Abstract
These NCCN Guidelines Insights highlight the important updates/changes specific to the 2016 version of the NCCN Clinical Practice Guidelines in Oncology for Multiple Myeloma. These changes include updated recommendations to the overall management of multiple myeloma from diagnosis and staging to new treatment options.
Overview
Multiple myeloma (MM) is characterized by the neoplastic proliferation of plasma cell clones producing monoclonal immunoglobulin. These plasma cell clones proliferate in the bone marrow and cause skeletal damage, the hallmark of MM. Other MM-related complications include hypercalcemia, renal insufficiency, anemia, and infections. The American Cancer Society has estimated that 30,330 new cases of MM will occur in the United States in 2016, with an estimated 12,650 deaths.1 MM accounts for approximately 1.8% of all cancers and slightly more than 15% of all hematologic malignancies in the United States.1
The NCCN MM Guidelines Panel has developed guidelines for the management of patients with various plasma cell dyscrasias, including solitary plasmacytoma, smoldering myeloma, MM, systemic light chain amyloidosis, and Waldenström’s macro-globulinemia. The NCCN Guidelines are updated annually, or sometimes more often if new, high-quality clinical data become available.
This NCCN Guidelines Insights report focuses on the updates to the 2016 version of the NCCN Guidelines for MM, which include the addition of (1) the new diagnostic criteria and the revised International Staging System (ISS) developed by the International Myeloma Working Group (IMWG), (2) new regimen options for the treatment of newly diagnosed MM, and (3) recent FDA-approved novel drug–containing regimens for the treatment of relapsed/refractory MM.
Diagnostic Criteria
The CRAB criteria that define MM include hypercalcemia (>11.5 mg/dL), renal insufficiency (creatinine >2 mg/dL), anemia (hemoglobin <10 g/dL or 2 g/dL < normal), and presence of bone lesions. The IMWG recently updated the definition of MM to include biomarkers in addition to existing requirements of CRAB features.2 The MM-defining biomarkers identified by the IMWG include one or more of the following: 60% or more clonal plasma cells in the bone marrow, involved/uninvolved free light chain ratio of 100 or more, and/or MRI with more than one focal lesion (involving bone or bone marrow).2 Additionally, the IMWG clarified that the presence of one or more osteolytic lesions seen on skeletal radiography, whole-body MRI, or PET/CT fulfils the criteria for bone disease.2
The criteria set by the IMWG for patients with smoldering (asymptomatic) disease include serum monoclonal protein (IgG or IgA) of 30 g/L or greater and/or clonal bone marrow plasma cells 10% to 60%, and absence of myeloma-defining events or amyloidosis.2 The updated IMWG diagnostic criteria help to initiate therapy before the occurrence of end-organ damage based on the presence of specific biomarkers, and also allow the use of sensitive imaging criteria to diagnose MM, including PET/CT and MRI.2 Patients with high-risk smoldering myeloma who meet the above criteria can be started on therapy without waiting for CRAB features to appear.
The NCCN panel included the updated diagnostic criteria for defining smoldering myeloma and MM in the 2016 version of the NCCN Guidelines (see MYEL-B; page 392).
MYEL-B.
DEFINITION OF MULTIPLE MYELOMA (SMOLDERING AND ACTIVE)
Staging
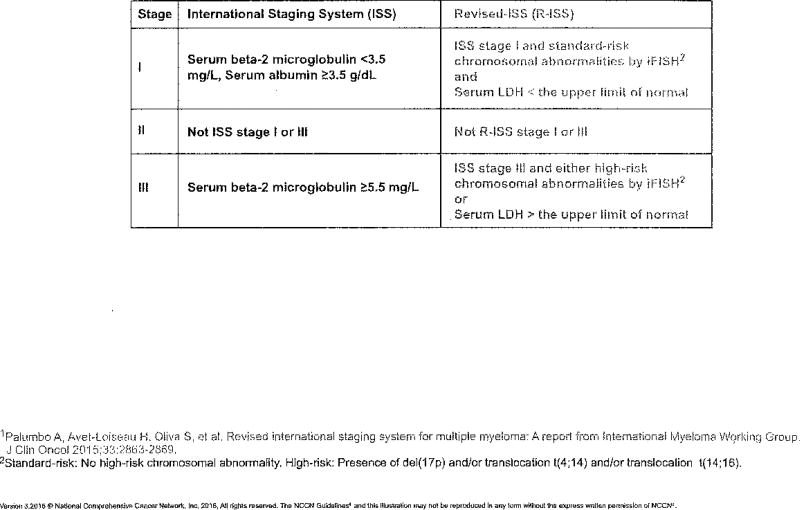
After diagnosis, patients with active myeloma are categorized according to stage based on either the Durie-Salmon staging system or the ISS.3 The Durie-Salmon staging system is based on tumor cell density in the bone marrow and measures end-organ damage (renal insufficiency, anemia, hypercalcemia, lytic bone lesions) and immunoglobulin levels.4 The ISS system is based on levels of serum β-2 microglobulin and serum albumin to divide disease burden into 3 stages with different prognostic significance.3 Compared with the Durie-Salmon staging system, the ISS is based on easily obtained laboratory measures, is able to predict prognosis and overall survival (OS), and is easier to use for patients with previously untreated MM.
