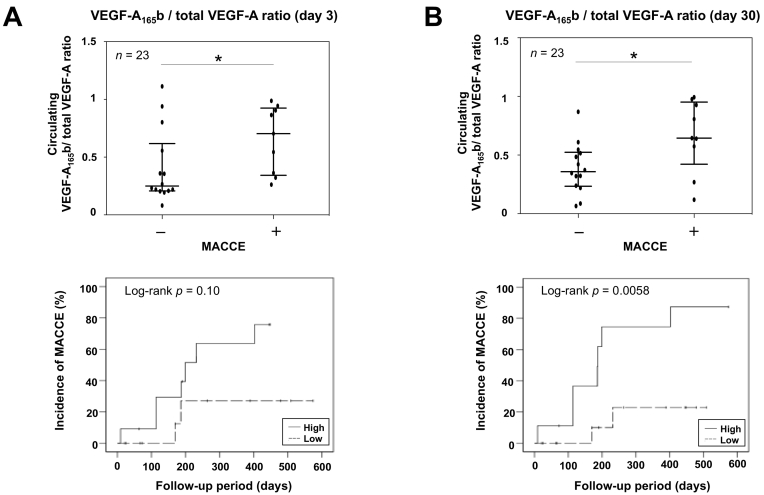
Next, we assessed the comparisons of total VEGF-A, VEGF-A165b, and the ratio at days 1, 3, 7, and 30 after PCI in patients with and without MACCEs. The levels of total VEGF-A tended to be lower in patients with MACCEs than in those without. The ratio was significantly higher at days 3 and 30 after PCI in patients with MACCEs (Fig. 2). In light of the significant differences in the ratio at days 3 and 30 after PCI, we established cut-off values for MACCEs using ROC curve analysis at that time. The cut-off values for the ratio at days 3 and 30 were 0.36 (area under the ROC curve [AURC] = 0.76, p = 0.010) and 0.57 (AURC = 0.79, p = 0.015), respectively. According to the cut-off values, the patients were divided into two groups, and the Kaplan-Meier analysis revealed that the cumulative incidences of MACCEs were significantly higher in patients with a high ratio of VEGF-A165b to VEGF-A at day 30 (0.87 vs. 0.25, p = 0.0058) (Fig. 2).
Fig. 2.
Comparisons of the ratio of VEGF-A165b to total VEGF-A according to the incidence of major adverse cardiac and cerebrovascular events (MACCEs), and cumulative incidence of MACCEs according to the cut-off value of the ratio at day 3 (A) and 30 (B) after PCI. The Kaplan-Meier analysis was performed to evaluate the cumulative incidence of MACCEs, and comparisons were assessed using the log-rank test. The values of median (interquartile range) in patients with and without MACCEs were as follows: A. Patients without MACCEs: 0.25 (0.21–0.56), Patients with MACCEs: 0.70 (0.36–0.91). B. Patients without MACCEs: 0.36 (0.24–0.52), Patients with MACCEs: 0.64 (0.57–0.93). ⁎p < 0.05 (Mann Whitney U test).
4. Discussion
The main findings of the present study are as follows: (1) the serial changes in total VEGF-A peaked at day 7 after PCI for AMI, which is in agreement with the findings of previous studies [21,22], whereas the ratio of VEGF-A165b to total VEGF-A changed conversely; (2) total VEGF-A levels tended to be lower and the ratio of VEGF-A165b to total VEGF-A tended to be higher in patients with MACCEs than in those without; and (3) the patients with a high ratio of VEGF-A165b to total VEGF-A at day 30 after PCI were significantly associated with the cumulative incidence of MACCEs after AMI.
Previously, our group reported that low plasma levels of VEGF-A on day 7 after AMI were associated with a significantly increased risk for MACCEs [4]. In contrast, Heeschen et al. showed that an elevation of the serum VEGF level was associated with a higher incidence of death or MI in patients with acute coronary syndrome [23]. The discrepancy in these results highlighted the need for more detailed evaluation of the properties of VEGF-A. Pine et al., reported that evaluated the association among the level of plasma pro- and anti-angiogenic isoform of VEGF-A, and the severity of diabetic retinopathy (DR) in patients with type 2 diabetes. They showed that the difference and imbalance between these VEGF-A isoforms concentrations might be better explanatory markers for predict an adverse prognosis of DR compared with either VEGF isoform [24]. In our study, we did not measure angiogenic VEGF-A165a isoform because it is impossible to obtain an angiogenic VEGF-A165a isoform specific ELISA kit that is accurately evaluated from a commercial product. Hence, we focused on the ratio of VEGF-A165b to total VEGF-A to evaluate the quality of VEGF-A and the association with MACCEs. Because the ratio of VEGF-A165b to total VEGF-A demonstrated significant differences between the patients with MACCEs and those without, the assessment of VEGF-A165b combined with total VEGF-A may be a valuable tool in the evaluation of the quality of VEGF-A.
