Abstract
Diseases affecting the brain contribute to a substantial proportion of morbidity and mortality in the general population. Conditions such as stroke, dementia and cognitive impairment have a prominent impact on global public health. Despite the heterogeneous clinical manifestations of these conditions and their diverse prognostic implications, current evidence supports a role for cardiovascular disease as a common pathophysiological ground. Brain white matter hyperintensities (WMH) are patchy white matter signal hyperintensity on T2-weighted magnetic resonance imaging sequences commonly found in elderly individuals. WMH appear to have a vascular pathogenesis and have been shown to confer an increased risk of stroke and cognitive decline. Indeed, they were proposed as a marker for central nervous system frailty. Cardiovascular diseases seem to play a key role in the etiology of WMH. Carotid atherosclerosis and atrial fibrillation were shown to be associated with higher WMH burden, while adequate blood pressure control has been reported reducing WMH progression. Aim of the present work is to review the available evidence linking WMH to cardiovascular disease, highlighting the complex interplay between cerebral and cardiovascular health.
1. Introduction
Diseases affecting the central nervous system exact a high toll from the general population [1]. Stroke and dementia combined are currently the leading cause of disability worldwide, accounting for over 140,000 Disability-Adjusted Life Years (DALY), i.e. years of good health lost globally, in 2016 [1]. In spite of the diverse pathological and clinical manifestations, current evidence suggests that cardiovascular disease may provide a cardinal pathophysiological background to both these conditions [2]. Interestingly, impairment of cerebral vascular health leads to pathological alterations of the cerebral parenchyma long before the clinical manifestation of neurological deficits or cognitive decline [2,3]. Among the subclinical cerebral alterations, white matter hyperintensities (WMH) appear of particular clinical interest. WMH are defined as patchy areas of signal hyperintensity scattered in the deep or periventricular white matter evident on brain magnetic resonance imaging (MRI) T2-weighted or Fluid Attenuation Inversion Recovery (FLAIR) sequences, as shown in Fig. 1 [4]. They are a common finding in MRI scans of asymptomatic individuals, and their prevalence was shown to increase with age. Approximately 11–21% of otherwise healthy subjects with mean age of 64 years, and around 64–94% of those with mean age of 82 years are affected by WMH [5,6]. The prognostic relevance of WMH is well established, being associated with reduced ability to carry out daily activities and with gait and mood disturbances [7]. Eventually, it confers a significantly higher risk of stroke, dementia and death [3]. Aim of the present review is to summarize currently available clinical evidence linking cardiovascular disease and WMH.
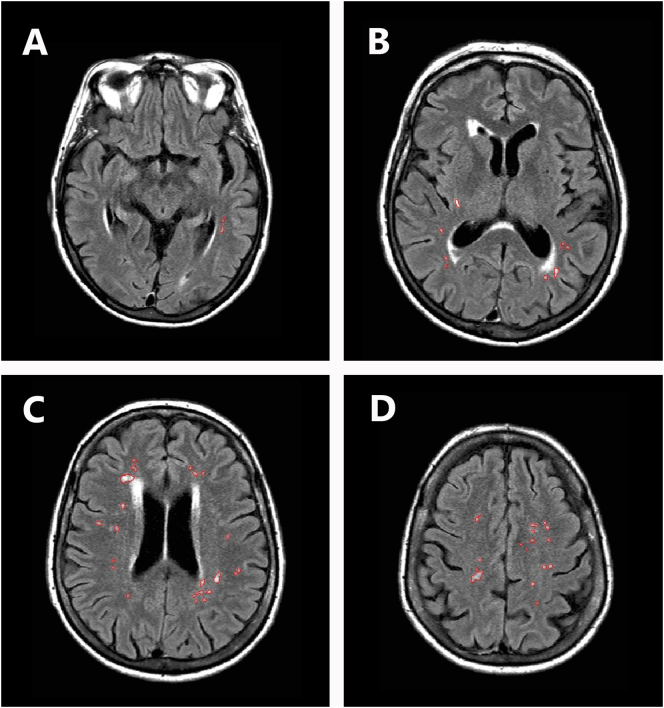
Fig. 1.
White matters hyperintensities in a representative subject. Panels A–D show fluid attenuated inversion recovery (FLAIR) sequence scans of the same patient at different levels. White matter hyperintensities are outlined in red.
2. WMH pathology and putative pathophysiology
Surprisingly few pathological studies have looked into the histological details of WMH [8]. Furthermore, the reliability of these studies appears to be hampered by the difficulty in matching MRI images with their tissue counterpart in post-mortem evaluation [8] and by potential sampling bias [9] and artifacts due to tissue processing [10]. Currently WMH are considered a rather heterogeneous pathological entity, the heterogeneity reflecting different stages of the disease [11]. Earlier reports focused on demyelination and axonal loss in WMH [12], with white matter cavitation and reduced glial density [13]. Subsequent reports focused on chronic edema due to a primary blood brain barrier dysfunction [8], which is supported by the evidence of increased albumin concentration within the interstitial space [14]. Indeed, more recent clinical data on dynamic MRI demonstrated increased gadolinium leakage into WMH, supporting in vivo the hypothesis of increased blood brain barrier permeability [15]. Interestingly, however, no disruption of endothelial tight junctions has been proven [14], yet endothelial cells were shown to express markers of activation [16]. The pathological complexity of WMH is reflected by current uncertainties in their etiology. The evidence of tortuous arterioles [8] and reduced cerebral blood flow [9], alongside with the histological evidence of venular disease and stenosis [17], suggests a cardinal role for ischemia, which is further supported by immunohistochemical and gene expression array studies [18]. Subsequent studies suggest a role also for immune and inflammatory activation [18], which was also supported by a recent population genetic study [19]. In a more recent report on 3248 participants in the Framingham Heart Study, whole blood gene expression profile demonstrated a more prominent expression of inflammation-related genes [20]. Higher neutrophil-to-lymphocyte ratio were demonstrated in subjects with a higher burden of WMH in a large cohort of otherwise healthy individuals, suggesting a shift in cell-mediated immune response [21].
