Abstract
A better understanding of long-term functional recovery process for patients with traumatic brain injury (TBI) facilitates effective rehabilitations. The aim of this study was to classify and characterize patients with moderate-to-severe TBI based on their functional trajectories up to 5 years post-injury. The study included 121 patients with moderate-to-severe TBIs (International Classification of Diseases, Tenth Revision [ICD-10], S06.0–S06.9), 16–55 years of age, and admitted at Trauma Referral Hospital within 24 h of injury between 2005 and 2007. Demographics and injury characteristics were documented at the admission, and functional status was recorded at 3 months and 1 and 5 years post-injury using Functional Independence Measure motor (FIM-M) and cognitive (FIM-C) subscales. We used group-based trajectory models to classify patients' functional trajectories over a 5-year period. For FIM-M, three trajectories were identified: 8.2% of patients showed stable low recovery (13.6 ± 1.5, 17.9 ± 8.8, and 21.0 ± 17.9), 9.2% elevated good recovery (35.8 ± 14.5, 75.5 ± 12.4, and 85.5 ± 8.1), and 82.6% stable good recovery (89.0 ± 3.6, 90.3 ± 1.9, and 90.8 ± 1.0) at the three follow-up points, respectively. For FIM-C, four trajectories were revealed: 4.1% of patients showed stable low recovery (5.0 ± 0, 5.0 ± 0, and 5.0 ± 0), 12.6% delayed moderate recovery (8.9 ± 3.5, 20.6 ± 4.6, and 28.3 ± 3.8), 28.7% elevated good recovery (27.0 ± 3.8, 30.4 ± 7.3, and 31.1 ± 2.3), and 54.6% stable good recovery (32.8 ± 2.3, 34.6 ± 1.0, and 34.7 ± 1.0). The results suggest that three FIM-M and four FIM-C trajectories described various patterns of functional recovery 5 years after moderate-to-severe TBI, with stable good recovery being the most common trajectory. Identifying and characterizing the trajectory memberships should enable targeted rehabilitation programs, inform patient-centered care, and improve long-term outcomes.
Keywords: : functional independent measurements, outcome trajectory, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of long-term disability affecting all ages and demographics.1–3 In the United States alone, it is estimated that 3.2 million people are living with TBI-related disability subsequent to a TBI-related hospitalization.1,4–6 Similarly, in the European Union, approximately 7.7 million people are living with disabilities caused by TBI.7,8 The disability post-TBI, particularly for persons with moderate-to-severe injuries, tends to be multi-dimensional, including cognitive and physical difficulties that could potentially last for a lifetime.1,9
A rich body of literature on long-term outcomes post-TBI has shown that a significant number of TBI survivors carry functional disability long after the initial injury and such disability varies significantly among individuals.9–18 Moreover, studies have suggested that patients with moderate-to-severe TBI could experience a slow or plateaued recovery in the functional measures of the Extended Glasgow Outcome Scale (GOS-E)18 or the Functional Independence Measures (FIM™),19,20 approximately 1 year or even earlier post-injury.21,22 These findings point to the chronic effects of TBI that can affect a person's health and social environment long after acute medical treatment and rehabilitation,23 raising an important question as to whether patients with disability receive the support and rehabilitation they need over time.
Whereas research results generated so far have helped describe the long-term recovery process for patients with moderate-to-severe TBI, further identification of subgroups of patients with continuing needs of assistance would be an important step to facilitate more effective rehabilitation intervention programs. From a clinical point of view, this means 1) allocation of more rehabilitation (and research) resources to patients targeted with less optimistic recovery trajectories to enhance rehabilitation to further improve recovery programs, 2) development of tailored rehabilitation intervention programs targeting the unmet needs and relevant clinical trajectories beyond the post-acute phase, and 3) further research, preferably randomized controlled trials, to evaluate the effectiveness of patient-centered rehabilitation programs and cost-effectiveness in different recovery trajectory groups. These are continuous and parallel processes. To better understand the heterogeneity of long-term physical and cognitive functional recovery processes for patients with TBI, this study applied a group-based trajectory modeling (GBTM) approach to classify and characterize patients with moderate-to-severe TBI based on their functional trajectories during the first 5 years post-injury. Given the distinctiveness of cognitive and physical recovery, we examined the trajectories for these functions separately and compared the baseline and in-hospital care characteristics between the identified trajectories.
Methods
Design and participants
The study was a prospective cohort study conducted in the Eastern Norway, Oslo University Hospital. The hospital provides healthcare services for approximately one half of the Norwegian population (2.8 million residents). Patients with moderate-to-severe TBIs who were consecutively admitted to Oslo University Hospital were screened between 2005 and 2007 for study eligibility. Details of the original design were described elsewhere.13 Briefly, the study inclusion criteria were 1) age between 16 and 55 years, 2) residents of the east region of Norway, 3) Glasgow Coma Scale (GCS)24 3–12, and 4) admitted with International Classification of Diseases, Tenth Revision (ICD-10) diagnoses S06.0–S06.9 within 24 h of injury. Although 160 patients met the inclusion criteria, 27 were excluded because of serious pre-injury comorbidities (e.g., neurological disorders or injuries, severe psychiatric disorders), coexisting conditions (e.g., spinal cord injuries), and substance abuse disorder. This study further excluded 12 subjects who later withdrew or were lost to follow-up; therefore, 121 participants were included in the present study.
