Synopsis
Obesity is common in women of childbearing age and management of this population around the time of pregnancy involves specific challenges. Weight and medical comorbidities should be optimized both prior to and during pregnancy. During pregnancy, gestational weight gain should be limited, comorbidities should be appropriately screened for and managed, and fetal health should be monitored. Consideration should be given to the optimal timing of delivery, and to reducing surgical and anesthetic complications. In the postpartum period, breastfeeding and weight loss should be promoted. Maternal obesity is associated with adverse metabolic effects in offspring, promoting an intergenerational cycle of obesity.
Keywords: Obesity, pregnancy, pre-conception, intergenerational obesity
INTRODUCTION
The high rates of obesity in women of child bearing age, has made obesity the most common medical problem in pregnancy. According to the 2013–2014 NHANES data, 37% of women between the ages of 20–39 have obesity and rates continue to rise.1 The rates vary dramatically by ethnic group, from 10% of Asian women, 33% of non-Hispanic white women, 43% of Hispanic women and 57% of non-Hispanic black women.1 Women with obesity are at a much higher rate of poor obstetrical outcomes across the continuum of reduced fertility, pregnancy complications and postpartum adverse events, of which some, but not all, are preventable through targeted medical care. The lack of good evidence for intervention, has resulted in differences across national clinical practice guidelines.2
In addition to being more at risk, women with obesity may suffer discrimination and humiliation at a time that should be joyful. In a study of obstetrics providers, 31% identified that they had made derogatory comments about obese women to colleagues and 66% felt more derogatory comments are made about women with obesity than those without obesity.3 Providers who care for women with obesity who of child bearing age need to identify strategies and tools that promote open, non-judgmental communication about the risks in pregnancy and provide safer care through adequate resources, specialized equipment and structured protocols.4–7
GOALS AND STRATEGIES
Preconception
Improve Fertility
Obesity in females is associated with subfertility and with a longer time to achieve pregnancy.8–10 Although partially explained by the higher prevalence of the polycystic ovarian syndrome (PCOS),11 which is characterized by anovulation and hyperandrogenism,12 the association between infertility and obesity exists even in women with ovulatory menstrual cycles.8–10 In addition, women with obesity have higher miscarriage rates13 and those using assisted reproductive technologies (ART) such as in-vitro fertilization (IVF) seem to have decreased pregnancy and live birth rates compared to those with normal BMI.14 Thus, obesity is associated with numerous factors that decrease the likelihood of achieving and maintaining a pregnancy.
There is a paucity of rigorous studies evaluating interventions to improve fertility in women with obesity. Observational evidence suggests that lifestyle interventions may improve pregnancy and live birth rates prior to undergoing ART.15 One multicenter randomized controlled trial (RCT) evaluated the effect of a 6 month lifestyle intervention followed by 18 months of infertility treatment in infertile women with BMI >29, compared to a control group receiving 24 months of prompt infertility treatment.16 The control group had a higher frequency of the primary outcome, a vaginal term birth of a healthy singleton, than the intervention group (35.2% vs. 27.1%), although women in the intervention group were more likely to achieve conception without infertility treatments. In contrast, in women with obesity and PCOS, post hoc data aggregation from two separate RCTs suggests that lifestyle intervention for weight loss prior to ovulation induction with clomiphene citrate increases live birth rate compared to immediate ovulation induction.17 Observational studies suggest that bariatric surgery improves fertility in women with obesity,18 however infertility is not considered an indication for bariatric surgery.19,20 Thus, infertility treatments are most effective at achieving live birth in women with obesity, however, in younger women in whom there is less urgency to conceive, weight loss via lifestyle interventions is a reasonable first step for infertility management, as it is likely associated with other benefits.
Preconception Weight Loss
Guidelines advise weight loss prior to conception for women with obesity.21,22 The majority of evidence supporting this recommendation comes from studies of women who have undergone bariatric surgery. Pregnant women who have previously undergone bariatric surgery are less likely to develop gestational diabetes mellitus (GDM), hypertensive disorders of pregnancy (HDP), postpartum hemorrhage, and fetal macrosomia, compared to controls who have not undergone bariatric surgery.23 However, the risks of preterm birth and having a small for gestational age (SGA) newborn are increased.23–25 Given that interventions during pregnancy have been found to be relatively ineffective in preventing comordities such as GDM,26 studies are currently underway to evaluate whether pre-pregnancy lifestyle interventions will be more effective in reducing adverse pregnancy outcomes in women with obesity.27
Current guidelines recommend a waiting period of 12–24 months following bariatric surgery before conceiving.19,22,28 Reduced absorption of oral hormonal contraception may occur following bariatric surgery,20 and alternate forms of contraception should be prescribed.19 Women who have previously undergone bariatric surgery require careful monitoring during pregnancy, as there is a risk of nutrient deficiencies21and anatomic surgical complications in this population.22 The benefits of postponing pregnancy to undertake bariatric surgery must also be weighed against the risk of declining fertility as maternal age increases.22
Congenital Anomaly Reduction
There is a “dose-dependent” increase in the rate of congenital anomalies among offspring of women with obesity. There are more neutral tube defects (OR 1.87; 95%CI 1.62–2.15), heart defects (OR 1.15; 1.07–1.23; including left and right ventricular outflow defects and hypoplastic left heart syndrome, but not conotruncal defects), cleft lip and/or palate (OR 1.20; 95% CI 1.09–1.31), anorectal atresia (OR 1.48; 95% CI 1.12–1.97), hydrocephaly (OR 1.68; 95% CI 1.19–2.36) and limb reduction anomalies (OR 1.34; 95% CI 1.03–1.73).29–31 The exact cause for this increased risk remains debatable, but includes abnormal glucose metabolism and nutrient deficiencies.32
Targeted management strategies are needed, but evidence for these remains elusive. Human organogenesis is largely complete by 9 weeks gestational age. While most women do access prenatal care in the first trimester, true prevention must begin pre-conceptually. Most importantly, abnormal glucose metabolism must be identified and glycemic control optimized prior to conception. Folic acid supplementation has been shown to reduce congenital anomalies. Women with obesity are less likely to take preconception supplementation which may be related to other health behaviours or unplanned pregnancies.33 Also, BMI may affect the distribution of folate, with obese women having lower serum levels relative to RBC folate levels.34 While further study into optimal nutritional supplementation is needed for women with obesity, current guidelines differ in recommendations with some suggesting 5 mg and others recommending 0.4 mg of folate.2,35 Consideration should be given to recommending additional folic acid compared to normal weight for three months prenatally and until 12 weeks’ gestation and vitamin D (10µg daily during pregnancy).35,36
Detect and Optimize comorbidities
Women with obesity are more likely to have other comorbidities including T2DM, hypertension, hyperlipidemia, sleep apnea and nonalcoholic steatohepatitis (NASH) which may or may not have been previously identified and treated. Each of these conditions can contribute to poor obstetrical outcomes and may worsen as part of the normal physiological changes in pregnancy. It is essential that the woman be screened for these prior to pregnancy allowing for investigations to be completed and treatment adjusted for upcoming planned pregnancy. Although a detailed discussion is out of scope for this article, key points for optimizing outcomes for each of these conditions are listed in Box 1.
Box 1. Key points for screening for and optimizing preexisting comorbidities.
- Hyperglycemia
- Screen: using HgA1c and/or fasting glucose prior to pregnancy.