3. Cardiovascular risk factors and WMH
Several large, general population based epidemiological studies have demonstrated that cardiovascular risk factors, aside for contributing substantially to the risk of stroke, negatively influence global brain health [22,23]. Indeed, the cumulative exposure to multiple vascular risk factors, including smoking, hypertension, diabetes, obesity and physical inactivity was associated to poor performance on neurocognitive tests [24]. Among cardiovascular risk factors, hypertension was shown to be more strongly associated with WMH, possibly damaging cerebral microcirculation. An early report from Strassburger and colleague showed that hypertensive subjects had a higher burden of WMH in the deep white matter, but not in the periventricular white matter, when compared to age matched normotensive individuals [25]. Wiseman et al. on the other hand showed that hypertensive subjects had a more severe WMH involvement throughout the whole white matter when compared to healthy individuals [26]. Furthermore, a report from the Framingham Offspring Cohort Study on 1352 subjects free of dementia was able to demonstrate that prolonged hypertension strongly associates with an accelerated progression of WMH [27]. Another report from the Framingham Offspring Cohort Study comprising 1814 subjects demonstrated that both hypertension and smoking are associated with a higher WMH burden [28]. While current evidence support a role for hypertension and smoking in WMH pathogenesis, the role of dyslipidemia appears not to be well understood. In a large Chinese cohort comprising 4683 hospitalized subjects, high low-density lipoprotein (LDL) cholesterol was found to be associated with high WMH [29]. On the other hand, a report from Jimenez-Conde and colleagues on a cohort of 1135 ischemic stroke patients shows that subjects with hyperlipidemia had a lower burden of WMH [30]. Diabetes mellitus is currently regarded as a strong risk factor for cardiovascular disease. A recent proof of principle study including 60 type 2 diabetes mellitus patients and 54 matched non diabetic controls failed to identify any difference in terms of WMH volume, but identified a peculiar shape and distribution of WMH lesions in diabetics [31]. The Authors hypothesize that these differences may be an expression of different microvascular affections in diabetic patients. Table 1 summarizes these findings. Interestingly, initial evidence is currently available showing that high intensity management of cardiovascular risk factors, including hypertension [32] and dyslipidemia [33], is able to slow down the progression of WMH compared to untreated controls.
Table 1.
Summary of the main studies evaluating the association between white matter hyperintensities (WMH) and cardiovascular disease.
| Authors and year | Sample size | Results | Reference |
|---|---|---|---|
| Cardiovascular risk factors | |||
| Strassburger et al., 1997 | 27 treated hypertensive subjects, 20 age-matched normotensive subjects | Hypertensive subjects had a more severe involvement of deep white matter with WMH, while periventricular WMH were comparable to normotensive subjects | [25] |
| Wiseman et al., 2004 | 103 hypertensive, 51 normotensive subject | Hypertensive subjects have a higher WMH burden throughout the entire brain | [26] |
| Debette et al., 2011 | 1352 free living, non-demented subjects | Mid-life exposure to hypertension associates with accelerated rates of WMH progression | [27] |
| Jeerakathil et al., 2004 | 1814 free living subjects | Age, smoking and history of cardiovascular disease independently predicted WMH burden | [28] |
| Lin et al., 2017 | 4863 hospitalized subjects | Heavy drinking and high LDL cholesterol are associated to high burden of WMH | [29] |
| Jimenez-Conde et al., 2010 | 1315 ischemic stroke patients | Hyperlipidemia, i.e. total cholesterol > 220 mg/dL or current statin use daily triglycerides > 150 mg/dL, was associated with less WMH | [30] |
| De Bresser et al., 2018 | 60 patients with diabetes type 2, 54 healthy matched controls | No difference in terms of WMH volume between diabetics and controls. Diabetics had more non-punctate lesions and punctate eccentric deep WMH when compared to controls | [31] |
| Carotid atherosclerosis | |||
| Rundek et al., 2017 | 1166 stroke-free subjects | WMH burden was associated with a larger common carotid diameter and reduced carotid elasticity. | [35] |
| Della-Morte et al., 2018 | 1299 subjects | WMH volume was directly correlated with carotid intima-media thickness. | [36] |
| Moroni et al., 2016 | 5306 free living subjects | Meta-analysis of observational studies. The presence of carotid artery plaque is associated to an OR of WMH of 1.42 | [41] |
| Ataf et al., 2006 | 57 stroke patients undergoing carotid endarterectomy | Plaque features of vulnerability on histology were associated with higher WMH burden in the subtended hemisphere | [42] |
| Baradaran et al., 2017 | Systematic review | The Authors conclude that there is insufficient and contradicting evidence linking carotid plaque to ipsilateral hemispherical WMH burden. | [43] |
| Ammirati et al., 2017 | 67 asymptomatic subjects with intermediate carotid stenosis | Hypertension, presence of a plaque ulcer and the degree of stenosis were independent predictors of WMH, evaluated quantitatively. | [44] |
