Abstract
In recent years, several randomized controlled trials evaluating pharmaceutical treatments for traumatic brain injury (TBI) have failed to demonstrate efficacy over placebo, with both active and placebo arms improving at comparable rates. These findings could be viewed in opposing ways, suggesting on the one hand failure of the tested outcome, but on the other, representing evidence of robust placebo effects in TBI. In this article, we examine several of the primary psychological processes driving placebo effects (verbal suggestion, cognitive re-framing, interpersonal interactions, conditioning, therapeutic alliance, anxiety reduction) as well as placebo neurobiology (top-down cortical regulation, reward system activation, dopaminergic and serotonergic neurotransmission). We then extrapolate from the literature to explore whether something inherent in TBI makes it particularly responsive to placebos. Viewed as such here, placebos may indeed represent a powerful and effective treatment for a variety of post-TBI complaints.
Keywords: : dopamine, placebo effects, reward system, TBI, verbal suggestion
Introduction
In recent years, several of the largest randomized controlled trials (RCTs) evaluating pharmaceutical treatments for traumatic brain injury (TBI) have been unable to demonstrate efficacy over placebo. In such studies, both active and placebo arms have improved at comparable rates.1–4 These findings could be viewed in opposing ways, suggesting on the one hand failure of the tested outcome, but representing on the other, evidence of robust placebo effects in the TBI population. Of late, placebos have boasted a 66.7% response rate in a study of amantadine for post-TBI irritability,2 a 49.3% response rate in a study of rivastigmine for post-TBI cognitive impairment,5 and a 43% 4 and 32%1 response rate in two studies of sertraline for post-TBI depression. Although the mechanisms underlying placebo responses are still not well understood, viewed as such here, placebos appear quite effective for treating a variety of post-TBI symptoms.
A widely cited, seminal paper published in the 1950s reported placebo effects of ∼35% across a range of conditions.6 The more recent placebo literature, however, argues against placebos exerting a uniform influence across all diagnostic categories and treatments. Rather, certain disease entities such as pain, depression, fatigue, menopausal hot flashes, benign prostatic hyperplasia, and Parkinson's disease might respond especially well to placebos.7–9 Disorders with self-rated versus objectively measured outcomes may also be more susceptible to placebo effects;10–13 however, objective improvements in response to placebos have certainly been documented,14 and even when only subjective responses are generated, compelling evidence suggests simultaneous involvement of neurobiological substrates.15 Placebo responses in RCTs that test medications acting on the central nervous system (CNS) also appear to be more potent that those acting on other organ systems.16 In this article, we will explore whether placebo effects hold particular relevance for TBI as well.
Research into the psychological and neurobiological mechanisms underlying placebo effects (along with its nocebo counterpart) has grown considerably in past decades. Placebos are now in large part conceptualized as a learned response.17 Prior therapeutic encounters can become associated with the psychosocial context of an intervention such that subsequently, contextual variables (e.g., interaction with a clinician, the paraphernalia of medicine) alone can produce a positive (placebo) or negative (nocebo) effect.18 Some of the main processes by which one learns to respond to placebos include suggestion, cognitive re-framing, elements of the therapeutic relationship, recollections of prior experience, social learning from others, conditioning, hope, anxiety, and stress reduction.16–23 Some argue that the various placebo-generating processes described here share a final, common pathway of shaping expectation,17,20 although an added level of complexity arises from neuroimaging and behavioral evidence, suggesting that placebo responses can be elicited unconsciously.24–28
The neurobiological and physiological underpinnings of the placebo response are also under investigation. Current research suggests that placebo responses are initiated through cognitive and affective processes occurring in the dorsolateral prefrontal cortex (dLPFC), orbital prefrontal cortex (OFC), and anterior cingulate cortex (ACC).7,20 After higher-order meaning-making occurs in the cortex, placebo responses are propagated through dopaminergic reward systems in the ventral striatum.29 The end-organ effects of placebo responses ultimately diverge based on disease process and expected outcome, triggering, for example, release of endogenous opioids in the case of pain, release of dopamine from the dorsal striatum with Parkinson's disease, or increased serotonergic activity with depression.7 Possible genetic signatures that influence response to placebos may also exist.30,31
To date, no studies have directly evaluated the role of placebo effects in acute or post-acute TBI trials. In this article, we will review some of the basic theories of placebo and extrapolate from existing, relevant literature to explore whether there is something inherent to TBI—its symptoms or treatments—that makes it particularly responsive to placebos. Our discussion will touch upon many of the primary psychological and neurobiological mechanisms implicated in placebo effects. In a forthcoming article, we engage similar questions with regard to nocebos in TBI.
Psychological Processes
Suggestion
Placebo responses in laboratory experiments are commonly elicited through the pairing of a verbal suggestion with an inert intervention. Verbal suggestion—“this pill will make you feel better”—helps set up an explicit, conscious expectation for healing. Expectation generated by an intervention can also be implicit, as when taking a pill unconsciously brings to mind prior personal experiences or cultural beliefs associating ingestion of medication with symptom improvement.
In one trial directly demonstrating the impact of verbal suggestion, the effectiveness of an opioid agonist was doubled when paired with a promise of potent analgesia, but negated when paired with a warning that the agent could exacerbate pain.32 With verbal suggestion, both the logical content and emotional delivery appear to matter. During a local anesthetic injection, subjects who heard a gentle, reassuring message that a “numbing” medication would make them “comfortable” during their upcoming procedure rated the painfulness of the injection substantially lower than subjects told they would feel a “big bee sting,” which would be “the worst part of the procedure.”33
Implicit societal assumptions also impact the therapeutic effectiveness of placebo interventions. Studies have shown that two placebo pills are more effective than one placebo pill,34 placebos labeled with a name brand work better than unbranded placebos,35 and expensive placebos are more effective than inexpensive placebos.36,37 Such findings reflect societal values (whether valid or not) associating quantity, exclusivity, and expense with quality.
The contemporary esteem for advanced technology is another societal bias impacting placebo effectiveness of late. Often, the more sophisticated the technology and the more extravaganza involved, the greater the probability for a placebo response to happen.23,38–40 Accordingly, a review of placebo responses in migraine treatments showed superiority of sham acupuncture and sham surgery over inert oral medications.41,42 In another review weighing various placebo treatments across a range of diagnostic categories, however, the efficacy of more or less “intensive” placebos was similar.43
No studies to date have directly evaluated placebo effects—effects beyond spontaneous remission and regression to the mean—in the TBI population. Yet, in several RCTs testing the efficacy of active pharmaceuticals, participants in placebo arms have improved at considerable rates.1–4 In these trials, subjects told that they would be randomized to receive either an active medication or a placebo reported symptom improvement. Beyond pharmaceutical interventions, the treatment of TBI in some cases may also benefit from an “enhanced” (via advanced technology) placebo response as described, via procedural intervention and medical devices; for example, botulinum toxin injections for post-traumatic headaches or transcranial magnetic stimulation (TMS) for cognitive or mood symptoms after TBI.