- Prior to pregnancy:
-
◦target A1c of <7% to reduce congenital anomalies
-
◦Discontinue medications without known safety profile including SGLT2 inhibitors, DPP4 inhibitors, GLP-1 analogues
-
◦Consider discontinuing sulfonlyureas and metformin (unless metformin being used for ovulation induction)
-
◦Optimize insulin and self management skills
-
◦Assess for microvascular complications
-
◦
- Hypertension58
- Screen: if indicated, screen for secondary causes of hypertension
- Prior to pregnancy:
- Discontinue medications without known safety profile including ACE Inhibitors, Angiotensin receptor blockers, aldosterone antagonists
- Consider using labetalol, methyldopa, nifidepine
- Hyperlipidemia
- Screen: reevaluate indication for treatment as safety during pregnancy not established
- Prior to pregnancy:
- Optimize nutritional management, especially for cases of severe hypertriglyceridemia
- Consider discontinuing lipid lowering agents unless there is a clear indication
Optimize: lifestyle modifications, if treated medically with pioglitazone or other agents consider discontinuation if there is no safety data in pregnancy
Pregnancy
Limit Gestational Weight Gain
Excess GWG is associated with adverse outcomes including increased risk of developing GDM, T2DM and HDP,37 elevated infant birth weight and adiposity, and increased risk of metabolic syndrome and childhood obesity in offspring.38 Women with pre-pregnancy overweight and obesity are more likely to gain excess weight during pregnancy.39,40 One Canadian study found that 47% of normal weight women compared to 78% of overweight and 72% of women with obesity exceeded recommended GWG.40
The Institute of Medicine (IOM) recommends gestational weight gain ranges based on maternal pre-pregnancy BMI (see Table 1), with less weight gain recommended for higher BMI categories.41 A systematic review found that in women with obesity, less GWG than that recommended by the IOM is associated with increased risk of preterm birth and having a small for gestational age infant, but also with reduced risk of macrosmoia, HDP, and cesarean delivery.42 Some studies have even suggested that weight loss in women with obesity during pregnancy may be associated with some reduced adverse outcomes.43,44 Professional guidelines state that while recommendations must be individualized, women with obesity who are gaining less weight than recommended by the IOM need not increase their weight gain if fetal growth is adequate.45
Table 1.
Recommended Gestational Weight Gain by Pre-pregnancy BMI
| Prepregnancy BMI |
BMI (kg/m2) |
Total Weight Gain Range (lbs) |
Rates of Weight Gain 2nd and 3rd Trimester (Mean Range in lbs/wk) |
|---|---|---|---|
| Underweight | <18.5 | 28–40 | 1 (1–1.3) |
| Normal Weight | 18.5–24.9 | 25–35 | 1 (0.8–1) |
| Overweight | 25.0–29.9 | 15–25 | 0.6 (0.5–0.7) |
| Obese (Includes all classes) | ≥30.0 | 11–20 | 0.5 (0.4–0.6) |
From Committee to Reexamine IOM Pregnancy Weight Guidelines. Institute of Medicine and National Research Council. Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: The National Academies Press; 2009; with permission.
GWG targets should be calculated and discussed with women early in pregnancy.21,46 A recent systematic review and meta-analysis evaluated RCTs of antenatal interventions for preventing excess GWG and found that diet, exercise or diet plus exercise interventions reduced the risk of excess GWG by an average of 20% (RR 0.80, 95% CI 0.73–0.87).47 The results also suggested a lower risk of cesarean section, maternal hypertension, macrosomia, and newborn respiratory distress syndrome in mothers who received the interventions. Pregnant women with obesity should receive diet and exercise counseling to assist with managing GWG.21 After the treating clinician has ruled out any contraindications to exercise,48 an eventual goal of moderate-intensity exercises for 20–30 minutes/day on most days of the week can be advised.48 An RCT evaluating the use of metformin vs. placebo starting at 12–18 weeks gestation in pregnant women with obesity but without diabetes resulted in reduced median GWG in the metformin group (4.6 kg vs. 6.3 kg) but no difference in the primary outcome of neonatal birth-weight z score. However, there was no significant difference in neonatal or obstetrical outcomes, and thus, the use of metformin to reduce GWG in women with obesity cannot be routinely recommended.
Screen early for hyperglycemia
Women with obesity who are not known to have T2DM should be screened at the first antenatal visit for hyperglycemia. There are two strategies for testing glucose levels in early pregnancy – using the non-pregnancy recommended screening tests (fasting plasma glucose (FPG) or A1C) or using the typical 24–28 week GDM screening criteria.49 There has been no rigorous validation that criteria accepted for the diagnosis of GDM in the second or third trimester are appropriate for use in the first trimester. A fasting glucose >7.0 mmol/l or A1c >=6.5% should be diagnosed as likely overt diabetes and treatment implemented. However, both FPG and A1C decrease early in pregnancy and may lead to under-diagnosis of pre-existing diabetes. One study screened 16,122 women at a median of 47 days gestation, and found higher rates of major congenital anomalies (RR 2.67, 1.28–5.53), preeclampsia (RR 2.42, 1.28–5.53), shoulder dystocia (RR 2.47, 1.05–5.85) and perinatal death (RR 3.96,1.54–10.16) with an A1C of 5.9–6.4% in the first trimester.50 Although consideration can be given to treatment of women with HbA1c 5.9–6.4% in the first trimester, whether intervention earlier in pregnancy makes a difference remains unknown. Unfortunately the lack of rigourous data has resulted in different professional groups and organizations having different criteria for diagnosis of early dysglycemia.51,52 All women with overt diabetes diagnosed during pregnancy, should be retested postpartum as up to 41% will return to normal postpartum.53
Reduce Hypertensive Disorders of Pregnancy
Maternal obesity is associated with increased risk of preeclampsia and gestational hypertension, and the risk increases as BMI increases.9,54,55 Observational studies demonstrate an inverse association between maternal exercise and preeclampsia risk,56,57 and the risk of maternal hypertension, but not preeclampsia specifically, was reduced in RCTs of diet and/or exercise interventions during pregnancy.47 However, the role of exercise in pregnancy for preeclampsia prevention is felt to be unclear.58,59 Strategies for prevention of preeclampsia should be considered, including recommending both ASA 81mg daily (taken orally at bedtime from the time pregnancy is diagnosed until 37 weeks’ gestation) and adequate calcium intake.59,60
Improve Fetal Surveillance
Aneuploidy detection is now performed using either traditional first-trimester screening (IPS or its variations) or noninvasive prenatal testing (NIPT). Interestingly, the risk of Trisomy 21 is increased among women with obesity.61 A higher BMI does not affect the rate of positive first-trimester screening,61 making such testing appropriate. Fetal cell-free DNA levels are inversely proportional to gestational weight and mothers with obesity should be advised that there is a higher rate of insufficient DNA levels, necessitating a second blood draw for NIPT testing.62
The ability of screening ultrasound to detect genetics syndromes, fetal anomalies and non-reassuring fetal wellbeing status is substantially reduced in this population. In the FaSTER trial, maternal obesity was associated with a 10% higher false-negative rate for the detection of two more soft markers of aneuploidy, a lower rate of detection of congenital anomalies in general (aOR 0.7, 95% CI 0.6–0.9) and a lower detection rate of congenital heart anomalies (8.3% versus 21.6%).63 There is often an inability to both image completely and detect anomalies of cardiac and craniospinal structures in this population.64,65 The limitations of prenatal ultrasound screening should be recognized and the women counseled accordingly.
Fetal surveillance is often indicated in later gestation to ensure fetal wellbeing. Common indications include concurrent diabetes and hypertension, but consideration may also be given to performing biophysical profile or nonstress tests on women with obesity in general, given their increased risk of fetal distress and stillbirth. Although it may be more difficult to physically perform such testing on women with obesity, they are not more likely to have nonreactive non-stress tests or to require additional time to a normal non-stress test.
Minimize Risk of Preterm Birth
The risks of both overall preterm birth and extremely preterm births are increased among women with obesity. These preterm deliveries are often iatrogenic and related to need for delivery due to medical comorbidities like hypertension and diabetes. In general, obesity is associated with prolonged pregnancy, but there appears to be an interesting increase in spontaneous extremely preterm labour (<28 weeks’ gestation) as shown in a population-based cohort study of women from Sweden (see table 2).66 This rise was attributed to increased inflammatory markers and increased risk of intrauterine bacterial infection/chorioamnionitis.
Table 2.
Extremely Preterm Birth Among Women with Obesity in Sweden from 1992–2010
| BMI (kg/m2) | Rate (%) | Odds Ratio (95% CI) |
|---|---|---|
| 18.5–<25 | 0.17 | |
| 25–<30 | 0.21 | 1.26 (1.15–1.37) |
| 30–<35 | 0.27 | 1.58 (1.39–1.79) |
| 35–<40 | 0.35 | 2.01 (1.66–2.45) |
| 40+ | 0.52 | 2.99 (2.28–3.92) |
Adapted from Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA 2013;309(22):2364; with permission.