| Pico et al., 2002 | 640 healthy subjects aged 59–71 years | After 4 years follow up, subjects with baseline detection of carotid artery plaque had an OR of 1.70 for the progression to severe WMH, independently from age, sex and hypertension | [45] |
| Atrial fibrillation | |||
| Kobayashi et al., 2012 | 71 AFib (29 persistent and 42 paroxysmal) vs 71 age matched controls | Patients with Afib had more severe WMH involvement of the deep white matter, no difference was found in periventricular white matter. WMH involvement was evaluated with Fazekas score. | [50] |
| Gaita et al., 2013 | 90 paroxysmal Afib, 90 persistent Afib and 90 controls | Afib subjects had an OR of 11.2 for having WMH when compared to controls. Persistent Afib subjects had a higher number of WMH compared to paroxysmal Afib subjects (41.1 vs 33.2). | [51] |
| Mayasi et al., 2017 | 234 stroke patients (114 with Afib) | Afib subjects had an OR of 3.6 of having a higher burden of WMH selectively in the subcortical white matter of the anterior circulation. | [53] |
| Ntaios et al., 2015 | 1892 stroke patients (670 with Afib) | WMH is only associated with stroke recurrence in non-Afib patients (HR 1.8). | [54] |
| Heart failure | |||
| Vogels et al., 2007 | 58 HF patients, 48 subjects with cardiovascular disease and no HF, 42 healthy controls | HF subjects have more severe WMH involvement of the brain. LVEF is an independent predictor of structural brain alterations | [58] |
| Alosco et al., 2013 | 48 heart failure patients | Cardiac index is inversely associated to WMH burden | [59] |
| Alosco et al., 2013 | 69 heart failure patients | Blood flow velocity on transcranial Doppler imaging inversely correlates with WMH burden. A higher WMH burden associates with poorer cognitive performances on MMSE | [60] |
| Miscellaneous conditions | |||
| Ueno et al., 2010 | 115 stroke patients: 49 with PFO, 4 with ASA, 23 with PFO and ASA and 39 with neither PFO nor ASA |
Patients with PFO and ASA had significantly higher WMH burden than other subjects. | [63] |
| Dokumaci et al., 2017 | 10 children aged between 5 and 16 years affected by Eisenmenger syndrome and 10 age matched control | WMH were detectable in the periventricular area of 3 children | [64] |
| Bertholdt et al., 2014 | 30 neonates: 22 transposition of the great arteries, 6 univentricular hearts with hypoplastic aortic arches, 2 aortic arch obstructions. | 20% of neonates had WMH. 2 neonates had a progression of WMH after surgery. | [65] |
4. Carotid atherosclerosis and WMH
The carotid arteries provide the largest proportion of blood flow to the brain. Any disturbance in cerebral blood flow and resistances is likely to be reflected in a change in morphology and elastic properties of the carotid arteries [34]. In a recent sub-analysis including 1166 stroke free subjects from the Northern Manhattan Study, Rundek and colleagues described an association between increased diastolic common carotid artery diameter and eventually reduced carotid elasticity and a higher WMH burden [35].
Subclinical carotid atherosclerosis evaluated as carotid intima-media thickness (cIMT), a marker of increased cardiovascular risk was also shown to be associated with an increased WMH burden. In a cohort of 1229 participants in the Northern Manhattan Study, cIMT was directly correlated with WMH volume [36]. Significant stenosis of the carotid artery due to atherosclerosis is very common in the general population [37], while the presence of carotid artery atherosclerotic plaques in the absence of a hemodynamically significant stenosis appears to be even more widespread, reaching up to 40% of otherwise healthy individuals [38]. Carotid atherosclerosis has a causative role in the development of ischemic stroke, mainly through and athero-embolic mechanism [39]. Importantly however, carotid artery atherosclerosis may affect cerebral circulation in other ways, including the reduction of cerebral perfusion in case of flow limiting stenosis or as a source of micro-emboli [40]. The relation between carotid atherosclerosis and WMH is currently subject of intense debate. A recently published meta-analysis of cross-sectional studies by our group comprising 5306 otherwise healthy subjects showed that carotid atherosclerosis confers an odds ratio (OR) of 1.42 of having WMH [41]. The potential athero-embolic etiology of WMH is supported by data showing that plaque features of instability are in fact associated with high WMH burden. Indeed, Altaf and colleagues show a higher WMH burden in cerebral hemispheres subtended by vulnerable atherosclerotic plaques identified by histology [42]. On the other hand, a systematic review by Baradaran et al. identified inconclusive evidence linking carotid atherosclerotic plaques to ipsilateral increased burden of WMH, as would be expected in the case of a direct embolic pathogenesis [43]. A recent study by our group confirms the lack of association between carotid atherosclerotic plaques and ipsilateral WMH [44]. On the other hand, we were able to show that hypertension, presence of plaque ulcer and more severe stenosis were all independent predictors of global WMH burden [44]. Further studies may be necessary to clarify the role of carotid atherosclerosis in this setting: while plaque ulcers and stenosis may have a direct role in the pathogenesis of WMH, they can in fact merely reflect a higher systemic vascular impairment [44].
