Abstract
There is increasing evidence that a fine-tuned integrin cross talk can generate a high degree of specificity in cell adhesion, suggesting that spatially and temporally coordinated expression and activation of integrins are more important for regulated cell adhesive functions than the intrinsic specificity of individual receptors. However, little is known concerning the molecular mechanisms of integrin cross talk. With the use of β1-null GD25 cells ectopically expressing the β1A integrin subunit, we provide evidence for the existence of a cross talk between β1 and αV integrins that affects the ratio of αVβ3 and αVβ5 integrin cell surface levels. In particular, we demonstrate that a down-regulation of αVβ3 and an up-regulation of αVβ5 occur as a consequence of β1A expression. Moreover, with the use of GD25 cells expressing the integrin isoforms β1B and β1D, as well as two β1 cytoplasmic domain deletion mutants lacking either the entire cytoplasmic domain (β1TR) or only its “variable” region (β1COM), we show that the effects of β1 over αV integrins take place irrespective of the type of β1 isoform, but require the presence of the “common” region of the β1 cytoplasmic domain. In an attempt to establish the regulatory mechanism(s) whereby β1 integrins exert their trans-acting functions, we have found that the down-regulation of αVβ3 is due to a decreased β3 subunit mRNA stability, whereas the up-regulation of αVβ5 is mainly due to translational or posttranslational events. These findings provide the first evidence for an integrin cross talk based on the regulation of mRNA stability.
INTRODUCTION
Integrins form one family of cell adhesion receptors that play a prominent role in the adhesive interactions between cells and their surrounding extracellular matrix (ECM) (Hynes, 1992). All integrins are heterodimers composed of noncovalently linked α and β subunit transmembrane glycoproteins containing large extracellular domains, short transmembrane domains, and carboxyl-terminal cytoplasmic domains of variable length (Hynes, 1992). These adhesive receptors are endowed with both structural and regulatory functions, linking extracellular matrix to the actin cytoskeleton at focal adhesion sites and providing bidirectional transmission of signals across the plasma membrane (Schoenwaelder and Burridge, 1999; Critchley, 2000). The cytoplasmic domain of the β subunit has been shown to play a critical role in focal adhesion and actin stress fiber organization and both outside-in and inside-out integrin signaling (Liu et al., 2000).
Through their molecular interactions integrins regulate a number of critical cellular processes, including proliferation, differentiation, survival, migration, and gene expression (Giancotti, 1997; Giancotti and Ruoslahti, 1999). It is now clear that altered, modulated, or regulated adhesive interactions can change the way cells interact with their environment with dramatic consequences for both normal and pathological conditions. Cells can vary their adhesive properties by selectively expressing different integrins and by modulating their integrin specificity and affinity for ligands (Hynes, 1996). However, cells often display multiple integrins capable of interacting with a particular ECM protein and, conversely, individual integrins can recognize several extracellular matrix molecules (Hynes, 1992). Thus, integrin expression and ligand specificity are often apparently redundant, at least in terms of simple adhesion. The biological significance of this phenomenon is not clear yet; nevertheless, there is increasing evidence that individual integrin receptors mediate distinct functions and can convey unique information (Giancotti, 2000).
Most integrins belong to one of two major subfamilies defined by the β1 and αV subunits. The β1 subunit pairs with at least 12 different α subunits (α1-α11, αV) to comprise receptors for a variety of ECM proteins, including collagen, laminin, fibronectin, and vitronectin (Hynes, 1992). A large body of literature (Brakebusch et al., 1997; Giancotti, 1997; reviewed in Schoenwaelder and Burridge, 1999) has addressed the role of β1 integrins in mediating important cell adhesion and signal transduction events. Four different β1 isoforms have been identified (β1A, β1B, β1C, and β1D), which differ in their cytoplasmic domains and differentially affect many integrin functions (Belkin et al., 1997; Fornaro and Languino, 1997; Belkin and Retta, 1998; Pfaff et al., 1998; Retta et al., 1998).
The αV subunit is known to associate with at least five different β subunits (β1, β3, β5, β6, and β8). Among these αV integrins, αVβ3 and αVβ5 have been extensively studied. The αVβ3 integrin, in particular, has a relatively limited cellular and tissue distribution (Yamada et al., 1995), but its expression and activity are tightly regulated during a variety of biological processes, including cell proliferation and survival (Montgomery et al., 1994), wound healing (Clark et al., 1996a), angiogenesis (Brooks et al., 1994), bone remodeling (McHugh et al., 2000), tumor progression (Albelda et al., 1990) and metastasis (Yun et al., 1996). This integrin can bind to a variety of ECM proteins, including vitronectin, fibronectin, fibrinogen, thrombospondin, Von Willebrand factor, and denatured collagen (Kühn and Eble, 1994), and it is able to recruit cytoskeletal and signaling proteins to focal adhesion sites (Lewis et al., 1996). In addition, αVβ3 is one of the integrins that promotes the assembly of fibronectin matrix (Wennerberg et al., 1996; Wu et al., 1996; Retta et al., 1998).
In contrast to αVβ3, αVβ5 is among the most widely expressed integrins. This receptor can specifically and efficiently bind its ligand vitronectin but remains randomly distributed over the surface of the cells and does not trigger the assembly of focal adhesion structures (Wayner et al., 1991; Leavesley et al., 1992). Moreover, αVβ5 integrin has different requirements than αVβ3 for mediating adhesive events, such as cell spreading and migration, to the common ligand vitronectin (Klemke et al., 1994; Lewis et al., 1996), and it can induce differential biological responses (Friedlander et al., 1995).
Perturbation experiments with antibodies, blocking peptides, and antisense oligonucleotides demonstrated that both β1 and αV integrins play a primary role in important physiological and pathological processes (reviewed in Varner and Cheresh, 1996; Brakebusch et al., 1997; Bader et al., 1998). However, recent genetic analyses have clearly increased questions as to the primacy of these integrins, and instead have pointed to a cross talk model where spatiotemporal regulation, combinatorial expression, and activation of several integrin receptors generate a high degree of specificity in cell adhesion (Fassler et al., 1996; Hynes, 1996, 1999; Brakebusch et al., 1997; Bader et al., 1998; Hodivala-Dilke et al., 1999; McHugh et al., 2000). Several observations indicate the existence of a cross talk between β1 and αV integrins, which usually takes the form of one integrin influencing the functional behavior of another integrin expressed on the same cell (Yang and Hynes, 1996; Belkin et al., 1997; Retta et al., 1998; Blystone et al., 1999; Corbett and Schwarzbauer, 1999). However, in most cases, the mechanistic basis of this receptor cross talk is not completely understood, and it is unknown whether and how the integrin cross talk can regulate the ratio of integrin cell-surface expression levels.
GD25 cells, derived from β1-null mouse embryonic stem cells (Wennerberg et al., 1996), are a valuable model for examination of integrin cross talk. In fact, these cells express αVβ3, αVβ5, and α6β4 as major integrin complexes but do not express integrins of the β1 subfamily, thus permitting a variety of genetic experiments exploring the basis of integrin cross talk. We have previously transfected GD25 cells with cDNAs encoding for the isoform A, B, or D of the human β1 integrin subunit or two β1 mutants lacking either the entire cytoplasmic domain (β1TR) or only the cytoplasmic domain “variable” region that characterizes each isoform (β1COM) (Retta et al., 1998). With the use of these cells, we investigated the specific functional properties of the isoform B and D of the human β1 integrin subunit, showing the existence of a functional cross talk between these two β1 isoforms and the endogenous αV integrins. In particular, both β1B and β1D expression prevented different fibronectin (FN)-dependent αV integrin functions, including its ability to mediate cell adhesion, to localize to focal adhesions, and to assemble an FN matrix (Belkin et al., 1997; Retta et al., 1998).
In the present study, we show that the cross talk between β1 and αV integrins is mainly based on the regulation of β3 and β5 integrin subunit expression exerted by β1 integrins. In fact, the ectopic expression of either β1A, β1B, or β1D in GD25 cells induces a drastic down-regulation of β3 and an up-regulation of β5 integrin cell surface levels. Moreover, analysis of GD25 cells expressing β1 integrins lacking either the entire β1 cytoplasmic domain (β1TR) or only its variable region (β1COM) demonstrate that the “common” region of the β1 cytoplasmic domain is required for these effects. We further demonstrate that β1 exerts its control over αvβ3 expression level by modulating the β3 mRNA stability, whereas the up-regulation of αVβ5 is mainly due to translational or posttranslational events leading to an increased recruitment of the β5 subunit at the cell surface.