Process
All study participants provided a written informed consent at the study entry. For those who were unable to consent initially, the consents were first obtained from a close relative or legal guardian and then from the participant, when possible. If the participants were <18 years of age, their parents' consents were obtained. Baseline data on patient demographics, injury characteristics, and medical history were documented at the study admission. From the original study, 88 study participants had valid measures of post-traumatic amnesia (PTA) measurements and the measures were dichotomous at the mean value (i.e., 0–3 vs. >3 weeks). All patients had an acute computed tomography (CT) head scan followed by a second control scan between 6 and 12 h post-injury. All CT scans were assessed and categorized by the same neuroradiologist according to the Marshall CT classification,25 and the “worst” scan result was used for classification. Length of in-hospital stays (LOS) in days were recorded as LOS in the intensive care unit (ICU), total LOS in the trauma center, and rehabilitation unites. All patients received neurosurgical and/or ICU care in the acute setting whereas 60% of patients received post-acute brain injury rehabilitation services in order to optimize recovery, increase functional independence in personal and domestic activities of daily life, restore social participation, and minimize the distress of the patient as well as of the caregivers.26
Patients' functional status was followed and assessed at 3 months and 1 and 5 years post-injury at the outpatient department. The study was approved by the Regional Ethics Committee for Medical Research in the East of Norway and the Norwegian Data Inspectorate.
Outcome measurement
For this study, the outcome of interest was the patients' functional status up to 5 years post-injury, which was measured through the FIM instrument27 and by the FIM-certified raters. The instrument is a well-established and widely used tool for measuring the degree of a patient's disability and grade of assistance required to carry out activities of daily living (ADL). It includes 18 items, and each item is scored on a 7-point ordinal scale, where 1 indicates complete dependence and 7 indicates complete independence in ADL. The instrument can also be grouped into two subscales: FIM motor tasks (FIM-M) and FIM cognitive tasks (FIM-C). The FIM-M and FIM-C subscales include 13 items and five items, and the sum of each scale will be a value between 13 and 91 (FIM-M) or between 5 and 35 (FIM-C).
Statistical analysis
Two sets of analyses were carried out to classify the patient subgroups based on their functional trajectories during the first 5 years post-TBI and to characterize these subgroups. In the first set, GBTM was used through the SAS Proc Traj procedure28,29 to identify the patient subgroups who shared the same functional profiles (i.e., measures of FIM-M and FIM-C subscales) over time. Subjects with missing outcome data were included in the analysis, but only available data for each subject were used. Models with different trajectory shapes and a varying number of classes were compared to find the model that best fit the data. Polynomial orders, Bayesian Information Criterion (BIC), logged Bayes factor (2ΔBIC),, and substantive knowledge were used to determine the shapes and groups of the trajectories over the 5-year period.
In the Proc Traj procedure, the maximum polynomial order is a quadratic form, thus the model-building process began with a model consisting of one group with a quadratic degree polynomial and then increasing the group numbers until the number of groups that best fit the data was identified based on the model selection criteria. For the model-building process, BIC was used to assess the improvement in model fit gained by adding more parameters (e.g., more groups or more complexed trajectory shapes), but also penalizing models with more parameters. When two models were compared, the larger BIC value would be chosen. If the fit of two models was the same, the simpler model would be chosen.30 Further, 2ΔBIC was used to select a meaningful model improvement in the process. The 2ΔBIC, or an approximation of the logged Bayes factor, could be interpreted as the degree of evidence favoring the more complex model.30,31 When two models were compared, a difference of 10 in 2ΔBIC would be considered as a meaningful difference.32
In the second set of analyses to characterize the trajectory memberships, chi-square test or analysis of variance was used to compare the differences in demographic and injury characteristics measured at baseline and the FIM-M and FIM-C scores at 3 months and 1 and 5 years post-injury. Bonferroni's correction was applied to counteract type 1 errors attributed to multiple comparisons. For this analysis, subjects with missing outcome data were included in the analysis, but no imputation was done and only available data for each subject were used. The analyses for comparing membership trajectories were performed using SAS software (version 9.4; copyright [c] 2002–2012 by SAS Institute Inc., Cary, NC).
Results
The FIM total score for all study participants was 104.6 ± 34.4 at 3 months, 113.6 ± 27.1 at 1 year, and 118.3 ± 22.1 at 5 year post-injury.
Identification of subgroup functional trajectories
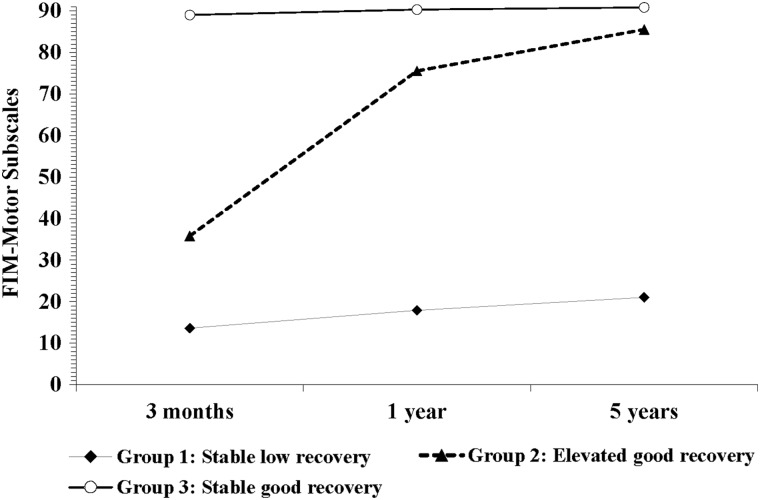
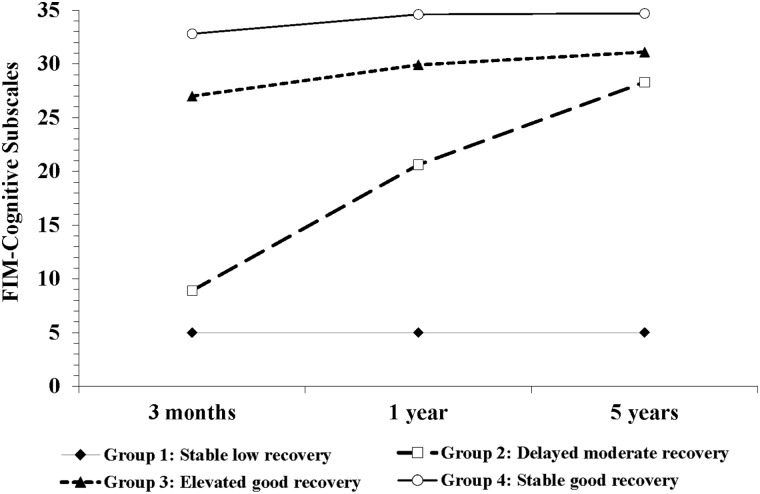
Three trajectories of physical functional recovery (FIM-M subscale) were identified at 3 months, 1 year, and 5 years (Fig. 1), with 8.2%, 9.2%, and 82.6% patients assigned to the three groups, respectively. Four trajectories of cognitive functional recovery (FIM-C subscale) were identified over the same three time points (Fig. 2), with 4.1%, 12.6%, 28.7%, and 54.6% patients assigned to the four groups, respectively.