To the best of our knowledge, only few studies have evaluated longitudinally the relation between carotid atherosclerosis and WMH. In particular, Pico et al. evaluated 640 healthy subjects after a follow up of 4 years, and identified the presence of carotid plaques at baseline as a significant independent predictor for the progression to severe WMH [45]. Further research, and in particular well designed prospective studies, are needed to eventually assess the putative causative role for carotid atherosclerosis in the development of WMH.
5. Atrial fibrillation and WMH
Atrial fibrillation (AFib) is the most common disorder of heart rhythm [46], with currently estimated prevalence increasing with age from around 1.5% between 50 and 59 years of age to approximately 23.5% in octogenarians [39,47]. To date, AFib accounts for over 5900 DALY, representing a major public health issue [1]. Aside from determining a risk of stroke of approximately 5-fold, AFib has been consistently associated with incidence of dementia [48,49] and eventually to silent cerebral damage [50]. Interestingly, a growing amount of evidence supports an association between AFib and WMH. Kobayashi et al. first reported increased WMH in AFib patients compared to age and sex matched controls in a cohort of 142 subjects [50]. In particular, AFib patients were shown to have a more severe involvement of deep white matter, while WMH involvement of periventricular white matter did not differ from controls. Gaita and colleagues reported an increased odds ratio for the presence of WMH in AFib subjects when compared to healthy controls (OR 11.2, 95% confidence interval 6 to 21) [51]. Interestingly, in the same cohort the number of WMH was found to be higher in subjects with persistent AFib with respect to subjects with paroxysmal AFib [51], which could be related to higher burden of thromboembolic events associated with persistent AFib [52]. On the other hand, in a more recent report on 234 patients suffering from stroke, AFib conferred a significantly higher risk of increased WMH burden, which was observed selectively in the anterior subcortical white matter (OR 3.6, 95% confidence interval 1.7–7.9) [53]. Due to the specificity of the WMH pattern and the lack of relation with embolic distribution, the Authors put forward the hypothesis that the link between AFib and WMH may extend beyond thromboembolism, and in fact may be due to a more global cardiovasculopathy [53]. Finally, a report by Ntaios and colleagues on 1892 stroke subjects show that while WMH strongly predict stroke recurrence in non-AFib subjects, in AFib subjects this association is not valid [54]. Table 1 summarizes the evidence available on the relation between WMH and AFib.
6. Heart failure and WMH
Heart failure (HF) is a clinical syndrome in which the heart cannot generate a blood flow adequate to the metabolic need of the organism, or can do so only at the expense of increased filling pressures [55]. HF has been consistently associated with cognitive impairment and eventually dementia [56], possibly due to the reduced cerebral perfusion that can be granted by the failing heart [57]. In an earlier report, Vogels and colleagues showed that HF patients had ha higher burden of WMH when compared to both healthy age and sex matched controls and to patients with established cardiovascular disease and no HF [58]. Interestingly, at multivariable regression analysis only age and left ventricular ejection function were associated to more severe cerebral alterations [58]. A lower cardiac index was shown to predict a more diffuse involvement of the brain by WMH [59]. Subsequent studies confirmed an association between reduced blood flow to the brain in HF patients and more severe WMH burden [60]. Furthermore, a higher burden of WMH was shown to associate with poorer cognitive performances and depression in HF subjects [60,61]. Table 1 summarizes the evidence presented.
7. WMH and miscellaneous cardiovascular conditions
The presence of patent foramen ovale (PFO) has been shown to be associated to the development of cryptogenic ischemic stroke, possibly due to paradoxical embolism [62]. An interesting report from Ueno et al. showed that stroke patients with both PFO and atrial septal aneurysm had increased WMH burden when compared to subjects with only PFO or with no atrial septal anomaly [63]. WMH were detected in children with Eisenmenger syndrome, a life threatening condition characterized by pulmonary hypertension and cyanosis in patients with congenital heart disease [64]. Complex congenital heart disease, including transposition of the great arteries or univentricular hearts, are associated with neonatal WMH [65]. The complex cardiac surgeries that these patients require, which involve prolonged cardiopulmonary bypass times, contribute to the progression of cerebral damage [65]. See Table 1 for an overview of the evidence presented.
8. Future perspectives
The complex interplay between the cardiovascular and the nervous systems is gaining increasing attention. In particular, novel studies are exploring the relation between potential etiopathogenic factors and WMH, while in other instances a pharmacotherapy aimed at reducing the occurrence and progression of WMH is being tested. A currently recruiting study is evaluating the relation between physical exercise and WMH (see ClinicalTrials NCT02729428). A study by our group, named the Imaging of the PLAque in the Carotid arteries (IMPLAC, ClinicalTrials.gov NCT03333330) has recently completed the 18 months follow up timeframe. The IMPLAC study recruited 67 subjects with an asymptomatic carotid artery plaque determining a stenosis <70%, and is expected to identify plaque characteristics specifically associated to a progression of WMH. A randomized controlled trial comparing the efficacy of aspirin versus that of cilostazol in preventing WMH progression has currently finished enrolling 255 subjects with pre-existent WMH. The follow up time will last 2 full years and results are expected to be presented by the end of 2018 (see ClinicalTrials.gov NCT01932203). In another currently recruiting study, the LEukoaraiosis and blOod pressure reduction in OLD people (LEOPOLD study ClinicalTrials.gov NCT02472028), 820 elderly subjects will be randomized to strict blood pressure control, i.e. with a systolic blood pressure target of <135 mm Hg vs standard care. The primary endpoint will be the progression of WMH at 36 months, with the results expected by the end of 2019 (Table 2).