MATERIALS AND METHODS
Antibodies and Reagents
The mouse anti-human β1 monoclonal antibody (mAb) TS2/16 was obtained from American Type Culture Collection (Manassas, VA). The rat anti-mouse α6 mAb GoH3 was a gift from A. Sonnenberg (The Netherlands Cancer Institute, Amsterdam, The Netherlands). The rabbit polyclonal antisera to αV, α3, and α5 integrin cytoplasmic domains, produced in our laboratory, were previously described (Retta et al., 1998). The polyclonal antisera to β3 and β5 were produced with the use of a previously described protocol (Defilippi et al., 1995). Briefly, rabbits were immunized against a GST-β3 fusion protein containing the cytoplasmic domain of the mouse β3 integrin subunit and against a synthetic peptide reproducing an amino acid sequence from the carboxy terminus of mouse β5 integrin subunit, respectively. The β5 peptide EKAQLKPPATSDA was synthesized by solid phase methods with the use of an LKB Biolynx synthesizer (Amersham Pharmacia Biotech AB, Uppsala, Sweden) and coupled to keyhole limpet hemocyanin with the use of glutaraldehyde. The mouse anti-paxillin mAb was purchased from Transduction Laboratories (Nottingham, United Kingdom). The affinity-purified rhodamine-labeled goat anti-mouse and goat anti-rabbit IgG were from Sigma (St. Louis, MO). Poly-l-lysine and monensin were from Sigma. Vitronectin and fibronectin were purified from human plasma as previously described (Balzac et al., 1994; Retta et al., 1999). Protein A-Sepharose and protein G-Sepharose were from Amersham Pharmacia Biotech AB.
Cells and Culture Conditions
The mouse GD25 fibroblast line, which lacks expression of β1 integrin heterodimers because of disruption of the β1 gene by homologous recombination, was established after differentiation of β1-null embryonic stem cells and immortalization with simian virus 40 large T antigen (Wennerberg et al., 1996). GD25 cells expressing the human β1A, β1B, or β1D integrin isoforms or the β1TR and β1COM human β1 mutants, lacking the entire cytoplasmic domain and the cytoplasmic domain variable region, respectively, were obtained as previously described (Belkin et al., 1997; Retta et al., 1998). To avoid selection for anomalous functional traits, no efforts were made to establish clonal cell lines; instead, bulk cell populations expressing β1 integrins were selected. Cells were cultured in DMEM (Invitrogen Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. The β1-expressing GD25 cells were cultured in the same medium plus 300 μg/ml hygromycin B (Roche Molecular Biochemicals, Mannheim, Germany). Cell populations expressing high levels of the β1 forms used were selected by the panning method and monitored by flow cytometry as described previously (Retta et al., 1998).
Biotinylation of Cell Surface Proteins
Adherent cells, grown to 80–90% confluence in 90-mm tissue culture dishes, were washed twice with ice-cold buffer A (1.3 mM CaCl2, 0.4 mM MgSO4, 5 mM KCl, 138 mM NaCl, 5,6 mM d-glucose, 25 mM HEPES, pH 7.4) and incubated with 0.5 mg/ml membrane-impermeable biotinylation reagent Sulfo-NHS-Biotin (Sigma) in buffer A at 4°C for 30 min. The reaction was quenched with DMEM containing 0.6% bovine serum albumin (BSA) and 25 mM HEPES, pH 7.4. The cells were then washed four times with ice-cold buffer A and lysed on ice in Tris-buffered saline (150 mM NaCl, 20 mM Tris-HCl, pH 7.4) containing 0.5% Triton X-100 and the protease inhibitors aprotinin (10 μg/ml), leupeptin (10 μg/ml), phenylmethylsulfonyl fluoride (1 mM), and benzamidine (1 mM) (all from Sigma). Cell lysates were centrifuged at 12,000 × g for 30 min at 4°C, and total protein concentration in the supernatants was determined with the use of a bicinchoninic acid protein assay (Pierce, Rockford, IL). Supernatants containing equal amounts of proteins were precleared with a mixture of protein A-Sepharose and protein G-Sepharose and used in immunoprecipitation experiments.
Immunoprecipitation and Analysis of Integrins
Integrins were immunoprecipitated from precleared cell lysate supernatants by incubation with appropriate dilutions of specific antibodies and a mixture of protein A-Sepharose and protein G-Sepharose beads for 1 h at 4°C. Complexes were washed four times with the lysis buffer then the proteins were eluted with Laemmli's sample buffer (62.5 mM Tris-HCl, pH 6.8, 20% glycerol, 2% SDS) and subjected to SDS-polyacrilamide (7.5%) gel electrophoresis under nonreducing conditions. To visualize the biotinylated proteins, the gel was electroblotted onto Hybond-C transfer membrane (Amersham Pharmacia Biotech AB). The blot was then blocked with 5% BSA in phosphate-buffered saline (PBS) for 1 h at 42°C, incubated with streptavidin-peroxidase (Sigma) (1:10.000 in PBS/1% BSA) for 1 h at room temperature, and further processed by the Western blotting enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia Biotech AB).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Northern Blot Analysis
Total RNA was isolated from 1 × 107 cultured cells with the use of the RNeasy Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. Where indicated, before RNA isolation confluent cells were detached by treatment with 5 mM EDTA in PBS, washed twice with serum-free DMEM, resuspended in the same medium containing 1 μM monensin, and plated for 2 h on tissue culture dishes that had been coated with 10 μg/ml polylysine, fibronectin, or vitronectin as previously described (Retta et al., 1998). A multiplex semiquantitative RT-PCR was used to detect the relative levels of β3 and β5 or β1 integrin mRNAs. cDNA was synthesized from 5 μg of cytoplasmic RNA with the use of the 1st Strand cDNA Synthesis kit (Roche Molecular Biochemicals), and subjected to 28 (β3/β5) or 32 (β1 and β1/β3/β5) PCR cycles. The reaction conditions and oligonucleotide PCR primers used were optimized so that the amplification products fell within the range of PCR amplification linearity. PCR was performed with each reaction mixture containing 5 μl of cDNA, 1× reaction buffer (Amersham Pharmacia Biotech AB), 1.5 mM MgCl2, 200 μM dNTP, 0.5 μM of each primer, and 2 U of Taq DNA polymerase (Amersham Pharmacia Biotech AB) in a total volume of 50 μl. The following stages were used for each PCR cycle: 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, with a prolonged extension stage of 72°C for 5 min after the final cycle. The primers were derived from nonhomologous regions of the mouse β3 and β5 and the human β1 cDNA sequences, and led to 705-, 570-, and 857-bp PCR products, respectively. PCR products were electrophoresed on 2% agarose gels and stained with ethidium bromide. Gels were photographed under UV light and intensities of the amplified cDNA fragments were quantitated with the use of a densitometric software (Molecular Analyst; Bio-Rad, Hemel Hempstead, United Kingdom). Molecular size standards (123-bp DNA ladder) were from Sigma.
For Northern blot hybridization, equal amounts of the purified total RNA (25 μg/lane) were separated by electrophoresis on a 1.2% agarose gel containing 1.8% formaldehyde and 1× FA Gel buffer [20 mM 3-(N-morpholino)propanesulfonic acid, 5 mM NaAc, 1 mM EDTA, pH 7.0], transferred to a Nytran SuPerCharge transfer membrane (Schleicher & Schuell, Dassel, Germany) with the use of the TurboBlotter blotting device accordingly to manufacturer's instructions (Schleicher & Schuell), and UV cross-linked to the membrane. The membrane was prehybridized by incubation in Church's buffer (0.5 M Na-phosphate buffer, 10 mg/ml BSA, 7% SDS, 1 mM EDTA, 0.1 mg/ml salmon sperm DNA, pH 7.4) for 8 h at 65°C and hybridized with 32P-labeled probes overnight at 65°C in Church's buffer. After hybridization, the membrane was washed once in 2× SSC + 0.1% SDS, once in 1× SSC + 0.1% SDS, once in 0.2× SSC + 0.1% SDS, and once in 0.1× SSC + 0.1% SDS for 15 min each at 65°C. The membrane was then exposed to x-ray film for 24–72 h at −80°C with an intensifying screen. Probes were synthesized by random priming with cDNA fragments of mouse β3 and β5 integrin subunits amplified by PCR and cloned in our laboratory. The same blots were rehybridized with a probe of the housekeeping gene β-actin to ensure equal loading.
