Abstract
Background
Whether an individually determined appropriate level of cardiac rehabilitation (CR) has a favorable effect on the renal function still remains unclarified. The aim of this study was to confirm the effect of CR on the estimated glomerular filtration rate (eGFR) using cystatin C, which is known to be unaffected by physical exercise.
Methods
The study population was comprised of 86 patients (61 males; average age 74 y/o) with a lower-moderate level of chronic kidney disease (CKD) who was admitted to our hospital for treatment of cardiovascular disease (CVD) and who participated in our 3-month CR program. The exercise capacity was assessed by cardiopulmonary exercise testing (CPX) and the eGFR was measured by a formula based on the serum cystatin C concentration (eGFRcys) in each patient both at the beginning and end of the CR.
Results
In the CVD patients with CKD, both the peak oxygen uptake (VO2) and peak work rate (WR) improved significantly after CR (15.0 ± 3 to 15.8 ± 3 ml/min/kg, p = 0.002. 65.5 ± 21 to 70.2 ± 25 W, p = 0.001). Regarding the renal function, the eGFRcys improved (45.2 ± 11 to 47.3 ± 13 ml/min/1.73 m2, p = 0.023), however, the eGFR assessed by the serum creatinine (eGFRcr) did not improve after CR (45.1 ± 12 to 44.9 ± 13 ml/min/1.73 m2, p = 0.834).
Conclusions
In CVD patients, a novel CR program significantly improved the exercise capacity. Further, CR was shown to have a favorable effect on the renal function when it was estimated by the eGFRcys.
Keywords: Cardiac rehabilitation, Chronic kidney disease, Cardiovascular disease, Cystatin C, Estimated glomerular filtration rate, Exercise capacity
Highlights
-
•
Cardiac rehabilitation improved the renal function using serum cystatin C.
-
•
Cardiac rehabilitation didn't change the renal function using serum creatinine.
-
•
Cardiac rehabilitation is most effective on moderate to severe CKD patients.
1. Introduction
Strenuous exercise has been shown to possess unfavorable effects on the renal function and can be attributed to the reduction in the renal blood flow and glomerular filtration rate (GFR) [[1], [2], [3]]. For that reason, patients with chronic kidney disease (CKD) are usually advised to avoid any excessive exercise. On the other hand, comprehensive cardiac rehabilitation (CR), including an appropriate level of exercise training, has been shown to improve a variety of coronary risk factors, exercise capacity, quality of life [4] and even the prognosis [5]. In patients with CKD, exercise training has been proved to improve the coronary risk factors [6] and exercise capacity [7]. However, the influence of CR on the renal function still remains controversial, i.e., some investigators have demonstrated no significant difference in the renal function before and after CR [8,9], while CR was found to be associated with an obvious improvement in the renal function in some other reports [[10], [11], [12]].
In clinical practice, the GFR is commonly calculated using a formula mainly constructed using the serum creatinine concentration (eGFRcr) and age. The eGFRcr is inferred to be affected by exercise training due to muscle growth, and thus the eGFRcr may underestimate the actual renal function particularly in the patients undergoing chronic exercise training.
Recently, attention has been directed to the observation that the eGFR, based on the serum concentration of cystatin-C (eGFRcys), may overcome the aforementioned shortcoming of the eGFRcr. Cystatin-C is a single chain protein produced by all nucleated human cells and is suggested to be superior to creatinine in estimating the GFR [13].
Consequently, the aim of the present study was to evaluate the effects of the CR on the renal function using the eGFRcys in patients with both cardiovascular disease (CVD) and CKD. Another aim was to compare the change in the eGFRcys and eGFRcr in the same patient population. The results of this study may suggest the possibility of the CR improving the renal function in these patient populations that can be adequately estimated using the eGFRcys.
2. Methods
2.1. Study population
There were 240 patients who were admitted to our hospital for the evaluation and treatment of CVD and participated in and completed our 3-month CR program from April 2014 until March 2016. Out of them, we excluded 7 patients who were dependent on hemodialysis at the entry of this study, and 147 with a normal kidney function defined as an eGFRcys ≧ 60 ml/min/1.73 cm2. Finally the study population was comprised of 86 patients with a mild-moderate level (G3-G4) of CKD (mean age: 74 ± 9 years, 61 male patients) (Fig. 1). We traced the patients' medical records retrospectively. This study has been approved by the research ethics committee of Tokai University.
Fig. 1.
Enrollment.
2.2. CR program
Our CR program in this study was a comprehensive program consisting of exercise therapy, nutritional guidance, medication teaching, and attending a course on cardiopulmonary resuscitation. In the exercise therapy, our physiotherapists taught the patients an individual way to do aerobic exercises with walking or bicycle ergometer exercise and resistance training for 40 min per lesson. The exercise intensity was determined individually at a heart rate at the anaerobic threshold level obtained in a symptom-limited cardiopulmonary exercise test or at a level from 11 to 13 of the 6–20 scale training rating (original Borg's scale [14,15]). They were also encouraged to walk at a prescribed heart rate or do resistance training for 30–60 min, 3–7 times a week at home.