Table 2.
Ongoing studies concerned with the relation between cardiovascular system and cardiovascular pharmacology and white matter hyperintensities. BP = blood pressure; WMH = white matter hyperintensity.
| Institution | Population | Sample size (expected) | Intervention | Endpoint | NCT |
|---|---|---|---|---|---|
| Assistance Publique - Hôpitaux de Paris | Elderly individuals (60–88 years of age) | 820 | 1:1 randomization to intense BP control (SBP < 135 mm Hg) vs standard care | WMH progression after 36 months | NCT02472028 |
| Inha University Hospital | Individuals aged 50–85 years | 255 | Randomization to aspirin 100 mg vs cilostazol 200 mg | WMH progression after 24 months | NCT01932203 |
| University of Wisconsin, Madison | Healthy individuals aged between 18 and 75 | 90 | – | Observational. Aims at correlating the degree of physical activity with the progression of WMH | NCT02729428 |
| Università Vita-Salute San Raffaele | Individuals with carotid artery plaques <70% | 67 | – | Observational. Aims at identifying plaque characteristics associated with WMH progression at 18 months | NCT03333330 |
9. Conclusions
WMH are common in the general population, and have a definite prognostic implication. Their pathophysiology is not yet resolved, but appears to involve both hypoperfusion and neuroinflammation. Cardiovascular risk factors and established cardiovascular disease mainly influence the former, and were shown to strongly associate with the presence and progression of WMH. While the nature of this association is yet to be define, current evidence underlines the importance to take into account in the evaluation of the cardiological patient also the complex interplay between the cardiovascular and the nervous system. Interestingly, initial evidence shows that treatment of the associated conditions may influence the progression of WMH. Finally, it must be further proven if WMH can be used as meaningful surrogate of brain damage in patients at risk for cardio or athero-thrombotic events (i.e. patients with carotid plaque or AFib) to be used as secondary outcome to validate treatments or medications in clinical trials [66]. Fig. 2 summarizes the possible elements involved in WMH pathogenesis, along with their mechanism of action.
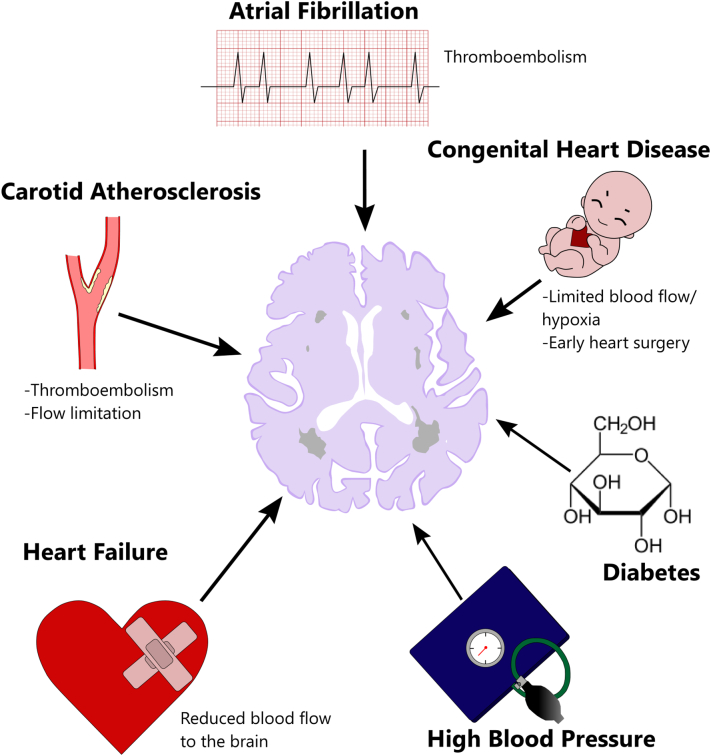
Fig. 2.
Summary of possible causative elements in white matter hyperintensities and possible mechanism of action.
Conflict of interest
The authors have no conflict of interest regarding the present work.