The IMWG recently developed the revised International Staging System (R-ISS),5 which is more robust in providing prognostic information compared with the ISS. The R-ISS incorporates factors included in the ISS (serum β-2 microglobulin and serum albumin), serum lactate dehydrogenase, and high-risk chromosomal abnormalities detected by Interphase fluorescence in situ hybridization.5
In the updated NCCN Guidelines for MM, the Durie-Salmon staging is no longer included. The 2 staging systems recommended by the NCCN panel for patients with newly diagnosed MM include the ISS and the R-ISS (see MYEL-A; page 391).
MYEL-A.
STAGING SYSTEMS FOR MULTIPLE MYELOMA1
New Treatment Options
The recently updated NCCN Guidelines have several new treatment options for both patients with newly diagnosed MM and those with relapsed/refractory disease.
New Regimens for Patients With Newly Diagnosed MM
Bortezomib/Lenalidomide/Dexamethasone
A phase I/II study with bortezomib, lenalidomide, and dexamethasone as primary therapy showed that this regimen is active and well tolerated in patients with newly diagnosed MM.6 In the phase II population, the rate of partial response (PR) or better was 100%, with 74% demonstrating a very good partial response (VGPR) or better, and 52% achieving a complete response (CR)/near CR.6 A post-hoc analysis showed a low risk of progression after 1 year of therapy initiation regardless of autologous stem cell transplantation (SCT) status. The 18-month progression-free survival (PFS) rate was 75% and the OS rate was 97% after lenalidomide, bortezomib, dexamethasone with or without autologous SCT.6
Two other phase II trials, IFM 20087 and EVOLUTION,8 also studied the efficacy of bortezomib, lenalidomide, and dexamethasone as primary therapy. In the IFM 2008 phase II trial, patients received bortezomib, lenalidomide, and dexamethasone as primary therapy followed by SCT. Patients subsequently received 2 cycles of bortezomib, lenalidomide, and dexamethasone as consolidation cycles and 1-year of lenalidomide as maintenance. The rate of VGPR or better at the completion of primary therapy was 58%. After transplantation and consolidation therapy the rates of VGPR or better were 70% and 87%, respectively. The phase II EVOLUTION trial was designed to examine the tolerability and efficacy of primary treatment with bortezomib, cyclophosphamide, lenalidomide, and dexamethasone versus bortezomib, lenalidomide, and dexamethasone versus cyclophosphamide, bortezomib, and dexamethasone (CyBorD) followed by maintenance with bortezomib in a randomized multicenter setting. The overall response rate (ORR) after primary treatment with bortezomib, lenalidomide, and dexamethasone followed by maintenance with bortezomib was 85% (51% VGPR or better; 24% CR) and the 1-year PFS rate was 83%.8
Recently, the randomized phase III trial, SWOG SO777, reported results of primary treatment with bortezomib, lenalidomide, and dexamethasone compared with lenalidomide plus dexamethasone.9 The ORR was higher for bortezomib, lenalidomide, and dexamethasone compared with lenalidomide and dexamethasone (71% vs 64%). Median PFS with the 3-drug combination was 43 versus 31 months for lenalidomide plus dexamethasone. Median OS was not reached for bortezomib, lenalidomide, and dexamethasone compared with 63 months for lenalidomide plus dexamethasone.9 As expected, grade 3 or higher neuropathy was more frequent in the bortezomib-containing arm (24% vs 5%; P<.0001).9
Because the phase III results confirmed that the addition of bortezomib to lenalidomide and dexamethasone as primary therapy in previously untreated MM results in a statistically significant and clinically meaningful improvement in PFS and better OS, the NCCN panel has included the bortezomib, lenalidomide, and dexamethasone regimen (category 1 recommendation) as a preferred primary treatment option for both transplant and nontransplant candidates (see MYEL-D; pages 393 and 394).
MYEL-D.