2.3. Cardiopulmonary exercise test
At the beginning and end of our 3-month CR program, a symptom limited exercise test was performed on a bicycle ergometer (Corival, Lode Co., Netherlands), which was coupled to a cardiopulmonary gas exchange system (Aero Monitor AE-310S, Minato medical science Co., Japan). The blood pressure was measured every minute and the 12-lead ECG was continuously monitored. The exercise protocol consisted of first a 4-minute resting period, followed by a 3-minute 10 or 20 watt warm up that was increased by 10 or 20 W per minute until one of the following occurred: (1) the patient requested to stop, (2) a plateau of the oxygen uptake, (3) an ischemic change in the electrocardiogram or symptoms, (4) an excessive rise or fall in the blood pressure, or (5) an onset of fatal arrhythmias. We measured the anaerobic threshold for an appropriate prescription of the exercise therapy, peak oxygen uptake (VO2), and peak work rate (WR) as an index of their exercise capacity. The slope of the relationship between the ventilation (VE) and VCO2 until the respiratory compensation point (VE/VCO2 slope) was determined as an index of respiratory inefficiency. The peak respiratory exchange ratio (RER: ratio of carbon dioxide output and oxygen uptake at peak exercise) was used as a measure of the patient effort during the testing, and a value >1.05 was considered to be sufficient.
2.4. Kidney function
We measured the renal function by the serum creatinine (Scr, mg/dl) and a serum cystatin C (Scys, mg/l) levels. In this study, the eGFR was evaluated by the Japanese version of the equation: eGFRcr [ml/min/1.73 m2] = 194 × Cr [mg/dl]−1.094 × age [y.o.]−0.287 (×0.739, if female), eGFRcys [ml/min/1.73 m2] in male = (104 × CysC [mg/l]−1.019 × 0.996age [y.o.]) − 8, in female = (104 × CysC [mg/l]−1.019 × 0.996age [y.o.] × 0.929) − 8.
2.5. Statistical analysis
Data are presented as the mean ± standard deviation or number (%). Data were analyzed for statistical significance using a Student's t-test for paired observations. All tests were assessed at a level of significance of a p value of <0.05. Statistical analyses were performed by SPSS, version16.0 software (SPSS Inc., Chicago, IL).
3. Results
3.1. Clinical characteristics of the participants
The demographic and clinical data are shown in Table 1. The mean age was 74 ± 9 years and approximately 71% of the patients were males. Basic heart disease consisted of angina pectoris in 31, myocardial infarctions in 22, silent myocardial ischemia in 15, non-ischemic heart failure in 18 and post-cardiac surgery in 30 patients, respectively. The patients had several coronary risk factors. More than a half of the patients were current smokers and/or had a history of smoking. Hypertension and dyslipidemia were also present in the majority of the patients.
Table 1.
Baseline characteristics. Data are expressed as the mean ± standard deviation or number (%).
| Total (N = 86) | |
|---|---|
| Age (years) | 74 ± 9 |
| Sex (M/F) | 61/25 |
| BMI (kg/m2) | 22.3 ± 3 |
| Angina pectoris | 31 (36%) |
| Myocardial infarction | 22 (26%) |
| Silent myocardial ischemia | 15 (17%) |
| Non-ischemic heart failure | 18 (21%) |
| Post-cardiac surgery | 30 (35%) |
| Coronary risk factor | |
| Smoking | 50 (58%) |
| Hypertension | 54 (63%) |
| Diabetes mellitus | 33 (38%) |
| Dyslipidemia | 52 (60%) |
| Family history | 16 (19%) |
| Medications | |
| ACE-I/ARB | 44 (51%) |
| Βeta-blocker | 58 (67%) |
| Diuretics | 63 (73%) |
| Calcium channel blocker | 25 (29%) |
| Inotropic agents | 0 (0%) |
| Blood exam | |
| Triglyceride (casual) (mg/dl) | 132 ± 55 |
| LDL-Chol (mg/dl) | 95 ± 32 |
| HDL-Chol (mg/dl) | 52 ± 15 |
| Blood glucose (casual) (mg/dl) | 130 ± 41 |
| HbA1c (%) | 6.2 ± 0.8 |
| BNP (pg/ml) | 224 ± 303 |
| Hb (g/dl) | 12.3 ± 1.7 |
| Scr (mg/dl) | 1.2 ± 0.4 |
| eGFRcr (ml/min/1.73 m2) | 45.1 ± 12 |
| Scys (mg/l) | 1.5 ± 0.4 |
| eGFRcys (ml/min/1.73 m2) | 45.2 ± 11 |
| CRP (mg/dl) | 0.32 ± 0.55 |
| CKD severity with eGFRcys | |
| G3a | 51 (59%) |
| G3b | 24 (28%) |
| G4 | 11 (13%) |
| Echocardiography | |
| LVDd (mm) | 49 ± 8 |
| LVEF (%) | 50.8 ± 13 |
| LVEF < 40 (%) | 19 (22%) |
| 40 ≦ LVEF < 60 (%) | 41 (48%) |
| LVEF ≧ 60 (%) | 26 (30%) |
| E/e′ | 12.5 ± 5 |
| E/e′ < 8 | 13 (15%) |
| 8 ≦ E/e′ ≦ 14 | 50 (58%) |
| E/e′ > 14 | 23 (27%) |
| MR ≧ 3°/4 | 0 (0%) |
| CPX at the beginning of CR | |
| Pre exercise | |
| Heart rate (bpm) | 74 ± 15 |
| Systolic BP (mm Hg) | 132 ± 26 |
| Peak exercise | |
| Heart rate (bpm) | 117 ± 23 |
| Systolic BP (mm Hg) | 169 ± 30 |
| AT (ml/min/kg) | 10.6 ± 2 |
| Peak VO2 (ml/min/kg) | 15.0 ± 3 |
| Peak WR (watt) | 65.5 ± 21 |
| VE/VCO2 slope | 38.9 ± 7 |
| Peak RER | 1.2 ± 0.1 |
| Amount of exercise during CR at outpatient visit | |
| Session attendance (times/3 months) | 6.6 |
Abbreviations: Scr, serum creatinine concentration; eGFRcr, estimated glomerular filtration rate based on Scr; Scys, serum concentration of cystatin-C; eGFRcys, eGFR based on Scys; LVDd, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; E/e′, the ratio of the mitral peak velocity during early filling (E) to the early diastolic mitral annular velocity (e′); AT, anaerobic threshold; Peak VO2, peak oxygen uptake; Peak WR, peak work rate; VE/VCO2 slope, The slope of the relationship between the ventilation (VE) and VCO2 up until the respiratory compensation point; Peak RER, ratio of the carbon dioxide output and oxygen uptake at peak exercise.