Evidence for prevention of preterm birth among women with obesity is limited. Optimizing underlying medical conditions like diabetes and hypertension and incorporating strategies for prevention of preeclampsia should be considered. In women who are symptomatic or who have had a prior preterm birth, confirmed bacterial vaginosis should be treated with oral Metronidazole or Clindamycin for seven days.
Labour and Delivery
Reduce still birth risk through timing of delivery
Determining the optimal timing of delivery of women with obesity is complex. Multiple studies have now shown a consistent increase in the risk of stillbirth among women with obesity, at all gestational ages. Overall, a 10-unit increase in pre-pregnancy BMI appears to be associated with a 1.5- to2-fold increase in stillbirth risk.67 Rates increase proportionately to BMI from an odds ratio of 1.37 (95% CI 1.02–1.85) for overweight women to an odds ratio of 5.04 (1.79–14.07) for women with a BMI higher than 50.68 The lowest rates of neonatal death and cerebral palsy are associated with delivery at 39 weeks’ gestation.69 The lowest rates of intrauterine demise and brachial plexus injury can be obtained by delivering earlier. A recent decision analysis suggested that the optimal gestational age of delivery may be 38 weeks – in the theoretical population of 100,000 singleton pregnancies in women with obesity, elective delivery at 38 weeks would prevent 203 intrauterine demises compared with expectant management until 41 weeks.69
When the decision to proceed with delivery, whether for medical indications or to reduce the risk of stillbirth, has been made, the mode of delivery must be considered. Among women who have obesity, term elective induction of labour appears to actually decrease the risk of Cesarean delivery, particularly in multiparous women, without increasing the risk of adverse outcomes, including operative vaginal delivery, lacerations or neonatal respiratory distress syndrome (Lee II).70 Thus, induction of labour at 38–39 weeks is currently preferred to elective Cesarean section for appropriate candidates.
Reduce Surgical Complications
Cesarean delivery is intuitively more difficult and risk-prone in women with obesity. Preparation is crucial – in addition to the usual requirements for Cesarean delivery, the availability of specialized equipment (eg. Retractors) is beneficial to both surgical team and patient. Surgeons should be familiar with and respect the weight capacity of wheelchairs, operating tables and other equipment, such as commodes. The presence of a large pannus can alter the anatomy of the abdominal wall considerably and strategies are needed to reduce risk (Table 3). As the umbilicus is usually more caudad than normal, the ideal position of the incision should be determined from more stable landmarks, including the symphysis pubis, the iliac wings and the fundus. The ideal choice of skin incision is still debated and should be individualized. A transverse skin incision can be made above or below the pannus and offers increased wound strength, reduced postoperative pain and improved respiratory status postpartum.71,72 However, a transverse incision makes retraction and delivery of the fetus more difficult because of the pannus. The climate underneath the pannus also results in frequent wound infection. A vertical skin incision allows for better visualization of the surgical field and easier wound care postpartum, but causes more pain, resulting in decreased respiratory effort.73 Vertical incisions do not necessarily decrease the risk of wound infection.73
Table 3.
Useful Strategies for Cesarean Section of Women with Obesity
| Strategy | Result |
|---|---|
| Pre-treatment of the skin under the pannus with antibacterial/antifungal dressings for 1–2 weeks | Improved skin health and decreased bacterial load |
| Skin cleansing with iodine or chlorhexidine | Decreased bacterial load |
| Antibiotics within 60 minutes of incision | Lower wound infection rates |
| Closure of the subcutaneous tissue with sutures when the fat thickness exceeds 2cm | Decreased wound disruption by 34%151 |
| Retraction of the pannus cephalad | Improved ease of transverse skin incision |
| Suturing of the skin incision | Decreased wound infection and wound separation rates152 |
Improve safety of Anaesthesia
Anesthetic risks are increased in women with obesity, for both regional techniques (epidural and spinal) and general anesthesia. Consultation with anesthesia during the third trimester is often very helpful to allow for risk assessment, additional testing (such as EKG or sleep studies) and patient counselling and expectation setting.
Placement of regional techniques is often challenging because the usual landmarks may be difficult to find due to adiposity. These challenges may lead to multiple attempts at needle insertion and, ultimately, a higher failure rate. Ultrasound-guided neuraxial analgesia is sometimes helpful.
General anesthesia is avoided whenever possible, but is more common with increasing obesity. Intubation is made more difficult by increased breast mass, increased chest diameter and exaggerated airway edema. These features result in a diagnosis of difficult intubation in up to 33% of women with obesity.74
Postpartum
Reduce Postpartum hemorrhage
The risk of postpartum hemorrhage is approximately doubled in women who are overweight or have obesity, an effect that is seen after both vaginal delivery (OR 2.11, 95% CI 1.54–2.89) and Cesarean section (OR 1.73, 95% CI 1.32–2.28).75 When the effects of perineal laceration and retained placenta are controlled for, the elevated risk can be attributed to uterine atony. The risk of postpartum hemorrhage is increased with increased infant birthweight, antepartum hemorrhage and Asian ethnicity.
Before delivery, iron stores should be optimized by providing oral or parenteral iron as needed. In addition to the usual practices of active management of the third stage, increased vigilance and preparation for postpartum hemorrhage are advised and additional uterotonics should be available, including Carbetocin, ergotamine, misoprostol and hemabate. Internal uterine compression is a third line treatment option.
Prevent Venous Thromboembolism
Women with a prepregnancy BMI > 30 kg/m2 who have undergone an emergency cesarean section are considered to be at high risk for postpartum venous thromboembolism (VTE).76,77 Guidelines recommend that either prophylactic low-molecular-weight-heparin (LMWH) or mechanical prophylaxis, such as elastic stockings or intermittent pneumatic compression, be used in this population while in hospital following delivery.76,77 In women with obesity who undergo a non-emergent cesarean section, VTE prophylaxis is recommended only in the presence of at least one additional risk factor for VTE, such as preeclampsia or fetal intrauterine growth restriction.76,77 LMWH is considered to be safe in women who are breastfeeding.76
Improve Breastfeeding rates
Guidelines recommend that babies be breastfed exclusively for the first 6 months of life, followed by ongoing breastfeeding up to at least 1–2 years of age.78,79 Breastfeeding is particularly beneficial for mothers with obesity, as it is associated with improved future cardiovascular risk in mothers,80 reduced risk of future T2DM,81,82 and decreased visceral adiposity in later life.83,84 Some,83,85 but not all86,87studies have reported less postpartum weight retention and future risk of obesity with breastfeeding.
Women with obesity are less likely than normal weight women to both initiate and maintain breastfeeding.88,89 This has been attributed to delayed onset of milk production, higher prevalence of insufficient breast glandular tissue, and psychosocial factors such as reduced confidence to breastfeed.90 There is some evidence that increased postpartum breastfeeding support can increase breastfeeding exclusivity and duration.90 The potential for breastfeeding challenges should be discussed with women prior to delivery, and resources such as lactation consultant services made available to assist with breastfeeding difficulties.