Moreover, we evaluated the cumulative incidence of MACCEs using the cut-off values of the ratio for MACCEs. The Kaplan-Meier analysis demonstrated significant differences using the cut-off values of the ratio at day 30 after AMI; however, no significant difference was shown using the cut-off value at day 3 after AMI. These results may suggest that a high residual VEGF-A165b in the subacute phase of AMI contributes to the incidence of MACCEs.
Healing after MI involves the accumulation of myeloid cells and the expression of surface glycoprotein lymphocyte antigen 6C (Ly-6C). Pro-inflammatory Ly-6C with high levels of monocytes/macrophages accumulates early (they peak around day 3 after AMI) in the myocardium and promotes the digestion of infarcted tissue and the removal of necrotic debris [25]. Owing to the effects of these factors, it may be difficult to elucidate significant differences using VEGF-A and VEGF-A165b at day 3 after AMI.
It has been proposed that the infarct size after AMI is associated with mortality [26,27]. Recently, Hueso et al. investigated the serial change of circulating VEGF-A165b in 50 patients after AMI, and they showed a negative correlation between the peak levels of VEGF-A165b reached at 24 h after AMI and the infarct size and LVEF assessed by cardiac magnetic resonance (CMR) imaging [17]. Therefore, we evaluated the infarct size using an estimation based on the AUC for CK and LVEF; however, we observed no significant correlation or differences between the patients with and without MACCEs in our study. These discrepancies may have occurred because our sample size was smaller than that study and our blood samples were not taken at 24 h after AMI. Moreover, the accuracy of the evaluation using the AUC for CK and LVEF using echocardiography was lower than that using CMR imaging. For these reasons, it was difficult to show the significant differences about the infarct size in this study.
Although details of the mechanisms linking VEGF-A or VEGF-A165b to MACCEs remain unclear in this study, deleterious effects such as insufficient neovascularization caused by a high residual VEGF-A165b level may inhibit the healing processes in the surviving myocardium and endothelial cells after AMI, and lead to adverse clinical outcomes after AMI.
There were several limitations in the present study. First, this study was conducted in a single center, and the sample size was relatively small. Thus, we were unable to include appropriate multivariate analyses, such as Cox proportional hazard regression analysis. Second, there were 8 (34.8%) samples at day 3 after AMI that included heparin because of the necessity for a continuous infusion of heparin treatment after PCI, which can reduce the serum VEGF level [28]. Finally, because this study enrolled only patients with AMI, we could not compare VEGF-A or VEGF-A165b levels with samples in healthy humans as a control group. Further studies are needed to clarify our results and the precise roles of VEGF-A and VEGF-A165b in patients after AMI.
5. Conclusion
The assessment of VEGF-A165b combined with VEGF-A is a valuable screening tool for predicting adverse clinical outcomes in patients after AMI.
Conflicts of Interest and Source of Funding
This work was supported by a grant-in-aid for Young Scientists B (#26860367) and grants from Uehara Memorial Foundation, Nagoya University Hospital Funding for Clinical Research, and Japan Foundation for Applied Enzymology (R. Kikuchi). Mitsui Life Social Welfare Foundation also supported this work (H. Ishii).
Acknowledgments
We express our sincere appreciation to all the patients, collaborating physicians, and other medical staff.
Contributor Information
Ryosuke Kikuchi, Email: ryosuke-k@med.nagoya-u.ac.jp.
Toyoaki Murohara, Email: murohara@med.nagoya-u.ac.jp.