MYELOMA THERAPY1–4
MYELOMA THERAPY1–4,9
Cyclophosphamide/Bortezomib/Dexamethasone
Data from 3 phase II studies involving patients with newly diagnosed MM have demonstrated high response rates with CyBorD as primary treatment.8,10,11 The study by Reeder et al10 conducted in the United States and Canada demonstrated an ORR of 88%, including a 61% rate of VGPR or better and a 39% rate of CR/near CR, with CyBorD as the primary regimen. The depth of response seen after primary treatment was maintained after transplant in those who underwent transplantation (70% rate of CR/near CR; 74% rate of VGPR or better).10 According to the long-term follow-up analysis, 5-year PFS and OS rates were 42% (95% CI, 31–57) and 70% (95% CI, 59–82), respectively.12
Analysis of the German DSMM XIa study also demonstrated high response rates with CyBorD as primary treatment (ORR, 84%; 74% PR rate; 10% CR rate), and that these high response rates were seen in patients with unfavorable cytogenetics.11 In the updated results of the phase II EVOLUTION study, primary treatment with CyBorD demonstrated a 75% ORR (22% CR, 41% VGPR or better) and a 1-year PFS rate of 93%.8
Twice-weekly bortezomib can be associated with toxicities that may limit efficacy caused by treatment delays or discontinuation. Therefore, Reeder et al13 modified the regimen to a weekly schedule of bortezomib. In the study, patients treated with weekly bortezomib experienced responses similar to those receiving the twice-weekly schedule (ORR, 93% vs 88%; VGPR, 60% vs 61%, respectively), experienced fewer grade 3 and 4 adverse events (37% and 3% vs 48% and 12%, respectively), and required fewer dose reductions of bortezomib and dexamethasone. However, neuropathy rates were the same in both cohorts, even though the total bortezomib dose per cycle was higher in the weekly compared with the twice-weekly schedule (6.0 mg/m2 vs 5.2 mg/m2, respectively).13
The role of CyBorD as initial therapy for transplant-ineligible patients with MM was studied in a small phase II trial (n=20), in which the median patient age was 76 years (range, 66–90 years). At a median follow-up of 9.5 months, the OS rate was 100%, and at median follow-up of 12 months, 5 patients had disease progression. With respect to toxicity, 6 patients experienced nonhematologic grade 3/4 adverse events (20%), including muscle weakness, sepsis, and pneumonia; neutropenia and thrombocytopenia were seen in 2 patients (10%).14
Based on data from the 3 phase II studies,8,10,11 the NCCN MM Panel included the combination of CyBorD as a category 2A recommendation to the list of primary treatment options available for transplant candidates. Additionally, based on results of the phase II data from Jimenez-Zepeda et al14 in patients ineligible for transplant and the results from the EVOLUTION trial8 that did not exclude transplant-ineligible patients, the NCCN panel included CyBorD as a primary therapy option (category 2A recommendation) for nontransplant candidates (see MYEL-D; pages 393 and 394).
Ixazomib/Lenalidomide/Dexamethasone
Ixazomib is an oral proteosome inhibitor that was recently FDA-approved in combination with lenalidomide and dexamethasone for the treatment of patients with MM who have received at least one previous therapy.
A phase I/II study was conducted to study the safety and efficacy of the all-oral combination of ixazomib with lenalidomide and dexamethasone in patients with newly diagnosed MM treated with combination lenalidomide and dexamethasone.15 The results of this trial show that the regimen was well tolerated and appeared active in newly diagnosed MM. Of the 64 patients in whom response could be evaluated, 37 (58%; 95% CI, 45–70) had a VGPR or better. Grade 3 or higher adverse events related to any drug in the combination were reported in 41 patients (63%). Adverse events included skin and subcutaneous tissue disorders (11 patients; 17%), neutropenia (8 patients; 12%), and thrombocytopenia (5 patients; 8%); drug-related peripheral neuropathy of grade 3 or higher occurred in 4 patients (6%).
Based on these phase II results and the fact that the combination of other proteosome inhibitors (bortezomib or carfilzomib) in combination with lenalidomide or dexamethasone has been shown to be effective as primary therapy in newly diagnosed MM,9,16–19 the NCCN panel has included ixazomib in combination with lenalidomide and dexamethasone as a treatment option (category 2A recommendation) for patients with newly diagnosed MM (see MYEL-D; pages 393 and 394).
New Treatment Options for Patients With Relapsed/Refractory MM
Carfilzomib/Dexamethasone
In the randomized, phase III, open-label, multicenter study ENDEAVOR, patients with relapsed/refractory MM who had received 1 to 3 previous treatments were randomly assigned to receive carfilzomib with dexamethasone or bortezomib with dexamethasone. Study results showed a 2-fold improvement in median PFS with carfilzomib and dexamethasone compared with bortezomib and dexamethasone (18.7 vs 9.4 months; hazard ratio [HR], 0.53; P<.0001).20 Adverse events (≥ grade 3) in the carfilzomib arm compared with the bortezomib arm included hypertension (8.9% vs 2.6%), dyspnea (5.6% vs 2.2%), cardiac failure (4.8% vs 1.8%), and acute renal failure (4.1% vs 2.6%).20
Based on the ENDEAVOR trial results,20 the NCCN panel included the combination of carfilzomib and dexamethasone as a treatment option (category 2A) for patients with relapsed/refractory MM (see MYEL-D; pages 393 and 394).