References
- 1.Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016Lancet (London, England) 2017;390(10100):1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardener H., Wright C.B., Rundek T., Sacco R.L. Brain health and shared risk factors for dementia and stroke. Nat. Rev. Neurol. 2015;11(11):651–657. doi: 10.1038/nrneurol.2015.195. [DOI] [PubMed] [Google Scholar]
- 3.Debette S., Markus H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341 doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw J.M., Smith E.E., Biessels G.J., Cordonnier C., Fazekas F., Frayne R. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ylikoski A., Erkinjuntti T., Raininko R., Sarna S., Sulkava R., Tilvis R. White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke. 1995;26(7):1171–1177. doi: 10.1161/01.str.26.7.1171. [DOI] [PubMed] [Google Scholar]
- 6.Garde E., Mortensen E.L., Krabbe K., Rostrup E., Larsson H.B. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet (London, England) 2000;356(9230):628–634. doi: 10.1016/S0140-6736(00)02604-0. [DOI] [PubMed] [Google Scholar]
- 7.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 8.Black S., Gao F., Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40(3 Suppl):S48–52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 9.Fernando M.S., O'Brien J.T., Perry R.H., English P., Forster G., McMeekin W. Comparison of the pathology of cerebral white matter with post-mortem magnetic resonance imaging (MRI) in the elderly brain. Neuropathol. Appl. Neurobiol. 2004;30(4):385–395. doi: 10.1111/j.1365-2990.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- 10.Love S., Miners J.S. White matter hypoperfusion and damage in dementia: post-mortem assessment. Brain Pathol. (Zurich, Switzerland) 2015;25(1):99–107. doi: 10.1111/bpa.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y., Wardlaw J.M. Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc. Neurol. 2016;1(3):83–92. doi: 10.1136/svn-2016-000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazekas F., Kleinert R., Offenbacher H., Schmidt R., Kleinert G., Payer F. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 13.Munoz D.G., Hastak S.M., Harper B., Lee D., Hachinski V.C. Pathologic correlates of increased signals of the centrum ovale on magnetic resonance imaging. Arch. Neurol. 1993;50(5):492–497. doi: 10.1001/archneur.1993.00540050044013. [DOI] [PubMed] [Google Scholar]
- 14.Simpson J.E., Wharton S.B., Cooper J., Gelsthorpe C., Baxter L., Forster G. Alterations of the blood-brain barrier in cerebral white matter lesions in the ageing brain. Neurosci. Lett. 2010;486(3):246–251. doi: 10.1016/j.neulet.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Li M., Zhang X., Shi Q., Yang S., Fan H. Higher blood-brain barrier permeability is associated with higher white matter hyperintensities burden. J. Neurol. 2017;264(7):1474–1481. doi: 10.1007/s00415-017-8550-8. [DOI] [PubMed] [Google Scholar]
- 16.Giwa M.O., Williams J., Elderfield K., Jiwa N.S., Bridges L.R., Kalaria R.N. Neuropathologic evidence of endothelial changes in cerebral small vessel disease. Neurology. 2012;78(3):167–174. doi: 10.1212/WNL.0b013e3182407968. [DOI] [PubMed] [Google Scholar]
- 17.Moody D.M., Brown W.R., Challa V.R., Anderson R.L. Periventricular venous collagenosis: association with leukoaraiosis. Radiology. 1995;194(2):469–476. doi: 10.1148/radiology.194.2.7824728. [DOI] [PubMed] [Google Scholar]
- 18.Wharton S.B., Simpson J.E., Brayne C., Ince P.G. Age-associated white matter lesions: the MRC cognitive function and ageing study. Brain Pathol. (Zurich, Switzerland) 2015;25(1):35–43. doi: 10.1111/bpa.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verhaaren B.F., Debette S., Bis J.C., Smith J.A., Ikram M.K., Adams H.H. Multiethnic genome-wide association study of cerebral white matter hyperintensities on MRI. Circ. Cardiovasc. Genet. 2015;8(2):398–409. doi: 10.1161/CIRCGENETICS.114.000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin H., Satizabal C., Xie Z., Yang Q., Huan T., Joehanes R. Whole blood gene expression and white matter hyperintensities. Mol. Neurodegener. 2017;12(1):67. doi: 10.1186/s13024-017-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nam K.W., Kwon H.M., Jeong H.Y., Park J.H., Kim S.H., Jeong S.M. High neutrophil to lymphocyte ratio is associated with white matter hyperintensity in a healthy population. J. Neurol. Sci. 2017;380:128–131. doi: 10.1016/j.jns.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Wolf P.A. Contributions of the Framingham Heart Study to stroke and dementia epidemiologic research at 60 years. Arch. Neurol. 2012;69(5):567–571. doi: 10.1001/archneurol.2011.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofman A., Brusselle G.G., Darwish Murad S., van Duijn C.M., Franco O.H., Goedegebure A. The Rotterdam Study: 2016 objectives and design update. Eur. J. Epidemiol. 2015;30(8):661–708. doi: 10.1007/s10654-015-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yano Y., Bakris G.L., Inokuchi T., Ohba Y., Tamaki N., Nagata M. Association of cognitive dysfunction with cardiovascular disease events in elderly hypertensive patients. J. Hypertens. 2014;32(2):423–431. doi: 10.1097/HJH.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 25.Strassburger T.L., Lee H.C., Daly E.M., Szczepanik J., Krasuski J.S., Mentis M.J. Interactive effects of age and hypertension on volumes of brain structures. Stroke. 1997;28(7):1410–1417. doi: 10.1161/01.str.28.7.1410. [DOI] [PubMed] [Google Scholar]