More than half of the patients took renin-angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers), diuretics, and beta blockers. No one took inotropic agents (Table 1). They were also able to maintain a good coronary risk control (serum triglycerides, cholesterol, and HbA1c) at the beginning of the CR (Table 1). With regards to the renal function, 51 patients (59%) had a grade G3a (mild-moderate grade of CKD; 45 ≦ eGFRcys < 60), 24 (28%) a grade G3b (moderate-severe grade of CKD; 30 ≦ eGFRcys < 45), and 11 patients (13%) had a grade G4 (severe grade of CKD): 15 ≦ eGFRcys < 30, respectively (Table 1). Further, most patients had a mildly reduced LV systolic function (LVEF < 40%: 19 pts, 40 < LVEF < 60%: 41 pts, and LVEF ≧ 60%: 26 pts) and diastolic function (E/e′ < 8: 13 pts, 8 ≦ E/e′ ≦ 14: 50 pts, and E/e′ > 14: 23pts) as shown in Table 1.
3.2. Exercise capacity
Most of the patients had a mild reduced exercise capacity and respiratory inefficiency detected by the cardiopulmonary exercise test at the beginning of the CR. Then, they also underwent an adequate inspection because of a peak RER (Table 1).
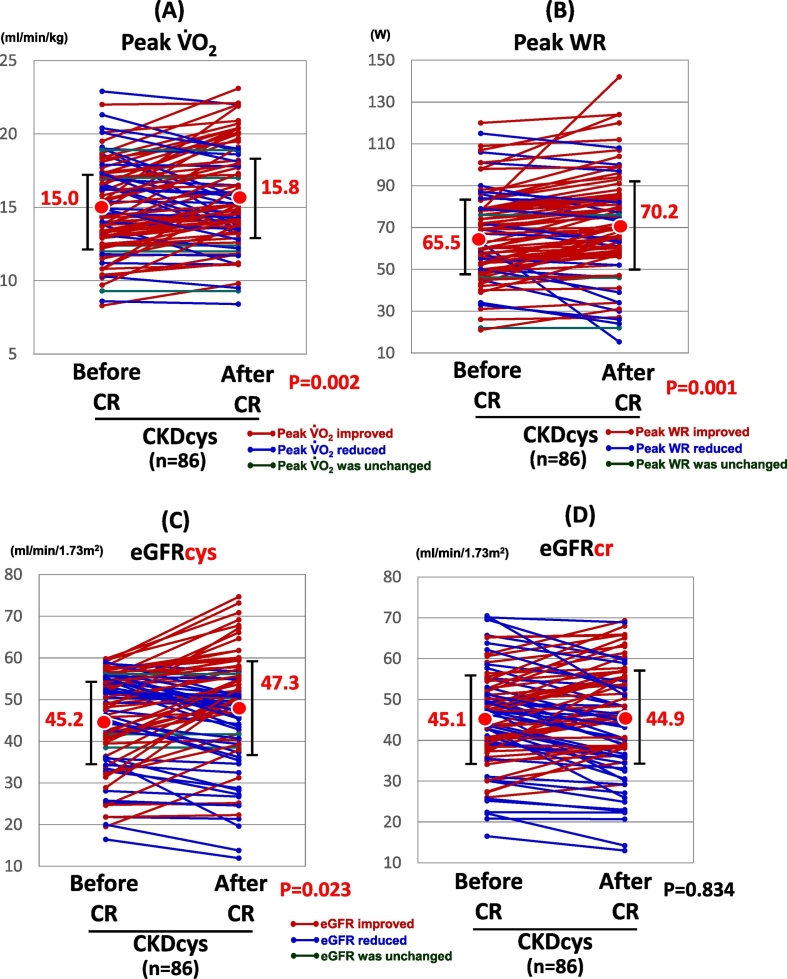
Our 3-month CR program improved their peak oxygen uptake (peak VO2), from 15.0 ml/min/kg at baseline to 15.8 ml/min/kg at the end of the study (p = 0.002, Fig. 2A). Further, the peak work rate (WR) also increased from 65.5 W at baseline to 70.2 W at the end of the study (p = 0.001, Fig. 2B).