Daratumumab
Daratumumab is a human IgG kappa monoclonal antibody that targets the CD38 surface protein on myeloma cells.21 It was FDA-approved for the treatment of patients with MM who have received at least 3 prior lines of therapy that included a proteasome inhibitor and an immunomodulatory agent or who are double-refractory to a proteosome inhibitor and an immunomodulatory agent. FDA approval was based on the results of a phase I/II study in which patients who had received more than 3 lines of therapy that included an immunomodulatory agent and a proteosome inhibitor or who were double-refractory to a proteosome inhibitor and an immunomodulatory agent were randomized to 2 different doses of daratumumab (8 mg/kg vs 16 g/kg). ORR was 29.2% (3 stringent CRs, 10 VGPRs, 18 PRs). Median response duration was 7.4 months and median time to progression was 3.7 months; the estimated 1-year OS rate was 65%.22 Adverse events reported included fatigue (39.6%), anemia (33.0%), nausea (29.2%), and thrombocytopenia (25.5%). Grade 1/2 infusion-related reactions were seen in 42.5% of patients, mainly during the first infusion. However, no study patients were discontinued due to infusion-related reactions.22
Based on these phase II results and the FDA approval, the panel added daratumumab as an option (category 2A) for the treatment of patients with MM who received at least 3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are double-refractory to a proteosome inhibitor and immunomodulatory agent (see MYEL-D; pages 393 and 394).
Elotuzumab/Lenalidomide/Dexamethasone
Elotuzumab is a humanized monoclonal antibody targeted against signaling lymphocytic activation molecule F7 (SLAMF7). SLAMF7, also called CS1 (cell-surface glycoprotein CD2 subset 1), is a glycoprotein expressed on myeloma and natural killer cells but not on normal tissues.23 The FDA has approved elotuzumab in combination with lenalidomide and dexamethasone for the treatment of patients with MM who have received 1 to 3 prior therapies. This is based on results of the phase III trial, ELOQUENT-2, which randomized 646 patients (ratio 1:1) to receive either elotuzumab in combination with lenalidomide and dexamethasone or lenalidomide and dexamethasone alone.24
The rates of PFS at the end of 1 and 2 years were higher for those receiving the elotuzumab-containing regimen (68% at 1 year and 41% at 2 years) compared with those receiving lenalidomide and dexamethasone alone (57% at 1 year and 27% at 2 years).24 Median PFS in the group receiving the elotuzumab-containing regimen was 19.4 versus 14.9 months in those receiving lenalidomide and dexamethasone alone (HR for progression or death in the elotuzumab group, 0.70; 95% CI, 0.57–0.85; P<.001), indicating a relative reduction of 30% in the risk of disease progression or death.24 Common grade 3 or 4 adverse events in both arms were lymphocytopenia, neutropenia, fatigue, and pneumonia. Infusion reactions occurred in 33 patients (10%) in the elotuzumab group and were grade 1 or 2 in 29 patients.
Based on these data and the FDA approval, the NCCN panel included elotuzumab in combination with lenalidomide and dexamethasone as a preferred regimen option (category 2A) for previously treated MM (see MYEL-D; pages 393 and 394).
Ixazomib/Lenalidomide/Dexamethasone
The double-blind, randomized, placebo-controlled phase III TOURMALINE-MM1 trial randomized 722 patients with relapsed and/or refractory multiple myeloma to a combination of ixazomib plus lenalidomide and dexamethasone or lenalidomide and dexamethasone alone (control group).25 This trial was designed based on the promising results of a phase I/II study15 (discussed in “New Treatment Options for Patients With Newly Diagnosed MM,” page 394).
The results of the TOURMALINE-MM1 trial show a significant improvement in PFS in patients treated with the ixazomib-containing regimen.25 After a median follow-up of almost 15 months, a 35% improvement in PFS was seen in the group treated with the ixazomib-regimen compared with the control group (HR, 0.742; P=.012). Median PFS was 20.6 months in the ixazomib-treated group versus 14.7 months in the group receiving lenalidomide and dexamethasone alone. In the ixazomib-treated group versus the control group, the ORR (78.3% vs 71.5%; P=.035) and CR rate (11.7% vs 6.6%; P=.019) were also improved. Of note, patients with high-risk cytogenetics enrolled in the trial receiving ixazomib had a similar HR for PFS as the entire study population (HR, 0.596 and 0.543, respectively). Grade 3 or greater adverse events were reported in 68% and 61% of patients in the ixazomib-treated and control groups, respectively. These included neutropenia (19% with ixazomib/lenalidomide/dexamethasone vs 16% with lenalidomide/dexamethasone), anemia (9% vs 13%), thrombocytopenia (13% vs 5%), and pneumonia (6% vs 8%). Serious adverse events were reported in 40% and 44% of patients in the ixazomib and placebo groups, respectively.25
Based on the results of the phase III TOURMALINE-MM1 trial,25 the NCCN panel included ixazomib/lenalidomide/dexamethasone as a preferred regimen option (categry 1) for previously treated MM (sec MYEL-D; pages 393 and 394).