Furthermore relevant to TBI, one might argue that by enhancing positive expectation, post-acute psychoeducational interventions for mild TBI may be, in part, capitalizing on placebo effects. In these initiatives, clinicians and researchers aim to mitigate some of the fear and negativity surrounding mild TBI through reassurance that symptoms are generally benign and temporary and/or by offering emotional support. Instructing patients to view their symptoms as such, rather than as indicative of danger or permanent brain injury, has been shown experimentally in three controlled trials, including two RCTs, to reduce subsequent symptom burden, lessen anxiety/distress levels, and improve everyday functioning compared with standard care.44–46 On the contrary, in two subsequent RCTs involving mild TBI patients with the greatest symptom burden shortly after injury, randomization to close follow-up with a physician did not provide any benefit over treatment as usual.47,48 In these studies, subjects in both the active and treatment-as-usual arms received written information on mild TBI shortly after injury, potentially confounding the results.
Interestingly, placebos have even been found to impact performance on cognitive testing. A group of healthy individuals randomized to take a placebo “cognitive enhancer” pill for 2 weeks outperformed a control group on a Stroop task and delayed recall task.16 Other studies in healthy populations have shown sham cognitive treatments to improve reaction time49 as well as performance on prospective memory50 and knowledge-based tasks.51 Similarly, in the TBI study on rivastigmine mentioned previously,5 nearly half of the participants in the active and placebo groups demonstrated improvements on sustained attention and verbal learning tasks. Although typically viewed as a more objective measurement than self-reported variables, neuropsychological test performance can, nevertheless, be impacted by numerous psychological and behavioral factors. In mild TBI, some of these factors may include expectation, effort, confidence, and mood.52,53 By providing reasonable expectation of improvement—which in and of itself has been associated with motivation and health-promoting behavioral change54—placebos may be well positioned to enhance neuropsychological test performance among those with TBI.
To broaden the impact of placebos and positive expectation in TBI rehabilitation even further, believing that one's post-TBI symptoms are transient or improving may lead one to make a greater effort in therapy or to be more willing to continue working or socializing, despite the added challenge after brain injury: behaviors that also in and of themselves promote recovery and well-being.55,56
Interpersonal factors
The interpersonal connection between patient and clinician also appears to play a role in placebo effects. Regarding the contribution of affect, a study on placebo acupuncture in irritable bowel syndrome showed additional therapeutic benefit when the treatment was delivered by an empathic versus a neutral provider.57,58 In a study evaluating the differential effects of combining a warm or cold delivery of positive or neutral expectations in a cohort of women with severe premenstrual pain, anxiety ratings improved only among those receiving a warm delivery of positive expectation.59 Several systematic reviews have similarly suggested that health outcomes can be affected by elements of the patient–clinician relationship.60–62
Cumulative exposure to healthcare providers has also been shown to be therapeutic. In a study using homeopathy to treat gastrointestinal reflux, longer clinic visits were found to be more beneficial than standard-length visits.63 In a study evaluating placebo effects across clinical trials of depression, a greater number of assessments was associated with larger placebo effects, presumably because of a greater cumulative exposure to nonspecific therapeutic effects.64
Interpersonal factors could influence post-acute TBI placebo responses in a variety of ways. Patients undergoing rehabilitation often have frequent contact with clinical providers (physical therapists, occupational therapists, speech language pathologists, as well as other clinicians), with repeated opportunities to derive nonspecific benefits. In this regard, the field of rehabilitation may share important similarities with psychotherapy, such that nonspecific effects—for example, therapeutic alliance and change processes—might theoretically drive a substantial proportion of clinical benefit.65,66
Additionally, simply being in a research study, even in the absence of direct clinical interaction, has been found to be therapeutic. In a study in which a placebo treatment was sent by mail, subjects nevertheless reported improvements in quality of life and psychological functioning.67 This phenomenon has been termed the “Hawthorne effect,” defined more specifically as the tendency of individuals to change their behavior in response to the additional attention paid to them throughout a study.68,69 Hawthorne effects are expected to play out in TBI trials as they do across other clinical entities.
In studies evaluating CNS medications affecting mood and behavior, as is commonly the case in TBI, additional interpersonal factors may contribute to the placebo effect. For example, in the study by Hammond and coworkers on amantadine and irritability,2 there was a robust improvement in irritability in both the placebo and treatment groups. Authors hypothesize that multiple interpersonal factors may have contributed to this effect, including frequent patient and provider interaction, emotional support, and encouragement from providers during the study period, as well as the effect of monitored patient behavior and patients' awareness of this monitoring. The authors also suggested that addressing patient care in the chronic phase of disease, as was the case in this study, provided a level of care beyond the typical acute rehabilitation course. Although these interpersonal factors were not an intentional part of the study intervention and were not formally quantified, they may have substantially affected the results. Similar effects have been noted in other studies seeking to address mood and behavior after TBI.1,4
Prior experience and classical conditioning
Learning based on prior experience is another psychological process underlying placebo effects. Through repeated exposures, individuals can learn to associate certain characteristics of a therapeutic intervention with symptom improvement. Subsequently, replacing a therapeutic intervention with an inert one (i.e., replacing a pill containing active ingredients with an identical sugar pill) can generate a placebo effect mimicking the effect of the previously administered drug. Classical conditioning operates in a similar, yet distinct manner. Here, an unconditioned stimulus (e.g., caffeine) which naturally causes an unconditioned response (e.g., alertness) is repeatedly paired with a conditioned stimulus (e.g., novel tasting drink), such that subsequently, the conditioned stimulus alone can provoke a conditioned response (alertness in response to a novel-tasting drink).