FIG. 1.
Three trajectories of the motor function 5 years after moderate-to-severe traumatic brain injury. The motor function was measured through the FIM-Motor subscale, and the trajectories were identified by a group-based modeling. FIM, Functional Independence Measure.
FIG. 2.
Four trajectories of the cognitive function 5 years after moderate-to-severe traumatic brain injury. The cognitive function was measured through the FIM-Cognitive subscale, and the trajectories were identified by a group-based modeling. FIM, Functional Independence Measure.
The model fitness statistics for identifying these trajectories are shown in Table 1. For the FIM-M subscale, the model with three-class memberships was selected as the best model because it presented the larger BIC value (−466.7) than the model with two-class memberships and showed a meaningful improvement over the two-class model (2ΔBIC = 62.2). Similarly, for the FIM-C subscale, both models with four- and five-class memberships presented larger BIC values and showed meaningful improvement than other four models (2ΔBIC >10), but the model with five-class memberships was considered less clinically meaningful. Therefore, the model with four-class memberships was considered as the best model.
Table 1.
Model Fit Statistics for Selecting the Optimal Number of Latent Trajectory Classes
| Model | BIC | Null model | 2*ΔBIC | % class membership |
|---|---|---|---|---|
| FIM-Motor subscale | ||||
| 1-Class | −550.0 | 100.0 | ||
| 2-Class | −497.8 | 1 | 104.4 | 14.0/86.0 |
| 3-Class | −466.7 | 2 | 62.2 | 8.2/9.2/82.6 |
| FIM-Cognition subscale | ||||
| 1-Class | −957.8 | 100.0 | ||
| 2-Class | −682.7 | 1 | 550.2 | 17.3/82.7 |
| 3-Class | −673.1 | 2 | 19.2 | 4.4/15.7/79.9 |
| 4-Class | −639.5 | 3 | 67.2 | 4.1/12.6/28.7/54.6 |
| 5-Class | −629.7 | 4 | 19.6 | 4.1/12.1/17.1/29.9/36.9 |
| 6-Class | −634.8 | 5 | −10.2 | 4.1/11.3/12.8/7.1/27.4/37.3 |
2*ΔBIC = Logged Bayes Factor, or 2 times difference in BICs between an alternative (more complex) and a null (simpler) models.
The 3-Class for FIM-Motor subscale and 4-Class for FIM-Cognition subscale have the best model fit to describe the optimal number of latent trajectories.
FIM, Functional Independence Measure; BIC, Bayesian Information Criterion.
Comparisons of the FIM-M and FIM-C measures at 3 months and 1 and 5 years among the trajectory groups are shown in Table 2. For the FIM-M subscale, three trajectories of function were identified: Group 1 (8.2%) showed stable low recovery (13.6 ± 1.5, 17.9 ± 8.8, and 21.0 ± 17.9), group 2 (9.2%) showed elevated good recovery (35.8 ± 14.5, 75.5 ± 12.4, and 85.5 ± 8.1), and group 3 (82.6%) showed stable good recovery (89.0 ± 3.6, 90.3 ± 1.9, and 90.8 ± 1.0) at the three follow-up points, respectively. For the FIM-C subscale, four trajectories of function were revealed over time: Group1 (4.1%) showed stable low recovery (5.0 ± 0, 5.0 ± 0, and 5.0 ± 0), group 2 (12.6%) showed delayed moderate recovery (8.9 ± 3.5, 20.6 ± 4.6, and 28.3 ± 3.8), group 3 (28.7%) showed elevated good recovery (27.0 ± 3.8, 30.4 ± 7.3, and 31.1 ± 2.3), and group 4 (54.6%) showed stable good recovery (32.8 ± 2.3, 34.6 ± 1.0, and 34.7 ± 1.0) at the same three follow-up points.
Table 2.
Functional Outcomes by Trajectory Groups (n = 121)
| Trajectory groups* Mean (SD) | |||||
|---|---|---|---|---|---|
| FIM-Motor subscale | |||||
| Follow-up points | Group 1 8.2% | Group 2 9.2% | Group 3 82.6% | Total 100.0% | p value |
| 3 month | 13.6 (1.5)a,b,c | 35.8 (14.5)a,b,c | 89.0 (3.6)a,b,c | 77.7 (25.4) | <0.0001 |
| 1 year | 17.9 (8.8)a,b,c | 75.5 (12.4)a,b,c | 90.3 (1.9)a,b,c | 83.1 (20.6) | <0.0001 |
| 5 year | 21.0 (17.9)a,c | 85.5 (8.1)a,c | 90.8 (1.0)a,c | 86.4 (16.8) | <0.0001 |
| FIM-Cognitive subscale | ||||||
|---|---|---|---|---|---|---|
| Group 1 4.1% | Group 2 12.6% | Group 3 28.7% | Group 4 54.6% | Total 100.0% | P value | |
| 3 month | 5.0 (0)c,d | 8.9 (3.5)c,d | 27.0 (3.8)a,b,c,d | 32.8 (2.3)a,b,c,d | 27.0 (9.4) | <0.0001 |
| 1 year | 5.0 (0)a,b,c,d | 20.6 (4.6)a,b,c,d | 29.9 (3.1)a,b,c,d | 34.6 (1.0)a,b,c,d | 30.4 (7.3) | <0.0001 |
| 5 year | 5.0 (0)a,b,c,d | 28.3 (3.8)a,b,c,d | 31.1 (2.3)a,b,c,d | 34.7 (1.0) a,b,c,d | 31.9 (5.9) | <0.0001 |
For comparing the mean FIM-Motor subscales by the trajectory groups, the Bonferroni adjustment was set at the level of 0.008; whereas for comparing the Mean FIM-Cognitive subscales, the adjustment was set at the level of 0.004, for between-subjects differences respectively.