Ixazomib With or Without Dexamethasone
Data from 2 phase I studies of single-agent ixazomib in patients with relapsed/refractory MM established the maximum tolerated dose of ixazomib to be 2.0 mg/m2 on a twice-weekly schedule and 2.97 mg/m2 on a weekly schedule.26,27 The patients in these studies had multiple prior lines of therapy (median of 4 prior lines of therapy in both studies). In the study with the weekly schedule,26 the rate of PR or better (≥PR) was 27% among 30 evaluable patients. In the twice-weekly schedule, the rate of PR or better was 15% among 55 evaluable patients.27 Adverse events of grade 3 or greater were reported in 78% (drug-related in 62%) of patients on the twice-weekly schedule27 and 65% (drug-related in 53%) of patients on the weekly schedule.26 These included thrombocytopenia (37%), neutropenia (17%), and skin and subcutaneous tissue disorders (8%) on the twice-weekly schedule, and thrombocytopenia (33%), neutropenia (18%), and diarrhea (17%) on the weekly schedule. Peripheral neuropathy was reported in 17% of patients (drug-related in 12%), with no grade 3 events, on the twice-weekly schedule.27 On the weekly schedule, drug-related peripheral neuropathy was reported in 20% of patients (2% grade 3).26
Subsequently, phase II trials were designed to evaluate ixazomib with or without dexamethasone in patients with myeloma who had limited prior exposure to bortezomib.28,29
In one trial, patients (N=33) with relapsed MM received weekly ixazomib, 5.5 mg and had dexamethasone added for suboptimal response or disease progression (In 67% of patients). Six additional patients experienced a PR after addition of dexamethasone.28 The ORR (PR or better) with or without the addition of dexamethasone was 34%.28 Adverse events of grade 3 or greater were reported in 78%, with the most common adverse events including thrombocytopenia, fatigue, nausea, and diarrhea.28
Another phase II study (N=70) evaluated 2 doses of weekly ixazomib (4 mg in arm A or 5.5 mg in arm B) with weekly dexamethasone (40 mg) in patients with relapsed MM not previously treated with a proteosome inhibitor (including bortezomib) or who received fewer than 6 cycles of therapy with bortezomib and had a PR or better with no progression at the time of discontinuation.29 The ORRs were 31% in arm A (95% CI, 17–49) and 51% (95% CI, 34–69) in arm B. Among the patients with no prior bortezomib exposure, the response rates were 38% for arm A and 52% for arm B.29 The most common toxicities reported in this trial were fatigue, thrombocytopenia, diarrhea, and nausea, with more grade 3 toxicities in arm B. Peripheral neuropathy, possibly related to ixazomib, was seen in 55% (only grade 1 or 2) of patients in arm A and 43% (2 patients with grade 3) in arm B.29
Based on these phase I/II trial data, the NCCN panel included ixazomib with or without dexamethasone as a treatment option for patients with relapsed/refractory MM (category 2A) who have received at least one prior therapy (see MYEL-D; pages 393 and 394).
Pomalidomide/Dexamethasone
A European multicenter, single-arm, open-label phase IIIb trial evaluated the safety and efficacy of pomalidomide and low-dose dexamethasone in a large patient population (N=604).30 The median PFS reported was 4.2 months and OS was 11.9 months. Despite whether the patients received prior lenalidomide or bortezomib, the PFS, OS, and ORR reported were similar.30
These data are consistent with the previous phase III data from the pivotal MM-003 trial.31 The NCCN panel has now included pomalidomide plus dexamethasone as a category 1 therapeutic option in patients who have received at least 2 prior therapies, including an immunomodulatory agent and bortezomib, and have demonstrated disease progression on or within 60 days of completion of the last therapy. For steroid-intolerant individuals, the NCCN panel suggests considering pomalidomide monotherapy (see MYEL-D; pages 393 and 394).
Panobinostat/Bortezomib/Dexamethasone
Panobinostat is a pan-deacetylase inhibitor that epigenetically modulates class I and II histone deacetylase (HDAC) enzymes.32 The FDA has approved the use of panobinostat in combination with bortezomib and dexamethasone for patients with relapsed/refractory MM who have had at least 2 prior therapies with regimens containing an immunomodulatory agent and bortezomib. The approval was based on the results of the randomized, placebo-controlled phase III PANORAMA-1 study. The study randomized 768 patients with MM who had received prior treatment with an immunomodulatory agent and bortezomib to receive bortezomib and dexamethasone along with either panobinostat or placebo. The results showed an improved median PFS with panobinostat-containing regimen compared with the control arm (11.99 months [95% CI, 10.33–12.94 months] vs 8.08 months [95% CI, 7.56–9.23 months]; HR, 0.63; 95% CI, 0.52–0.76; P<.0001) along an increased depth of response.33 The final OS data from this study are not yet available.