Many examples of learning through experience and classical conditioning exist in the literature. In a commonly employed placebo analgesia paradigm, subjects rate the painfulness of a noxious stimulus, and then receive a placebo intervention paired with a surreptitiously lowered noxious stimulus, so as to suggest the benefit of the inert treatment. When subjects are later retested with the original intensity of a noxious stimulus, a placebo response is demonstrated.70,71 A similar paradigm has been used to study motor outcomes in Parkinson's disease. In one such study, Parkinson's patients were given either no medication or varying durations of daily apomorphine injections prior to implantation of electrodes for deep brain stimulation. After the electrodes were placed, the patients received a placebo injection. Those who had been preconditioned by apomorphine demonstrated a placebo response both clinically and in terms of electrical firing at the single neuron level. Those who had not been preconditioned did not exhibit a placebo response.14
Variations of conditioning paradigms have considered the impact of additive and contradictory learning. In preconditioning trials in which previous exposure contributes to conditioning, learning through experience can be dose dependent, based on the number of prior exposures. Following preconditioning with a dopaminergic agent and subsequent replacement of the active agent with a placebo in a Parkinson's trial, the extent and duration of the placebo effect correlated with the number of prior exposures to the active pharmacological agent.14,72 Regarding contradictory learning, when healthy normal subjects preconditioned to believe an inert cream had analgesic properties were later told that the cream was inert, previously generated placebo responses were negated.73 Conversely, in a separate study, preconditioned placebo responses persisted following a “placebo reveal” if prior exposure to the active agent was prolonged (on the order of days) but not brief (a single exposure).74
Preconditioning protocols have also been found to be much less effective if a subject is first exposed to an unsuccessful treatment attempt.75 Ineffective prior treatment with an inert “analgesic” patch was associated with reduced placebo analgesia in response to an inert ointment, demonstrating that treatment history with one modality can transfer to another.76
Accumulating evidence suggests that one main difference between learning from prior experience and conditioning is that the former works on conscious and the latter works on unconscious physiological processes. Accordingly, subjects in one multi-arm study underwent preconditioning to suggest efficacy of a placebo treatment followed by opposite instructions: that the intervention was in fact a placebo. The authors of this study found that opposite instructions could negate placebo effects for pain and motor functioning, but not for neuroendocrine and hormonal secretion. According to their interpretation, conscious physiological processes (pain and motor function) are mediated primarily by expectation, whereas unconscious physiological processes (neuroendocrine functioning) are mediated by conditioning.24 A separate study analogously demonstrated that the immune system activity (another physiologic system not under conscious control) could be experimentally manipulated through classical conditioning, but not verbal suggestion.77
In post-acute TBI trials and in all of rehabilitation, there may be opportunities to utilize both prior learning and classical conditioning to produce a desired effect. TMS, for example, a noninvasive technique for neuromodulation used in rehabilitation, has elicited strong placebo analgesia when tested in clinical trials. This may occur largely through unconscious conditioned learning promoted by the technique itself, as sensory and auditory stimulation are involved when a subject is exposed to a sham coil.78
Neurobiological Processes
Placebo physiology
Research into the neurobiological mechanisms of placebo is continually advancing. The CNS is generally designated as the primary mediator of placebo effects.16 Initial placebo-related brain activity has been documented in regions such as the dlPFC, OFC, and ACC7,29,79,80 areas, with multiple cognitive and affective functions, including awareness, insight, expectation modulation, learning, and memory. This is where individuals first encounter and make meaning of verbal and contextual cues. Whether impairments in higher-order cognition reduce responsiveness to placebos remains to be determined. Although a decline in placebo responsiveness was noted in one study involving Alzheimer's patients with severe frontal lobe dysfunction,81 placebo effects have been consistently documented among individuals with genetically determined intellectual disability.82,83 In these latter studies, the persistence of placebo effects despite cognitive limitations was attributed to expectation, implicit learning, and placebo-by-proxy via clinicians and family members.82,83
The expectations ensuing after this higher-order processing secondarily trigger dopaminergic reward pathways in the nucleus accumbens of the ventral striatum.79,84 “Reward” here could mean food, money, or the prospect of relief from suffering.29 Evidence of activation of dopaminergic reward pathways has been best studied in Parkinson's disease and the placebo analgesia literature. Accordingly, in a positron emission tomography (PET)/functional MRI (fMRI) study, both a monetary reward task and placebo analgesia paradigm activated similar brain regions involving the nucleus accumbens.85
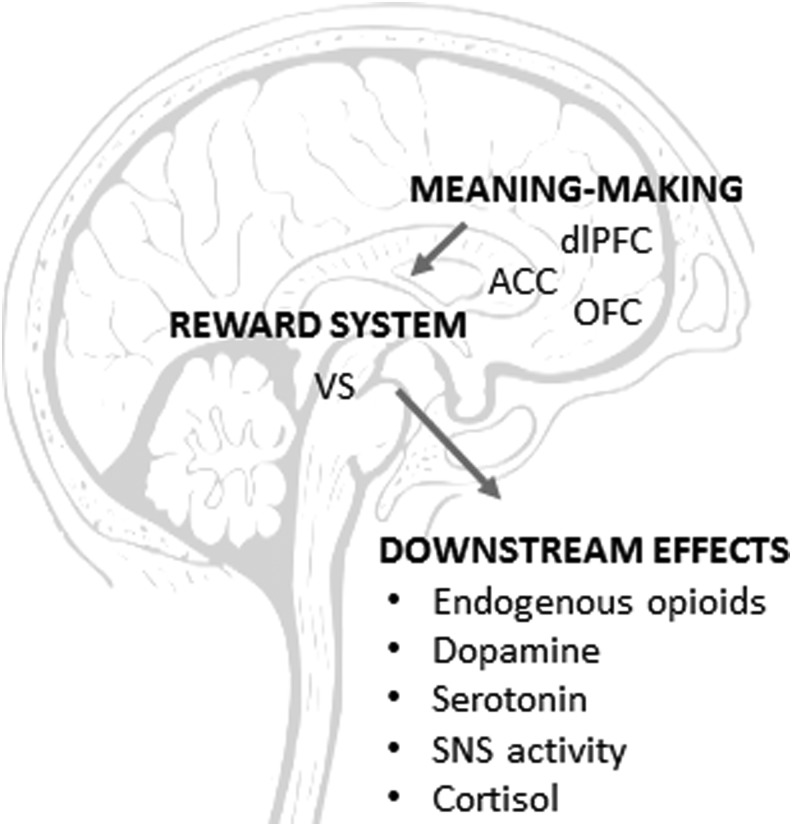
These reward pathways ultimately trigger various, disease-specific downstream physiological effects.7,79 In Parkinson's disease, there is further dopamine release in the nigrostriatum, leading to improved motor functioning.86 In placebo analgesia, there is release of endogenous opioids.87 In depression, reward pathways are hypothesized to subsequently impact serotonergic and μ-opioid activity in amygdala and limbic serotonergic pathways.7,88 Other placebo end-organ effects include changes in the cardiovascular and autonomic nervous system, gastrointestinal system, and respiratory function.89 A summary of the major components of placebo neurobiology is presented in Figure 1.
FIG. 1.
Placebo neurobiology. Placebo responses are initiated through higher-order cognitive and affective processes occurring in the dorsolateral prefrontal cortex (dlPFC), orbitofrontal cortex (OFC), and anterior cingulate cortex (ACC). They are next propagated through dopaminergic reward systems in the ventral striatum (VS). Placebos ultimately trigger various end-organ effects, including modulation of endogenous opioids, dopamine, serotonin, sympathetic nervous system (SNS) activity and cortisol.
A neurobiological framework, integrating the abovementioned literature, has been proposed to account for why Parkinson's disease, pain, and depression may be especially responsive to placebos. In each of these three disease categories, cognitive and emotional processes activate top-down cortical regulation, which may involve the reward system. Neurotransmitter or neuropeptide dysfunction also play a central role.7
With regard to placebo neurobiology in relation to TBI, several points deserve consideration. First, how or whether impairments in higher-order cognition affect placebo responsiveness is not yet known. Although one might expect placebo responses to be attenuated following severe post-TBI cognitive deficits, this is called into question by the studies showing persistence of placebo effects among those with lifelong intellectual disabilities.82,83
The central role of dopamine in placebo neurotransmission likely does, however, hold direct relevance to TBI. Brain regions densely populated with dopamine receptors are commonly disrupted after TBI. Loss of dopaminergic tone in frontostriatal regions has been associated with post-TBI cognitive dysfunction,90 injury to mesocorticolimbic circuits has been associated with emotional and behavioral dysregulation following TBI,91 and damage in reward circuitry has been associated with deficits in motivated behavior after TBI.92 Given the central role of dopamine release in both the generation of the placebo response and TBI outcomes, individuals with a history of brain injury may be particularly responsive to placebos. Some have further associated abnormalities in serotonergic tone with TBI,92 although the evidence of this link is less strong than for dopamine.