Significant difference from group 1; bsignificant difference from group 2; csignificant difference from group 3; dsignificant difference from group 4.
SD, standard deviation; FIM, Functional Independence Measure.
Comparison of trajectory memberships
To characterize the trajectory groups, the baseline demographics and characteristics of injuries and in-hospital care by the trajectory groups were compared as shown in Table 3. For four trajectories of cognitive functional groups, no significant differences were observed in the patients' mean age, sex, cause of injury, and mean measures of injury severity score and abbreviated injury scales among the groups. However, statistically significant differences were observed only in PTA (< = 3 vs. >3 weeks), GCS measures (severe TBI, GCS 3–8 vs. moderate TBI, GCS 9–12), and CT classifications (diffuse injury I–II, diffuse injury with swelling or shift III–IV, or mass lesion with or without evacuation V–VI), although some patients do not have information on PTA. In general, severe TBI, as defined by the GCS at study admission (GCS 3–8), was associated with the stable low and delayed moderate recovery trajectories (i.e., groups 1 and 2); whereas moderate TBI (GCS 9–12) was associated with the elevated and stable good recovery trajectories (i.e., groups 3 and 4). For the worst CT classification at study admission, all patients that were assigned to the stable low recovery trajectory (group 1) had CT classification III–IV; the majority of patients in delayed moderate recovery trajectory (group 2) had CT classification III–VI; and the stable good recovery trajectory (group 4) tended to have CT classification I–II or V–VI. The characteristics of in-hospital care (i.e., the length of ICU, trauma center, and rehabilitation unit stays), which again were indicators of injury severity, were significantly different among the trajectory groups. Apparently, the longer the patients had to stay in these care units, the worse the recovery trajectory memberships to which they would be assigned. Similar trends were observed in the baseline demographics and characteristics of injuries and in-hospital cares by three motor functional recovery trajectories.
Table 3.
Baseline and In-Hospital Care Characteristics by Trajectory Groups (n = 121)
| Trajectory groups N (%) | |||||
|---|---|---|---|---|---|
| FIM-Motor subscale | |||||
| Characteristics | Group 1 8.2% | Group 2 9.2% | Group 3 82.6% | Total 100% | p value |
| Demographics | |||||
| Agea | 29.5 (12.5) | 33.8 (14.7) | 32.4 (11.4) | 32.3 (11.7) | 0.74 |
| Sex | |||||
| Males | 6 (6.5) | 6 (6.5) | 81 (87.1) | 93 (76.9) | 0.65 |
| Females | 2 (7.1) | 3 (10.7) | 23 (82.1) | 28 (23.1) | |
| Cause of injury | |||||
| Motor vehicle accidents | 7 (10.0) | 7 (10.0) | 56 (80.0) | 70 (57.9) | 0.38 |
| Fall | 1 (3.5) | 1 (3.5) | 27 (93.1) | 29 (24.0) | |
| Violence/others | 0 (0) | 1 (4.6) | 21 (95.5) | 22 (18.2) | |
| Injury severity | |||||
| GCS | |||||
| 3–8 | 8 (9.0) | 9 (10.1) | 72 (89.9) | 89 (73.6) | 0.03 |
| 9–12 | 0 (0) | 0 (0) | 32 (100.0) | 32 (26.5) | |
| Injury severity scorea | 42.4 (12.2) | 34.4 (9.5) | 30.0 (13.5) | 31.1 (13.5) | 0.03 |
| Abbreviated injury scalea | 4.9 (0.4) | 4.7 (0.7) | 4.4 (0.9) | 4.4 (0.9) | 0.29 |
| PTAb | |||||
| < = 3 weeks | 0 (0.0) | 0 (0.0) | 51 (100.0) | 51 (58.0) | 0.002 |
| >3 weeks | 0 (0.0) | 7 (18.9) | 30 (81.1) | 37 (42.1) | |
| CT classification | |||||
| Diffuse injury (I–II) | 1 (2.2) | 0 (0.0) | 44 (97.8) | 45 (38.8) | 0.01 |
| Diffuse injury with swelling or shift (III–IV) | 5 (9.1) | 8 (14.6) | 42 (76.4) | 55 (47.4) | |
| Mass lesion with/out evacuation (V–VI) | 2 (12.5) | 1 (6.3) | 13 (81.3) | 16 (13.8) | |
| In-hospital care | |||||
| Length of ICU stay (days)a | 23.8 (6.2) | 13.9 (8.6) | 7.4 (7.4) | 9.8 (8.3) | <0.0001 |
| Length of trauma center stay (days)a | 40.3 (15.3) | 19.6 (13.4) | 9.8 (10.3) | 14.0 (13.4) | <0.0001 |
| Length of rehabilitation stay (days)a | 95.3 (34.9) | 68.8 (39.9) | 20.8 (31.2) | 35.6 (41.2) | <0.0001 |
| FIM-Cognitive subscale | ||||||
|---|---|---|---|---|---|---|
| Group 1 4.1% | Group 2 12.6% | Group 3 28.7% | Group 4 54.6% | Total 100% | p value | |
| Demographics | ||||||
| Agea | 27.0 (5.4) | 35.1 (14.9) | 31.6 (11.4) | 32.4 (11.5) | 32.3 (11.7) | 0.66 |
| %Sex | ||||||
| Males | 3 (3.2) | 8 (8.6) | 20 (21.5) | 62 (66.7) | 93 (76.9) | 0.33 |
| Females | 1 (3.6) | 4 (14.3) | 9 (32.1) | 14 (50.0) | 28 (23.1) | |
