Abstract
Background
Biventricular pacing has been shown to increase both cardiac contractility and coronary flow acutely but the causal relationship is unclear. We hypothesised that changes in coronary flow are secondary to changes in cardiac contractility. We sought to examine this relationship by modulating coronary flow and cardiac contractility.
Methods
Contractility and lusitropy were altered by varying the location of pacing in 8 patients. Coronary autoregulation was transiently disabled with intracoronary adenosine. Simultaneous coronary flow velocity, coronary pressure and left ventricular pressure data were measured in the different pacing settings with and without hyperaemia and wave intensity analysis performed.
Results
Multisite pacing was effective at altering left ventricular contractility and lusitropy (pos. dp/dtmax −13% to +10% and neg. dp/dtmax −15% to +17% compared to baseline). Intracoronary adenosine decreased microvascular resistance (362.5 mm Hg/s/m to 156.7 mm Hg/s/m, p < 0.001) and increased LAD flow velocity (22 cm/s vs 45 cm/s, p < 0.001) but did not acutely change contractility or lusitropy. The magnitude of the dominant accelerating wave, the Backward Expansion Wave, was proportional to the degree of contractility as well as lusitropy (r = 0.47, p < 0.01 and r = −0.50, p < 0.01). Perfusion efficiency (the proportion of accelerating waves) increased at hyperaemia (76% rest vs 81% hyperaemia, p = 0.04). Perfusion efficiency correlated with contractility and lusitropy at rest (r = 0.43 & −0.50 respectively, p = 0.01) and hyperaemia (r = 0.59 & −0.6, p < 0.01).
Conclusions
Acutely increasing coronary flow with adenosine in patients with systolic heart failure does not increase contractility. Changes in coronary flow with biventricular pacing are likely to be a consequence of enhanced cardiac contractility from resynchronization and not vice versa.
Keywords: Wave intensity analysis, Model, Coronary flow, Dyssynchrony, Cardiac resynchronisation therapy
1. Introduction
1.1. Background
Cross-talk between the coronary vasculature and cardiac muscle has been proposed as a theory to understand the simultaneous effects of coronary blood flow on cardiac contractility and vice versa [1].
Reduced coronary flow in the Left Anterior Descending (LAD) Artery and corresponding perfusion defects have been noted in patients with dyssynchronous left ventricular activation [[2], [3], [4]]. Furthermore, Cardiac Resynchronization Therapy (CRT) has been shown to increase LAD flow and correct these perfusion defects [5]. It is unclear whether the observed increases in LAD flow are a bystander effect of electrical resynchronization or whether the increase in coronary flow is mechanistically important in mediating some of the physiological effects of CRT, resulting in the recruitment of hibernating myocardium [6,7].
Coronary wave intensity can be calculated from simultaneously acquired coronary flow and pressure measurements. It provides temporal information on the nature and origin of forces that drive and impede myocardial perfusion [8]. This makes it an ideal tool to investigate cardiac-coronary coupling, specifically the relationship between changes in LAD flow, acute left ventricular (LV) contractility and electrical activation. Typically, as many as six waves have been described with the vast majority of flow being driven by a dominant backward travelling expansion wave (BEW) that “sucks” blood through the coronary artery and a dominant forward compression wave (FCW) which derives from the ejection of blood from the left ventricle into the aorta and down the coronary arteries [8,9]. The effect of changes in both contractility and microvascular resistance on coronary wave energy have previously been studied in a canine model and used to identify the causes of the systolic impediment to coronary flow velocity [10]. Kyriacou et al. demonstrated in a group of patients with CRT that an acute increase in contractility was associated with an increase in the BEW in the left main coronary artery [11]. This is the only clinical study to date where coronary wave energy and coronary flow have been correlated with changes in acute LV contractility. Contractility and microvascular resistance have not previously been simultaneously modulated in humans and hence their relative impact on wave energy remains unclear.
1.2. Hypothesis
We hypothesised that increased LAD coronary flow with hyperaemia would not affect LV contractility. We also sought to characterise the relationship between changes in acute LV contractility and lusitropy with the dominant wave energies driving coronary flow velocity in patients with dyssynchronous heart failure.
2. Methods
2.1. Study design and patient population
We investigated a group of patients with a CRT device as a clinical model that allowed manipulation of both coronary blood flow and contractility. Patients who had been implanted with a CRT device and were known to have unobstructed coronary arteries at invasive angiography were invited to take part.