The regimen containing panobinostat is associated with significant toxicity. Serious adverse events were reported in 228 (60%) of 381 patients in the panobinostat group and 157 (42%) of 377 patients in the placebo group. Common grade 3/4 adverse events were seen more in the panobinostat group versus the control group including thrombocytopenia (67% vs 31%), lymphopenia (53% vs 40%), diarrhea (26% vs 8%), fatigue (4% vs 2%), and peripheral neuropathy (18% vs 5%).33
The PANORAMA-2 is a phase II single arm, multicenter trial that evaluated combination of panobinostat with bortezomib and dexamethasone in patients who had relapsed disease that was refractory to bortezomib (N=55).34 Patients on this study achieved an ORR of 34.5% with the panobinostat-containing regimen.34 The median PFS was 5.4 months, and OS had not been reached at a median follow-up of 8.3 months.34 Common grade 3/4 adverse events included thrombocytopenia (63.6%), fatigue (20%), and diarrhea (20%).34
Based on this evidence and the FDA approval, the NCCN panel has included panobinostat in combination with bortezomib and dexamethasone as a category 1 option for patients who have received at least 2 prior therapies, including an immunomodulator and bortezomib (see MYEL-D; pages 393 and 394). A recent subgroup analysis of the PANORAMA-1 trial further provides support for use of this combination in this patient population.35 The results of the analysis clearly demonstrate a PFS benefit of 7.8 months with bortezomib and dexamethasone and panobinostat among patients who received 2 or more prior regimens, including bortezomib and an immunomodulatory drug.35
Panobinostat/Carfilzomib
A multicenter, phase I/II study assessed the safety and efficacy of the combination of panobinostat and carfilzomib in patients with relapsed/refractory MM who experienced relapse after at least one prior treatment.36 The phase I of the study was to determine the maximum tolerable dose of panobinostat and carfilzomib. The primary end point of die phase II was ORR.
No dose-limiting toxicities were observed at any of the planned dose levels in the phase I study. Of the 42 evaluable patients in phase II, the ORR was 67% and the clinical benefit rate was 79%.36 The ORR was 67% for patients’ refractory to prior proteasome inhibitor treatment and 75% for patients’ refractory to prior immune modulating drug treatment. At a median follow up of 17 months, median PFS was 7.7 months.36 Grade 3/4 treatment-related adverse events included thrombocytopenia (38%), neutropenia (21%), fatigue (11%), anemia (9%), hypertension (9%), and diarrhea (7%).36
The maximum tolerated dose of carfilzomib and panobinostat was not reached with the 4 dosing schedules in the first phase I study36; 2 additional dosing schedules were evaluated. The maximum planned dose from the first study was 30 mg of panobinostat plus 20/45 mg/m2 of carfilzomib. In this study,37 the dose of carfilzomib was escalated to 20/56 mg/m2 in one cohort. Because of dose reductions of panobinostat in the first study, the second cohort in this study explored 20 mg of panobinostat and carfilzomib 20/56 mg/m2. The most common adverse events of grade 3 or higher were thrombocytopenia (31%), fatigue (4%), and diarrhea (4%). The ORR was 82% (34% VGPR or better and 48% PR). The clinical benefit rate was 91 %.
Based on promising phase I/Il data, the NCCN panel has added panobinostat in combination with carfilzomib as a treatment option (category 2A) for patients with previously treated MM (see MYEL-D; pages 393 and 394).
Conclusions
These NCCN Guidelines Insights highlight the important updates/changes specific to the management of MM in the most recent version of the NCCN Guidelines. The NCCN Guidelines are in continuous evolution. They are updated annually, or sometimes more often if new high-quality clinical data become available in the interim. The recommendations in the NCCN Guidelines, with few exceptions, are based on the evidence from clinical trials. Expert medical clinical judgment is required when applying these guidelines in the context of individual clinical circumstances to provide optimal care. The physician and the patient have the responsibility to jointly explore and select the most appropriate option from among the available alternatives. When possible, consistent with NCCN philosophy, the panel strongly encourages patient/physician participation in prospective clinical trials.
NCCN: Continuing Education.
Accreditation Statement
This activity has been designed to meet the educational needs of physicians, nurses, and pharmacists involved in the management of patients with cancer. There is no fee for this article. The National Comprehensive Cancer Network (NCCN) is accredited by the ACCME to provide continuing medical education for physicians. NCCN designates this journal-based CE activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
NCCN is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center`s Commission on Accreditation.
NCCN designates this educational activity for a maximum of 1.0 contact hour. Accreditation as a provider refers to recognition of educational activities only; accredited status does not imply endorsement by NCCN or ANCC of any commercial products discussed/displayed in conjunction with the educational activity. Kristina M. Gregory, RN, MSN, OCN, is our nurse planner for this educational activity.