Reduce Postpartum Weight Retention
Postpartum weight retention is of particular concern in women with obesity who are planning future pregnancies.21 In a Swedish population-based cohort study that evaluated BMI changes between first and second pregnancies, the risk of stillbirth in the second pregnancy was found to increase linearly with interpregnancy increase in BMI.91 Similarly, interpregnancy weight gain is associated with increased risk of gestational hypertension and preeclampsia.92 Weight gain between pregnancies is associated with an increased risk of GDM in a subsequent pregnancy, while a weight loss of just 10 pounds is associated with reduced GDM risk.93 Weight loss between pregnancies in women with obesity decreases the risk of having a large-for-gestational age offspring in next pregnancy,94 and improves chances of a vaginal delivery after a previous cesarean section.95
A recent systematic review found that dietary interventions and diet with exercise interventions improved postpartum weight loss, as opposed to exercise only interventions.96 There was no evidence that these interventions had any adverse effect on maternal breastfeeding success. It is recommended that postpartum contraception and planning for future pregnancies be encouraged in women with obesity so that weight can be optimized between pregnancies.21
NEW EVIDENCE LINKING MATERNAL OBESITY AND LONG-TERM IMPLICATIONS TO OFFSPRING
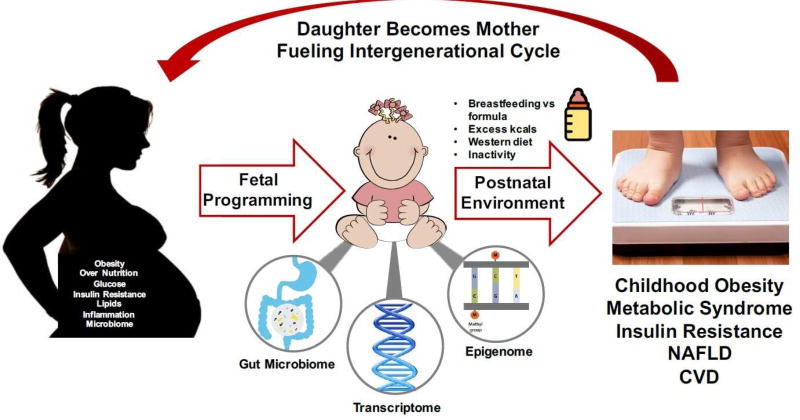
The number of infants and young children with overweight or obesity has tripled between 1990 to 201297 and is rising in parallel with rates of maternal obesity. Given that half of childhood obesity occurs by age 5, early life events may be contributing to pediatric obesity development.98 Many studies have reported strong associations between intrauterine exposure to maternal obesity and excess GWG with adiposity at birth99,100 and offspring development of obesity in childhood101,102 and adulthood103 (Figure 1). For example, increased adiposity at birth (but not birth weight) was correlated to adiposity at 6–11 years of age by DXA in offspring from a cohort of 89 mothers with either normal glucose tolerance or GDM.102 Childhood adiposity did not correlate with maternal GDM exposure but instead was strongly related to maternal obesity with an OR ~5.5.
Figure 1.
Maternal obesity is associated with adverse metabolic effects in offspring, promoting an intergenerational cycle of obesity.
In utero exposure to maternal obesity also increases obesity co-morbidities in offspring such as insulin resistance and changes in mitochondrial function,104–106 cardiovascular disease,107,108 and nonalcoholic fatty liver disease (NAFLD).109–111 NAFLD affects ~34% of children with obesity ages 3–18 and half have already progressed to the more severe NASH at time of diagnosis.112,113 Newborns at ~2 weeks of age who were born to mothers with obesity and GDM mothers demonstrated 68% more intrahepatic fat compared to the newborns from normal weight mothers and was correlated with maternal BMI (r = 0.05, p = 0.02).110 Whether early deposition of lipid in the fetal liver could prime it to be more susceptible to the postnatal influences of an unhealthy lifestyle resulting in NAFLD is unknown. The powerful influence of an intrauterine environment characterized by nutrient excess and obesity is also underscored by the marked decrease in the risk of obesity in children born to women with obesity who underwent bariatric surgery before their pregnancy as compared to their siblings who were born prior to their mother receiving bariatric surgery.114,115
An intrauterine environment characterized by nutrient excess or obesity imparts long-term programming of offspring obesity risk,116 especially in babies born large for gestational age (LGA). Umbilical cord derived mesenchymal stem cells from infants born to mothers with obesity demonstrated greater capacity to develop into adipocytes. These findings suggest that progenitor cells that differentiate into various tissue types such as adipose tissue, skeletal muscle, or chondrocytes may already be detrimentally programmed in utero.117
Epigenetics as a Mechanism and Indicator of Fetal Programming
Metabolic programming can occur via gene-environment interactions that may produce epigenetic events. These intrauterine exposures may silence or augment gene expression to impact fetal brain function and organ development which impart risk for developing chronic disease(s).118–121 Maternal nutrition can alter DNA methylation in infant tissues such as buccal cells,122 umbilical cord blood,123,124 and umbilical cord.125 Preconception maternal diet and nutritional status may be key determinants of the fetal epigenome [30, 31].126,127 Studies on mother-infant dyads in the Gambia found that seasonal variation (rainy versus dry) in maternal diet at the time of conception altered DNA methylation at metastable epialleles (MEs) in infants at 2–8 months of age.126,127 These changes correlated with maternal plasma levels of key methyl-donor pathways (e.g. methionine, choline, folate, homocysteine, B vitamins). DNA methylation at MEs is stochastically established during very early embryogenesis and are particularly sensitive to early maternal exposures, like nutrition and obesity. This early establishment of methyl groups at specific MEs allows methylation to be stably maintained systemically across cell lineages during differentiation. However, it still remains unclear whether maternal obesity, GWG, and maternal nutrition can permanently modify methylation patterns resulting in lasting changes in gene expression.
Potential Role of the Gut Microbiome in Maternal and Infant Obesity
Recently the gut microbiome has garnered significant attention as another mode of transmitting obesity risk from mothers to offspring. A seminal study described the effect on a germ free mouse when a first trimester (insulin sensitive) versus third trimester (insulin resistant) human microbiome was transplanted into a germ free mouse.128 The mouse receiving the 3rd trimester microbiome became fatter and demonstrated insulin resistance and gut dysbiosis compared to the mouse who received the first trimester microbiome. The exposure of the fetal intestine to maternal microbes at childbirth and possibly through amniotic fluid is an important contributor to gut maturation and, by extension, to infant health. Animal and human data strongly suggest that the composition of the neonatal gut microbiota is dependent both on maternal obesity and maternal diet during pregnancy and lactation129 as well as mode of delivery.130
The microbiome plays a major role in nutrition, extraction of energy, metabolism, protection against pathogens, resistance to infections, and immune system development. Studies in non-human primates have shown distinct effects of maternal high fat diet on offspring microbiota, as well as a decrease in overall bacterial diversity when compared to primates fed a control diet.129,131,132 While the exact implications of these changes in microbiota are not fully known, decreased bacterial diversity is associated with adiposity, insulin resistance, dyslipidemia, and low grade inflammation in humans.133,134 Another provocative study in primates has shown that after weaning, dysbiosis is only partially corrected by a controlled low fat diet,131,135 demonstrating the lasting effects of a high fat maternal diet on the microbiome of the offspring. Human studies showed that the microbiome from newborns at 2 weeks of age born to mothers with obesity exhibited less gammaproteobacteria, an early colonizing bacteria essential for the development of immune tolerance and a higher trend in bacilli class in the firmacutes phylum, a high consumer of choline, which may be highly relevant since low choline is associated with the development of NAFLD.136 The breast milk of women with obesity contains higher levels of insulin and leptin which may be able to pass through more permeable intestinal gap junctions in the newborn, potentially affecting appetite regulation, microbiome development, immune tolerance, and infant body composition and growth.136
Early microbes from infants born to women with obesity or GDM may contribute to long-term health risks by triggering pro-inflammatory remodeling of the innate and adaptive immune system as well as other organs and tissues in the neonate.137 Dysbiosis of the gut microbiome has been correlated with NAFLD in children and adults. However, if and how the early life microbial composition influences hepatic fat accumulation and inflammation before the disease occurs is unclear. Attempts to alter the infant microbiome by modifying the maternal microbiome by diet changes or pre- or probiotics in pregnancy have shown mixed results in prevention of GDM and GWG.138,139 A large Australian RCT enrolling more than 500 women (SPRING) was recently completed using the same probiotic and the results should be available soon.140
It is clear the –omics such as epigenetics, microbiome, and transcriptomics can advance our understanding of how obesity risk is transmitted to offspring. Metabolomics is another rising –omics platform that can provide information on macro- and micro-nutrient fluxes through metabolic pathways that can be altered by maternal diet or obesity, which could affect the offspring.141,142 There is also emerging evidence driven by animal studies for paternal obesity influencing offspring risk.143,144 Clinical studies suggest that sperm are altered by obesity145 such that fathers may no longer be out of the loop in being metabolically accountable to their offspring!