Cardiac contractility and lusitropy were altered by pacing from different points in the left and right ventricles. The first derivative of LV pressure was used to assess contractility and relaxation (pos. dp/dtmax and neg. dp/dtmax respectively). Microvascular resistance was altered by inducing hyperaemia with intracoronary adenosine.
The study received approval from the Local Research Ethics Committee (Rec no. 11/LO/1232) and was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent prior to taking part in the study.
2.2. Invasive protocol
Patients received dual antiplatelet therapy prior to the procedure and received unfractionated heparin to keep an activated clotting time > 250 s. Arterial access was gained via femoral and radial arteries. A 0.014″ Doppler wire (ComboWire™ model 9500, Volcano Corporation) was advanced to the proximal LAD to make simultaneous measurements of intracoronary pressure and Doppler flow velocity. A Primewire (Volcano Corporation) was placed in the LV cavity to measure pos. dp/dtmax and neg. dp/dtmax.
Acute contractility was modulated by pacing the ventricle from different points using the in situ CRT device. The site of ventricular stimulation was altered allowing atrio-ventricular synchronous pacing from the right ventricle alone, the left ventricle alone and biventricularly via the epicardial pacing lead. In 5 of the 8 patients, we also used a roving endocardial pacing catheter to perform LV endocardial pacing, which additionally allowed atrial synchronous biventricular endocardial pacing (with both a septal and lateral position used for the endocardial component) and simultaneous right ventricular endo, LV endo and LV epicardial pacing. All studies were performed with pacing at 10 beats above the intrinsic atrial rate to control for the Bowditch effect [12]. An atrially paced, ventricular sensed rhythm was used as the baseline in patients with in tact AV nodes. An atrially paced, right ventricular paced rhythm was used as the baseline in patients with AV block.
Intracoronary adenosine was given as a bolus dose of 36 microgrammes to induce hyperaemia, at each pacing protocol.
2.3. Data selection and beat analysis
The first three to five beats recorded after a change in pacing protocol were selected for analysis according to our previously published protocol [13]. A period of at least 10 s was allowed for stabilisation with each new pacing parameter.
2.4. Wave intensity analysis
Signals were sampled at 200 Hz and the raw data was exported to a custom-made study manager programme (Academic Medical Center, Amsterdam, Netherlands) for data extraction of selected beats at each different condition. Wave intensity analysis was then applied to the coronary data using custom-made software, “Cardiac Waves” (King's College London, London, United Kingdom). Details of the methodology used to perform wave intensity analysis have been previously described [14]. Briefly, a Savitzky–Golay convolution method was adopted using a polynomial filter to refine the derivatives of the intracoronary pressure and velocity signals. The selected three to five consecutive cardiac cycles were gated to the ECG R wave peak, with ensemble averaging of aortic pressure, distal coronary pressure (Pd), Average Peak velocity (APV) and heart rate. Net coronary wave intensity (dI) was calculated from the time derivatives (dt) of ensemble-averaged coronary pressure and flow velocity (U) as follows: dI = dPd/dt × dU/dt. Net wave intensity was then separated into forward and backward components.
The area beneath the 4 most prominent wave intensity peaks identified were analysed and included in this article. These are 1) the positive, aorta-derived FCW, occurring at the onset of systole, 2) the negative backward compression wave (BCW) also occurring at the onset of systole, 3) the negative BEW, the first backward wave occurring after the onset of ventricular relaxation, identified by the onset of diastole and 4) the positive Forward Expansion Wave (FEW) (see Fig. 1).
Fig. 1.