Fig. 2.
The change in the exercise capacity and renal function after CR.
Abbreviations: CKDcys, CKD as defined with the aid of the eGFRcys.
3.3. Renal function parameters
The temporal course of the renal function in 86 CVD patients using cystatin C and creatinine, is comparatively shown in Fig. 2C and D. The eGFRcys improved after the comprehensive CR (45.2 vs. 47.3 ml/min/1.73 m2, p = 0.023), while there was no significant change in the eGFRcr after the CR (45.1 vs. 44.9 ml/min/1.73 m2, p = 0.834). eGFRcys was improved in 44 patients, while it was aggravated in 38 patients. In the remaining 4 patients, it did not change after the CR.
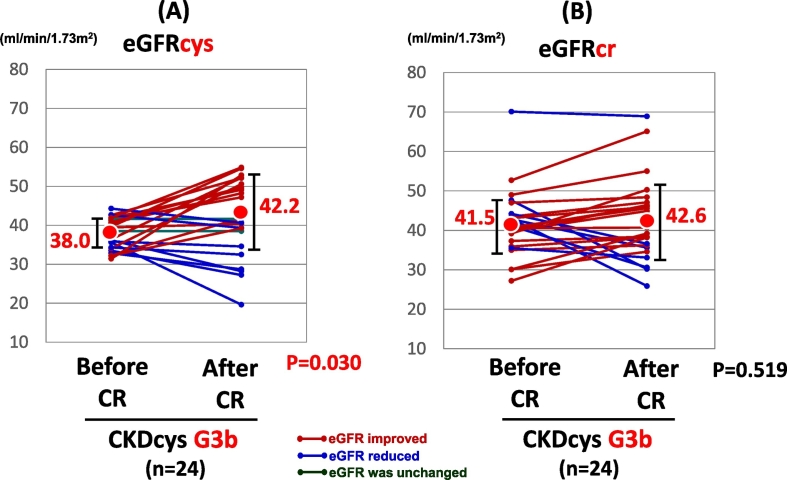
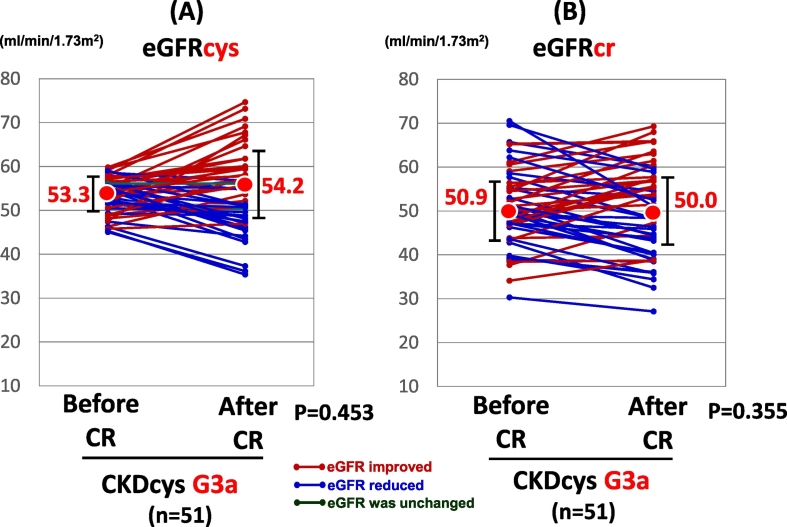
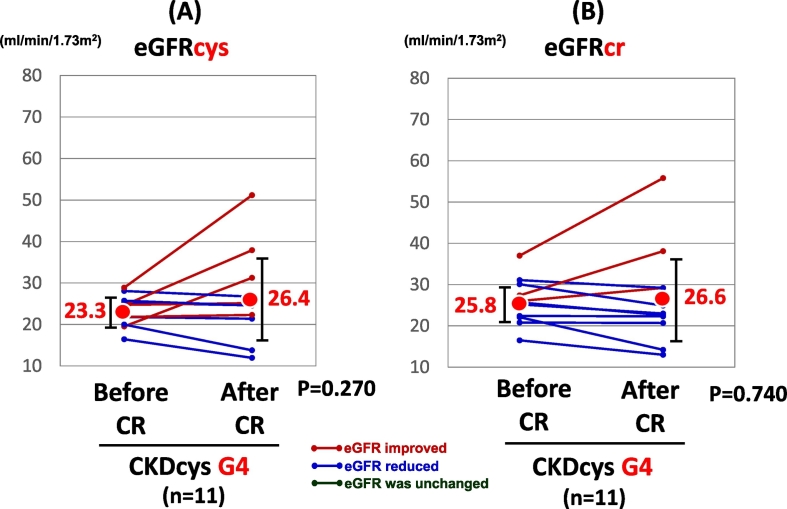
When the patients were divided into 3 groups according to the grade of renal dysfunction, i.e., G3a, G3b, and G4, there was no significant change in the CKDcys and CKDcr after the CR in the patients with G3a or G4 grade renal dysfunction. In CKD G3a group, eGFRcys was improved in 25 patients, while it was reduced in 24 patients. In the remaining 2 patients, it did not change after the CR. In CKD G4 groups, eGFRcys was improved in 5 patients, while it was aggravated in 6 patients after the CR. On the other hand, the eGFRcys exhibited a significant increase in the patients with a G3b CKD grade at the end of the CR (38.0 vs. 42.2 ml/min/1.73 m2, p = 0.030, Fig. 3A), even though the eGFRcr did not change significantly (41.5 vs. 42.6 ml/min/1.73 m2, p = 0.519, Fig. 3B). eGFRcys was improved in 14 patients, while it was reduced in 8 patients. In the remaining 2 patients, it did not change after the CR.