| Cause of injury | ||||||
| Motor vehicle accidents | 4 (5.7) | 9 (12.9) | 18 (25.7) | 39 (55.7) | 70 (57.9) | 0.19 |
| Fall | 0 (0) | 2 (6.9) | 9 (31.0) | 18 (62.1) | 29 (24.0) | |
| Violence/others | 0 (0) | 1 (4.6) | 2 (9.1) | 19 (86.4) | 22 (18.2) | |
| Injury severity | ||||||
| GCS | ||||||
| 3–8 | 4 (4.5) | 12 (13.5) | 25 (28.1) | 48 (53.9) | 89 (73.6) | 0.005 |
| 9–12 | 0 (0) | 0 (0) | 4 (12.5) | 28 (87.5) | 32 (26.5) | |
| Injury severity scorea | 45.3 (3.3) | 33.5 (8.3) | 32.0 (11.9) | 29.7 (14.6) | 31.1 (13.5) | 0.12 |
| Abbreviated injury scalea | 5.0 (0.0) | 4.8 (0.6) | 4.7 (0.6) | 4.3 (1.0) | 4.4 (0.9) | 0.11 |
| PTAb | ||||||
| < = 3 weeks | 0 (0.0) | 0 (0) | 11 (21.6) | 40 (78.4) | 51 (58.0) | <0.0001 |
| >3 weeks | 0 (0.0) | 7 (18.9) | 17 (46.0) | 13 (35.1) | 37 (42.1) | |
| CT classification | ||||||
| Diffuse injury (I–II) | 0 (0) | 2 (2.2) | 12 (26.7) | 32 (71.1) | 45 (38.8) | 0.008 |
| Diffuse injury with swelling or shift (III–IV) | 4 (7.3) | 8 (14.6) | 14 (25.5) | 29 (52.7) | 55 (47.4) | |
| Mass lesion with/out evacuation (V–VI) | 0 (0) | 3 (18.8) | 3 (18.8) | 10 (62.5) | 16 (13.8) | |
| In-hospital care | ||||||
| Length of ICU stay (days)a | 23.8 (6.2) | 13.9 (8.6) | 12.6 (7.4) | 7.4 (7.4) | 9.8 (8.3) | <0.0001 |
| Length of trauma center stay (days)a | 40.3 (15.3) | 19.6 (13.4) | 19.2 (13.4) | 9.8 (10.3) | 14.0 (13.4) | <0.0001 |
| Length of rehabilitation stay (days)a | 95.3 (34.9) | 68.8 (39.9) | 52.2 (45.1) | 20.8 (31.2) | 35.6 (41.2) | <0.0001 |
Mean and standard deviation.
Eighty-eight patients had measures of PTA.
GCS, Glasgow Coma Scale; PTA, post-traumatic amnesia; CT, computed tomography; ICU, intensive care unit; FIM, Functional Independence Measure.
Discussion
To our knowledge, the present study is the first to examine FIM trajectories longitudinally up to 5 years after moderate-to-severe TBI in Scandinavian countries. Using the FIM-M and FIM-C subscales and GBTM, this study identified three motor functional and four cognitive trajectories. Regarding the motor function trajectories (Fig. 1), the high motor function group (group 3) had a relatively stable motor score at approximately 90 across all three follow-up points. The low motor function group (group 1) started with total need of assistance at 3 months, had some improvement up to 1 year, and slight improvement up to 5 years, whereas group 2 started with moderate assistance at 3 months before a speedy improvement up to 1 year, and improvement continued at the 5-year follow-up toward a modified and complete independence. Regarding the cognitive function trajectories (Fig. 2), the high cognitive function group (group 4) showed a rather steady modified or complete independence in activities measured at 3-month and 1- and 5-year follow-up points. Group 2 was the one with the most improvement, which started with a low 3-month score, before having a steep improvement up to 1 year and the improvement continued up to 5 years post-injury. Group 3 began with the need of supervision at 3-month measurements and then improved to modified independence at 1- and 5-year follow-ups. On the other hand, the small proportion of patients in the low cognitive function group (group 1) had a stable need of total assistance measured across all follow-up points without improvement.
With respect to the demographic and injury characteristics at acute hospital admission and their relation to the identified functional trajectories, our results suggest that the lower cognitive and motor functional trajectories were mostly associated with injury severity-related characteristics. For instance, patients who shared the lower cognitive trajectory or FIM-C measures post-injury were in general more likely to have severe TBI, longer PTA time, and require longer rehabilitation stay and overall hospital stays. Moreover, patients who were differentiated to the lower cognitive trajectory subgroups were more likely to have diffuse injury with shifted or decompressed cisterns, whereas patients with higher cognitive trajectories were more likely to have simple diffuse injuries or mass lesions with or without surgical evacuations. Similar patterns were also observed with regard to the relation between injury characteristics and motor function trajectories or FIM-M measures, although more than 90% of patients had good physical recovery at 1 year post-injury.