With this lens, we can revisit the trials on amantadine for irritability2 and sertraline for mood1,4 following TBI. In theory, medications such as amantadine or sertraline may neurobiologically improve irritability and mood by acting on dopaminergic and serotonergic pathways among those with potentially disrupted neurotransmission, but by activating the same neurotransmitter systems, so too do placebos. In the treatment arms of such studies, placebo effects may either act alone to drive symptom improvement, or act synergistically with the active drug to promote an even greater effect.
Placebo genetics and the “placebome”
Recent studies suggest that placebo responses may in part be genetically predetermined. At the genomic level, studies of a “placebome”—genome-related mediators affecting one's response to placebos—are emerging.30 A recent placebome network analysis demonstrated proximity of a validated module to molecular pathways involved in certain diseases, symptoms, and drug classes. These included CNS disorders such as Parkinson's disease, migraine, and epilepsy; symptoms of pain, headache, nausea, fatigue, and anorexia; as well as medications in the categories of CNS depressants, neuroprotective agents, and dopamine uptake inhibitors.31 As it is a CNS disorder commonly accompanied by headache and fatigue for which centrally acting agents are often utilized, one might imagine placebo-related genetics holding relevance for TBI as well.
Regarding the specific genes implicated in placebo responses, those involved in dopamine, opioid, and serotonin signaling appear to be most influential.20 Catechol-O-methyltransferase (COMT) is an enzyme involved in dopamine catabolism. At codon 158 of the rs4680 single nucleotide polymorphism (SNP), a valine-methionine substitution can occur (val158met), affecting COMT and, therefore, dopaminergic activity.93,94 The number of val158met alleles has been associated with a heightened placebo response in irritable bowel syndrome95 and placebo analgesia in healthy subjects.96 Monoamine oxidase A (MAO-A) is another dopamine metabolizer implicated in placebo effects. In a study on placebos in depression, a MAO-A guanine to thiamine polymorphism was found to correspond with increased placebo responsiveness.97
Serotonergic-related gene polymorphisms have been linked to placebo relief among those suffering from social anxiety. Possessing the long allele of serotonin-transporter-linked polymorphic region (5-HTTLPR), a polymorphic region of SLC6A4, a gene coding for the serotonin transporter, has been associated with attenuated amygdala activity in response to placebo treatment.98 A large analysis of multiple SNPs in multiple genes showed a relationship between placebo antidepressant response and genetic variants in both serotonin (tryptophan hydroxylase 2 [TPH2], 5-HTTLPR) and dopaminergic pathway genes (MAO-A).99
Some of these same genetic variants have been implicated in TBI outcomes in the post-acute and chronic stages. The COMT val158met allele, for example, has been associated with perseveration on neuropsychological testing in a cohort of returning veterans,100 and performance on nonverbal learning tasks among those with a history of mild TBI.101 Among those with TBI and comorbid depression, a relationship between val158met (along with the ankyrin repeat and kinase domain containing 1 [ANKKI] Taq1a allele, also involved in dopaminergic activity) and frontal lobe performance has been noted.102 The val158met allele has also been associated with post-traumatic stress disorder (PTSD) incidence, and thereby functional outcomes among those with a history of mild TBI.103 On the other hand, a study evaluating the relationship between val158met and TBI outcomes in the acute stage was negative.104
Only a small handful of studies have evaluated associations between 5-HTTLPR genetic variants and outcomes following TBI. Although one study showed a significant relationship between serotonergic alleles and post-traumatic depression within the 1st year after severe TBI,105 these findings were not replicated in a second cohort of individuals with varying injury severities.106 Elsewhere in a study on returning veterans, 5-HTTLPR polymorphisms were associated with resilience and perceptions of limitations.107
Although a direct link among genetic variants, TBI, and placebo response has not yet been made, their potential interactions cannot be ignored, and warrant further understanding.
Stress
Stress pathophysiology and autonomic activity are additional end-organ mechanisms through which placebos can exert their effects.108 In the CNS, placebo anxiolytics have been associated with decreased stress-related activity in the amygdala,98 as well as reduced hypervigilance and arousal as measured by electroencephalography (EEG) and skin conductance.109 Placebo analgesia has also been shown to reduce plasma cortisol levels.110
Regarding autonomic effects, placebo analgesia has been associated with reduced heart rate via β-adrenergic activity,111 and both placebo anxiolytics112 and placebo analgesics113 have been shown to alter heart rate variability. Placebos have not significantly affected stress-related or autonomic variables in all studies, however.114
One can once again imagine applications to TBI here. TBI can impair the central autonomic system, including the insula, and hypothalamus and its descending brainstem tracts,115 and has been associated with autonomic disturbances varying on a spectrum of intensity from paroxysmal sympathetic hyperactivity115 to reduced heart rate variability.116,117 In the mild TBI literature, post-concussive symptoms have been show to co-vary with daily stress levels.118 Experimentally inducing stress through cognitively challenging tasks has further been shown to exacerbate post-concussive symptoms as well as information processing speed.119 In this way, placebos may be well positioned to help modulate the stress, anxiety, and alterations in autonomic activity occurring after TBI.
Conclusion
Placebo is not no treatment, but rather a series of psychological and neurobiological actions that deserve attention when considering TBI treatment. In this article, we argue that placebo effects hold particular relevance for researchers and clinicians working in the field of TBI. Verbal suggestion, prior experience, social learning, conditioning, interpersonal interactions, and expectation all influence placebo in TBI as they do in other disease entities. Placebos may impact TBI outcomes in a variety of ways, by leading one to view his or her condition more favorably, alleviating anxiety, or inspiring greater commitment to rehabilitation efforts. TBI also shares many neurobiological pathways implicated in placebo physiology, such as top-down cortical regulation, reward system activation, and neurotransmitter activity. Placebo may indeed be a very powerful and effective treatment in the TBI population and in rehabilitation medicine at large.