CONCLUSION
It is extremely important for clinicians to discuss pregnancy plans with women with obesity well in advance of conception in order to ensure that medical comorbidities and medications can be optimized before pregnancy. Weight management prior to pregnancy should be promoted with the aim of improving both maternal and fetal health. During pregnancy, careful screening for maternal and fetal complications should take place, with consideration given to preventative strategies where appropriate. Recognition of the increased risk of stillbirth in this population should lead to careful consideration of the risks and benefits of induction of labour around 38–39 weeks of gestation. In the postpartum period, promotion of breastfeeding and reducing postpartum weight retention should be recommended to women with obesity to improve future health and reduce adverse events in future pregnancies.
There is increasing recognition of the adverse metabolic effects on the offspring of women with obesity, which has large implications for future generations. Further research is needed to determine how best to attenuate these negative effects in order to halt and hopefully reverse the increasing prevalence of obesity and metabolic disease.
Key points.
Weight and obesity-related comorbidities should be optimized in women with obesity prior to conception.
Special considerations in pregnant women with obesity include optimization of gestational weight gain, prevention and management of gestational diabetes and hypertensive disorders of pregnancy, and being aware of risks to fetal health.
Labour and delivery in women with obesity carries increased risk of surgical and anesthetic complications.
Postpartum considerations in women with obesity include prevention of complications, reduction of postpartum weight retention, and breastfeeding promotion.
There is emerging evidence of adverse metabolic effects on the offspring of women with obesity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors have no relevant conflicts of interest to disclose.
Contributor Information
Heidi Pauline Dutton, University of Ottawa, 1967 Riverside Dr., Ottawa On Canada, K1h7W9, hdutton@toh.ca, 613 738 8400 ext 81946.
Sarah Jean Borengasser, University of Colorado – Anschutz, 12631 E. 17th Ave. Mailstop F561, Aurora, CO 80045, USA, sarah.borengasser@ucdenver.edu, 303 724 9550.
Laura Marie Gaudet, University of Ottawa, 1053 Carling Ave, Ottawa On Canada, K1Y 4E9, lagaudet@ohri.ca, 613 737 8899 ext 73056.
Linda A Barbour, Professor of Endocrinology and Maternal-Fetal Medicine, University of Colorado School of Medicine, 12801 E 17th Ave RC1 South Room 7103, Aurora, CO 80405, Lynn.barbour@ucdenver.edu, 303 724 3921.
Erin Joanne Keely, University of Ottawa, 1967 Riverside Dr., Ottawa On Canada, K1h7W9, ekeely@toh.ca, 613 738 8400 ext 81941.
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kominiarek MA, Chauhan SP. Obesity Before, During, and After Pregnancy: A Review and Comparison of Five National Guidelines. Am J Perinatol. 2016;33(5):433–441. doi: 10.1055/s-0035-1567856. [DOI] [PubMed] [Google Scholar]
- 3.Grohmann B, Brazeau-Gravelle P, Momoli F, Moreau K, Zhang T, Keely EJ. Obstetric healthcare providers' perceptions of communicating gestational weight gain recommendations to overweight/obese pregnant women. Obstetric Medicine. 2012;5(4) doi: 10.1258/om.2012.120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinsdale S, Branch K, Cook L, Shucksmith J. "As soon as you've had the baby that's it…" a qualitative study of 24 postnatal women on their experience of maternal obesity care pathways. BMC Public Health. 2016;16:625. doi: 10.1186/s12889-016-3289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heslehurst N, Lang R, Rankin J, Wilkinson JR, Summerbell CD. Obesity in pregnancy: a study of the impact of maternal obesity on NHS maternity services. BJOG. 2007;114(3):334–342. doi: 10.1111/j.1471-0528.2006.01230.x. [DOI] [PubMed] [Google Scholar]
- 6.Mills A, Schmied VA, Dahlen HG. 'Get alongside us', women's experiences of being overweight and pregnant in Sydney, Australia. Matern Child Nutr. 2013;9(3):309–321. doi: 10.1111/j.1740-8709.2011.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canadian Obesity Network. [Accessed May 27, 2017];5 A's of healthy pregnancy weight gain. http://www.obesitynetwork.ca/pregnancy.
- 8.Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414–420. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nohr EA, Timpson NJ, Andersen CS, Davey Smith G, Olsen J, Sorensen TI. Severe obesity in young women and reproductive health: the Danish National Birth Cohort. PLoS One. 2009;4(12):e8444. doi: 10.1371/journal.pone.0008444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324–328. doi: 10.1093/humrep/dem371. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Blasco F, Botella-Carretero JI, San Millan JL, Escobar-Morreale HF. Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch Intern Med. 2006;166(19):2081–2086. doi: 10.1001/archinte.166.19.2081. [DOI] [PubMed] [Google Scholar]
- 12.Rotterdam ESHRE/ASRM-sponsored PCOS consensus working group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril. 2008;90(3):714–726. doi: 10.1016/j.fertnstert.2007.07.1290. [DOI] [PubMed] [Google Scholar]
- 14.Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421–439. doi: 10.1016/j.rbmo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Sim KA, Partridge SR, Sainsbury A. Does weight loss in overweight or obese women improve fertility treatment outcomes? A systematic review. Obes Rev. 2014;15(10):839–850. doi: 10.1111/obr.12217. [DOI] [PubMed] [Google Scholar]
- 16.Mutsaerts MA, van Oers AM, Groen H, et al. Randomized Trial of a Lifestyle Program in Obese Infertile Women. N Engl J Med. 2016;374(20):1942–1953. doi: 10.1056/NEJMoa1505297. [DOI] [PubMed] [Google Scholar]
- 17.Legro RS, Dodson WC, Kunselman AR, et al. Benefit of Delayed Fertility Therapy With Preconception Weight Loss Over Immediate Therapy in Obese Women With PCOS. J Clin Endocrinol Metab. 2016;101(7):2658–2666. doi: 10.1210/jc.2016-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milone M, De Placido G, Musella M, et al. Incidence of Successful Pregnancy After Weight Loss Interventions in Infertile Women: a Systematic Review and Meta-Analysis of the Literature. Obes Surg. 2016;26(2):443–451. doi: 10.1007/s11695-015-1998-7. [DOI] [PubMed] [Google Scholar]
- 19.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 2013;21(Suppl 1):S1–27. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 105: bariatric surgery and pregnancy. Obstet Gynecol. 2009;113(6):1405–1413. doi: 10.1097/AOG.0b013e3181ac0544. [DOI] [PubMed] [Google Scholar]
- 21.American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 156: Obesity in Pregnancy. Obstet Gynecol. 2015;126(6):e112–126. doi: 10.1097/AOG.0000000000001211. [DOI] [PubMed] [Google Scholar]
- 22.Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: a committee opinion. Fertil Steril. 2015;104(5):1116–1126. doi: 10.1016/j.fertnstert.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Yi XY, Li QF, Zhang J, Wang ZH. A meta-analysis of maternal and fetal outcomes of pregnancy after bariatric surgery. Int J Gynaecol Obstet. 2015;130(1):3–9. doi: 10.1016/j.ijgo.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Kjaer MM, Lauenborg J, Breum BM, Nilas L. The risk of adverse pregnancy outcome after bariatric surgery: a nationwide register-based matched cohort study. Am J Obstet Gynecol. 2013;208(6):464, e461–465. doi: 10.1016/j.ajog.2013.02.046. [DOI] [PubMed] [Google Scholar]
- 25.Roos N, Neovius M, Cnattingius S, et al. Perinatal outcomes after bariatric surgery: nationwide population based matched cohort study. BMJ. 2013;347:f6460. doi: 10.1136/bmj.f6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmons D. Prevention of gestational diabetes mellitus: Where are we now? Diabetes Obes Metab. 2015;17(9):824–834. doi: 10.1111/dom.12495. [DOI] [PubMed] [Google Scholar]
- 27. [Accessed May 29, 2017];Lifestyle intervention in preparation for pregnancy. https://clinicaltrials.gov/ct2/show/NCT03146156?term=catalano&rank=4.
- 28.Royal College of Obstetricians and Gynaecologists. [Accessed Apr 27, 2017];Scientific Impact Paper #17: The role of reproductive surgery in improving reproductive health. 2015 https://www.rcog.org.uk/globalassets/documents/guidelines/scientific-impact-papers/sip_17.pdf.