Measurement of mRNA Stability
The measurement of mRNA stability was performed as described by Xu and Clark (1996). Briefly, cells were divided into three plates and cultured in 10% FBS/DMEM for 24 h before the addition of 60 μM 5,6-dichloro-1-β-d-ribofuranosyl-benzimidazole (DRB; Sigma), an inhibitor of transcription initiation. After addition of DRB, the cells were collected at 0, 4, 8, 12, and 24 h for RNA analysis. Total RNA isolation and Northern analysis were performed as described above.
Immunofluorescence Microscopy
Immunofluorescence studies were performed as described previously (Retta et al., 1996). Briefly, cells were seeded onto fibronectin-coated glass coverslips and allowed to spread for 3 h in complete culture medium. Cells were then washed with cold PBS, fixed for 10 min with 3.7% paraformaldehyde in PBS, permeabilized with ice-cold 0.5% Triton X-100, 3.7% paraformaldehyde in PBS for 5 min, and incubated with 1% BSA in PBS for 30 min. To localize αV and β1 integrins, the cells were stained with the rabbit antiserum to αV (1:200 in PBS/1% BSA) or the mAb TS2/16 to β1 (10 μg/ml in PBS/1% BSA). Bound primary antibodies were visualized by appropriate rhodamine-labeled secondary antibodies (1:100). Photographs were taken on an Olympus BX-60 epifluorescence microscope.
RESULTS
Expression of β1 Integrins Affects Subcellular Localization of αV Integrins
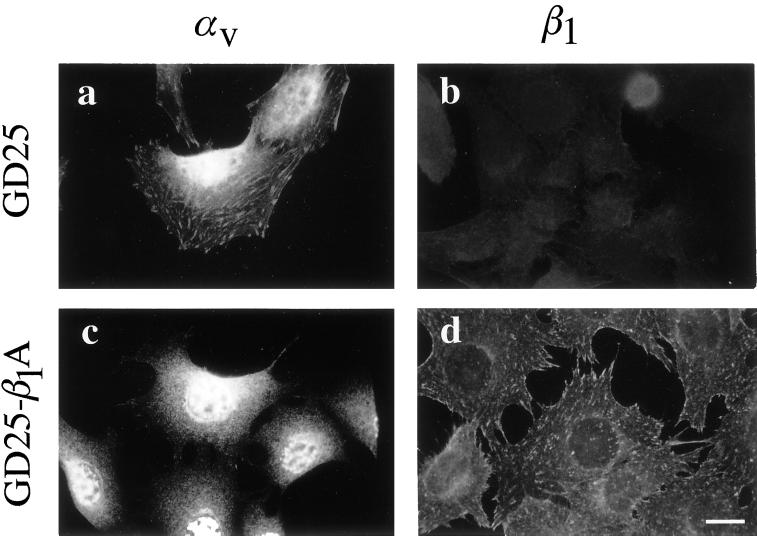
To determine the distribution of αV and β1 integrin heterodimers on GD25 and GD25-β1A cells attached to fibronectin, indirect immunofluorescence experiments with specific antibodies were performed. GD25 cells, which do not express β1 integrin heterodimers (Figure 1b), formed αV-containing prominent focal adhesions when allowed to attach and spread on coverslips coated with fibronectin (Figure 1a), consistent with the reported ability of αVβ3 to localize to focal adhesions in these cells (Wennerberg et al., 1996; Retta et al., 1998). In contrast, the amount of αV-containing focal adhesions was consistently reduced on GD25-β1A cells attached to fibronectin (Figure 1c), whereas β1A-containing focal adhesions were abundant (Figure 1d).
Figure 1.
Subcellular localization of αV and β1 integrins on GD25 and GD25-β1A cells plated on fibronectin. GD25 (a and b) and GD25-β1A (c and d) cells were allowed to attach and spread on coverslips coated with fibronectin (30 μg/ml in PBS) for 3 h at 37°C. Cells were then fixed, permeabilized, and incubated with primary antibodies against αV (rabbit anti-αV) and β1 (mAb TS2/16) integrin subunits. The αV and β1 antibody–antigen complexes were then detected with rhodamine-conjugated anti-rabbit and anti-mouse secondary antibodies, respectively. Representative fields were photographed with the use of an Olympus BM11 microscope fitted with epifluorescence. Notice that β1 integrins displace αV integrins from focal adhesions. Bar, 15 μm.
Thus, β1A, by localizing to focal adhesions, displaces the αV-containing heterodimers from these structures. Interestingly, we have previously shown that the expression of two other human β1 isoforms, namely, β1B, that does not localize to focal adhesions, and β1D, that is efficiently targeted to focal adhesions, also causes the delocalization of αV heterodimers on the cell surface (Belkin et al., 1997; Retta et al., 1998). Taken together, these data indicate that in GD25 cells cultured on fibronectin αVβ3 takes over the function of β1 integrins in mediating focal adhesion assembly; however, when expressed, β1 integrins behave as trans-dominant molecules with respect to αV integrins.
Expression of the β1 Integrin Subunit in GD25 Cells Induces Drastic Reduction of Surface Level of αVβ3 and an Up-Regulation of αVβ5
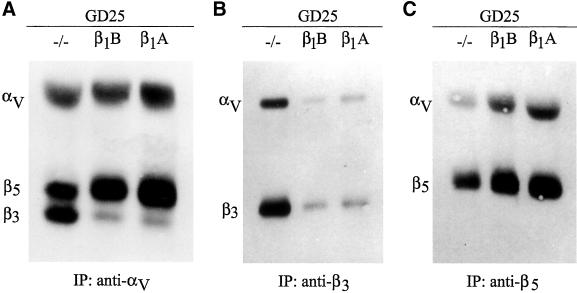
Previous results showed that transfection of GD25 cells with cDNA constructs of human β1 integrin led to surface expression of the β1 integrin subunit associated with the endogenous α3, α5, and α6 subunits but not with the αV subunit (Retta et al., 1998). In addition, no obvious differences in αV integrin expression were seen by immunoprecipitation from 125I-surface–labeled GD25 and GD25-β1A cells with an anti-αV antiserum (Retta et al., 1998). To understand the cellular mechanism(s) controlling the effect of β1 over αV integrins, we analyzed more in detail GD25 and GD25-β1A cells for the expression levels of their αV integrin heterodimers, namely, αVβ3 and αVβ5. Untransfected or β1A-transfected GD25 cells were surface-labeled with Sulfo-NHS-Biotin then αVβ3 and αVβ5 integrins were immunoprecipitated from nonionic detergent cell extracts and analyzed by Western blot. As expected, a polyclonal serum to the αV integrin subunit coimmunoprecipitated αV together with its associated β3 and β5 subunits (Figure 2A). The biotinylated αV, β3, and β5 proteins resulted as distinct bands in Western blots and, surprisingly, we noticed that, whereas expression of the αV subunit did not change significantly, the relative amounts of β3 and β5 proteins in β1A-expressing GD25 cells were clearly different from those of untransfected GD25 cells (Figure 2A). With the use of antibodies specific for β3 and β5 subunits, we confirmed this evidence: β3 protein levels were much lower in GD25-β1A than in GD25 cells, whereas the opposite was true for β5 protein levels (Figure 2, B and C). Thus, although the expression of the human β1A integrin isoform in GD25 cells did not modify the surface expression level of the αV integrin subunit, it led to a down-regulation and an up-regulation of the levels of its associated β3 and β5 subunits, respectively. These data suggest that the trans-dominant effect of β1 integrin isoforms over the subcellular localization of αV integrins in GD25-β1 cells is due to a switching of the relative amounts of the cell-surface expression levels of αVβ3 and αVβ5 integrins.