Recently, several U.S.-based, large-size longitudinal studies also looked at the long-term recovering trajectories for patients with TBI. Two studies from the U.S. National Traumatic Brain Injury Model Systems described temporal patterns of global outcomes after moderate or severe TBI using GOS-E33 and Disability Rating Scale (DRS).34 Both applied an individual growth curve modeling (GCM) approach to model the interindividual differences in recovery over time. The results suggested that the trajectory of GOS-E scores is best described with a model of quadratic change, in which scores initially increase and peak approximately 10 years after the first GOS-E assessment and then decrease.33 Baseline age, race, and FIM score at rehabilitation admission and rehabilitation length of stay were found to not only influence the trajectory of GOS-E, but also the trajectory of DRS.33,34 Given that GCM approach models mean trends in changes across time and individual departures from the average trend, it may not be sufficient to capture interindividual variability if all individuals in a population are not truly following a similar recovery trend. Thus, the results of these two studies may have a limited implication for individuals outside the model system setting or following different recovery trajectories.
Another recent U.S. national study35 applied latent class mixture modeling (or GBTM)29,36 to describe the FIM motor and cognitive trajectories among 16,583 individuals who received inpatient rehabilitation services for TBI. The study identified three unique trajectories for both motor and cognitive FIM scores across the period of inpatient rehabilitation admission, discharge, and study follow-up. The average study follow-up time was 103 ± 23 days post-discharge. The results also showed that individuals in the lower motor trajectory were more likely to be racial/ethnic minority and had older age, Medicare or Medicaid coverage, comorbid conditions, open TBI, and greater duration from injury date to rehabilitation admission date. Similarly, individuals in the lower cognitive trajectory were more likely to be male and racial/ethnic minority and have younger age, Medicare or Medicaid, comorbid conditions, and greater duration admission. Of notable limitations, the study did not include any direct measure of injury severity such as GCS, PTA, or CT/magnetic resonance imaging findings, making it difficult to distinguish the heterogeneity of the targeted patient population at acute phase and limit the external applicability of the study results.
Similar to the modeling approach by Howrey and colleagues,35 our study also applied GBTM to identify the trajectories of FIM-M and FIM-C measures up to 5-years post-injury. Comparing with the GCMs, an important distinction of GBTMs is that the model does not assume a one-size-fits-all model for characterizing the development change over time. The underlining model assumption is that the population is composed of a mixture of distinct groups defined by their development trajectories. Therefore, GBTMs may appear more attractive to clinical researchers as they provide empirical means of identifying patients' subgroups after both typical and atypical courses of development.37 It is worth noting another related modeling approach that is commonly used to describe the development trajectories in research: the growth mixture modeling (GMM) approach.38 GMM applies a finite mixture modeling to GCM so that two or more GCMs are used to model separate populations following a different development trajectory. Comparisons of techniques and usages among these modeling approaches are discussed in detail by Daniel S. Nagin and Candice L. Odgers.37
In addition to the modeling approach, compared with the study by Howrey and colleagues,35 our study found that acute GCS score, CT classification of injury severity, longer PTA time, and lengthy hospital stays (ICU, trauma center, and rehabilitation units) were the most important factors associated with long-term motor and cognitive function trajectories. However, age and sex were not found to be significantly associated with function trajectories in the present study. Our study sample was relatively small with a skewed gender distribution (77% males) and limited age span (16–55 years), which could, in part, explain the lack of association. Further, the functional trajectories modeled in this study had a very limited follow-up period, which makes it difficult to describe functional status beyond that time period. Our study found that the largest improvement in function was from 3 months to 1 year (e.g., FIM-M and FIM-C measures from the patients who were assigned to group 2 trajectories), and after 1 year such improvement was at a much slower pace up to the 5-year follow-up. However, additional long-term prospective studies are warranted to replicate these findings in larger samples.
Several other studies have also examined long-term FIM-M and FIM-C measures post-TBI. A study by Nakase-Richardson and colleagues20 followed 108 individuals with severe TBI from the rehabilitation discharge to the 1-, 2-, and 5-year follow-up points. The average FIM-M scores were improved from 25 at discharge to 77 at 1-year follow-up, with some further improvement to 81 at 2-year and 82 at 5-year follow-ups. The average FIM-C scores improved from 10 at discharge to 25 at 1-year follow-up, before stabilizing at 27 at both 2- and 5-year follow-ups. These average scores are rather similar to those of our study population. A large-size U.S. study39 followed 1-year survivors after moderate-to-severe TBI (n = 7728) up to 20 years post-injury. They found an average long-term FIM total score of 114.0 (standard deviation [SD], 19.6), but only 16% of individuals in total had reached the maximum score of 126. For comparison, the average FIM total score of our study population was 118.3 (SD, 22.1) at a 5-year follow-up. Another U.S./Danish study22 investigated individual growth curves of FIM total scores in 202 individuals with severe TBI during inpatient rehabilitation, and found that a slower recovery was associated with older age, longer coma, and interruptions to rehabilitation.
The current study is an extension of a TBI research project. Several limitations inherent from the original design need to be acknowledged when interpreting the results. First, the original study was initiated in 2005, and results might have been different if the study had been initiated in more recent years. However, it is worth mentioning that our study population received standard acute care and in-hospital rehabilitation services40 during the acute and post-acute phase, and that those services have been provided in our hospital consistently during the last 10 years. Moreover, our study results were compatible with the recent study finding from a large-size U.S. patient population35 and used a similar analytical approach, albeit with different lengths of follow-up. Our study results further supplemented long-term typical and atypical functional recovery processes after moderate and severe TBI. In a clinical setting, the results also support assessment of FIM during sub- and post-acute phase (e.g., 3 and 12 months post-injury) to enable early trajectory classification in individuals with moderate and severe TBI to facilitate better patient centered-care and tailored rehabilitation programs.