- 29.Madsen NL, Schwartz SM, Lewin MB, Mueller BA. Prepregnancy body mass index and congenital heart defects among offspring: a population-based study. Congenit Heart Dis. 2013;8(2):131–141. doi: 10.1111/j.1747-0803.2012.00714.x. [DOI] [PubMed] [Google Scholar]
- 30.Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- 31.Mills JL, Troendle J, Conley MR, Carter T, Druschel CM. Maternal obesity and congenital heart defects: a population-based study. Am J Clin Nutr. 2010;91(6):1543–1549. doi: 10.3945/ajcn.2009.28865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brite J, Laughon SK, Troendle J, Mills J. Maternal overweight and obesity and risk of congenital heart defects in offspring. Int J Obes (Lond) 2014;38(6):878–882. doi: 10.1038/ijo.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masho SW, Bassyouni A, Cha S. Pre-pregnancy obesity and non-adherence to multivitamin use: findings from the National Pregnancy Risk Assessment Monitoring System (2009–2011) BMC Pregnancy Childbirth. 2016;16(1):210. doi: 10.1186/s12884-016-1002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tinker SC, Hamner HC, Berry RJ, Bailey LB, Pfeiffer CM. Does obesity modify the association of supplemental folic acid with folate status among nonpregnant women of childbearing age in the United States? Birth Defects Res A Clin Mol Teratol. 2012;94(10):749–755. doi: 10.1002/bdra.23024. [DOI] [PubMed] [Google Scholar]
- 35.Royal College of Obstetricians and Gynaecologists. Management of women with obesity in pregnancy. [Accessed 27 May 2017];Joint Guideline. 2010 https://www.rcog.org.uk/globalassets/documents/guidelines/cmacercogjointguidelinemanagementwomenobesitypregnancya.pdf.
- 36.Mojtabai R. Body mass index and serum folate in childbearing age women. Eur J Epidemiol. 2004;19(11):1029–1036. doi: 10.1007/s10654-004-2253-z. [DOI] [PubMed] [Google Scholar]
- 37.Ferraro ZM, Contador F, Tawfiq A, Adamo KB, Gaudet L. Gestational weight gain and medical outcomes of pregnancy. Obstet Med. 2015;8(3):133–137. doi: 10.1177/1753495X15591320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adamo KB, Ferraro ZM, Brett KE. Pregnancy is a critical period for prevention of obesity and cardiometabolic risk. Canadian Journal of Diabetes. 2012;36:133–141. [Google Scholar]
- 39.Weisman CS, Hillemeier MM, Downs DS, Chuang CH, Dyer AM. Preconception predictors of weight gain during pregnancy: prospective findings from the Central Pennsylvania Women's Health Study. Womens Health Issues. 2010;20(2):126–132. doi: 10.1016/j.whi.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferraro ZM, Barrowman N, Prud'homme D, et al. Excessive gestational weight gain predicts large for gestational age neonates independent of maternal body mass index. J Matern Fetal Neonatal Med. 2012;25(5):538–542. doi: 10.3109/14767058.2011.638953. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen KM, Yaktine AL, editors. Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): 2009. [Google Scholar]
- 42.Kapadia MZ, Park CK, Beyene J, Giglia L, Maxwell C, McDonald SD. Can we safely recommend gestational weight gain below the 2009 guidelines in obese women? A systematic review and meta-analysis. Obes Rev. 2015;16(3):189–206. doi: 10.1111/obr.12238. [DOI] [PubMed] [Google Scholar]
- 43.Bogaerts A, Ameye L, Martens E, Devlieger R. Weight loss in obese pregnant women and risk for adverse perinatal outcomes. Obstet Gynecol. 2015;125(3):566–575. doi: 10.1097/AOG.0000000000000677. [DOI] [PubMed] [Google Scholar]
- 44.Blomberg M. Maternal and neonatal outcomes among obese women with weight gain below the new Institute of Medicine recommendations. Obstet Gynecol. 2011;117(5):1065–1070. doi: 10.1097/AOG.0b013e318214f1d1. [DOI] [PubMed] [Google Scholar]
- 45.American College of Obstetricians and Gynecologists. ACOG Committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol. 2013;121(1):210–212. doi: 10.1097/01.aog.0000425668.87506.4c. [DOI] [PubMed] [Google Scholar]
- 46.Davies GA, Maxwell C, McLeod L, et al. SOGC Clinical Practice Guidelines: Obesity in pregnancy. No. 239, February 2010. Int J Gynaecol Obstet. 2010;110(2):167–173. doi: 10.1016/j.ijgo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;(6):CD007145. doi: 10.1002/14651858.CD007145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet Gynecol. 2015;126(6):e135–142. doi: 10.1097/AOG.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 49.McIntyre HD, Sacks DA, Barbour LA, et al. Issues With the Diagnosis and Classification of Hyperglycemia in Early Pregnancy. Diabetes Care. 2016;39(1):53–54. doi: 10.2337/dc15-1887. [DOI] [PubMed] [Google Scholar]
- 50.Hughes RC, Moore MP, Gullam JE, Mohamed K, Rowan J. An early pregnancy HbA1c >/=5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care. 2014;37(11):2953–2959. doi: 10.2337/dc14-1312. [DOI] [PubMed] [Google Scholar]
- 51.Barbour LA. Unresolved controversies in gestational diabetes: implications on maternal and infant health. Curr Opin Endocrinol Diabetes Obes. 2014;21(4):264–270. doi: 10.1097/MED.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 52.American College of Obstetricians and Gynecologists. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013;122(2 Pt 1):406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 53.Wong T, Ross GP, Jalaludin BB, Flack JR. The clinical significance of overt diabetes in pregnancy. Diabet Med. 2013;30(4):468–474. doi: 10.1111/dme.12110. [DOI] [PubMed] [Google Scholar]
- 54.Robinson HE, O'Connell CM, Joseph KS, McLeod NL. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106(6):1357–1364. doi: 10.1097/01.AOG.0000188387.88032.41. [DOI] [PubMed] [Google Scholar]
- 55.O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 56.Sorensen TK, Williams MA, Lee IM, Dashow EE, Thompson ML, Luthy DA. Recreational physical activity during pregnancy and risk of preeclampsia. Hypertension. 2003;41(6):1273–1280. doi: 10.1161/01.HYP.0000072270.82815.91. [DOI] [PubMed] [Google Scholar]
- 57.Saftlas AF, Logsden-Sackett N, Wang W, Woolson R, Bracken MB. Work, leisure-time physical activity, and risk of preeclampsia and gestational hypertension. Am J Epidemiol. 2004;160(8):758–765. doi: 10.1093/aje/kwh277. [DOI] [PubMed] [Google Scholar]
- 58.American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 59.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P, Committee SHG. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(7):575–576. doi: 10.1016/S1701-2163(15)30533-8. [DOI] [PubMed] [Google Scholar]
- 60.Cantu JA, Jauk VR, Owen J, et al. Is low-dose aspirin therapy to prevent preeclampsia more efficacious in non-obese women or when initiated early in pregnancy? J Matern Fetal Neonatal Med. 2015;28(10):1128–1132. doi: 10.3109/14767058.2014.947258. [DOI] [PubMed] [Google Scholar]
- 61.Hildebrand E, Kallen B, Josefsson A, Gottvall T, Blomberg M. Maternal obesity and risk of Down syndrome in the offspring. Prenat Diagn. 2014;34(4):310–315. doi: 10.1002/pd.4294. [DOI] [PubMed] [Google Scholar]
- 62.Wang E, Batey A, Struble C, Musci T, Song K, Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013;33(7):662–666. doi: 10.1002/pd.4119. [DOI] [PubMed] [Google Scholar]
- 63.Aagaard-Tillery KM, Flint Porter T, Malone FD, et al. Influence of maternal BMI on genetic sonography in the FaSTER trial. Prenat Diagn. 2010;30(1):14–22. doi: 10.1002/pd.2399. [DOI] [PubMed] [Google Scholar]
- 64.Dashe JS, McIntire DD, Twickler DM. Effect of maternal obesity on the ultrasound detection of anomalous fetuses. Obstet Gynecol. 2009;113(5):1001–1007. doi: 10.1097/AOG.0b013e3181a1d2f5. [DOI] [PubMed] [Google Scholar]
- 65.Dashe JS, McIntire DD, Twickler DM. Maternal obesity limits the ultrasound evaluation of fetal anatomy. J Ultrasound Med. 2009;28(8):1025–1030. doi: 10.7863/jum.2009.28.8.1025. [DOI] [PubMed] [Google Scholar]
- 66.Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA. 2013;309(22):2362–2370. doi: 10.1001/jama.2013.6295. [DOI] [PubMed] [Google Scholar]