Figure 2.
Surface expression of αv, β3 and β5 integrins in GD25 and GD25-β1 cells. Integrin heterodimers were immunoprecipitated from surface biotinylated untransfected (−/−) or β1A- and β1B-transfected GD25 cells with polyclonal antibodies specific for αv (A), β3 (B), and β5 (C) integrin subunits, respectively. After separation by nonreducing SDS-PAGE and Western blot, the immunoprecipitated proteins were detected with the use of peroxidase-conjugated streptavidin and ECL as described in MATERIALS AND METHODS. Notice that, although the expression of either integrin β1 A or B isoforms does not alter significantly the expression level of the αV integrin subunit, it induces a net change of the αVβ3/αVβ5 ratio.
Expression of β1 Integrins Differentially Regulates mRNA Steady-State Levels of β3 and β5 Integrin Subunits
To address at what level the control of β3 and β5 protein expression in GD25-β1 cells was exercised, we first compared mRNA steady-state levels of these two integrin subunits in GD25-β1A with those of untransfected GD25 cells, with the use of both RT-PCR and Northern blot procedures.
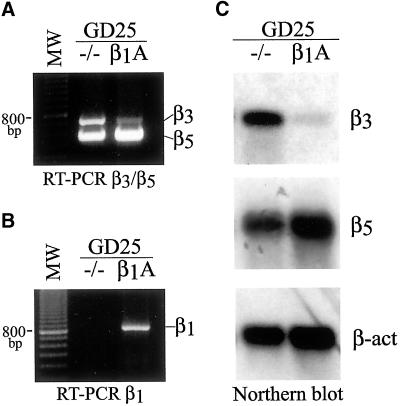
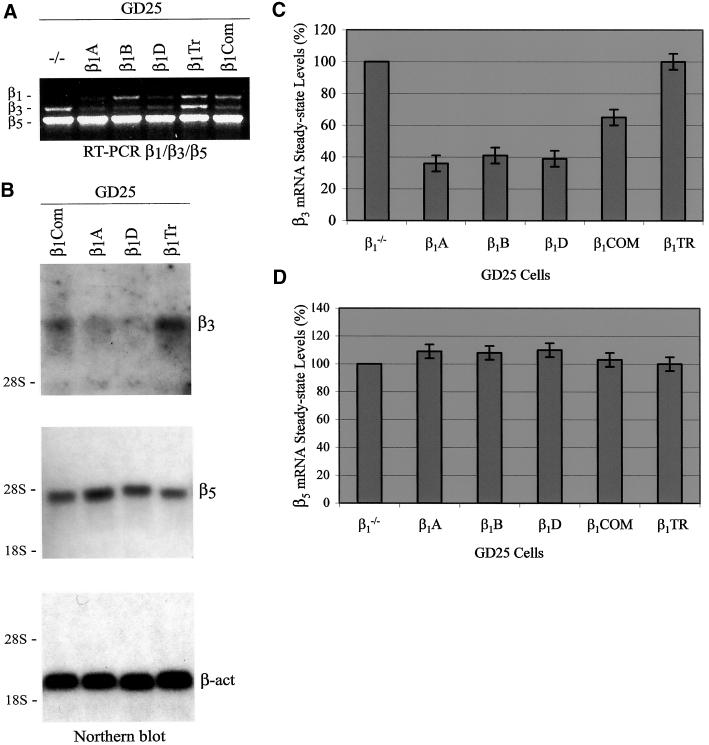
A duplex RT-PCR assay with two sets of primers was developed for the simultaneous detection of the relative levels of β3 and β5 integrin subunit mRNAs. As shown in Figure 3A, a great difference in β3 mRNA steady-state levels was observed between GD25 and GD25-β1A cells. Interestingly, the lower mRNA levels of β3 in GD25-β1A compared with those in GD25 cells reflected what we observed at the level of protein cell-surface expression (compare with Figure 2, A and B). On the contrary, there was little difference in β5 mRNA levels among untransfected and β1-transfected GD25 cells, with a small elevation observed in GD25-β1A cells (Figure 3A). RT-PCR for β1 mRNA was performed as control (Figure 3B). Thus, the presence of β1 integrins in GD25 cells differently modulates β3 and β5 mRNA expression.
Figure 3.
β1 integrins differentially regulate mRNA steady-state levels of β3 and β5 integrin subunits. Total RNA was isolated from 1 × 107 cultured GD25 (−/−) and GD25-β1A cells, and β3 and β5 mRNAs were evaluated by RT-PCR and Northern blot analyses as described in MATERIALS AND METHODS. (A) Duplex RT-PCR assay for the simultaneous detection of β3 and β5 mRNAs. (B) RT-PCR for β1 mRNA performed as control. Molecular size standards (123-bp DNA ladder) are shown on the left. (C) Northern blot: equal amounts of total RNA (25 μg/lane) were probed sequentially by 32P-labeled mouse integrin β3 and β5 cDNA fragments, and by a 32P-labeled β-actin probe as a control for RNA loading. Notice that the presence of β1 integrins causes a marked down-regulation of β3 and a little up-regulation of β5 mRNA expression levels.
Northern blot analysis demonstrated that our cDNA probes to β3 and β5 specifically recognized mRNAs of ∼6.6 and 3.5 kb, respectively, consistent with what has been previously described (Yamada et al., 1995). As resulted from this analysis, the mRNA steady-state level of β3 was much lower in GD25-β1A than in GD25 cells (Figure 3C, β3), thus reflecting the difference observed by RT-PCR and protein analysis (see above). In addition, the Northern blot analysis confirmed that the difference in β5 mRNA steady-state levels between GD25 and GD25-β1A cells (Figure 3C, β5) did not fully correlate with the difference in β5 cell-surface expression level (compare with Figure 2, A and C). Thus, in GD25-β1A cells the down-regulation of αVβ3 cell-surface expression strictly correlates with the down-regulation of β3 mRNA steady-state level, whereas the up-regulation of αVβ5 is mainly due to translational or posttranslational events leading to an increase of β5 subunit cell-surface recruitment.
β1 Effect over αV Integrins Occurs Irrespective of the Type of β1 Isoform and Is Dependent on the Presence of the β1 Cytoplasmic Domain Common Region
We have previously characterized some of the functional properties of β1B and β1D integrin isoforms, comparing these properties with those of the common β1A isoform. In particular, we have shown that the unique cytoplasmic sequences of β1B and β1D endow these molecules with distinctive functional properties with respect to a number of cellular functions (Balzac et al., 1994; Belkin et al., 1997; Belkin and Retta, 1998; Calìet al., 1998; Retta et al., 1998).
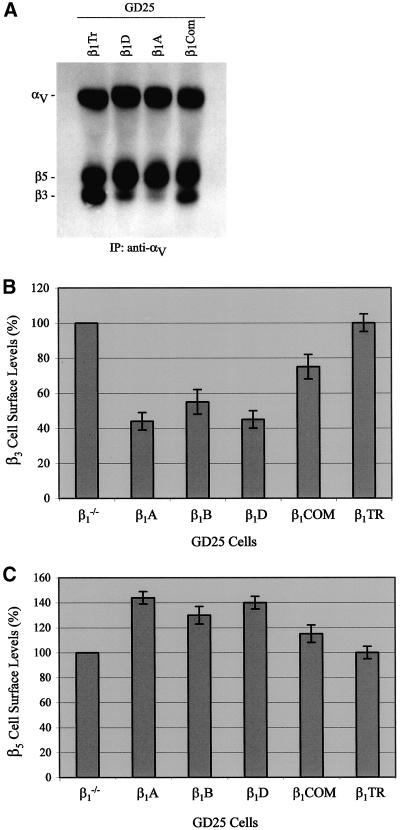
To analyze more in detail the effects of β1 over β3 and β5 integrins, we tested GD25 cells expressing β1B and β1D isoforms as well as two β1 deletion mutants lacking almost the entire cytoplasmic domain (β1TR) or the cytoplasmic domain variable region (β1COM) (Retta et al., 1998). Untransfected or β1-transfected GD25 cells were surface-labeled with Sulfo-NHS-Biotin, and the expression of αVβ3 and αVβ5 integrin heterodimers was examined by immunoprecipitation and Western blot analysis as described (see MATERIALS AND METHODS). The αV subunit was expressed at a constant level in all the examined cells (Figures 2A and 4A), whereas a down-regulation of β3 and an up-regulation of β5 cell-surface levels were seen in either β1B- (Figures 2 and 4, B and C) or β1d-expressing GD25 cells (Figure 4, A–C). Moreover, the expression of the β1COM cytoplasmic domain mutant induced similar effects, although to a lower extent (Figure 4, A–C). However, GD25-β1TR cells, expressing a β1 mutant carrying the deletion of the cytoplasmic domain (β1TR), behaved equivalently to the β1-deficient GD25 cells (Figure 4, A–C).