Fig. 3.
The change in the renal function of CKD G3b group after CR.
Abbreviations: CKDcys, CKD as defined with the aid of the eGFRcys.
4. Discussion
The main findings of the present study were as follows: (1) the exercise capacity in the CVD patients with CKD improved by the end of the CR, (2) the eGFRcys in the CVD patients with CKD also increased by the end of the CR, and (3) in the subgroup analysis of the grade of CKD, the eGFRcys significantly increased in the patients with G3b CKD, whereas it did not change in those with either G3a or G4.
To the best of our knowledge, this is the first study to clearly demonstrate that the kidney function assessed by Scys improved in CVD patients with CKD after CR. Several previous studies have reported that exercise training did not worsen the renal function [8,9], but did not improve it either, with exercise in the CVD patients with CKD. One of the reasons for the discrepancy in those results compared to our data might be the use of the serum creatinine in the evaluation of the renal function in the previous studies.
The serum creatinine is the most common marker of the renal function [16]. However, the serum creatinine is influenced by the age, sex, BMI and eating cooked meat. Because creatine is converted into creatinine in the muscles [[17], [18], [19]]. Resistance training, a part of comprehensive cardiac rehabilitation, is likely to induce muscle growth resulting in an increase in the muscle mass [20,21]. For that reason, physical training may increase the serum creatinine level at the end of the CR, and thus the eGFR may consequently decrease when it is assessed using the serum creatinine.
On the other hand, the most useful marker for the evaluation of the renal function during the CR is the ‘serum cystatin C’. There is almost no relationship between the muscle mass and serum cystatin C because cystatin C is produced by all investigated nucleated cells in humans [[22], [23], [24]]. Therefore, it is recommended that the serum cystatin C should be alternatively used for the evaluation of the change in the eGFR due to exercise training to eliminate the influence of the change in the amount of the muscle mass.
Strenuous exercise has previously been considered to have a deteriorative effect on the renal function [[1], [2], [3]]. When we exercise, an increased body temperature may be one of the causes of transient renal dysfunction, since a fever produces albuminuria. Moreover, renal vasoconstriction during exercise, which is characterized by an increased filtration fraction, suggests a relatively more marked constriction of the efferent glomerular arterioli, resulting in an increased filtration pressure. Further, severe exercise leads to an increased glomerular permeability and partial tubular reabsorption inhibition to proteins [25,26]. However, no investigators have shown any adverse effects of symptom limited exercise training on the long-term renal function in CKD patients.
Although the mechanisms underlying the improvement in the renal function remain unclear, there have been several previous suggestions regarding this. Deterioration of the renal function is affected by hypertension, hyperlipidemia, and diabetes mellitus. One study reported that normalizing the blood pressure leads to a decrease in the proteinuria of CRF patients [27]. Some other studies suggested that hyperlipidemia might play a role in the renal functional deterioration and an increase in high-density lipoprotein cholesterol (HDL) causes an improvement in the renal function [28,29]. With regard to diabetes mellitus, one report indicated that an improvement in the insulin sensitivity was an independent predictor of an increased eGFR [30]. A comprehensive CR program is widely known to improve the coronary risk factors such as hypertension, hyperlipidemia, and diabetes mellitus. Therefore, because CR gives rise to favorable changes in the coronary risk factors, it is inferred to be a potent mechanism of an improvement in the kidney function.
In a sub-analysis of the CKD severity classification, an improvement in the eGFRcys was observed only in CVD patients with CKD G3b, which corresponds to the moderate to severe CKD, but it was not the case in patients with a G3a or G4 grade. In the CKD G3a patients, they might have had little space for a beneficial change in the eGFRcys with CR because there was a mild reduction in the kidney function at the beginning of the CR. In CKD G4 patients, it might be difficult to improve the renal dysfunction because almost all those patients had multiple coronary risk factors and accumulated irreversible renal damage.
The most important finding in this study was that the comprehensive CR program might improve both the exercise capacity and renal function in CVD patients with a moderate to severe level of CKD. We still tend to avoid exercise training in this category of patients, because of the obsolete knowledge that exercise may worsen the renal function. It could be recommended that an adequate level of exercise (symptom-limited) could be applied safely and effectively even in patients with CVD with moderate to severe CKD, however, the influence on the long-term prognosis remains unclarified.