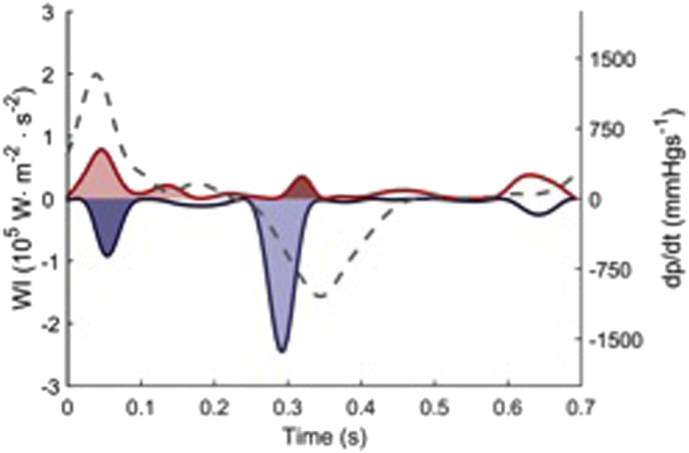
Wave intensity analysis of a single heartbeat. Forward waves are red and backward waves are blue. The accelerating waves (BEW and FCW) are shaded light and the decelerating waves (BCW, FEW) are shaded dark. The hatched black line corresponds to changes in LV pressure on the right hand axis.
Coronary Flow Velocity Reserve was calculated as the ratio between APV at rest and hyperaemia. Microvascular resistance (MR) was calculated as the ratio of the Pd and APV.
We also calculated coronary perfusion efficiency (P.E). This is a metric which quantifies accelerating wave energy as a proportion of total coronary wave energy generated by the FCW and BEW which are the waves that theoretically drive rather than impede coronary flow velocity [15]. PE is calculated using the magnitude of the areas under the curve (AUCs) of the component waves i.e.
2.5. Statistical analysis
Statistical analysis was performed using IBM SPSS 21.0. Baseline demographics are presented as either mean & standard deviations or as proportions. Baseline clinical haemodynamic data are presented as median with interquartile ranges. Normally distributed data are compared using paired and independent student t-tests and non-normally distributed data using the non-parametric equivalents. Similarly, Pearson and Spearman correlation coefficients are quoted to express associations between variables for normal and non-normally distributed data respectively.
3. Results
3.1. Patient population and baseline haemodynamic data
Acute contractility and coronary data were obtained in 39 different pacing settings in the LAD from 8 patients (see demographics in Table 1). There were no complications arising from the study protocol.
Table 1.
Demographic data of patients.
| Characteristics | Mean (±S.D) or proportion |
|---|---|
| Age (y) | 56.9 (10.6) |
| Male | 7/8 |
| QRS duration pre CRT (ms) | 150.8 (23.4) |
| Ejection fraction (%) | 23 (10.8) |
| NYHA class | 2.7 (0.49) |
| Ace inhibitors/angiotensin blockers | 8/8 |
| Betablockers | 8/8 |
| Aldosterone receptor antagonists | 6/8 |
Baseline pos. dp/dtmax was 1110 mm Hg/s (IQR 711 to 1177 mm Hg/s) and baseline neg. dp/dtmax was −912 mm Hg/s (IQR −861 to −1214 mm Hg/s). Baseline APV was 17.4 cm/s (IQR 13.7 to 24.7 cm/s) and CFVR was 2.3 (IQR 1.5 to 2.7).
Pacing via different modes led to significant variation in contractility and lusitropy. The percentage change from baseline in pos. dp/dtmax varied between −13% (IQR -7% to −14%) and + 10% (7.5% to 14.5%) and in neg. dp/dtmax between −15% (IQR -13% to −22%) and + 17% (IQR 9% to 33.8%). The effect of different pacing stimuli on wave energies are shown in Table 2.
Table 2.
The effect of hyperaemia on the 4 dominant coronary wave energies.
| Rest median (IQR) | Hyperaemia median (IQR) | |
|---|---|---|
| FCW LAD | 2774 (1501 to 3614) p < 0.01 vs hyperaemia |
5622 (3753 to 9756) |
| BCW LAD | 2370 (1815 to 3784) p < 0.01 vs hyperaemia |
3703 (2178 to 5137) |
| BEW LAD | 7118 (4713 to 9098) p < 0.01 vs hyperaemia |
12,068 (8880 to 18,103) |
| FEW LAD | 176 (35 to 493) p < 0.01 vs hyperaemia |
393 (201 to 1017) |
3.2. Variation in coronary flow and LV contractility
Across the range of pacing settings, there was a slight reduction in LV contractility (pos. dp/dtmax) with hyperaemia vs rest (1037 ± 336 mm Hg to 1056 ± 330 mm Hg, p = 0.05) and no significant change in lusitropy (neg. dp/dtmax, −908 ± 263 mm Hg versus −905 ± 261 mm Hg, p = 0.25). Coronary flow velocity in the LAD increased significantly with hyperaemia (22.2 cm/s ± 8.6 to 45.3 cm/s ± 18.1, p < 0.001) due to a fall in microvascular resistance (362.5 mm Hg/s/m ± 125.5 to 156.7 mm Hg/s/m ± 50.2, p < 0.001). Resting microvascular resistance correlated with neg. dp/dtmax (r = −0.46, p < 0.01) but not pos. dp/dtmax r = 0.25, p = 0.13. There was no correlation between minimal microvascular resistance and contractility indices. The comparison of coronary wave energies at rest and hyperaemia are also shown in Table 2.