Author Disclosure Statement
No competing financial interests exist
References
- 1.Ashman T.A., Cantor J.B., Gordon W.A., Spielman L., Flanagan S., Ginsberg A., Engmann C., Egan M., Ambrose F., and Greenwald B. (2009). A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch. Phys. Med. Rehabil. 90, 733–740 [DOI] [PubMed] [Google Scholar]
- 2.Hammond F.M., Sherer M., Malec J.F., Zafonte R.D., Whitney M., Bell K., Dikmen S., Bogner J., Mysiw J., and Pershad R. (2015). Amantadine effect on perceptions of irritability after traumatic brain injury: results of the amantadine irritability multisite study. J. Neurotrauma 32, 1230–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver J.M., Koumaras B., Chen M., Mirski D., Potkin S.G., Reyes P., Warden D., Harvey P.D., Arciniegas D., Katz D.I., and Gunay I. (2006). Effects of rivastigmine on cognitive function in patients with traumatic brain injury. Neurology 67, 748–755 [DOI] [PubMed] [Google Scholar]
- 4.Fann J.R., Bombardier C.H., Temkin N., Esselman P., Warms C., Barber J., and Dikmen S. (2017). Sertraline for major depression during the year following traumatic brain injury: a randomized controlled trial. J. Head Trauma Rehabil 32, 332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silver J.M., Koumaras B., Chen M., Mirski D., Potkin S.G., Reyes P., Warden D., Harvey P.D., Arciniegas D., Katz D.I., and Gunay I. (2006). Effects of rivastigmine on cognitive function in patients with traumatic brain injury. Neurology 67, 748–755 [DOI] [PubMed] [Google Scholar]
- 6.Beecher H. (1955). The powerful placebo. J. Am. Med. Assoc. 159, 1602–1606 [DOI] [PubMed] [Google Scholar]
- 7.Diederich N.J., and Goetz C.G. (2008). The placebo treatments in neurosciences: new insights from clinical and neuroimaging studies. Neurology 71, 677–684 [DOI] [PubMed] [Google Scholar]
- 8.Sorokin I., Schatz A., and Welliver C. (2015). Placebo medication and sham surgery responses in benign prostatic hyperplasia treatments: implications for clinical trials. Curr. Urol. Rep. 16, 73. [DOI] [PubMed] [Google Scholar]
- 9.Freeman E.W., Ensrud K.E., Larson J.C., Guthrie K.A., Carpenter J.S., Joffe H., Newton K.M., Sternfeld B., and LaCroix A.Z. (2015). Placebo improvement in pharmacologic treatment of menopausal hot flashes: time course, duration, and predictors. Psychosom. Med. 77, 167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hróbjartsson A., and Gøtzsche P.C. (2004). Is the placebo powerless? update of a systematic review with 52 new randomized trials comparing placebo with no treatment. J. Intern. Med. 256, 91–100 [DOI] [PubMed] [Google Scholar]
- 11.Kaptchuk T.J., and Miller F.G. (2015). Placebo effects in medicine. N. Engl. J. Med. 373, 8–9 [DOI] [PubMed] [Google Scholar]
- 12.Wise R.A., Bartlett S.J., Brown E.D., Castro M., Cohen R., Holbrook J.T., Irvin C.G., Rand C.S., Sockrider M.M., and Sugar E.A. (2009). Randomized trial of the effect of drug presentation on asthma outcomes. J. Allergy Clin. Immunol. 124, 436–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wechsler M., and Kelley J. (2011). Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N. Engl. J. Med. 365, 119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedetti F., Frisaldi E., Carlino E., Giudetti L., Pampallona A., Zibetti M., Lanotte M., and Lopiano L. (2016). Teaching neurons to respond to placebos. J. Physiol. 594, 5647–5660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finniss D.G., Kaptchuk T.J., Miller F., and Benedetti F. (2010). Biological, clinical, and ethical advances of placebo effects. Lancet 375, 686–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oken B.S. (2008). Placebo effects: clinical aspects and neurobiology. Brain 131, 2812–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colloca L., and Miller F.G. (2011). How placebo responses are formed: a learning perspective. Philos. Trans. R. Soc. London 366, 1859–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wager T.D., and Atlas L.Y. (2015). The neuroscience of placebo effects: Connecting context, learning and health. Nat. Rev. Neurosci. 16, 403–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benedetti F., and Amanzio M. (2011). The placebo response: how words and rituals change the patient's brain. Patient Educ. Couns. 84, 413–419 [DOI] [PubMed] [Google Scholar]
- 20.Colagiuri B., Schenk L.A., Kessler M.D., Dorsey S.G., and Colloca L. (2015). The placebo effect: from concepts to genes. Neuroscience 307, 171–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson J.J., Ritenbaugh C., and Nichter M. (2009). Reconsidering the placebo response from a broad anthropological perspective. Cult. Med. Psychiatry 33, 112–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballou S., Kaptchuk T., Hirsch W., Nee J., Iturrino J., Hall K., Kelley J., Cheng V., Kirsch I., Jacobson E., Conboy L., Lembo A., and Davis R. (2017). Open-label versus double-blind placebo treatment in irritable bowel syndrome: study protocol for a randomized controlled trial. Trials 18, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaptchuk T.J. (2002). The placebo effect in alternative medicine: Can the performance of a healing ritual have clinical significance? Ann. Intern. Med. 136, 817–825 [DOI] [PubMed] [Google Scholar]
- 24.Benedetti F., Pollo A., Lopiano L., Lanotte M., Vighetti S., and Rainero I. (2003). Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J. Neurosci. 23, 4315–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen K.B., Kaptchuk T.J., Kirsch I., Raicek J., Lindstrom K.M., Berna C., Gollub R.L., Ingvar M., and Kong J. (2012). Nonconscious activation of placebo and nocebo pain responses. Proc. Natl. Acad. Sci. 109, 15,959–15,964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen K.B., Kaptchuk T.J., Chen X., Kirsch I., Ingvar M., Gollub R.L., and Kong J. (2015). A neural mechanism for nonconscious activation of conditioned placebo and nocebo responses. Cereb. Cortex 25, 3903–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen K., Kirsch I., Odmalm S., Kaptchuk T.J., and Ingvar M. (2015). Classical conditioning of analgesic and hyperalgesic pain responses without conscious awareness. Proc. Natl. Acad. Sci. 112, 7863–7867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bąbel P., Bajcar E.A., Adamczyk W., Kicman P., Lisińska N., Świder K., and Colloca L. (2017). Classical conditioning without verbal suggestions elicits placebo analgesia and nocebo hyperalgesia. PLoS One 12, e0181856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de la Fuente-Fernández R. (2009). The placebo-reward hypothesis: dopamine and the placebo effect. Parkinsonism Relat. Disord. 15, Suppl. 3, S72–74 [DOI] [PubMed] [Google Scholar]