4.1. Limitations
There were several limitations to the present study. First, this study had a small sample size, especially of the severe CKD patients. Further studies are needed to investigate the effect of exercise training on the renal function in severe CKD patients. Second, the present study did not have a control group that did not participate in the CR. Third, we did not have long-term follow up data. Therefore, the longitudinal effect of the CR on the renal function and prognosis remains unknown.
5. Conclusions
In conclusion, in the CVD patients with moderate to severe CKD, the CR program resulted in a significant improvement in both the exercise capacity and renal function. Further, a measurement of the serum cystatin C may be useful for evaluating the renal function in the CVD patients with CKD.
Acknowledgments
Acknowledgement
The authors express their special gratitude to Mr. John Martin for his linguistic assistance.
Declaration of conflicting interests
Dr. Kobayashi reports personal fees from Fukuda Denshi CC, Kowa Co. Ltd., Boehringer Ingelheim Japan, Medtronic Japan, Daiichi-Sankyo CC, and Pfizer outside the submitted work. Dr. Ikari reports personal fees from the TERUMO CORPORATION, AstraZeneca K.K., Abbott Vascular, Otsuka Pharmaceutical Co, Ltd., and Sanofi K.K., grants and personal fees from Daiichi Sankyo Co., Ltd., Bayer Yakuhin, Ltd., and grants from St. Jude Medical Japan, Co. Ltd., and Eisai Co. Ltd. outside the submitted work. The other authors have nothing to disclose.
Footnotes
These authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Appendix A
Fig. A.1.
The change in the renal function of CKD G3a group after CR.
Abbreviations: CKDcys, CKD as defined with the aid of the eGFRcys.
Fig. A.2.
The change in the renal function of CKD G4 group after CR.
Abbreviations: CKDcys, CKD as defined with the aid of the eGFRcys.
Table A.1.
Baseline characteristics of CKD subgroups.
| CKD G3a (N = 51) | CKD G3b (N = 24) | CKD G4 (N = 11) | |
|---|---|---|---|
| Age (years) | 73 ± 9 | 77 ± 7 | 77 ± 7 |
| Sex (M/F) | 35/16 | 16/8 | 10/1 |
| BMI (kg/m2) | 22.3 ± 3 | 22.8 ± 3 | 21.3 ± 2 |
| Angina pectoris | 18 (35%) | 10 (42%) | 3 (27%) |
| Myocardial infarction | 16 (31%) | 4 (17%) | 2 (18%) |
| Silent myocardial ischemia | 9 (18%) | 4 (17%) | 2 (18%) |
| Non-ischemic heart failure | 11 (22%) | 5 (21%) | 2 (18%) |
| Post-cardiac surgery | 16 (31%) | 8 (33%) | 6 (55%) |
| Coronary risk factor | |||
| Smoking | 29 (57%) | 12 (50%) | 9 (82%) |
| Hypertension | 29 (57%) | 16 (67%) | 9 (82%) |
| Diabetes mellitus | 16 (31%) | 12 (50%) | 5 (45%) |
| Dyslipidemia | 29 (57%) | 16 (67%) | 7 (64%) |
| Family history | 11 (22%) | 4 (17%) | 1 (9%) |
| Medications | |||
| ACE-I/ARB | 24 (47%) | 15 (63%) | 5 (45%) |
| Βeta-blocker | 35 (69%) | 13 (54%) | 10 (91%) |
| Diuretics | 34 (67%) | 20 (83%) | 9 (82%) |
| Calcium channel blocker | 11 (22%) | 9 (38%) | 5 (45%) |
| Inotropic agents | 0 (0%) | 0 (0%) | 0 (0%) |
| Blood exam | |||
| Triglyceride (casual) (mg/dl) | 133 ± 57 | 133 ± 53 | 124 ± 50 |
| LDL-Chol (mg/dl) | 93 ± 33 | 98 ± 29 | 99 ± 32 |
| HDL-Chol (mg/dl) | 54 ± 16 | 46 ± 12 | 55 ± 14 |
| Blood glucose (casual) (mg/dl) | 128 ± 38 | 128 ± 41 | 148 ± 75 |
| HbA1c (%) | 6.2 ± 0.8 | 6.2 ± 0.6 | 6.0 ± 0.8 |
| BNP (pg/ml)a | 215 ± 247 | 137 ± 119 | 455 ± 579 |
| Hb (g/dl)b | 12.8 ± 1.6 | 12.1 ± 1.4 | 10.7 ± 1.7 |
| Scr (mg/dl)c | 1.0 ± 0.2 | 1.2 ± 0.2 | 2.0 ± 0.3 |
| eGFRcr (ml/min/1.73 m2)c | 50.9 ± 8.9 | 41.5 ± 8.4 | 25.8 ± 5.3 |
| Scys (mg/l)c | 1.2 ± 0.1 | 1.6 ± 0.1 | 2.4 ± 0.3 |
| eGFRcys (ml/min/1.73 m2)c | 53.3 ± 4.0 | 38.0 ± 3.9 | 23.3 ± 3.6 |
| CRP (mg/dl)d | 0.19 ± 0.20 | 0.43 ± 0.66 | 0.67 ± 0.98 |
| Echocardiography | |||
| LVDd (mm) | 51 ± 8 | 47 ± 6 | 49 ± 8 |
| LVEF (%) | 50 ± 13 | 54 ± 11 | 49 ± 14 |
| LVEF < 40 (%) | 13 (25%) | 2 (8%) | 4 (36%) |
| 40 ≦ LVEF < 60 (%) | 25 (49%) | 13 (54%) | 3 (27%) |
| LVEF ≧ 60 (%) | 13 (25%) | 9 (38%) | 4 (36%) |
| E/e′e | 11.8 ± 4 | 12.1 ± 4 | 16.4 ± 8 |
| E/e′ < 8 | 8 (16%) | 4 (17%) | 1 (9%) |
| 8 ≦ E/e′ ≦ 14 | 31 (61%) | 13 (54%) | 4 (36%) |
| E/e′ > 14 | 12 (24%) | 7 (29%) | 6 (55%) |