3.3. Relationship between coronary wave indices and LV haemodynamics
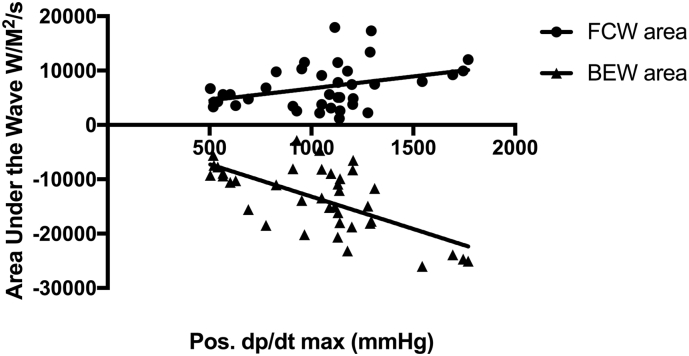
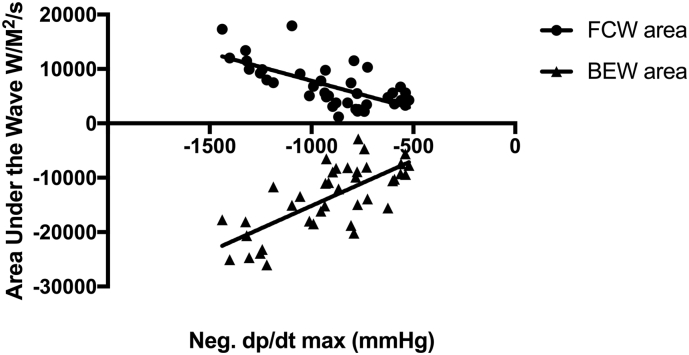
BEW magnitude correlated with the degree of contractility and lusitropy (r = 0.47, p < 0.01 and r = 0.50, p < 0.01 respectively) at rest. In contrast, there was no relationship between the magnitude of the FCW and LV indices. The magnitude of both BEW and FCW at hyperaemia correlated with the degree of contractility (FCW, r = 0.37, p = 0.02; BEW, r = −0.65, p < 0.01) and lusitropy (FCW, r = −0.67, p < 0.01, BEW, r = 0.72, p < 0.01 for each) (see Fig. 2, Fig. 3).
Fig. 2.
The correlations between the forward compression wave (FCW) and Backward Expansion Wave (BEW) with pos. dp/dtmax at hyperaemia.
Fig. 3.
The correlations between the forward compression wave (FCW) and Backward Expansion Wave (BEW) with neg. dp/dtmax at hyperaemia.
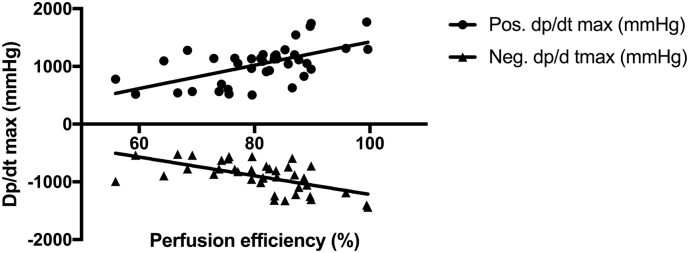
Perfusion efficiency (proportion of accelerating wave energy) was 76.2% ± 7.0% at rest and 80.8% ± 9.8% at hyperaemia (p = 0.04). Perfusion efficiency correlated with contractility and lusitropy at rest (r = 0.43, p < 0.01 and r = −0.50, p = 0.01) and more so during hyperaemia (r = 0.59, p < 0.01 and r = −0.6, p < 0.01) (see Fig. 4).
Fig. 4.