- 30.Hall K.T., Loscalzo J., and Kaptchuk T.J. (2015). Genetics and the placebo effect: the placebome. Trends Mol. Med. 21, 285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R., Hall K., Giulianini F., Passow D., Kaptchuk T., and Loscalzo J. (2017). Network analysis of the genomic basis of the placebo effect. JCI Insight 2, 93911, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bingel U., Wanigasekera V., Wiech K., Ni Mhuircheartaigh R., Lee M.C., Ploner M., and Tracey I. (2011). The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci. Transl. Med. 3, 70ra14. [DOI] [PubMed] [Google Scholar]
- 33.Varelmann D., Pancaro C., Cappiello E.C., and Camann W.R. (2010). Nocebo-induced hyperalgesia during local anesthetic injection. Anesth. Analg. 110, 868–870 [DOI] [PubMed] [Google Scholar]
- 34.Rickels K., Hesbacher P., and Weise C. (1970). Pills and improvement: a study of placebo response in psychoneurotic outpatients. Psychopharmacologia 16, 318–328 [DOI] [PubMed] [Google Scholar]
- 35.Branthwaite A., and Cooper P. (1981). Analgesic effects of branding in treatment of headaches. Br. Med. J. 282, 1576–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waber R., Shiv B., Carmon Z., and Ariely D. (2008). Commercial features of placebo and therapeutic efficacy. J. Am. Med. Assoc. 299, 1016–1017 [DOI] [PubMed] [Google Scholar]
- 37.Espay A.J., Norris M.M., Eliassen J.C., Dwivedi A., Smith M.S., Banks C., Allendorfer J.B., Lang A.E., Fleck D.E., Linke M.J., and Szaflarski J.P. (2015). Placebo effect of medication cost in Parkinson disease: a randomized double-blind study. Neurology 84, 794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaptchuk T.J. (2006). Sham device v inert pill: randomised controlled trial of two placebo treatments. Br. Med. J. 332, 391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaptchuk T.J., Goldman P., Stone D.A., and Stason W.B. (2000). Do medical devices have enhanced placebo effects? J. Clin. Epidemiol. 53, 786–792 [DOI] [PubMed] [Google Scholar]
- 40.Kaptchuk T.J. (2011). Placebo studies and ritual theory: a comparative analysis of Navajo, acupuncture and biomedical healing. Philos. Trans. R. Soc. 366, 1849–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linde K., Niemann K., and Meissner K. (2010). Are sham acupuncture interventions more effective than (other) placebos? A re-analysis of data from the cochrane review on placebo effects. Forsch. Komplementarmed. 17, 259–264 [DOI] [PubMed] [Google Scholar]
- 42.Meissner K., Fässler M., Rücker G., Kleijnen J., Hróbjartsson A., Schneider A., Antes G., and Linde K. (2013). Differential effectiveness of placebo treatments. JAMA Intern. Med. 173, 1941–1951 [DOI] [PubMed] [Google Scholar]
- 43.Fässler M., Meissner K., Kleijnen J., Hróbjartsson A., and Linde K. (2015). A systematic review found no consistent difference in effect between more and less intensive placebo interventions. J. Clin. Epidemiol. 68, 442–451 [DOI] [PubMed] [Google Scholar]
- 44.Ponsford J., Willmott C., Rothwell A., Cameron P., Kelly A.-M., Nelms R., and Curran C. (2002). Impact of early intervention on outcome following mild head injury in adults. J. Neurol. Neurosurg. Psychiatry 73, 330–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bell K.R., Hoffman J.M., Temkin N.R., Powell J.M., Fraser R.T., Esselman P.C., Barber J.K., and Dikmen S. (2008). The effect of telephone counselling on reducing post-traumatic symptoms after mild traumatic brain injury: a randomised trial. J. Neurol. Neurosurg. Psychiatry 79, 1275–1281 [DOI] [PubMed] [Google Scholar]
- 46.Suffoletto B., Wagner A.K., Arenth P.M., Calabria J., Kingsley E., Kristan J., and Callaway C.W. (2013). Mobile phone text messaging to assess symptoms after mild traumatic brain injury and provide self-care support: a pilot study. J. Head Trauma Rehabil. 28, 302–312 [DOI] [PubMed] [Google Scholar]
- 47.Matuseviciene G., Borg J., Stalnacke B.-M., Ulfarsson T., and de Boussard C. (2013). Early intervention for patients at risk for persisting disability after mild traumatic brain injury: a randomized, controlled study. Brain Inj. 27, 318–324 [DOI] [PubMed] [Google Scholar]
- 48.Matuseviciene G., Eriksson G., and DeBoussard C.N. (2016). No effect of an early intervention after mild traumatic brain injury on activity and participation: a randomized controlled trial. J. Rehabil. Med. 48, 19–26 [DOI] [PubMed] [Google Scholar]
- 49.Buckalew L. (1972). An analysis of experimental components in a placebo effect. Psychol. Rec. 22, 113–119 [Google Scholar]
- 50.Parker S., Garry M., Einstein G.O., and McDaniel M.a. (2011). A sham drug improves a demanding prospective memory task. Memory 19, 606–12 [DOI] [PubMed] [Google Scholar]
- 51.Weger U.W., and Loughnan S. (2013). Mobilizing unused resources: using the placebo concept to enhance cognitive performance. Q. J. Exp. Psychol. 66, 23–28 [DOI] [PubMed] [Google Scholar]
- 52.Silver J.M. (2015). Invalid symptom reporting and performance: what are we missing? NeuroRehabilitation 36, 463–469 [DOI] [PubMed] [Google Scholar]
- 53.Lange R.T., Iverson G.L., Brooks B.L., and Ashton Rennison V.L. (2010). Influence of poor effort on self-reported symptoms and neurocognitive test performance following mild traumatic brain injury. J. Clin. Exp. Neuropsychol. 32, 961–972 [DOI] [PubMed] [Google Scholar]
- 54.Crum A., and Phillips D.J. (2015). Self-fulfilling prophesies, placebo effects, and the social-psychological creation of reality. In Emerging Trends in the Social and Behavioral Sciences (Scott R.A. and Kosslyn S.M., eds.) [Google Scholar]
- 55.Silverberg N.D., and Iverson G.L. (2013). Is rest after concussion “The best medicine?”: recommendations for activity resumption following concussion in athletes, civilians, and military service members. J. Head Trauma Rehabil. 28, 250–259 [DOI] [PubMed] [Google Scholar]
- 56.DiFazio M., Silverberg N.D., Kirkwood M.W., Bernier R., and Iverson G.L. (2016). Prolonged activity restriction after concussion. Clin. Pediatr. (Phila). 55, 443–451 [DOI] [PubMed] [Google Scholar]
- 57.Kelley J.M., Lembo A.J., Ablon J.S., Villanueva J.J., Conboy L.A., Levy R., Marci C.D., Kerr C.E., Kirsch I., Jacobson E.E., Riess H., and Kaptchuk T.J. (2009). Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosom. Med. 71, 789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaptchuk T.J., Kelley J.M., Conboy L.A., Davis R.B., Kerr C.E., Jacobson E.E., Kirsch I., Schyner R.N., Nam B.H., Nguyen L.T., Park M., Rivers A.L., McManus C., Kokkotou E., Drossman D.A., Goldman P., and Lembo A.J. (2008). Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 336, 999–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verheul W., Sanders A., and Bensing J. (2010). The effects of physicians' affect-oriented communication style and raising expectations on analogue patients' anxiety, affect and expectancies. Patient Educ. Couns. 80, 300–306 [DOI] [PubMed] [Google Scholar]