Figure 4.
Comparative analysis of surface expression of αv integrins in GD25 cells expressing different integrin β1 forms. (A) αv integrin heterodimers were immunoprecipitated from surface biotinylated GD25 cells expressing either the β1A or β1D isoforms or two β1 deletion mutants, lacking the entire cytoplasmic domain (β1TR) or the cytoplasmic domain variable region (β1COM), with the use of a polyclonal antibody against the αv subunit. After separation by nonreducing SDS-PAGE and Western blot, the immunoprecipitated proteins were detected with the use of peroxidase-conjugated streptavidin and ECL as described in MATERIALS AND METHODS. (B and C) Scanning densitometry analysis of β3 (B) and β5 (C) integrins as detected by Western blot. Data are displayed as percentage of the control (GD25) and are representative of three independent experiments. Notice that the β1 effect over the αVβ3/αVβ5 ratio occurs irrespective of the type of the β1 isoform and is dependent on the presence of the β1 cytoplasmic domain common region.
The spectrum of relative β3 integrin levels in untransfected or β1-transfected GD25 cells compared well with our subsequent mRNA analysis. In fact, when we analyzed by RT-PCR and Northern blot the mRNA steady-state level of the β3 subunit in GD25 cells expressing either β1B, β1D, β1COM or β1TR, we found that in GD25-β1B and GD25-β1D cells it was as low as in GD25-β1A cells, whereas in GD25-β1COM cells it was also reduced but to a lower extent (Figure 5, A–C). On the contrary, the β3 mRNA level in GD25-β1TR was higher and similar to that of β1-deficient GD25 cells (Figure 5, A–C). On the other hand, although a little increase of β5 mRNA steady-state level was observed in GD25 cells expressing β1B, β1D, or β1COM (Figure 5, B and D), it did not fully reflect the high increase observed at the β5 protein level in the same cells.
Figure 5.
Comparative analysis of β3 and β5 mRNA steady-state levels in GD25 cells expressing different integrin β1 forms. Total RNA was isolated from 1 × 107 cultured cells as described in MATERIALS AND METHODS. (A) Multiplex RT-PCR assay for the simultaneous detection of β1, β3, and β5 mRNAs in GD25 cells expressing either the β1A, β1B, or β1D isoforms or two β1 deletion mutants lacking the entire cytoplasmic domain (β1TR) or the cytoplasmic domain variable region (β1COM). (B) Northern blot: equal amounts of total RNA (25 μg/lane) were probed sequentially by 32P-labeled mouse integrin β3 and β5 cDNA fragments and by a 32P-labeled β-actin probe as a control for RNA loading. The positions of 28s and 18s rRNAs are indicated as markers for RNA sizes. (C and D) Scanning densitometry analysis of β3 and β5 mRNA levels as detected by Northern blot. Northern signals were normalized to β-actin and displayed as percentage of the control (GD25). Data are representative of three independent experiments. Notice that the down-regulation of β3 mRNA steady-state level occurs irrespective of the type of the β1 isoform and is dependent on the presence of the β1 cytoplasmic domain common region.
These results indicate that the β1-dependent modulation of β3 and β5 integrin subunit expression was not confined to GD25-β1A cells, but that it was also present in GD25 cells expressing two other β1 isoforms. In addition, the fact that a down-regulation of β3 and an up-regulation of β5 were also observed in GD25-β1COM, but not in GD25-β1TR cells, strongly suggests that the control of the expression level of β3 and β5 integrin subunits was dependent on the presence of the β1 cytoplasmic domain common region.
Cell Adhesion to ECM Proteins Is not Required for β1 Effect on β3 mRNA Steady-State Level
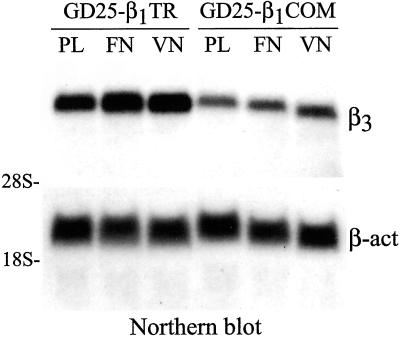
To determine whether cell adhesion to ECM proteins was required for β1 effect over β3 expression levels, we performed Northern blot analysis of β3 mRNA steady-state level in cells plated on tissue culture dishes coated with either polylysine or two ECM proteins, namely, fibronectin and vitronectin. GD25-β1TR and GD25-β1COM cells were cultured to confluence in complete culture medium and then resuspended in serum-free medium, containing 1 μM monensin, and allowed to attach and spread on polylysine-, fibronectin-, and vitronectin-coated dishes for 2 h at 37°C before RNA isolation for Northern blot analysis.
The results, shown in Figure 6, indicate that the β3 mRNA steady-state level was constitutively low in GD25-β1COM cells compared with that of GD25-β1TR cells. Similar results were obtained by comparing β3 mRNA steady-state levels in cells kept in suspension in serum-free medium for up to 2 h with those of long-term adherent cells. Thus, these data suggest that ligation of ECM proteins is not required for β1 effect over β3 expression.
Figure 6.
Cell adhesion to ECM proteins is not required for β1 effect on β3 mRNA steady-state level. GD25-β1TR and GD25-β1COM cells were cultured to confluence in complete culture medium. Cells were then resuspended in serum-free medium containing 1 μM monensin and allowed to attach and spread on polylysine- (PL), FN-, or vitronectin (VN)-coated tissue culture dishes for 2 h at 37°C before lysis. Total RNA was isolated as described in MATERIALS AND METHODS, and equal amounts (25 μg/lane) were analyzed for β3 mRNA steady-state level by Northern blot hybridization with the use of 32P-labeled mouse integrin β3 cDNA fragments as probe. Equal loading was confirmed by hybridization of the same blot with a 32P-labeled probe for β-actin. The positions of 28s and 18s rRNAs are indicated as markers for RNA sizes. Notice that the β3 mRNA steady-state level is constitutively low in GD25-β1COM compared with that of GD25-β1TR cells.
De Novo Surface Expression of β1-associated α Subunits Does Not Affect the Level of β3 Integrin Subunit
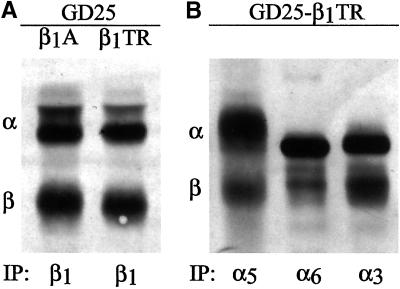
Because human β1 expression in GD25 cells leads to the assembly and cell-surface recruitment of integrin complex with endogenous α3, α5, and α6 subunits (Retta et al., 1996), it was possible that these β1-associated α subunits could play a direct role in the down-regulation of αVβ3 integrin.
To exclude this possibility we took advantage of GD25-β1TR cells by extending our observations with this cell line. The expression of the β1TR mutant at the surface of GD25-β1TR cells was comparable with that of the β1A isoform in GD25-β1A cells, as previously determined by flow cytometry analysis (Retta et al., 1998). In addition, by immunoprecipitation experiments we did not see any detectable change in the pattern of α subunits associated with β1TR in GD25 cells compared with GD25-β1A cells (Figure 7). Nevertheless, the presence of β1TR integrin heterodimers did not lead to any detectable effect over αVβ3 or αVβ5 integrins (Figures 4, A–C, and 5, A–D). Taken together, these data strongly suggest that the down-regulation of β3 is not directly due to de novo surface expression of the β1-associated α subunits and confirm that for the effect of β1 integrins over αVβ3/αVβ5 integrin ratio a β1 subunit carrying, at least, the common region of the cytoplasmic domain is required.