Increased perfusion efficiency in the LAD correlates with increased acute contractility and lusitropy at hyperaemia.
3.4. Summary of main findings
The main findings of this study are
-
1)
Ventricular stimulation from different sites is an effective means of modulating contractility and lusitropy in patients who have impaired left ventricular function and can be used in an experimental model to examine coronary-cardiac coupling.
-
2)
The magnitude of the dominant accelerating wave, the BEW, and total accelerating wave energy correlate with the degree of left ventricular lusitropy and contractility.
-
3)
Increasing coronary flow by pharmacological hyperaemia has no acute effect on LV contractility indices.
4. Discussion
We believe this is the first description of a clinical model based on the simultaneous use of multisite pacing and adenosine induced hyperaemia to assess the effects of cardiac contractility and microvascular resistance on coronary wave energy. The large changes in coronary flow created by directly reducing microvascular resistance have no effect on indices of acute contractility, although the latter have been shown to have a significant effect on the coronary wave intensity profile. We have previously demonstrated that acutely increased LAD flow velocity correlates with increased pos. dp/dtmax in CRT patients compared to right ventricular pacing, although the mechanistic relationship between LV contractility and coronary flow was unclear at that stage [13]. Our current study provides clarity on the causal relationship and suggests that the previously observed changes in LAD flow are a consequence of resynchronization rather than being a determinant of the acute response in LV contractility following CRT [16]. It has previously been shown in an animal model that there is a minimum perfusion requirement, within the pathophysiological limits seen in vivo, to allow a positive acute response to CRT [6]. Our data suggest that where this perfusion threshold is met, a further increase in coronary blood flow velocity will not result in increased contractility.
It is possible that adenosine induced increase in flow velocity represents a distinct entity to the autoregulatory changes that may occur at the time of a pacing-mediated increase in contractility. For instance, the hyperaemic response will result in a matched increase in myocardial venous emptying and thus any changes in contractility as a result of the Gregg effect through stretch induced calcium release and increased calcium sensitivity may not occur. However our model is unlikely to be affected by either the Anrep and Gregg effect as each pacing protocol lasted for no more than 30 s and both the Anrep and Gregg effects take longer to exert their influence on cardiac contractility [17].
Conventional interpretation of the dominant wave energy profiles in the coronary arteries links them to left ventricular contractility and lusitropy. This relationship has previously been confirmed in vivo, in the LMCA, although most studies reporting changes in coronary wave energy have investigated more distal epicardial arteries [11,14]. The most dominant wave is the BEW, an accelerating wave in early diastole that coincides with the rapid fall in ventricular pressure. We have demonstrated that the magnitude of the BEW is proportional to the degree of lusitropy as quantified by the neg. dp/dtmax in the left ventricle at rest. The fact that the BEW magnitude also correlates well with pos. dp/dtmax suggests that contractility and lusitropy are coupled in this population.
The other important accelerating wave is the FCW, which corresponds temporally to peak aortic pressure and systolic coronary blood flow. Whilst left ventricular contractility is a determinant of peak aortic pressure, at rest we did not find a correlation between pos. dp/dtmax and the FCW magnitude, suggesting that this relationship may be more complex as has been suggested in previous animal studies [10]. Indeed, these studies have failed to show a strong correlation between LV contractility in the LAD and our findings are in keeping with this. An increase in the FCW has been noted in the aorta alongside a presumed increase in LV contractility with dobutamine but this has not been demonstrated in the coronary circulation and ours is the first in human experiment to provide a detailed examination of these relationships when contractility is being measured [18].
It may be that the lack of correlation between the FCW and contractility is due to the CRT model itself, in that LBBB ventricular conduction is asynchronous and as such this may disrupt the correlation between contractility and the FCW via the LAD [19]. From the clinical perspective, it is clear that the changes in LV contractility are not the result of changes in coronary flow and it appears that the noted changes in WIA are a passive phenomenon related to changes in LV haemodynamics.
At hyperaemia both the FCW and BEW correlated with both pos. dp/dtmax and neg. dp/dtmax. Both the FCW and BEW are thought to be generated by changes in intracavity LV pressure. If one removes the insulator effects of the microvasculature with adenosine then the waves should better correlate with acute measures of contractility and lusitropy. This would be particularly the case with the BEW which “sucks” blood during diastole towards the LV cavity via the microvasculature from the epicardial arteries. If the resistance in the microvasculature is removed with adenosine then an impediment to this relationship is removed. Indeed, this may account for the stronger correlations being seen with the BEW than the FCW as well as the improved correlations noted at hyperaemia.