- 60.Blasi Z. D, Harkness E., Ernst E., Georgiou A., and Kleijnen J. (2001). Influence of context effects on health outcomes: a systematic review. Lancet 357, 757–762 [DOI] [PubMed] [Google Scholar]
- 61.Kelley J.M., Kraft-Todd G., Schapira L., Kossowsky J., and Riess H. (2014). The influence of the patient-clinician relationship on healthcare outcomes: a systematic review and meta-analysis of randomized controlled trials. PLoS One 9, e94207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Birkhäuer J., Gaab J., Kossowsky J., Hasler S., Krummenacher P., Werner C., and Gerger H. (2017). Trust in the health care professional and health outcome: a meta-analysis. PLoS One 12, e0170988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dossett M.L., Mu L., Davis R.B., Bell I.R., Lembo A.J., Kaptchuk T.J., and Yeh G.Y. (2015). Patient-provider interactions affect symptoms in gastroesophageal reflux disease: a pilot randomized, double-blind, placebo-controlled trial. PLoS One 10, e0136855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papakostas G.I., Ostergaard S.D., and Iovieno N. (2015). The nature of placebo response in clinical studies of major depressive disorder. J. Clin. Psychiatry 76, 456–466 [DOI] [PubMed] [Google Scholar]
- 65.Grencavage L.M., and Norcross J.C. (1990). Where are the commonalities among the therapeutic common factors? Prof. Psychol. Res. Pract. 21, 372–378 [Google Scholar]
- 66.Miciak M., Gross D.P., and Joyce A. (2012). A review of the psychotherapeutic “common factors” model and its application in physical therapy: the need to consider general effects in physical therapy practice. Scand. J. Caring Sci. 26, 394–403 [DOI] [PubMed] [Google Scholar]
- 67.Bouchet C., Guillemin F., and Briançon S. (1996). Nonspecific effects in longitudinal studies: impact on quality of life measures. J. Clin. Epidemiol. 49, 15–20 [DOI] [PubMed] [Google Scholar]
- 68.McCambridge J., Witton J., and Elbourne D.R. (2014). Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J. Clin. Epidemiol. 67, 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parsons H. (1974). What happened at Hawthorne? Science 183, 922–932 [DOI] [PubMed] [Google Scholar]
- 70.Voudouris N.J., Peck C.L., and Coleman G. (1990). The role of conditioning and verbal expectancy in the placebo response. Pain 43, 121–128 [DOI] [PubMed] [Google Scholar]
- 71.Voudouris N.J., Peck C.L., and Coleman G. (1989). Conditioned response models of placebo phenomena: further support. Pain 38, 109–116 [DOI] [PubMed] [Google Scholar]
- 72.Colloca L., Petrovic P., Wager T.D., Ingvar M., and Benedetti F. (2010). How the number of learning trials affects placebo and nocebo responses. Pain 151, 430–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montgomery G.H., and Kirsch I. (1997). Classical conditioning and the placebo effect. Pain 72, 107–113 [DOI] [PubMed] [Google Scholar]
- 74.Schafer S.M., Colloca L., and Wager T.D. (2015). Conditioned placebo analgesia persists when subjects know they are receiving a placebo. J. Pain 16, 412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colloca L., and Benedetti F. (2006). How prior experience shapes placebo analgesia. Pain 124, 126–133 [DOI] [PubMed] [Google Scholar]
- 76.Kessner S., Wiech K., Forkmann K., Ploner M., and Bingel U. (2013). The effect of treatment history on therapeutic outcome: an experimental approach. JAMA Intern. Med. 173, 1468–1469 [DOI] [PubMed] [Google Scholar]
- 77.Albring A., Wendt L., Benson S., Witzke O., Kribben A., Engler H., and Schedlowski M. (2012). Placebo effects on the immune response in humans: the role of learning and expectation. PLoS One 7, e49477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.André-Obadia N., Magnin M., and Garcia-Larrea L. (2011). On the importance of placebo timing in rTMS studies for pain relief. Pain 152, 1233–1237 [DOI] [PubMed] [Google Scholar]
- 79.Lidstone S.C. (2014). Great expectations: the placebo effect in Parkinson's disease. Handb. Exp. Pharmacol. 225, 139–147 [DOI] [PubMed] [Google Scholar]
- 80.Zubieta J.K., and Stohler C.S. (2009). Neurobiological mechanisms of placebo responses. Ann. N. Y. Acad. Sci. 1156, 198–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benedetti F., Arduino C., Costa S., Vighetti S., Tarenzi L., Rainero I., and Asteggiano G. (2006). Loss of expectation-related mechanisms in Alzheimer's disease makes analgesic therapies less effective. Pain 121, 133–144 [DOI] [PubMed] [Google Scholar]
- 82.Jensen K.B., Kirsch I., Pontén M., Rosén A., Yang K., Gollub R.L., Des Portes V., Kaptchuk T.J., and Curie A. (2017). Certainty of genuine treatment increases drug responses among intellectually disabled patients. Neurology 88, 1912–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Curie A., Yang K., Kirsch I., Gollub R.L., Des Portes V., Kaptchuk T.J., and Jensen K.B. (2015). Placebo responses in genetically determined intellectual disability: a meta-analysis. PLoS One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De La Fuente-Fernández R., Schulzer M., and Stoessl A.J. (2004). Placebo mechanisms and reward circuitry: clues from Parkinson's disease. Biol. Psychiatry 56, 67–71 [DOI] [PubMed] [Google Scholar]
- 85.Scott D.J., Stohler C.S., Egnatuk C.M., Wang H., Koeppe R.A., and Zubieta J.K. (2007). Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 55, 325–336 [DOI] [PubMed] [Google Scholar]
- 86.de la Fuente-Fernández R., Ruth T.J., Sossi V., Schulzer M., Calne D.B., and Stoessl A. J. (2001). Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science 293, 1164–1166 [DOI] [PubMed] [Google Scholar]
- 87.Scott D.J., Stohler C.S., Egnatuk C.M., Wang H., Koeppe R. A, and Zubieta J.-K. (2008). Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry 65, 220–231 [DOI] [PubMed] [Google Scholar]
- 88.Peciña M., and Zubieta J.K. (2015). Molecular mechanisms of placebo responses in humans. Mol. Psychiatry 7, 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meissner K. (2014). Placebo responses on cardiovascular, gastrointestinal, and respiratory organ functions. Handb. Exp. Pharmacol. 225, 183–203 [DOI] [PubMed] [Google Scholar]
- 90.Bales J.W., Wagner A.K., Kline A.E., and Dixon C.E. (2009). Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci. Biobehav. Rev. 33, 981–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mega M.S., and Cummings J.L. (1994). Frontal-subcortical circuits and neuropsychiatric disorders. J. Neuropsychiatry Clin. Neurosci. 6, 358–370 [DOI] [PubMed] [Google Scholar]
- 92.McAllister T. (2013). Emotional and behavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry 7, 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yavich L., Forsberg M.M., Karayiorgou M., Gogos J.A., and Mannisto P.T. (2007). Site-specific role of Catechol-O-Methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J. Neurosci. 27, 10,196–10,209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meyer-Lindenberg A., Kohn P.D., Kolachana B., Kippenhan S., McInerney-Leo A., Nussbaum R., Weinberger D.R., and Berman K.F. (2005). Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat. Neurosci. 8, 594–596 [DOI] [PubMed] [Google Scholar]