National Comprehensive Cancer Network is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. NCCN designates this continuing education activity for 1.0 contact hour(s) (0.1 CEUs) of continuing education credit in states that recognize ACPE accredited providers. This is a knowledge-based activity. UAN: 0836-0000-16-004-H01-P
All clinicians completing this activity will be issued a certificate of participation. To participate in this journal CE activity: 1) review the learning objectives and author disclosures; 2) study the education content; 3) take the posttest with a 66% minimum passing score and complete the evaluation at http://education.nccn.org/node/78264; and 4) view/print certificate.
Release date: April 10, 2016; Expiration date: April 10, 2017
Learning Objectives
Upon completion of this activity, participants will be able to:
Integrate into professional practice the updates to NCCN Guidelines for Multiple Myeloma
Describe the rationale behind the decision-making process for developing the NCCN Guidelines for Multiple Myeloma
NCCN Categories of Evidence and Consensus.
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management for any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
Acknowledgments
This activity is supported by educational grants from AstraZeneca, Bayer Healthcare Pharmaceuticals Inc., Bristol-Myers Squibb, Clovis Oncology, Foundation Medicine, Genentech, Novartis Oncology, Otsuka America Pharmaceutical, Inc., Seattle Genetics, Inc., and Takeda Oncology; support provided by Actelion Pharmaceuticals US, Inc., and by an independent educational grant from Astellas and Medivation, Inc.
Footnotes
The full and most current version of these NCCN Guidelines are available at NCCN.org.
Disclosure of Relevant Financial Relationships
Editor:
Kerrin M. Green, MA, Assistant Managing Editor, JNCCN—Journal of the National Comprehensive Cancer Network, has disclosed that she has no relevant financial relationships.
CE Authors:
Deborah J. Moonan, RN, BSN, Director, Continuing Education, NCCN, has disclosed that she has no relevant financial relationships.
Ann Gianola, MA, Manager, Continuing Education Accreditation & Program Operations, NCCN, has disclosed that she has no relevant financial relationships.
Kristina M. Gregory, RN, MSN, OCN, Vice President, Clinical Information Operations, NCCN, has disclosed that she has no relevant financial relationships.
Rashmi Kumar, PhD, Senior Manager, Clinical Content, NCCN, has disclosed that she has no relevant financial relationships.
Individuals Who Provided Content Development and/or Authorship Assistance:
Kenneth C. Anderson, MD, Panel Chair, has disclosed that he is a scientific advisor for Acetylon Pharmaceuticals, Inc., Bristol-Myers Squibb Company, Celgene Corporation, Gilead Sciences, Inc., Millennium Pharmaceuticals, Inc., and OncoPep, and receives other financial benefits from Acetylon Pharmaceuticals, Inc. and OncoPep.
Shaji K. Kumar, MD, Panel Member, has disclosed that he receives grant/research support from Celgene Corporation, Takeda Pharmaceutical Company Ltd, Novartis, AbbVie, Janssen Pharmaceuticals, Inc., Sanofi, and Merck & Co., and receives consulting fees from Takeda Pharmaceutical Company Ltd, Amgen Inc., Janssen Pharmaceuticals, Inc., Bristol-Myers Squibb Company, GlycoMimetics, Inc., Skyline Diagnostics, NOXXON Pharma, and Kesios Therapeutics Ltd.
Dorothy A. Shead, MS, Clinical Information Operations, NCCN, has disclosed that she has no relevant financial relationships.