A further explanation for the better correlation of the BEW than the FCW to LV haemodynamics may simply relate to the differing order of magnitude of the two waves, with the area under the curve of the BEW being typically 3 times larger than the FCW making it less prone to noise and sampling error within the experiment. This might account for less pronounced and clear correlations in the data.
Further research is therefore required to elucidate the FCW and pos. dp/dtmax relationship.
The ratio of accelerating to decelerating wave energy can be regarded as a measure of myocardial perfusion efficiency and this metric has previously been applied to understanding altered coronary wave profiles in symptomatic aortic stenosis and in hypertrophic obstructive cardiomyopathy [15,20]. Our data show that enhanced contractility and lusitropy at rest are associated with greater perfusion efficiency and that these correlations are once again stronger at hyperaemia. These findings are consistent with previous work that has shown biventricular pacing to be highly efficient when it increases cardiac contractility with favourable changes in cardiac work at minimal cost with regard to oxygen demand [[21], [22], [23]]. It should be noted that our data show improved perfusion efficiency rather than coronary flow per se.
5. Study limitations
Due to the complexity of the procedure, the number of patients investigated was small which limits the statistical power of the study and may affect the generalizability of our conclusions. This limitation applies to similar complex invasive clinical studies [11]. The use of pos. dp/dtmax and neg. dp/dtmax to assess the acute haemodynamic response of CRT is well validated in CRT research [24]. Whilst other measures of contractility can be used, the use of a conductance catheter for example would have increased the procedural burden to an unacceptably high level. Furthermore, whilst peak measures may vary to some extent, the variability would not be sufficient to negate our chief clinical finding that a significant increase in coronary flow does not result in a significant increase in LV haemodynamics. Nonetheless it is important that future studies consider experimental methodologies that might allow other measures of contractility and relaxation to be assessed. Finally, we have looked at the relationship between coronary haemodynamics and acute changes in contractility and lusitropy. Whether these conclusions can be applied to interpreting the chronic remodeling and long-term changes in myocardial perfusion in patients undergoing CRT needs further exploration.
We calculated wave speed using the sum of squares method; however, during hyperaemia, the wave speed estimated using this method might differ from the true wave speed [25].
6. Clinical and research implications
-
•
Changes in coronary wave energy and flow velocity seen with different types of pacing do not cause changes in acute cardiac contractility but conversely, the changes in coronary blood flow and wave intensity are the result of changing contractile force. Thus whilst there may be a minimal perfusion required for CRT to exert its benefits, it is unlikely that treatments aimed at increasing coronary flow will improve ventricular function per se in patients with unobstructed coronary arteries [6].
-
•
Varying the site and mode of pacing, using a multisite model, successfully alters acute left ventricular contractility and lusitropy in patients with contractile dyssynchrony. This model could be used to elucidate the mechanism and evaluate the efficacy of potential therapies in future.
7. Conclusions
We report the successful implementation of a novel model in heart failure patients that allows the acute manipulation of the two main determinants of coronary flow (LV contractility and microvascular resistance).
Increasing coronary flow did not result in increased contractility in this cohort of patients with normal coronary arteries and systolic heart failure, whereas altering LV pacing site did. We can conclude that any observed changes in coronary haemodynamics with biventricular pacing are likely a consequence of improved cardiac contractility from resynchronization-related cardiac output changes, rather than the cause of enhanced cardiac contractility.