Second, although the study population was representative to the patients with moderate-to-severe TBI from the southeast region of Norway, the inclusion and exclusion criteria from the original study, particularly the patients' age range at the study admission (between 16 and 55 years old) and geographical setting, may limit this study's ability to generalize the finding to a broader patient population and healthcare settings. Third, the present study only captured acute treatment and in-hospital rehabilitation service provided to this cohort during the acute and post-acute phase. Ideally, the course and content of rehabilitation provided in a 5-year period should be described given that these services would impact the study outcomes. However, this was beyond the scope of this study. Finally, the sample size was too small to test predictors in the trajectories. The total sample size was 121, and only 94 patients had outcome data at all three follow-up time points. Therefore, the study could be statistically underpowered. Future study with a larger sample and prospective design is needed to verify the findings of this study and to account for factors other than baseline characteristics, such as information on the access to care, current rehabilitation practice, and family and societal support during long-term follow-up.
Conclusion
The findings from the present study support the notion that patients with moderate-to-severe TBI follow different recovery trajectories, with the stable good recovery being most common (FIM-M subscale, 82.6%; FIM-C subscale, 54.6%). The largest improvement in functional ability was found from 3 months to 1 year post-injury; thereafter, the function generally improved at a much slower pace up to the 5-year follow-up. Identifying and characterizing patient subgroups with specific functional trajectories and in need of assistance and intervention after acute and subacute care is an important first step to build efficient intervention programs that are tailored to meet individual patient needs, inform patient-centered care, and ultimately improve long-term outcomes post-TBI.
Acknowledgments
Special thanks are given to Tone Jerstad (neuroradiologist, Oslo University Hospital, Ulleval) for the CT assessments and Morten Hestnes (Trauma Register, Oslo University Hospital, Ulleval) for the extraction of trauma scores.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Centers for Disease Control and Prevention. (2015). Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention: Atlanta, GA [Google Scholar]
- 2.Nguyen R., Fiest K.M., McChesney J., Kwon C.S., Jette N., Frolkis A.D., Atta C., Mah S., Dhaliwal H., Reid A., Pringsheim T., Dykeman J., and Gallagher C. (2016). The international incidence of traumatic brain injury: a systematic review and meta-analysis. Can. J. Neurol. Sci. 43, 774–785 [DOI] [PubMed] [Google Scholar]
- 3.Maas A. (2016). Traumatic brain injury: changing concepts and approaches. Chin. J. Traumatol. 19, 3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selassie A.W., Zaloshnja E., Langlois J.A., Miller T., Jones P., and Steiner C. (2008). Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 23, 123–131 [DOI] [PubMed] [Google Scholar]
- 5.Zaloshnja E., Miller T., Langlois J.A., and Selassie A.W. (2008). Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil. 23, 394–400 [DOI] [PubMed] [Google Scholar]
- 6.Corrigan J.D., Selassie A.W., and Orman J.A. (2010). The epidemiology of traumatic brain injury. J. Head Trauma Rehabil. 25, 72–80 [DOI] [PubMed] [Google Scholar]
- 7.Langlois J.A., and Sattin R.W. (2005). Traumatic brain injury in the United States: research and programs of the Centers for Disease Control and Prevention (CDC). J Head Trauma Rehabil 20, 187–188 [DOI] [PubMed] [Google Scholar]
- 8.Roozenbeek B., Maas A.I., and Menon D.K. (2013). Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 9, 231–236 [DOI] [PubMed] [Google Scholar]
- 9.Schwab K.A., Gudmudsson L.S., and Lew H.L. (2015). Long-term functional outcomes of traumatic brain injury, in: Handbook of Clinical Neurology, Vol. 128 (3rd series), Traumatic Brain Injury, Part II. Grafman J., and Salazar A.M. (eds), Elsevier: New York, NY: [DOI] [PubMed] [Google Scholar]
- 10.Hammond F.M., Grattan K.D., Sasser H., Corrigan J.D., Rosenthal M., Bushnik T., and Shull W. (2004). Five years after traumatic brain injury: a study of individual outcomes and predictors of change in function. NeuroRehabilitation 19, 25–35 [PubMed] [Google Scholar]
- 11.5LeBlanc J., de Guise E., Gosselin N., and Feyz M. (2006). Comparison of functional outcome following acute care in young, middle-aged and elderly patients with traumatic brain injury. Brain Inj. 20, 779–790 [DOI] [PubMed] [Google Scholar]
- 12.Cameron C.M., Purdie D.M., Kliewer E.V., and McClure R.J. (2008). Ten-year outcomes following traumatic brain injury: a population-based cohort. Brain Inj. 22, 437–449 [DOI] [PubMed] [Google Scholar]
- 13.Andelic N., Sigurdardottir S., Schanke A.K., Sandvik L., Sveen U., and Roe C. (2010). Disability, physical health and mental health 1 year after traumatic brain injury. Disabil. Rehabil. 32, 1122–1131 [DOI] [PubMed] [Google Scholar]
- 14.Sigurdardottir S., Andelic N., Roe C., and Schanke A.K. (2014). Identifying longitudinal trajectories of emotional distress symptoms 5 years after traumatic brain injury. Brain Inj. 28, 1542–1550 [DOI] [PubMed] [Google Scholar]
- 15.Andelic N., Soberg H.L., Berntsen S., Sigurdardottir S., and Roe C. (2014). Self-perceived health care needs and delivery of health care services 5 years after moderate-to-severe traumatic brain injury. PM R. 6, 1013–21; quiz, 1021. [DOI] [PubMed] [Google Scholar]