Rashmi Kumar, PhD, Senior Manager, Clinical Content, NCCN, has disclosed that she has no relevant financial relationships.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 3.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 4.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–2869. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roussel M, Lauwers-Cances V, Robillard N, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myelome. J Clin Oncol. 2014;32:2712–2717. doi: 10.1200/JCO.2013.54.8164. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119:4375–4382. doi: 10.1182/blood-2011-11-395749. [DOI] [PubMed] [Google Scholar]
- 9.Durie B, Hoering A, Rajkumar SV, et al. Bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT): results of the randomized phase III trial SWOG SO777 [abstract] Blood. 2015;126:25. doi: 10.1038/s41408-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23:1337–1341. doi: 10.1038/leu.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einsele H, Liebisch P, Langer C, et al. Velcade, intravenous cyclophosphamide and dexamethasone (VCD) induction for previously untreated multiple myeloma (German DSMM XIa Trial) [abstract] Blood. 2009;114 Abstract 131. [Google Scholar]
- 12.Reeder CB, Reece DE, Kukreti V, et al. Long-term survival with cyclophosphamide, bortezomib and dexamethasone induction therapy in patients with newly diagnosed multiple myeloma. Br J Haematol. 2014;167:563–565. doi: 10.1111/bjh.13004. [DOI] [PubMed] [Google Scholar]
- 13.Reeder CB, Reece DE, Kukreti V, et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood. 2010;115:3416–3417. doi: 10.1182/blood-2010-02-271676. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez-Zepeda VH, Duggan P, Neri PE, Bahlis NJ. Cyclophosphamide, bortezomib and dexamethasone (CyBORD) is a feasible and active regimen for non-transplant eligible, multiple myeloma patients [abstract] Blood. 2014;124:5751. [Google Scholar]
- 15.Kumar SK, Berdeja JG, Niesvizky R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503–1512. doi: 10.1016/S1470-2045(14)71125-8. [DOI] [PubMed] [Google Scholar]
- 16.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–1809. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dytfeld D, Jasielec J, Griffith KA, et al. Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99:e162–164. doi: 10.3324/haematol.2014.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korde N, Zingone A, Kwok M, et al. Phase II clinical and correlative study of carfilzomib, lenalidomide, and dexamethasone (CRd) in newly diagnosed Multiple Myeloma (MM) patients [abstract] Blood. 2012;120 Abstract 732. [Google Scholar]
- 19.Korde N, Zingone A, Kwok M, et al. Phase II clinical and correlative study of carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended dosing (CRD-R) induces high rates of MRD negativity in newly diagnosed multiple myeloma (MM) patients [abstract] Blood. 2013;122 Abstract 538. [Google Scholar]
- 20.Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 21.Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207–1219. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 22.Lonial S, Weiss B, Usmani S, et al. Phase II study of daratumumab (DARA) monotherapy in patients with ≥3 lines of prior therapy or double refractory multiple myeloma (MM): 54767414MMY2002 (Sirius) [abstract] J Clin Oncol. 2015;33(15 Suppl) Abstract LBA8512. [Google Scholar]
- 23.Hsi ED, Steinle R, Balasa B, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14:2775–2784. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lonial S, Dimopoulos MA, Palumbo A, et al. ELOQUENT-2: a phase III, randomized, open-label study of lenalidomide (Leu)/dexamethasone (dex) with/without elotuzumab (Elo) in patients (pts) with relapsed/refractory multiple myeloma (RRMM) [abstract] J Clin Oncol. 2015;33(Suppl) Abstract 8508. [Google Scholar]
- 25.Moreau P, Masszi T, Grzasko N, et al. Ixazomib, an investigational oral proteasome inhibitor (PI), in combination with lenalidomide and dexamethasone (lRd), significantly extends progression-free survival (PFS) for patients (Pts) with relapsed and/or refractory multiple myeloma (RRMM): the phase 3 TOURMALINE-MM1 study. Blood. 2015;126:727–727. [Google Scholar]
- 26.Kumar SK, Bensinger WI, Zimmerman TM, et al. Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma. Blood. 2014;124:1047–1055. doi: 10.1182/blood-2014-01-548941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson PG, Baz R, Wang M, et al. Phase 1 study of twice-weekly ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma patients. Blood. 2014;124:1038–1046. doi: 10.1182/blood-2014-01-548826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar SK, LaPlant B, Roy V, et al. Phase 2 trial of ixazomib in patients with relapsed multiple myeloma not refractory to bortezomib. Blood Cancer J. 2015;5:e338. doi: 10.1038/bcj.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar SK, Laplant BR, Reeder CB, et al. Randomized phase 2 trial of two different doses of ixazomib in patients with relapsed multiple myeloma not refractory to bortezomib. Blood. 2015;126:3050–3050. doi: 10.1038/bcj.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimopoulos MA, Palumbo A, Weisel K, et al. Safety and efficacy in the stratus (MM-010) trial, a single-arm phase 3b study evaluating pomalidomide + low-dose dexamethasone in patients with refractory or relapsed and refractory multiple myeloma; Presented at the American Society of Hematology Annual Meeting; December 6–9, 2014; San Francisco, California. Abstract 80. [Google Scholar]
- 31.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;4:1055–1066. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 32.Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009;280:233–241. doi: 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 33.San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15:1195–1206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 34.Richardson PG, Schlossman RL, Alsina M, et al. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood. 2013;122:2331–2337. doi: 10.1182/blood-2013-01-481325. [DOI] [PubMed] [Google Scholar]
- 35.Richardson PG, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone in previously treated multiple myeloma: outcomes by prior treatment. Blood. 2016;127:713–721. doi: 10.1182/blood-2015-09-665018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berdeja JG, Hart LL, Mace JR, et al. Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma. Haematologica. 2015;100:670–676. doi: 10.3324/haematol.2014.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berdeja JG, Gregory TK, Matous J, et al. A phase I/II study of the combination of panobinostat (PAN) and carfilzomib (CFZ) in patients (pts) with relapsed or relapsed/refractory multiple myeloma (MM) [Abstract] J Clin Oncol. 2015;33(Suppl) Abstract 8513. [Google Scholar]