Figure 7.
Pattern of α subunits associated with the β1TR cytoplasmic domain deletion mutant. (A) β1 Integrins immunoprecipitated from surface biotinylated β1A- and β1TR-transfected GD25 cells with the mAb TS2/16. (B) Integrin heterodimers immunoprecipitated from surface biotinylated GD25-β1TR cells with the mAb GoH3 against α6 and polyclonal antibodies against α3 and α5 integrin subunits. After separation by nonreducing SDS-PAGE and Western blot, the immunoprecipitated proteins were detected with the use of peroxidase-conjugated streptavidin and ECL as described in MATERIALS AND METHODS. Notice that the β1TR mutant correctly associates with three major α subunits at the surface of GD25-β1TR cells.
Expression of β1 Integrins Induces a Marked Decrease in β3 mRNA Stability
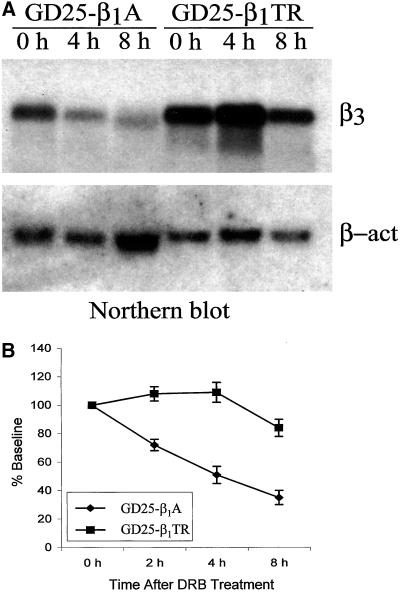
Because modulation of mRNA stability is a potential regulatory mechanism for integrin expression (Sachs, 1993; Feng et al., 1999), we next asked whether the changes in mRNA steady-state levels were due to changes in integrin mRNA stability. The rate of turnover of β3 and β5 mRNAs was determined by inhibition of RNA synthesis with 60 μM DRB followed by quantitative blot hybridization analysis of β3 and β5 mRNA as a function of time. In GD25-β1A cells grown on tissue culture dishes a clear decrease of β3 mRNA stability was detected compared with GD25 cells (Figure 8A). The β3 mRNA decayed with an apparent half-life of >8 h in GD25 cells but <4 h in GD25-β1A cells (Figure 8, A and B). In contrast, the stability of β5 mRNA was much higher than that of β3, and no significant difference was observed when GD25 and GD25-β1A cells were compared. Therefore, the effects of β1 expression over β3 and β5 mRNA levels clearly involve a regulation of β3, but not β5, mRNA stability
Figure 8.
The presence of the β1 cytoplasmic domain doubles the decay rate of the β3 mRNA in GD25 cells. GD25-β1A and GD25-β1TR cells grown to confluence were divided into three 10-cm Petri dishes and cultured in DMEM containing 10% FBS for 24 h before the addition of 60 μM DRB, an inhibitor of transcription initiation. After addition of DRB, the cells were collected at 0, 4, and 8 h for RNA analysis. Total RNA isolation and Northern analysis were performed as described in MATERIALS AND METHODS. (A) Northern blot: equal amounts of total RNA (25 μg/lane) were probed sequentially by 32P-labeled mouse integrin β3 and β-actin cDNA fragments. (B) Scanning densitometry analysis of β3 mRNA levels as detected by Northern blot. β3 Northern signals were normalized to β-actin and displayed as percentage of the baseline (time 0). Data presented are the mean values ± SE of three independent experiments. Notice the lower stability of β3 integrin subunit mRNA in GD25-β1A than in GD25-β1TR cells.
DISCUSSION
There is increasing evidence that a coordinated cross talk between integrin receptors is crucial for an integrated and functional response of a single cell to the extracellular environment (Porter and Hogg, 1997; Blystone et al., 1999; Hynes, 1999). However, the molecular mechanisms of integrin cross talk remain mostly undetermined.
Previously, we showed that the expression of either β1B or β1D integrin isoforms in β1-null GD25 cells prevented different FN-dependent functions of endogenous αV integrins, including their ability to mediate cell adhesion, to localize to focal adhesions, and to assembly an FN matrix, thus indicating the existence of a functional cross talk between these two β1 isoforms and αV integrins (Belkin et al., 1997; Retta et al., 1998). The present study was undertaken to examine this integrin cross talk and establish the regulatory mechanism(s) whereby β1 integrins exert their trans-acting functions. The main findings are that 1) de novo expression of the β1 integrin subunit in β1-null GD25 cells induces a drastic down-regulation of αVβ3 and an up-regulation of αVβ5 integrin cell surface levels; 2) this β1 effect occurs irrespective of the type of β1 isoform but is dependent on the presence of the common region of the β1 cytoplasmic domain; and 3) the down-regulation of αVβ3 is due to a decreased mRNA stability of the β3 subunit, whereas the up-regulation of αVβ5 is mainly due to translational or posttranslational events. These findings provide the first evidence of a cross talk between β1 and αV integrins based on mechanisms of control of mRNA and protein levels.
Expression of the β1 Integrin Subunit in GD25 Cells Induces a Drastic Reduction of Surface Level of αvβ3 and an Up-Regulation of αvβ5
Despite the apparent high degree of integrin-ligand binding redundancy (Hynes, 1992), the localization of distinct integrins to focal adhesions is usually very restricted (Fath et al., 1989). In GD25 cells two integrins are believed to be able to localize to focal adhesions on fibronectin, namely, αVβ3 and, upon β1 ectopic expression, α5β1 (Wennerberg et al., 1996; Belkin et al., 1997; Retta et al., 1998). However, whereas αV integrins can take over some FN-dependent functions in the absence of β1 integrins (Wennerberg et al., 1996; Retta et al., 1998), immunofluorescence analyses of β1A-transfected and untransfected GD25 cells plated on fibronectin show that β1 integrins clearly dominate upon αV integrins in localizing to focal adhesions. This phenomenon is mainly due to a cross talk between β1 and αV integrins that occurs at the level of expression on the cell surface. In fact, although the presence of β1 integrins in GD25 cells does not affect the total amount of αV integrins, it causes a clear rearrangement of the relative cell surface levels of β3 and β5 subunits, leading to a marked down-regulation of αVβ3 and a correspondent up-regulation of αVβ5.