- 67.Carmichael SL, Blumenfeld YJ, Mayo J, et al. Prepregnancy Obesity and Risks of Stillbirth. PLoS One. 2015;10(10):e0138549. doi: 10.1371/journal.pone.0138549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacob L, Kostev K, Kalder M. Risk of stillbirth in pregnant women with obesity in the United Kingdom. Obes Res Clin Pract. 2016;10(5):574–579. doi: 10.1016/j.orcp.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 69.Lee VRLB, Anjali K, Little S, Nicholson J, Caughey AB. Optimal timing of delivery in obese women: A decision analysis. Obstet Gynecol. 2014;123(S1):152S–153S. [Google Scholar]
- 70.Lee VR, Darney BG, Snowden JM, et al. Term elective induction of labour and perinatal outcomes in obese women: retrospective cohort study. BJOG. 2016;123(2):271–278. doi: 10.1111/1471-0528.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alanis MC, Villers MS, Law TL, Steadman EM, Robinson CJ. Complications of cesarean delivery in the massively obese parturient. Am J Obstet Gynecol. 2010;203(3):271, e271–277. doi: 10.1016/j.ajog.2010.06.049. [DOI] [PubMed] [Google Scholar]
- 72.Alexander CI, Liston WA. Operating on the obese woman--A review. BJOG. 2006;113(10):1167–1172. doi: 10.1111/j.1471-0528.2006.01073.x. [DOI] [PubMed] [Google Scholar]
- 73.Wall PD, Deucy EE, Glantz JC, Pressman EK. Vertical skin incisions and wound complications in the obese parturient. Obstet Gynecol. 2003;102(5 Pt 1):952–956. doi: 10.1016/s0029-7844(03)00861-5. [DOI] [PubMed] [Google Scholar]
- 74.Tan T, Sia AT. Anesthesia considerations in the obese gravida. Semin Perinatol. 2011;35(6):350–355. doi: 10.1053/j.semperi.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 75.Fyfe EM, Thompson JM, Anderson NH, Groom KM, McCowan LM. Maternal obesity and postpartum haemorrhage after vaginal and caesarean delivery among nulliparous women at term: a retrospective cohort study. BMC Pregnancy Childbirth. 2012;12:112. doi: 10.1186/1471-2393-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e691S–736S. doi: 10.1378/chest.11-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36(6):527–553. doi: 10.1016/s1701-2163(15)30569-7. [DOI] [PubMed] [Google Scholar]
- 78.World Health Organization. Breastfeeding. [Accessed December 6, 2016];Health Topics. http://www.who.int/topics/breastfeeding/en/
- 79.American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 80.Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113(5):974–982. doi: 10.1097/01.AOG.0000346884.67796.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294(20):2601–2610. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 82.Liu B, Jorm L, Banks E. Parity, breastfeeding, and the subsequent risk of maternal type 2 diabetes. Diabetes Care. 2010;33(6):1239–1241. doi: 10.2337/dc10-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Natland ST, Nilsen TI, Midthjell K, Andersen LF, Forsmo S. Lactation and cardiovascular risk factors in mothers in a population-based study: the HUNT-study. Int Breastfeed J. 2012;7(1):8. doi: 10.1186/1746-4358-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McClure CK, Schwarz EB, Conroy MB, Tepper PG, Janssen I, Sutton-Tyrrell KC. Breastfeeding and subsequent maternal visceral adiposity. Obesity (Silver Spring) 2011;19(11):2205–2213. doi: 10.1038/oby.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baker JL, Gamborg M, Heitmann BL, Lissner L, Sorensen TI, Rasmussen KM. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr. 2008;88(6):1543–1551. doi: 10.3945/ajcn.2008.26379. [DOI] [PubMed] [Google Scholar]
- 86.Coitinho DC, Sichieri R, D'Aquino Benicio MH. Obesity and weight change related to parity and breastfeeding among parous women in Brazil. Public Health Nutr. 2001;4(4):865–870. doi: 10.1079/phn2001125. [DOI] [PubMed] [Google Scholar]
- 87.Palmer JR, Kipping-Ruane K, Wise LA, Yu J, Rosenberg L. Lactation in Relation to Long-Term Maternal Weight Gain in African-American Women. Am J Epidemiol. 2015;181(12):932–939. doi: 10.1093/aje/kwv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thompson LA, Zhang S, Black E, et al. The association of maternal pre-pregnancy body mass index with breastfeeding initiation. Matern Child Health J. 2013;17(10):1842–1851. doi: 10.1007/s10995-012-1204-7. [DOI] [PubMed] [Google Scholar]
- 89.Amir LH, Donath S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy Childbirth. 2007;7:9. doi: 10.1186/1471-2393-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bever Babendure J, Reifsnider E, Mendias E, Moramarco MW, Davila YR. Reduced breastfeeding rates among obese mothers: a review of contributing factors, clinical considerations and future directions. Int Breastfeed J. 2015;10:21. doi: 10.1186/s13006-015-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cnattingius S, Villamor E. Weight change between successive pregnancies and risks of stillbirth and infant mortality: a nationwide cohort study. Lancet. 2016;387(10018):558–565. doi: 10.1016/S0140-6736(15)00990-3. [DOI] [PubMed] [Google Scholar]
- 92.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368(9542):1164–1170. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- 93.Glazer NL, Hendrickson AF, Schellenbaum GD, Mueller BA. Weight change and the risk of gestational diabetes in obese women. Epidemiology. 2004;15(6):733–737. doi: 10.1097/01.ede.0000142151.16880.03. [DOI] [PubMed] [Google Scholar]
- 94.Jain AP, Gavard JA, Rice JJ, Catanzaro RB, Artal R, Hopkins SA. The impact of interpregnancy weight change on birthweight in obese women. Am J Obstet Gynecol. 2013;208(3):205, e201–207. doi: 10.1016/j.ajog.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 95.Callegari LS, Sterling LA, Zelek ST, Hawes SE, Reed SD. Interpregnancy body mass index change and success of term vaginal birth after cesarean delivery. Am J Obstet Gynecol. 2014;210(4):330, e331–337. doi: 10.1016/j.ajog.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 96.Amorim AR, Linne YM, Lourenco PM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev. 2007(3):CD005627. doi: 10.1002/14651858.CD005627.pub2. [DOI] [PubMed] [Google Scholar]
- 97.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370(17):1660–1661. doi: 10.1056/NEJMc1402397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015;101(2):302–309. doi: 10.3945/ajcn.114.094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nicklas JM, Barbour LA. Optimizing Weight for Maternal and Infant Health - Tenable, or Too Late? Expert Rev Endocrinol Metab. 2015;10(2):227–242. doi: 10.1586/17446651.2014.991102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Olson CM, Strawderman MS, Dennison BA. Maternal weight gain during pregnancy and child weight at age 3 years. Matern Child Health J. 2009;13(6):839–846. doi: 10.1007/s10995-008-0413-6. [DOI] [PubMed] [Google Scholar]
- 102.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reynolds RM, Osmond C, Phillips DI, Godfrey KM. Maternal BMI, parity, and pregnancy weight gain: influences on offspring adiposity in young adulthood. J Clin Endocrinol Metab. 2010;95(12):5365–5369. doi: 10.1210/jc.2010-0697. [DOI] [PubMed] [Google Scholar]
- 104.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nicholas LM, Morrison JL, Rattanatray L, Zhang S, Ozanne SE, McMillen IC. The early origins of obesity and insulin resistance: timing, programming and mechanisms. Int J Obes (Lond) 2016;40(2):229–238. doi: 10.1038/ijo.2015.178. [DOI] [PubMed] [Google Scholar]
- 106.Barbour LA. Changing perspectives in pre-existing diabetes and obesity in pregnancy: maternal and infant short- and long-term outcomes. Curr Opin Endocrinol Diabetes Obes. 2014;21(4):257–263. doi: 10.1097/MED.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 107.Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet. 2004;363(9421):1642–1645. doi: 10.1016/S0140-6736(04)16210-7. [DOI] [PubMed] [Google Scholar]