Conflict of interests
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgement of grants
SC is a St Jude Medical Research Fellow, ZC and BM have received grants from the British Heart Foundation, KDS has received grants from HRUK, NIHR and Wellcome, TJ was a Medtronic Research Fellow, SN has received grants from Boston Scientific, St Jude Medical, British Heart Foundation and Medtronic, CR has received grants from Boston Scientific, Livanova, St Jude Medical, British Heart Foundation and Medtronic. DP has received project grants from the British Heart Foundation. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- 1.Westerhof N., Boer C., Lamberts R.R., Sipkema P. Cross-talk between cardiac muscle and coronary vasculature. Physiol. Rev. 2006;86:1263–1308. doi: 10.1152/physrev.00029.2005. [DOI] [PubMed] [Google Scholar]
- 2.Ogano M., Iwasaki Y.-K., Tanabe J., Takagi H., Umemoto T., Hayashi M. Cardiac resynchronization therapy restored ventricular septal myocardial perfusion and enhanced ventricular remodeling in patients with non-ischemic cardiomyopathy presenting with left bundle branch block: ventricular septal redistribution by CRT in LBBB. Heart Rhythm. 2014 doi: 10.1016/j.hrthm.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Lindner O., Vogt J., Kammeier A., Wielepp P., Holzinger J., Baller D. Effect of cardiac resynchronization therapy on global and regional oxygen consumption and myocardial blood flow in patients with non-ischaemic and ischaemic cardiomyopathy. Eur. Heart J. 2005;26:70–76. doi: 10.1093/eurheartj/ehi046. [DOI] [PubMed] [Google Scholar]
- 4.Knaapen P., van Campen L.M.C., de Cock C.C., Götte M.J.W., Visser C.A., Lammertsma A.A. Effects of cardiac resynchronization therapy on myocardial perfusion reserve. Circulation. 2004;110:646–651. doi: 10.1161/01.cir.0000138108.68719.c1. [DOI] [PubMed] [Google Scholar]
- 5.Deftereos S., Giannopoulos G., Kossyvakis C., Raisakis K., Kaoukis A., Driva M. Differential effect of biventricular and right ventricular DDD pacing on coronary flow reserve in patients with ischemic cardiomyopathy. J. Cardiovasc. Electrophysiol. 2010;21:1233–1239. doi: 10.1111/j.1540-8167.2010.01827.x. [DOI] [PubMed] [Google Scholar]
- 6.Svendsen M., Prinzen F.W., Das M.K., Berwick Z., Rybka M., Tune J.D. Bi-ventricular pacing improves pump function only with adequate myocardial perfusion in canine hearts with pseudo-left bundle branch block. Exp. Biol. Med. (Maywood) 2012;237:644–651. doi: 10.1258/ebm.2012.012023. [DOI] [PubMed] [Google Scholar]
- 7.Claridge S., Chen Z., Jackson T., Sammut E., Sohal M., Behar J. Current concepts relating coronary flow, myocardial perfusion and metabolism in left bundle branch block and cardiac resynchronisation therapy. Int. J. Cardiol. 2015;181:65–72. doi: 10.1016/j.ijcard.2014.11.194. [DOI] [PubMed] [Google Scholar]
- 8.Davies J.E., Whinnett Z.I., Francis D.P., Manisty C.H., Aguado-Sierra J., Willson K. Evidence of a dominant backward-propagating “suction” wave responsible for diastolic coronary filling in humans, attenuated in left ventricular hypertrophy. Circulation. 2006;113:1768–1778. doi: 10.1161/CIRCULATIONAHA.105.603050. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y.H., Anderson T.J., Parker K.H., Tyberg J.V. Wave-intensity analysis: a new approach to coronary hemodynamics. J. Appl. Physiol. 2000;89:1636–1644. doi: 10.1152/jappl.2000.89.4.1636. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y.H., Anderson T.J., Parker K.H. Effects of left ventricular contractility and coronary vascular resistance on coronary dynamics. Am. J. Physiol. Heart Circ. Physiol. 2004;286:1590–1595. doi: 10.1152/ajpheart.01100.2001. [DOI] [PubMed] [Google Scholar]
- 11.Kyriacou A., Whinnett Z.I., Sen S., Pabari P.A., Wright I., Cornelussen R. Improvement in coronary blood flow velocity with acute biventricular pacing is predominantly due to an increase in a diastolic backward-travelling decompression (suction) wave. Circulation. 2012;126:1334–1344. doi: 10.1161/CIRCULATIONAHA.111.075606. [DOI] [PubMed] [Google Scholar]