- 26.Wiseman R.M., Saxby B.K., Burton E.J., Barber R., Ford G.A., O'Brien J.T. Hippocampal atrophy, whole brain volume, and white matter lesions in older hypertensive subjects. Neurology. 2004;63(10):1892–1897. doi: 10.1212/01.wnl.0000144280.59178.78. [DOI] [PubMed] [Google Scholar]
- 27.Debette S., Seshadri S., Beiser A., Au R., Himali J.J., Palumbo C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeerakathil T., Wolf P.A., Beiser A., Massaro J., Seshadri S., D'Agostino R.B. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35(8):1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 29.Lin Q., Huang W.Q., Ma Q.L., Lu C.X., Tong S.J., Ye J.H. Incidence and risk factors of leukoaraiosis from 4683 hospitalized patients: a cross-sectional study. Medicine. 2017;96(39) doi: 10.1097/MD.0000000000007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jimenez-Conde J., Biffi A., Rahman R., Kanakis A., Butler C., Sonni S. Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke. Stroke. 2010;41(3):437–442. doi: 10.1161/STROKEAHA.109.563502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Bresser J., Kuijf H.J., Zaanen K., Viergever M.A., Hendrikse J., Biessels G.J. White matter hyperintensity shape and location feature analysis on brain MRI; proof of principle study in patients with diabetes. Sci. Rep. 2018;8(1):1893. doi: 10.1038/s41598-018-20084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira J.P., Kearney Schwartz A., Watfa G., Zohra L., Felblinger J., Boivin J.M. Memory alterations and white matter hyperintensities in elderly patients with hypertension: the ADELAHYDE-2 Study. J. Am. Med. Dir. Assoc. 2017;18(5):451.e13–451.e25. doi: 10.1016/j.jamda.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Xiong Y., Wong A., Cavalieri M., Schmidt R., Chu W.W., Liu X. Prestroke statins, progression of white matter hyperintensities, and cognitive decline in stroke patients with confluent white matter hyperintensities. Neurotherapeutics. 2014;11(3):606–611. doi: 10.1007/s13311-014-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Squair J.W., Field T.S., Phillips A.A. Journal club: relationship between carotid arterial properties and cerebral white matter hyperintensities. Neurology. 2018;90(7):338–340. doi: 10.1212/WNL.0000000000004964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rundek T., Della-Morte D., Gardener H., Dong C., Markert M.S., Gutierrez J. Relationship between carotid arterial properties and cerebral white matter hyperintensities. Neurology. 2017;88(21):2036–2042. doi: 10.1212/WNL.0000000000003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Della-Morte D., Dong C., Markert M.S., Elkind M.S.V., Sacco R.L., Wright C.B. Carotid intima-media thickness is associated with white matter hyperintensities: the Northern Manhattan Study. Stroke. 2018;49(2):304–311. doi: 10.1161/STROKEAHA.117.018943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Weerd M., Greving J.P., Hedblad B., Lorenz M.W., Mathiesen E.B., O'Leary D.H. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke. 2010;41(6):1294–1297. doi: 10.1161/STROKEAHA.110.581058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabayan B., van Buchem M.A., Sigurdsson S., Zhang Q., Meirelles O., Harris T.B. Cardiac and carotid markers link with accelerated brain atrophy: the AGES-Reykjavik Study (Age, Gene/Environment Susceptibility-Reykjavik) Arterioscler. Thromb. Vasc. Biol. 2016;36(11):2246–2251. doi: 10.1161/ATVBAHA.116.308018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markus H.S., King A., Shipley M., Topakian R., Cullinane M., Reihill S. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol. 2010;9(7):663–671. doi: 10.1016/S1474-4422(10)70120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moroni F., Ammirati E., Magnoni M., D'Ascenzo F., Anselmino M., Anzalone N. Carotid atherosclerosis, silent ischemic brain damage and brain atrophy: a systematic review and meta-analysis. Int. J. Cardiol. 2016;223:681–687. doi: 10.1016/j.ijcard.2016.08.234. [DOI] [PubMed] [Google Scholar]
- 42.Altaf N., Daniels L., Morgan P.S., Lowe J., Gladman J., MacSweeney S.T. Cerebral white matter hyperintense lesions are associated with unstable carotid plaques. Eur. J. Vasc. Endovasc. Surg. 2006;31(1):8–13. doi: 10.1016/j.ejvs.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 43.Baradaran H., Mtui E.E., Richardson J.E., Delgado D., Gupta A. Hemispheric differences in leukoaraiosis in patients with carotid artery stenosis: a systematic review. Clin. Neuroradiol. 2017;27(1):7–13. doi: 10.1007/s00062-015-0402-2. [DOI] [PubMed] [Google Scholar]
- 44.Ammirati E., Moroni F. Relation between characteristics of carotid atherosclerotic plaques and brain white matter hyperintensities in asymptomatic patients. 2017;7(1):10559. doi: 10.1038/s41598-017-11216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pico F., Dufouil C., Levy C., Besancon V., de Kersaint-Gilly A., Bonithon-Kopp C. Longitudinal study of carotid atherosclerosis and white matter hyperintensities: the EVA-MRI cohort. Cerebrovasc. Dis. 2002;14(2):109–115. doi: 10.1159/000064741. [DOI] [PubMed] [Google Scholar]