A great deal of experimental work has shown that integrin expression is highly dynamic during development (reviewed in Darribere et al., 2000; Tarone et al., 2000). In particular, it has been suggested that the presence and functions of the αV integrins are developmentally controlled by differential temporal and spatial regulation of its β subunits (Yamada et al., 1995), whereas there are reports showing that a balanced ratio of integrin receptors is crucial for the maintenance of the differentiation state of a particular cell (Carroll et al., 1995; Sastry et al., 1996). However, genetic ablation experiments have shown that the absence of some widely expressed integrins that were believed to be key regulators of development and differentiation has resulted into mild or late phenotypes. In particular, it has come out that processes such as myogenesis, vasculogenesis, and angiogenesis, which through antibody or peptide perturbation experiments were shown to be dependent on specific β1 or αV integrins, can actually proceed without these integrins (Bader et al., 1998; Hirsch et al., 1998), suggesting that there might be some overlapping or compensatory functions between different integrins. A very striking example of this point comes from a thorough study of compound mutations showing that gene knockouts of αV and α5 integrin subunits have synergistic effects when combined pairwise and result in a phenotype similar to that of FN-null mutation, suggesting that α5β1 and αV integrins normally overlap or can compensate each other in mesodermal development (Yang et al., 1999). Other examples derive from in vitro experiments showing that in cultured cells αV integrins are able to compensate for the loss of the α5β1 fibronectin receptors (Wennerberg et al., 1996; Yang and Hynes, 1996; Retta et al., 1998). On the other hand, αVβ3 has previously been shown to negatively regulate α5β1-mediated cell migration (Bilato et al., 1997; Simon et al., 1997; Blystone et al., 1999) and phagocytosis (Blystone et al., 1994, 1999). Notably, the above-cited reports are all examples of functional cross talk between αV and β1 integrins, apparently without quantitative up-regulation of integrin levels. Our present report now demonstrates that, besides the existence of a functional compensation of αV integrins for the lack of certain β1 integrin functions, a cross talk mechanism occurs that regulates the ratio of αVβ3 and αVβ5 on the cell surface upon β1 integrin expression. Taken together, these observations suggest that the cross talk between β1 and αV integrins must be bidirectional, articulate, and based on integrated and tightly regulated mechanisms. Several examples support the existence of such a composite cross talk between integrins. One example comes from our recent report showing that ectopic expression of α7B integrin subunit in Chinese hamster ovary cells leads to down-regulation of both cell surface expression and ligand binding affinity of the integrin α5β1 (Tomatis et al., 1999). Another straightforward example derives from analysis of the expression of β1 splice variants during mouse muscle development: genetic analysis by homologous recombination has demonstrated that mice lacking β1D, the only β1 isoform in adult muscles, develop normally without gross apparent defects; in this case, however, the β1A isoform is not down-regulated as in wild-type muscles and can apparently compensate both in function and in level for the absence of β1D (Baudoin et al., 1998). A similar phenomenon might likely occur during β1-null myoblast differentiation where an up-regulation of αVβ3, both in level and function, could give a reason for the unexpected mild phenotype (Hirsch et al., 1998). Interestingly, αVβ3 has been recently shown to be up-regulated in β1-null cardiac cells compared with wild-type cells (Guan et al., 2001). On the other hand, a switch from αVβ5 to αVβ3 integrins has been observed to occur in important biological processes, including wound healing (Clark et al., 1996a,b) and tumor progression in situ (Marshall et al., 1991; Li et al., 1998). Thus, taken together with the above-mentioned observations, our results support the hypothesis that the enigma of the recurrent discrepancy between blocking experiments and genetic analyses (Hynes, 1996; Brakebusch et al., 1997; Bader et al., 1998; Hirsch et al., 1998; McHugh et al., 2000) could be explained by taking into account that a compensatory up-regulation both in level and function between integrins can occur.
Expression of β1 Integrins Differentially Regulates mRNA Steady-State Levels of β3 and β5 Integrin Subunits
The expression of integrins can be modulated by a variety of agents, including proinflammatory cytokines, growth factors, hormones, extracellular matrix components, and pharmacological agents (Delcommenne and Streuli, 1995; Kim and Yamada, 1997). In particular, the integrin αVβ3 has been shown to be up-regulated by transforming growth factor-β1, platelet-derived growth factor-BB (Janat et al., 1992), basic fibroblast growth factor (Sepp et al., 1994), vitamin D (Medhora et al., 1993), fibronectin (Feng et al., 1999), and phorbol esters (Swerlick et al., 1992), and down-regulated by tumor necrosis factor-α, interferon-γ (Defilippi et al., 1991), and collagen (Feng et al., 1999). Mechanisms regulating integrin expression include regulation of protein levels by transcriptional or posttranscriptional events, alternative splicing of mRNA, and mobilization of preexisting intracellular stores (Xu and Clark, 1996; Kim and Yamada, 1997). However, little is known regarding integrin cross talk mechanisms that modulate integrin ratios in individual cells. Here, we show that the expression of β1 integrins in GD25 cells regulates the cell surface levels of αVβ3 and αVβ5 by two distinct mechanisms. In particular, the decreased expression of integrin αVβ3 in GD25-β1A cells clearly involves an increased decay rate of β3 subunit mRNA. On the contrary, because the up-regulation of the β5 protein level we observed was not reflected at the mRNA level, the enhanced cell surface expression of αVβ5 is probably mainly due to the mobilization to the cell surface of intracellular stores of the β5 subunit. This is consistent with previous reports showing the presence of intracellular pools of β subunits and indicating the availability of the α subunit as a rate-limiting step in integrin complex assembly and cell-surface expression (Swerlick et al., 1992).
The regulation of mRNA stability is a very important mechanism of posttranscriptional regulation of gene expression, and evidence exists for both a wide range of half-lives for different mRNAs in the same cells and different half-lives for the same mRNA in the same cell under different circumstances (Sachs, 1993). Interestingly, it has been previously suggested that coordinate signals from ECM molecules and growth factors can modulate the mRNA decay rate of specific integrins (Xu and Clark, 1996). In addition, a recent report shows that the β3 mRNA stability can be increased by cell interaction with fibrin but not with collagen (Feng et al., 1999). Because most extracellular matrix proteins signal through integrins, which have also been shown to physically associate and act synergistically with growth factor receptors (Giancotti and Ruoslahti, 1999), it is possible to hypothesize a scenario where the expression of a specific integrin can influence the expression of another integrin by affecting its mRNA stability, either directly or with the cooperation of an associated growth factor receptor. This could be a way for a rapid change of integrin ratios in response to a variation of the extracellular environment, as it occurs during tissue formation or repair. Interestingly, αVβ3 expression has been shown to increase focally and transiently during cutaneous wound repair (Feng et al., 1999). On the other hand, the existence of this regulatory integrin cross talk could explain the compensatory up-regulation of αVβ3 in the absence of β1 integrins, highlighting the usefulness of stabilizing specific integrin mRNA only when it is needed.
β1 Effect over β3 Expression Is Dependent on the Presence of the β1 Cytoplasmic Domain Common Region
Our results clearly demonstrate that the control of β3/β5 ratio is β1-dependent, and neither confined to a particular cell population nor restricted to a specific β1 isoform. Instead, it requires the presence of the common region of the β1 cytoplasmic domain. In addition, the fact that the β1COM mutant does not contribute to cell adhesion (Retta et al., 1998), together with the observation that the β3 mRNA steady-state level is constitutively low in GD25-β1COM cells compared with GD25-β1TR cells, indicates that the binding of extracellular ligands is not required for β1 to regulate β3 expression. On the other hand, it is noteworthy that, although in long-term adherent cells the β1COM mutant was less effective than β1A in inducing an effect over the β3 mRNA steady-state level, no significant difference was observed when we compared β3 mRNA levels in GD25-β1COM and GD25-β1A cells either plated on polylysine or kept in suspension for 2 h (unpublished). These results could be explained by taking into account that, in contrast with the expression of β1A, the expression of β1COM in GD25 cells does not entirely prevent the localization of αV integrins to FN-dependent focal adhesions (Retta et al., 1998). Thus, a likely possibility is that the common region of the β1 cytoplasmic domain is able to constitutively induce a down-regulation of β3 mRNA steady-state level; however, the αVβ3 integrin, due to its ability to localize to focal adhesions in GD25-β1COM cells, counteracts this β1 constitutive action leading to a mild effect on β3 expression level. Interestingly, in accordance with a recent report (Feng et al., 1999), the higher β3 mRNA level in cells cultured on fibronectin or vitronectin than on polylysine (Figure 6) suggests that ECM proteins that are ligands for αVβ3 can sustain β3 mRNA steady-state level.
In conclusion, our results indicate a novel mechanism of integrin cross talk where one integrin can regulate the expression of another by modulating the decay rate of its mRNA. The biological implications of this integrin cross talk are potentially of high functional significance as a fine-tuned mechanism for selective and transient integrin expression in different extracellular contexts. Our attempt now will be to uncover β1-dependent events regulating the β3 mRNA steady-state levels.
ACKNOWLEDGMENTS
We thank Reinhard Fässler and Arnoud Sonnenberg for generously providing GD25 cells and mAb GoH3, respectively. The students Tiziana Spatola and Federica Logrand are gratefully acknowledged for helping in some experiments. We also thank Fiorella Balzac and Emilio Hirsch for helpful discussions. This work was supported by grants from the Italian Association for Cancer Research to G.T., from the University of Torino (ex 60%) to SFR and GT and from the University of Palermo (ex 60%) to GDL.