- 12.Bowditch H.P. 1871. On the Peculiarities of Excitability Which the Fibres of Cardiac Muscle Show1, 2. [Google Scholar]
- 13.Claridge S., Chen Z., Jackson T., De Silva K., Behar J., Sohal M. Effects of epicardial and endocardial cardiac resynchronization therapy on coronary flow: insights from wave intensity analysis. J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Silva K., Foster P., Guilcher A., Bandara A., Jogiya R., Lockie T. Coronary wave energy: a novel predictor of functional recovery after myocardial infarction. Circ. Cardiovasc. Interv. 2013;6:166–175. doi: 10.1161/CIRCINTERVENTIONS.112.973081. [DOI] [PubMed] [Google Scholar]
- 15.Lumley M., Williams R., Asrress K.N., Arri S., Briceno N., Ellis H. Coronary physiology during exercise and vasodilation in the healthy heart and in severe aortic stenosis. J. Am. Coll. Cardiol. 2016;68:688–697. doi: 10.1016/j.jacc.2016.05.071. [DOI] [PubMed] [Google Scholar]
- 16.Fang F., Chan J.Y.-S., Lee A.P.-W., Sung S.-H., Luo X.-X., Jiang X. Improved coronary artery blood flow following the correction of systolic dyssynchrony with cardiac resynchronization therapy. Int. J. Cardiol. 2013;167:2167–2171. doi: 10.1016/j.ijcard.2012.05.094. [DOI] [PubMed] [Google Scholar]
- 17.Lamberts R.R., Van Rijen M.H.P., Sipkema P., Fransen P., Sys S.U., Westerhof N. Coronary perfusion and muscle lengthening increase cardiac contraction: different stretch-triggered mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1515–22. doi: 10.1152/ajpheart.00113.2002. [DOI] [PubMed] [Google Scholar]
- 18.Fok H., Guilcher A., Li Y., Brett S., Shah A., Clapp B. Augmentation pressure is influenced by ventricular contractility/relaxation dynamics: novel mechanism of reduction of pulse pressure by nitrates. Hypertension. 2014;63:1050–1055. doi: 10.1161/HYPERTENSIONAHA.113.02955. [DOI] [PubMed] [Google Scholar]
- 19.Skalidis E.I., Kochiadakis G.E., Koukouraki S.I., Parthenakis F.I., Karkavitsas N.S., Vardas P.E. Phasic coronary flow pattern and flow reserve in patients with left bundle branch block and normal coronary arteries. J. Am. Coll. Cardiol. 1999;33:1338–1346. doi: 10.1016/s0735-1097(98)00698-6. [DOI] [PubMed] [Google Scholar]
- 20.Raphael C.E., Cooper R., Parker K.H., Collinson J., Vassiliou V., Pennell D.J. Mechanisms of myocardial ischemia in hypertrophic cardiomyopathy: insights from wave intensity analysis and magnetic resonance. J. Am. Coll. Cardiol. 2016;68:1651–1660. doi: 10.1016/j.jacc.2016.07.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson G.S., Berger R.D., Fetics B.J., Talbot M., Spinelli J.C., Hare J.M. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102:3053–3059. doi: 10.1161/01.cir.102.25.3053. [DOI] [PubMed] [Google Scholar]
- 22.Kyriacou A., Pabari P.A., Mayet J., Peters N.S., Davies D.W., Lim P.B. Cardiac resynchronization therapy and AV optimization increase myocardial oxygen consumption, but increase cardiac function more than proportionally. Int. J. Cardiol. 2013 doi: 10.1016/j.ijcard.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steendijk P., Tulner S.A., Bax J.J., Oemrawsingh P.V., Bleeker G.B., van Erven L. Hemodynamic effects of long-term cardiac resynchronization therapy: analysis by pressure-volume loops. Circulation. 2006;113:1295–1304. doi: 10.1161/CIRCULATIONAHA.105.540435. [DOI] [PubMed] [Google Scholar]
- 24.Duckett S.G., Ginks M., Shetty A.K., Bostock J., Gill J.S., Hamid S. Invasive acute hemodynamic response to guide left ventricular lead implantation predicts chronic remodeling in patients undergoing cardiac resynchronization therapy. J. Am. Coll. Cardiol. 2011;58:1128–1136. doi: 10.1016/j.jacc.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 25.Rolandi M.C., De Silva K., Lumley M., Lockie T.P.E., Clapp B., Spaan J.A.E. Wave speed in human coronary arteries is not influenced by microvascular vasodilation: implications for wave intensity analysis. Basic Res. Cardiol. 2014;109:405–411. doi: 10.1007/s00395-014-0405-1. [DOI] [PubMed] [Google Scholar]