| MR ≧ 3°/4 | 0 (0%) | 0 (0%) | 0 (0%) |
| CPX at the beginning of CR | |||
| Pre exercise | |||
| Heart rate (bpm) | 75 ± 16 | 77 ± 12 | 66 ± 14 |
| Systolic BP (mm Hg) | 131 ± 29 | 127 ± 18 | 145 ± 20 |
| Peak exercise | |||
| Heart rate (bpm) | 122 ± 25 | 115 ± 17 | 103 ± 24 |
| Systolic BP (mm Hg) | 171 ± 31 | 166 ± 32 | 171 ± 17 |
| AT (ml/min/kg) | 10.7 ± 2 | 10.7 ± 2 | 9.8 ± 2 |
| Peak VO2 (ml/min/kg)f | 15.7 ± 3 | 14.0 ± 3 | 13.9 ± 3 |
| Peak WR (watt) | 68.5 ± 23 | 61.6 ± 17 | 59.9 ± 17 |
| VE/VCO2 slope | 38.4 ± 7 | 38.7 ± 6 | 41.3 ± 5 |
| Peak RER | 1.17 ± 0.1 | 1.19 ± 0.1 | 1.22 ± 0.1 |
| Amount of exercise during CR at outpatient visit | |||
| Session attendance (times/3 months) | 6.2 | 7.5 | 6.5 |
CKD G3a vs CKD G4, CKD G3b vs CKD G4, p = 0.014.
CKD G3a vs CKD G4, CKD G3b vs CKD G4, p = 0.000.
CKD G3a vs CKD G3b vs CKD G4, p = 0.000.
CKD G3a vs CKD G4, p = 0.014.
CKD G3a vs CKD G4, CKD G3b vs CKD G4, p = 0.020.
CKD G3a vs CKD G3b, p = 0.036.
References
- 1.Poortmans J.R., Mathieu N., Plaen P.D. Influence of running different distances on renal glomerular and tubular impairment in humans. Eur. J. Appl. Physiol. 1996;72:522–527. doi: 10.1007/BF00242285. [DOI] [PubMed] [Google Scholar]
- 2.Clorius J.H., Mandelbaum A., Hupp T., Reinbold F., Zuna I., Denk S. Exercise activates renal dysfunction in hypertension. Am. J. Hypertens. 1996;9:653–661. doi: 10.1016/0895-7061(96)00036-2. [DOI] [PubMed] [Google Scholar]
- 3.Castenfors J. Renal function during prolonged exercise. Ann. N. Y. Acad. Sci. 1977;301:151–159. doi: 10.1111/j.1749-6632.1977.tb38194.x. [DOI] [PubMed] [Google Scholar]
- 4.Belardinelli R., Paolini I., Cianci G., Piva R., Georgiou D., Purcaro A. Exercise training intervention after coronary angioplasty: the ETICA trial. J. Am. Coll. Cardiol. 2001;37:1891–1900. doi: 10.1016/s0735-1097(01)01236-0. [DOI] [PubMed] [Google Scholar]
- 5.Piepoli M.F., Davos C., Francis D.P., Coats A.J. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;24 doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkataraman R., Sanderson B., Bitter V. Outcomes in patients with chronic kidney disease undergoing cardiac rehabilitation. Am. Heart J. 2005;150:1140–1146. doi: 10.1016/j.ahj.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 7.Eidemak I., Haaber A.B., Rasmussen B.F., Kanstrup I.-L., Strandgaard S. Exercise training and the progression of chronic renal failure. Nephron. 1997;75:36–40. doi: 10.1159/000189497. [DOI] [PubMed] [Google Scholar]
- 8.Kohzuki M., Kamimoto M., Wu X.-M., Xu H.-L., Kawamura T., Mori N. Renal protective effects of chronic exercise and antihypertensive therapy in hypertensive rats with chronic renal failure. J. Hypertens. 2001;19:1877–1882. doi: 10.1097/00004872-200110000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Clyne N., Ekholm J., Jogestrand T., Lins L.-E., Pehrsson S.K. Effects of exercise training in predialytic uremic patients. Nephron. 1991;59:84–89. doi: 10.1159/000186524. [DOI] [PubMed] [Google Scholar]
- 10.Takaya Y., Kumasaka R., Arakawa T., Ohara T., Nakanishi M., Noguchi T. Impact of cardiac rehabilitation on renal function in patients with and without chronic kidney disease after acute myocardial infarction. Circ. J. 2014;78:377–384. doi: 10.1253/circj.cj-13-0779. [DOI] [PubMed] [Google Scholar]
- 11.Greenwood S.A., Koufaki P., Mercer T.H., MacLaughlin H.L., Rush R., Lindup H. Effect of exercise training on estimated GFR, vascular health, and cardiorespiratory fitness in patients with CKD: a pilot randomized controlled trial. Am. J. Kidney Dis. 2015;65:425–434. doi: 10.1053/j.ajkd.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Toyama K., Sugiyama S., Oka H., Sumida H., Ogawa H. Exercise therapy correlates with improving renal function through modifying lipid metabolism in patients with cardiovascular disease and chronic kidney disease. J. Cardiol. 2010;56:142–146. doi: 10.1016/j.jjcc.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Gastro S., Fusco S., Corica F., Rosignuolo M., Marino A., Montesanto A. Estimating glomerular filtration rate in older people. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/916542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borg G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 15.Scherr J., Wolfarth B., Christle J.W., Pressler A., Wagenpfeil S., Halle M. Associations between Borg's rating of perceived exertion and physiological measures of exercise intensity. Eur. J. Appl. Physiol. 2013;113:147–155. doi: 10.1007/s00421-012-2421-x. [DOI] [PubMed] [Google Scholar]