- 16.Corrigan J.D., Cuthbert J.P., Harrison-Felix C., Whiteneck G.G., Bell J.M., Miller A.C., Coronado V.G., and Pretz C.R. (2014). US population estimates of health and social outcomes 5 years after rehabilitation for traumatic brain injury. J. Head Trauma Rehabil. 29, E1–E9 [DOI] [PubMed] [Google Scholar]
- 17.Dams-O'Connor K., Pretz C., Billah T., Hammond F.M., and Harrison-Felix C. (2015). Global outcome trajectories after TBI among survivors and nonsurvivors: a National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems study. J. Head Trauma Rehabil. 30, E1–E10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forslund M.V., Roe C., Perrin P.B., Sigurdardottir S., Lu J., Berntsen S., and Andelic N. (2017). The trajectories of overall disability in the first 5 years after moderate and severe traumatic brain injury. Brain Inj. 31, 329–335 [DOI] [PubMed] [Google Scholar]
- 19.Hall K.M., Bushnik T., Lakisic-Kazazic B., Wright J., and Cantagallo A. (2001). Assessing traumatic brain injury outcome measures for long-term follow-up of community-based individuals. Arch. Phys. Med. Rehabil. 82, 367–374 [DOI] [PubMed] [Google Scholar]
- 20.Nakase-Richardson R., Whyte J., Giacino J.T., Pavawalla S., Barnett S.D., Yablon S.A., Sherer M., Kalmar K., Hammond F.M., Greenwald B., Horn L.J., Seel R., McCarthy M., Tran J., and Walker W.C. (2012). Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI Model Systems Programs. J. Neurotrauma 29, 59–65 [DOI] [PubMed] [Google Scholar]
- 21.Sandhaug M., Andelic N., Langhammer B., and Mygland A. (2015). Functional level during the first 2 years after moderate and severe traumatic brain injury. Brain Inj. 29, 1431–1438 [DOI] [PubMed] [Google Scholar]
- 22.Hart T., Kozlowski A.J., Whyte J., Poulsen I., Kristensen K., Nordenbo A., and Heinemann A.W. (2014). Functional recovery after severe traumatic brain injury: an individual growth curve approach. Arch. Phys. Med. Rehabil. 95, 2103–2110 [DOI] [PubMed] [Google Scholar]
- 23.Corrigan J.D., and Hammond F.M. (2013). Traumatic brain injury as a chronic health condition. Arch. Phys. Med. Rehabil. 94, 1199–1201 [DOI] [PubMed] [Google Scholar]
- 24.Teasdale G., and Jennett B. (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 25.Marshall L.F., Marshall S.B., Klauber M.R., Van Berkum Clark M., Eisenberg H., Jane J.A., Luerssen T.G., Marmarou A., and Foulkes M.A. (1992). The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma 9, Suppl. 1, S287–S292 [PubMed] [Google Scholar]
- 26.Sandhaug M., Andelic N., Vatne A., Seiler S., and Mygland A. (2010). Functional level during sub-acute rehabilitation after traumatic brain injury: course and predictors of outcome. Brain Inj. 24, 740–747 [DOI] [PubMed] [Google Scholar]
- 27.Uniform Data System for Medical Rehabilitation (UDSMR). (2012). Uniform Data System for Medical Rehabilitation. 2012. The FIMTM Instrument: Its Background, Structure, and Usefulness. Uniform Data System for Medical Rehabilitation (UDSMR): Buffalo, NY [Google Scholar]
- 28.Jones B.L., and Nagin D.S. (2007). Advances in group-based trajectory modeling and an SAS procedure for estimating them. SMR. 35, 542–571 [Google Scholar]
- 29.Jones B., Nagin D., and Roeder K. (2001). A SAS procedure based on mixture models for estimating developmental trajectories. SMR. 29, 374–393 [Google Scholar]
- 30.Kass R.E., and R.E. (1995). Bayes factors. J. Am. Stat. Assoc. 90, 773–795 [Google Scholar]
- 31.Jeffreys H. (1961). Theory of Probability, 3rd ed. Oxford University Press: London [Google Scholar]
- 32.Nagin D.S. (2014). Group-based trajectory modeling: an overview. Ann. Nutr. Metab. 65, 205–210 [DOI] [PubMed] [Google Scholar]
- 33.Pretz C.R., and Dams-O'Connor K. (2013). Longitudinal description of the glasgow outcome scale-extended for individuals in the traumatic brain injury model systems national database: a National Institute on Disability and Rehabilitation Research traumatic brain injury model systems study. Arch. Phys. Med. Rehabil. 94, 2486–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pretz C.R., Malec J.F., and Hammond F.M. (2013). Longitudinal description of the disability rating scale for individuals in the National Institute on Disability and Rehabilitation Research traumatic brain injury model systems national database. Arch. Phys. Med. Rehabil. 94, 2478–2485 [DOI] [PubMed] [Google Scholar]
- 35.Howrey B.T., Graham J.E., Pappadis M.R., Granger C.V., and Ottenbacher K.J. (2017). Trajectories of functional change after inpatient rehabilitation for traumatic brain injury. Arch. Phys. Med. Rehabil. 98, 1606–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones B.L., and Nagin D.S. (2013). A note on a Stata plugin for estimating groupbased trajectory models. SMR. 42, 608–613 [Google Scholar]
- 37.Nagin D.S., and Odgers C.L. (2010). Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 6, 109–138 [DOI] [PubMed] [Google Scholar]
- 38.Muthen B., and Shedden K. (1999). Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics 55, 463–469 [DOI] [PubMed] [Google Scholar]
- 39.Brooks J.C., Strauss D.J., Shavelle R.M., Paculdo D.R., Hammond F.M., and Harrison-Felix C.L. (2013). Long-term disability and survival in traumatic brain injury: results from the National Institute on Disability and Rehabilitation Research Model Systems. Arch. Phys. Med. Rehabil. 94, 2203–2209 [DOI] [PubMed] [Google Scholar]
- 40.Andelic N., Bautz-Holter E., Ronning P., Olafsen K., Sigurdardottir S., Schanke A.K., Sveen U., Tornas S., Sandhaug M., and Roe C. (2012). Does an early onset and continuous chain of rehabilitation improve the long-term functional outcome of patients with severe traumatic brain injury? J. Neurotrauma 29, 66–74 [DOI] [PubMed] [Google Scholar]