References
- 1.Ferrara N., Gerber H.P., LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 2.Cochain C., Channon K.M., Silvestre J.S. Angiogenesis in the infarcted myocardium. Antioxid. Redox Signal. 2013;18:1100–1113. doi: 10.1089/ars.2012.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu J., Han X., Qi H., Yin J., Zhang Z. Correlation between vascular endothelial growth factor and long-term prognosis in patients with acute myocardial infarction. Exp. Ther. Med. 2016;12:475–479. doi: 10.3892/etm.2016.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsudaira K., Maeda K., Okumura N. Group NAMISN. Impact of low levels of vascular endothelial growth factor after myocardial infarction on 6-month clinical outcome. Results from the Nagoya Acute Myocardial Infarction Study. Circ. J. 2012;76:1509–1516. doi: 10.1253/circj.cj-11-1127. [DOI] [PubMed] [Google Scholar]
- 5.Neufeld G., Cohen T., Gengrinovitch S., Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 6.Peiris-Pagès M. The role of VEGF 165b in pathophysiology. Cell Adhes. Migr. 2012;6:561–568. doi: 10.4161/cam.22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolard J., Wang W.Y., Bevan H.S. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 8.Manetti M., Guiducci S., Romano E. Overexpression of VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, leads to insufficient angiogenesis in patients with systemic sclerosis. Circ. Res. 2011;109:e14–26. doi: 10.1161/CIRCRESAHA.111.242057. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S., Yoshihisa A., Yokokawa T. Association between levels of anti-angiogenic isoform of vascular endothelial growth factor A and pulmonary hypertension. Int. J. Cardiol. 2016;222:416–420. doi: 10.1016/j.ijcard.2016.07.277. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi R., Nakamura K., MacLauchlan S. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat. Med. 2014;20:1464–1471. doi: 10.1038/nm.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu L.H., Ganta V.C., Choi M.H., Chen G., Finley S.D., Annex B.H. A multiscale computational model predicts distribution of anti-angiogenic isoform VEGF(165b) in peripheral arterial disease in human and mouse. Sci. Rep. 2016;6:37030. doi: 10.1038/srep37030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganta V.C., Choi M., Kutateladze A., Annex B.H. VEGF165b modulates endothelial VEGFR1-STAT3 signaling pathway and angiogenesis in human and experimental peripheral arterial disease. Circ. Res. 2017;120:282–295. doi: 10.1161/CIRCRESAHA.116.309516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hueso L., Rios-Navarro C., Ruiz-Sauri A. Dynamics and implications of circulating anti-angiogenic VEGF-A165b isoform in patients with ST-elevation myocardial infarction. Sci. Rep. 2017;7:9962. doi: 10.1038/s41598-017-10505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latorre E., Pilling L.C., Lee B.P. The VEGFA156b isoform is dysregulated in senescent endothelial cells and may be associated with prevalent and incident coronary heart disease. Clin. Sci. (Lond.) 2018;132:313–325. doi: 10.1042/CS20171556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thygesen K., Alpert J.S., Jaffe A.S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 20.Dissmann R., Linderer T., Schröder R. Estimation of enzymatic infarct size: direct comparison of the marker enzymes creatine kinase and alpha-hydroxybutyrate dehydrogenase. Am. Heart J. 1998;135:1–9. doi: 10.1016/s0002-8703(98)70335-7. [DOI] [PubMed] [Google Scholar]
- 21.Soeki T., Tamura Y., Shinohara H., Sakabe K., Onose Y., Fukuda N. Serum hepatocyte growth factor predicts ventricular remodeling following myocardial infarction. Circ. J. 2002;66:1003–1007. doi: 10.1253/circj.66.1003. [DOI] [PubMed] [Google Scholar]
- 22.Kranz A., Rau C., Kochs M., Waltenberger J. Elevation of vascular endothelial growth factor—a serum levels following acute myocardial infarction. Evidence for its origin and functional significance. J. Mol. Cell. Cardiol. 2000;32:65–72. doi: 10.1006/jmcc.1999.1062. [DOI] [PubMed] [Google Scholar]
- 23.Heeschen C., Dimmeler S., Hamm C.W., Boersma E., Zeiher A.M., Simoons M.L. Investigators CcEA-PTiURa. Prognostic significance of angiogenic growth factor serum levels in patients with acute coronary syndromes. Circulation. 2003;107:524–530. doi: 10.1161/01.cir.0000048183.37648.1a. [DOI] [PubMed] [Google Scholar]
- 24.Paine S.K., Mondal L.K., Borah P.K., Bhattacharya C.K., Mahanta J. Pro- and antiangiogenic VEGF and its receptor status for the severity of diabetic retinopathy. Mol. Vis. 2017;23:356–363. [PMC free article] [PubMed] [Google Scholar]
- 25.Nahrendorf M., Pittet M.J., Swirski F.K. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schömig A., Kastrati A., Dirschinger J. Coronary stenting plus platelet glycoprotein IIb/IIIa blockade compared with tissue plasminogen activator in acute myocardial infarction. Stent versus thrombolysis for occluded coronary arteries in patients with acute myocardial infarction study investigators. N. Engl. J. Med. 2000;343:385–391. doi: 10.1056/NEJM200008103430602. [DOI] [PubMed] [Google Scholar]
- 27.Keeley E.C., Boura J.A., Grines C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 28.Tamura K., Nakajima H., Rakue H. Elevated circulating levels of basic fibroblast growth factor and vascular endothelial growth factor in patients with acute myocardial infarction. Jpn. Circ. J. 1999;63:357–361. doi: 10.1253/jcj.63.357. [DOI] [PubMed] [Google Scholar]