- 46.Go A.S., Hylek E.M., Phillips K.A., Chang Y., Henault L.E., Selby J.V. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 47.Wang T.J., Massaro J.M., Levy D., Vasan R.S., Wolf P.A., D'Agostino R.B. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290(8):1049–1056. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 48.Kwok C.S., Loke Y.K., Hale R., Potter J.F., Myint P.K. Atrial fibrillation and incidence of dementia: a systematic review and meta-analysis. Neurology. 2011;76(10):914–922. doi: 10.1212/WNL.0b013e31820f2e38. [DOI] [PubMed] [Google Scholar]
- 49.Santangeli P., Di Biase L., Bai R., Mohanty S., Pump A., Cereceda Brantes M. Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm. 2012;9(11):1761–1768. doi: 10.1016/j.hrthm.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi A., Iguchi M., Shimizu S., Uchiyama S. Silent cerebral infarcts and cerebral white matter lesions in patients with nonvalvular atrial fibrillation. J. Stroke Cerebrovasc. Dis. 2012;21(4):310–317. doi: 10.1016/j.jstrokecerebrovasdis.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Gaita F., Corsinovi L., Anselmino M., Raimondo C., Pianelli M., Toso E. Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. J. Am. Coll. Cardiol. 2013;62(21):1990–1997. doi: 10.1016/j.jacc.2013.05.074. [DOI] [PubMed] [Google Scholar]
- 52.Link M.S., Giugliano R.P., Ruff C.T., Scirica B.M., Huikuri H., Oto A. Stroke and mortality risk in patients with various patterns of atrial fibrillation: results from the ENGAGE AF-TIMI 48 Trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48) Circ. Arrhythm. Electrophysiol. 2017;10(1) doi: 10.1161/CIRCEP.116.004267. [DOI] [PubMed] [Google Scholar]
- 53.Mayasi Y., Helenius J., McManus D.D., Goddeau R.P., Jun-O'Connell A.H., Moonis M. Atrial fibrillation is associated with anterior predominant white matter lesions in patients presenting with embolic stroke. J. Neurol. Neurosurg. Psychiatry. 2018;89(1):6–13. doi: 10.1136/jnnp-2016-315457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ntaios G., Lip G.Y., Lambrou D., Papavasileiou V., Manios E., Milionis H. Leukoaraiosis and stroke recurrence risk in patients with and without atrial fibrillation. Neurology. 2015;84(12):1213–1219. doi: 10.1212/WNL.0000000000001402. [DOI] [PubMed] [Google Scholar]
- 55.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G., Coats A.J. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European journal of heart failure. 2016;18(8):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 56.Vogels R.L., Scheltens P., Schroeder-Tanka J.M., Weinstein H.C. Cognitive impairment in heart failure: a systematic review of the literature. Eur. J. Heart Fail. 2007;9(5):440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Meng L., Hou W., Chui J., Han R., Gelb A.W. Cardiac output and cerebral blood flow: the integrated regulation of brain perfusion in adult humans. Anesthesiology. 2015;123(5):1198–1208. doi: 10.1097/ALN.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 58.Vogels R.L., van der Flier W.M., van Harten B., Gouw A.A., Scheltens P., Schroeder-Tanka J.M. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur. J. Heart Fail. 2007;9(10):1003–1009. doi: 10.1016/j.ejheart.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Alosco M.L., Brickman A.M., Spitznagel M.B., Griffith E.Y., Narkhede A., Raz N. Independent and interactive effects of blood pressure and cardiac function on brain volume and white matter hyperintensities in heart failure. J. Am. Soc. Hypertens. 2013;7(5):336–343. doi: 10.1016/j.jash.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alosco M.L., Brickman A.M., Spitznagel M.B., Garcia S.L., Narkhede A., Griffith E.Y. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congest. Heart Fail. (Greenwich, Conn.) 2013;19(4):E29–34. doi: 10.1111/chf.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vogels R.L., Oosterman J.M., van Harten B., Gouw A.A., Schroeder-Tanka J.M., Scheltens P. Neuroimaging and correlates of cognitive function among patients with heart failure. Dement. Geriatr. Cogn. Disord. 2007;24(6):418–423. doi: 10.1159/000109811. [DOI] [PubMed] [Google Scholar]
- 62.Yaghi S., Bernstein R.A., Passman R., Okin P.M., Furie K.L. Cryptogenic stroke: research and practice. Circ. Res. 2017;120(3):527–540. doi: 10.1161/CIRCRESAHA.116.308447. [DOI] [PubMed] [Google Scholar]
- 63.Ueno Y., Shimada Y., Tanaka R., Miyamoto N., Tanaka Y., Hattori N. Patent foramen ovale with atrial septal aneurysm may contribute to white matter lesions in stroke patients. Cerebrovasc. Dis. (Basel, Switzerland) 2010;30(1):15–22. doi: 10.1159/000313439. [DOI] [PubMed] [Google Scholar]
- 64.Dokumaci D.S., Dogan F., Yildirim A., Boyaci F.N., Bozdogan E., Koca B. Brain metabolite alterations in Eisenmenger syndrome: evaluation with MR proton spectroscopy. Eur. J. Radiol. 2017;86:70–75. doi: 10.1016/j.ejrad.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Bertholdt S., Latal B., Liamlahi R., Pretre R., Scheer I., Goetti R. Cerebral lesions on magnetic resonance imaging correlate with preoperative neurological status in neonates undergoing cardiopulmonary bypass surgery. Eur. J. Cardiothorac. Surg. 2014;45(4):625–632. doi: 10.1093/ejcts/ezt422. [DOI] [PubMed] [Google Scholar]
- 66.Ammirati E., Scotti I., Camici P.G. Can silent brain lesions be a target to guide anticoagulation treatment in patients with low-risk atrial fibrillation to reduce cognitive impairment? J. Am. Coll. Cardiol. 2014;63(20):2174–2175. doi: 10.1016/j.jacc.2013.12.044. [DOI] [PubMed] [Google Scholar]