- 95.Hall K.T., Lembo A.J., Kirsch I., Ziogas D.C., Douaiher J., Jensen K.B., Conboy L.A., Kelley J.M., Kokkotou E., and Kaptchuk T.J. (2012). Catechol-O-Methyltransferase val158met polymorphism predicts placebo effect in irritable bowel syndrome. PLoS One 7, e48135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu R., Gollub R.L., Vangel M., Kaptchuk T., Smoller J.W., and Kong J. (2014). Placebo analgesia and reward processing: integrating genetics, personality, and intrinsic brain activity. Hum. Brain Mapp. 35, 4583–4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leuchter A.F., McCracken J.T., Hunter A.M., Cook I. A., and Alpert J.E. (2009). Monoamine oxidase a and catechol-o-methyltransferase functional polymorphisms and the placebo response in major depressive disorder. J. Clin. Psychopharmacol. 29, 372–377 [DOI] [PubMed] [Google Scholar]
- 98.Furmark T., Appel L., Henningsson S., Ahs F., Faria V., Linnman C., Pissiota A., Frans O., Bani M., Bettica P., Pich E.M., Jacobsson E., Wahlstedt K., Oreland L., Långström B., Eriksson E., and Fredrikson M. (2008). A link between serotonin-related gene polymorphisms, amygdala activity, and placebo-induced relief from social anxiety. J. Neurosci. 28, 13,066–13,074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tiwari A.K., Zai C.C., Sajeev G., Arenovich T., Müller D.J., and Kennedy J.L. (2013). Analysis of 34 candidate genes in bupropion and placebo remission. Int. J. Neuropsychopharmacol. 16, 771–781 [DOI] [PubMed] [Google Scholar]
- 100.Lipsky R.H., Sparling M.B., Ryan L.M., Xu K., Salazar A.M., Goldman D., and Warden D.L. (2005). Association of COMT Val158Met genotype with executive functioning following traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 17, 465–471 [DOI] [PubMed] [Google Scholar]
- 101.Winkler E.A., Yue J.K., McAllister T.W., Temkin N.R., Oh S.S., Burchard E.G., Hu D., Ferguson A.R., Lingsma H.F., Burke J.F., Sorani M.D., Rosand J., Yuh E.L., Barber J., Tarapore P.E., Gardner R.C., Sharma S., Satris G.G., Eng C., Puccio A.M., Wang K.K.W., Mukherjee P., Valadka A.B., Okonkwo D.O., Diaz-Arrastia R., and Manley G.T. (2016). COMT Val 158 Met polymorphism is associated with nonverbal cognition following mild traumatic brain injury. Neurogenetics 17, 31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myrga J.M., Juengst S.B., Failla M.D., Conley Y.P., Arenth P.M., Grace A.A., and Wagner A.K. (2016). COMT and ANKK1 genetics interact with depression to influence behavior following severe TBI: an initial assessment. Neurorehabil. Neural Repair 30, 920–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Winkler E.A., Yue J.K., Ferguson A.R., Temkin N.R., Stein M.B., Barber J., and Yuh E.L. (2017). COMT Val158Met polymorphism is associated with post-traumatic stress disorder and functional outcome following mild traumatic brain injury. J. Clin. Neurosci. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Willmott C., Withiel T., Ponsford J., and Burke R. (2014). COMT Val158Met and cognitive and functional outcomes after traumatic brain injury. J. Neurotrauma 31, 1507–1514 [DOI] [PubMed] [Google Scholar]
- 105.Failla M.D., Burkhardt J.N., Miller M. A., Scanlon J.M., Conley Y.P., Ferrell R.E., and Wagner A.K. (2013). Variants of SLC6A4 in depression risk following severe TBI. Brain Inj. 27, 696–706 [DOI] [PubMed] [Google Scholar]
- 106.Chan F., Lanctôt K.L., Feinstein A., Herrmann N., Strauss J., Sicard T., Kennedy J.L., McCullagh S., and Rapoport M.J. (2008). The serotonin transporter polymorphisms and major depression following traumatic brain injury. Brain Inj. 22, 471–479 [DOI] [PubMed] [Google Scholar]
- 107.Graham D.P., Helmer D.A., Harding M.J., Kosten T.R., Petersen N.J., and Nielsen D.A. (2013). Serotonin transporter genotype and mild traumatic brain injury independently influence resilience and perception of limitations in veterans. J. Psychiatr. Res. 47, 835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meissner K. (2011). The placebo effect and the autonomic nervous system: evidence for an intimate relationship. Philos. Trans. R. Soc. London 366, 1808–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meyer B., Yuen K.S.L., Ertl M., Polomac N., Mulert C., Buchel C., and Kalisch R. (2015). Neural mechanisms of placebo anxiolysis. J. Neurosci. 35, 7365–7373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peciña M., Azhar H., Love T.M., Lu T., Fredrickson B.L., Stohler C.S., and Zubieta J.-K. (2013). Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology 38, 639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pollo A., Vighetti S., Rainero I., and Benedetti F. (2003). Placebo analgesia and the heart. Pain 102, 125–133 [DOI] [PubMed] [Google Scholar]
- 112.Darragh M., Vanderboor T., Booth R.J., Sollers J.J., and Consedine N.S. (2015). Placebo “serotonin” increases heart rate variability in recovery from psychosocial stress. Physiol. Behav. 145, 45–49 [DOI] [PubMed] [Google Scholar]
- 113.Aslaksen P.M., and Flaten M.A. (2008). The roles of physiological and subjective stress in the effectiveness of a placebo on experimentally induced pain. Psychosom. Med. 70, 811–818 [DOI] [PubMed] [Google Scholar]
- 114.Zimmermann-Viehoff F., Steckhan N., Meissner K., Deter H.C., and Kirschbaum C. (2016). Influence of a suggestive placebo intervention on psychobiological responses to social stress: a randomized controlled trial. J. Evid. Based Complement. Altern. Med. 21, 3–9 [DOI] [PubMed] [Google Scholar]
- 115.Takahashi C., Hinson H.E., and Baguley I.J. (2015). Autonomic dysfunction syndromes after acute brain injury. Handb. Clin. Neurol. 128, 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vistisen S.T., Hansen T.K., Jensen J., Nielsen J.F., and Fleischer J. (2014). Heart rate variability in neurorehabilitation patients with severe acquired brain injury. Brain Inj. 28, 196–202 [DOI] [PubMed] [Google Scholar]
- 117.Senthinathan A., Mainwaring L.M., and Hutchison M. (2016). Heart rate variability of athletes across concussion recovery milestones. Clin. J. Sport Med. 27, 288–295 [DOI] [PubMed] [Google Scholar]
- 118.Gouvier W.D., Cubic B., Jones G., Brantley P., and Cutlip Q. (1992). Postconcussion symptoms and daily stress in normal and head-injured college populations. Arch. Clin. Neuropsychol. 7, 193–211 [PubMed] [Google Scholar]
- 119.Hanna-Pladdy B., Berry Z.M., Bennett T., Phillips H.L., and Gouvier W.D. (2001). Stress as a diagnostic challenge for postconcussive symptoms: sequelae of mild traumatic brain injury or physiological stress response. Clin. Neuropsychol. 15, 289–304 [DOI] [PubMed] [Google Scholar]