- 16.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann. Intern. Med. 1999;16:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Verhave J.C., Fesler P., Ribstein J., du Cailar G., Mimran A. Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am. J. Kidney Dis. 2005;46:233–241. doi: 10.1053/j.ajkd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Baxmann A.C., Ahmed M.S., Marques N.C., Menon V.B., Pereira A.B., Kirsztajn G.M. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin. J. Am. Soc. Nephrol. 2008;3:348–354. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen F.K., Christensen C.K., Moqensen C.E., Andreasen F., Heiskov N.S. Pronounced in serum creatinine concentration after eating cooked meat. Br. Med. J. 1979;21:1049–1050. doi: 10.1136/bmj.1.6170.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams M.A., Haskell W.L., Ades P.A., Amsterdam E.A., Bittner V., Franklin B.A. Resistance exercise in individuals with and without cardiovascular disease: 2007 update. Circulation. 2007;116:572–584. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 21.Hunter G.R., McCarthy J.P., Bamman M.M. Effects of resistance training on older adults. Sports Med. 2004;34:329–348. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- 22.Simonsen O., Grubb A., Thysell H. The blood serum concentration of cystatin C (γ-trace) as a measure of the glomerular filtration rate. Scand. J. Clin. Lab. Invest. 1985;45:97–101. doi: 10.3109/00365518509160980. [DOI] [PubMed] [Google Scholar]
- 23.Grubb A., Simonsen O., Sturfelt G., Truedsson L., Thysell H. Serum concentration of cystatin C, factor D and β2-microglobulin as a measure of glomerular filtration rate. Acta Med. Scand. 1985;218:499–503. doi: 10.1111/j.0954-6820.1985.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 24.Tian S., Kusano E., Ohara T., Tabei K., Itou Y., Kawai T. Cystatin C measurement and its practical use in patients with various renal diseases. Clin. Nephrol. 1997;48:104–108. [PubMed] [Google Scholar]
- 25.Poortmans J.R., Brauman H., Staroukine M., Verniory A., Decaestecker C., Leclercq R. Indirect evidence of glomerular/tubular mixed-type postexercise proteinuria in healthy humans. Am. J. Phys. 1988;254:277–283. doi: 10.1152/ajprenal.1988.254.2.F277. [DOI] [PubMed] [Google Scholar]
- 26.Bergamaschi C.T., Boim M.A., Moura L.A., Picarro I.C., Schor N. Effects of long-term training on the progression of chronic renal failure in rats. Med. Sci. Sports Exerc. 1997;29:169–174. doi: 10.1097/00005768-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Pechter U., Ots M., Mesikepp S., Zilmer K., Kullissaar T., Vihalemm T. Beneficial effects of water-based exercise in patients with chronic kidney disease. Int. J. Rehabil. Res. 2003;26:153–156. doi: 10.1097/00004356-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Osato S., Onoyama K., Okuda S., Sanai T., Hori K., Fujishima M. Effect of swimming exercise on the progress of renal dysfunction in rat with focal glomerulosclerosis. Nephron. 1990;55:306–311. doi: 10.1159/000185980. [DOI] [PubMed] [Google Scholar]
- 29.Athyros V.G., Kakafika A.I., Papageorgiou A.A., Pagourelias E.D., Savvatianos S.D., Elisaf M. Statin-induced increase in HDL-C and renal function in coronary heart disease patients. Open Cardiovasc. Med. J. 2007;1:8–14. doi: 10.2174/1874192400701010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straznicky N.E., Grima M.T., Lambert E.A., Eikelis N., Dawood T., Lambert G.W. Exercise augments weight loss induced improvement in renal function in obese metabolic syndrome individuals. J. Hypertens. 2011;29:553–564. doi: 10.1097/HJH.0b013e3283418875. [DOI] [PubMed] [Google Scholar]