- 108.Reynolds RM, Allan KM, Raja EA, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539. doi: 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119(2):323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brumbaugh DE, Tearse P, Cree-Green M, et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatr. 2013;162(5):930–936. e931. doi: 10.1016/j.jpeds.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wesolowski SR, Kasmi KC, Jonscher KR, Friedman JE. Developmental origins of NAFLD: a womb with a clue. Nat Rev Gastroenterol Hepatol. 2017;14(2):81–96. doi: 10.1038/nrgastro.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Newton KP, Feldman HS, Chambers CD, et al. Low and High Birth Weights Are Risk Factors for Nonalcoholic Fatty Liver Disease in Children. J Pediatr. 2017 doi: 10.1016/j.jpeds.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goyal NP, Schwimmer JB. The Progression and Natural History of Pediatric Nonalcoholic Fatty Liver Disease. Clin Liver Dis. 2016;20(2):325–338. doi: 10.1016/j.cld.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kral JG, Biron S, Simard S, et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118(6):e1644–1649. doi: 10.1542/peds.2006-1379. [DOI] [PubMed] [Google Scholar]
- 115.Smith J, Cianflone K, Biron S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab. 2009;94(11):4275–4283. doi: 10.1210/jc.2009-0709. [DOI] [PubMed] [Google Scholar]
- 116.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 117.Boyle KE, Patinkin ZW, Shapiro AL, Baker PR, 2nd, Dabelea D, Friedman JE. Mesenchymal Stem Cells From Infants Born to Obese Mothers Exhibit Greater Potential for Adipogenesis: The Healthy Start BabyBUMP Project. Diabetes. 2016;65(3):647–659. doi: 10.2337/db15-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R711–722. doi: 10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.El Hajj N, Schneider E, Lehnen H, Haaf T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction. 2014;148(6):R111–120. doi: 10.1530/REP-14-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ganu RS, Harris RA, Collins K, Aagaard KM. Maternal diet: a modulator for epigenomic regulation during development in nonhuman primates and humans. Int J Obes Suppl. 2012;2(Suppl 2):S14–S18. doi: 10.1038/ijosup.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Teh AL, Pan H, Chen L, et al. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Res. 2014;24(7):1064–1074. doi: 10.1101/gr.171439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ollikainen M, Smith KR, Joo EJ, et al. DNA methylation analysis of multiple tissues from newborn twins reveals both genetic and intrauterine components to variation in the human neonatal epigenome. Hum Mol Genet. 2010;19(21):4176–4188. doi: 10.1093/hmg/ddq336. [DOI] [PubMed] [Google Scholar]
- 123.Cooper WN, Khulan B, Owens S, et al. DNA methylation profiling at imprinted loci after periconceptional micronutrient supplementation in humans: results of a pilot randomized controlled trial. FASEB J. 2012;26(5):1782–1790. doi: 10.1096/fj.11-192708. [DOI] [PubMed] [Google Scholar]
- 124.Khulan B, Cooper WN, Skinner BM, et al. Periconceptional maternal micronutrient supplementation is associated with widespread gender related changes in the epigenome: a study of a unique resource in the Gambia. Hum Mol Genet. 2012;21(9):2086–2101. doi: 10.1093/hmg/dds026. [DOI] [PubMed] [Google Scholar]
- 125.Lin X, Lim IY, Wu Y, et al. Developmental pathways to adiposity begin before birth and are influenced by genotype, prenatal environment and epigenome. BMC Med. 2017;15(1):50. doi: 10.1186/s12916-017-0800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Waterland RA, Kellermayer R, Laritsky E, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 2010;6(12):e1001252. doi: 10.1371/journal.pgen.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dominguez-Salas P, Moore SE, Baker MS, et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun. 2014;5:3746. doi: 10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chu DM, Meyer KM, Prince AL, Aagaard KM. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes. 2016;7(6):459–470. doi: 10.1080/19490976.2016.1241357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brumbaugh DE, Arruda J, Robbins K, et al. Mode of Delivery Determines Neonatal Pharyngeal Bacterial Composition and Early Intestinal Colonization. J Pediatr Gastroenterol Nutr. 2016;63(3):320–328. doi: 10.1097/MPG.0000000000001124. [DOI] [PubMed] [Google Scholar]
- 131.Prince AL, Antony KM, Ma J, Aagaard KM. The microbiome and development: a mother's perspective. Semin Reprod Med. 2014;32(1):14–22. doi: 10.1055/s-0033-1361818. [DOI] [PubMed] [Google Scholar]
- 132.Chu DM, Antony KM, Ma J, et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016;8(1):77. doi: 10.1186/s13073-016-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 134.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 135.Ma J, Prince AL, Bader D, et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. 2014;5:3889. doi: 10.1038/ncomms4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lemas DJ, Young BE, Baker PR, 2nd, et al. Alterations in human milk leptin and insulin are associated with early changes in the infant intestinal microbiome. Am J Clin Nutr. 2016;103(5):1291–1300. doi: 10.3945/ajcn.115.126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Soderborg TK, Borengasser SJ, Barbour LA, Friedman JE. Microbial transmission from mothers with obesity or diabetes to infants: an innovative opportunity to interrupt a vicious cycle. Diabetologia. 2016;59(5):895–906. doi: 10.1007/s00125-016-3880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr. 2010;103(12):1792–1799. doi: 10.1017/S0007114509993898. [DOI] [PubMed] [Google Scholar]
- 139.Wickens KL, Barthow CA, Murphy R, et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br J Nutr. 2017;117(6):804–813. doi: 10.1017/S0007114517000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nitert MD, Barrett HL, Foxcroft K, et al. SPRING: an RCT study of probiotics in the prevention of gestational diabetes mellitus in overweight and obese women. BMC Pregnancy Childbirth. 2013;13:50. doi: 10.1186/1471-2393-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sandler V, Reisetter AC, Bain JR, et al. Associations of maternal BMI and insulin resistance with the maternal metabolome and newborn outcomes. Diabetologia. 2017;60(3):518–530. doi: 10.1007/s00125-016-4182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hellmuth C, Lindsay KL, Uhl O, et al. Association of maternal prepregnancy BMI with metabolomic profile across gestation. Int J Obes (Lond) 2017;41(1):159–169. doi: 10.1038/ijo.2016.153. [DOI] [PubMed] [Google Scholar]
- 143.Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature. 2010;467(7318):963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 144.de Castro Barbosa T, Ingerslev LR, Alm PS, et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol Metab. 2016;5(3):184–197. doi: 10.1016/j.molmet.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Donkin I, Versteyhe S, Ingerslev LR, et al. Obesity and Bariatric Surgery Drive Epigenetic Variation of Spermatozoa in Humans. Cell Metab. 2016;23(2):369–378. doi: 10.1016/j.cmet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 146.Balserak B. Sleep disordered breathing in pregnancy. Breathe. 2015;11(4):268–277. doi: 10.1183/20734735.009215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rice JR, Larrabure-Torrealva GT, Luque Fernandez MA, et al. High risk for obstructive sleep apnea and other sleep disorders among overweight and obese pregnant women. BMC Pregnancy Childbirth. 2015;15:198. doi: 10.1186/s12884-015-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120(5):1085–1092. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28(4):637–653. doi: 10.1016/j.bpg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 150.Hagstrom H, Hoijer J, Ludvigsson JF, et al. Adverse outcomes of pregnancy in women with non-alcoholic fatty liver disease. Liver Int. 2016;36(2):268–274. doi: 10.1111/liv.12902. [DOI] [PubMed] [Google Scholar]
- 151.Chelmow DRE, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103(5 part 1):974–980. doi: 10.1097/01.AOG.0000124807.76451.47. [DOI] [PubMed] [Google Scholar]
- 152.Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, Macones GA, Odibo AO. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682–690. doi: 10.1097/AOG.0b013e31820ad61e. [DOI] [PubMed] [Google Scholar]