Abbreviations used:
- ECM
extracellular matrix
- FN
fibronectin
REFERENCES
- Albelda SM, Mette SA, Elder DE, Stewart R, Damjanovich L, Herlyn M, Buck CA. Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Balzac F, Retta SF, Albini A, Melchiorri A, Koteliansky VE, Geuna M, Silengo L, Tarone G. Expression of β1B integrin isoform in CHO cells results in a dominant negative effect on cell adhesion and motility. J Cell Biol. 1994;127:557–565. doi: 10.1083/jcb.127.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoin C, Goumans MJ, Mummery C, Sonnenberg A. Knockout and knockin of the β1 exon D define distinct roles for integrin splice variants in heart function and embryonic development. Genes Dev. 1998;12:1202–1216. doi: 10.1101/gad.12.8.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin AM, Retta SF. β1D integrin inhibits cell cycle progression in normal myoblasts and fibroblasts. J Biol Chem. 1998;273:15234–15240. doi: 10.1074/jbc.273.24.15234. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Retta SF, Pletjushkina OY, Balzac F, Silengo L, Fassler R, Koteliansky VE, Burridge K, Tarone G. Muscle β1D integrin reinforces the cytoskeleton-matrix link: modulation of integrin adhesive function by alternative splicing. J Cell Biol. 1997;139:1583–1595. doi: 10.1083/jcb.139.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilato C, Curto KA, Monticone RE, Pauly RR, White AJ, Crow MT. The inhibition of vascular smooth muscle cell migration by peptide and antibody antagonists of the αvβ3 integrin complex is reversed by activated calcium/calmodulin-dependent protein kinase II. J Clin Invest. 1997;100:693–704. doi: 10.1172/JCI119582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blystone SD, Graham IL, Lindberg FP, Brown EJ. Integrin αvβ3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor α5β1. J Cell Biol. 1994;127:1129–1137. doi: 10.1083/jcb.127.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blystone SD, Slater SE, Williams MP, Crow MT, Brown EJ. Molecular mechanism of integrin crosstalk: αvβ3 suppression of calcium/calmodulin-dependent protein kinase II regulates α5β1 function. J Cell Biol. 1999;145:889–897. doi: 10.1083/jcb.145.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Hirsch E, Potocnik A, Fassler R. Genetic analysis of β1 integrin function: confirmed, new and revised roles for a crucial family of cell adhesion molecules. J Cell Sci. 1997;110:2895–2904. doi: 10.1242/jcs.110.23.2895. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Calì G, Retta SF, Negri R, Damiano I, Gentile R, Tarone G, Nitsch L, Garbi C. β1B integrin interferes with matrix assembly but not with confluent monolayer polarity, and alters some morphogenetic properties of FRT epithelial cells. Eur J Cell Biol. 1998;75:107–117. doi: 10.1016/s0171-9335(98)80053-8. [DOI] [PubMed] [Google Scholar]
- Carroll JM, Romero MR, Watt FM. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 1995;83:957–968. doi: 10.1016/0092-8674(95)90211-2. [DOI] [PubMed] [Google Scholar]
- Clark RA, Ashcroft GS, Spencer MJ, Larjava H, Ferguson MW. Re-epithelialization of normal human excisional wounds is associated with a switch from αvβ5 to αvβ6 integrins. Br J Dermatol. 1996b;135:46–51. [PubMed] [Google Scholar]
- Clark RA, Tonnesen MG, Gailit J, Cheresh DA. Transient functional expression of αvβ3 on vascular cells during wound repair. Am J Pathol. 1996a;148:1407–1421. [PMC free article] [PubMed] [Google Scholar]
- Corbett SA, Schwarzbauer JE. β3 integrin activation improves αvβ3-mediated retraction of fibrin matrices. J Surg Res. 1999;83:27–31. doi: 10.1006/jsre.1998.5552. [DOI] [PubMed] [Google Scholar]
- Critchley DR. Focal adhesions—the cytoskeletal connection. Curr Opin Cell Biol. 2000;12:133–139. doi: 10.1016/s0955-0674(99)00067-8. [DOI] [PubMed] [Google Scholar]
- Darribere T, Skalski M, Cousin HL, Gaultier A, Montmory C, Alfandari D. Integrins: regulators of embryogenesis. Biol Cell. 2000;92:5–25. doi: 10.1016/s0248-4900(00)88760-2. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Retta SF, Olivo C, Palmieri M, Venturino M, Silengo L, Tarone G. p125FAK tyrosine phosphorylation and focal adhesion assembly: studies with phosphotyrosine phosphatase inhibitors. Exp Cell Res. 1995;221:141–152. doi: 10.1006/excr.1995.1361. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Truffa G, Stefanuto G, Altruda F, Silengo L, Tarone G. Tumor necrosis factor α and interferon gamma modulate the expression of the vitronectin receptor (integrin β3) in human endothelial cells. J Biol Chem. 1991;266:7638–7645. [PubMed] [Google Scholar]
- Delcommenne M, Streuli CH. Control of integrin expression by extracellular matrix. J Biol Chem. 1995;270:26794–26801. doi: 10.1074/jbc.270.45.26794. [DOI] [PubMed] [Google Scholar]
- Fassler R, Georges-Labouesse E, Hirsch E. Genetic analyses of integrin function in mice. Curr Opin Cell Biol. 1996;8:641–646. doi: 10.1016/s0955-0674(96)80105-0. [DOI] [PubMed] [Google Scholar]
- Fath KR, Edgell CJ, Burridge K. The distribution of distinct integrins in focal contacts is determined by the substratum composition. J Cell Sci. 1989;92:67–75. doi: 10.1242/jcs.92.1.67. [DOI] [PubMed] [Google Scholar]
- Feng X, Clark RA, Galanakis D, Tonnesen MG. Fibrin and collagen differentially regulate human dermal microvascular endothelial cell integrins: stabilization of αv/β3 mRNA by fibrin1. J Invest Dermatol. 1999;113:913–919. doi: 10.1046/j.1523-1747.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- Fornaro M, Languino LR. Alternatively spliced variants: a new view of the integrin cytoplasmic domain. Matrix Biol. 1997;16:185–193. doi: 10.1016/s0945-053x(97)90007-x. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct αv integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- Giancotti FG. Complexity and specificity of integrin signaling. Nat Cell Biol. 2000;2:E13–E14. doi: 10.1038/71397. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Guan K, Czyz J, Furst DO, Wobus AM. Expression and cellular distribution of α(v) integrins in β(1) integrin-deficient embryonic stem cell-derived cardiac cells. J Mol Cell Cardiol. 2001;33:521–532. doi: 10.1006/jmcc.2000.1326. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Lohikangas L, Gullberg D, Johansson S, Fassler R. Mouse myoblasts can fuse and form a normal sarcomere in the absence of β1 integrin expression. J Cell Sci. 1998;111:2397–2409. doi: 10.1242/jcs.111.16.2397. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Targeted mutations in cell adhesion genes: what have we learned from them? Dev Biol. 1996;180:402–412. doi: 10.1006/dbio.1996.0314. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Cell adhesion: old and new questions. Trends Cell Biol. 1999;9:33–37. [PubMed] [Google Scholar]
- Janat MF, Argraves WS, Liau G. Regulation of vascular smooth muscle cell integrin expression by transforming growth factor β1 and by platelet-derived growth factor-BB. J Cell Physiol. 1992;151:588–595. doi: 10.1002/jcp.1041510319. [DOI] [PubMed] [Google Scholar]
- Kim LT, Yamada KM. The regulation of expression of integrin receptors. Proc Soc Exp Biol Med. 1997;214:123–131. doi: 10.3181/00379727-214-44078. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Yebra M, Bayna EM, Cheresh DA. Receptor tyrosine kinase signaling required for integrin αvβ5-directed cell motility but not adhesion on vitronectin. J Cell Biol. 1994;127:859–866. doi: 10.1083/jcb.127.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn K, Eble J. The structural bases of integrin-ligand interactions. Trends Cell Biol. 1994;4:256–261. doi: 10.1016/0962-8924(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Leavesley DI, Ferguson GD, Wayner EA, Cheresh DA. Requirement of the integrin β3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol. 1992;117:1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JM, Cheresh DA, Schwartz MA. Protein kinase C regulates αvβ5-dependent cytoskeletal associations and focal adhesion kinase phosphorylation. J Cell Biol. 1996;134:1323–1332. doi: 10.1083/jcb.134.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen B, Blystone SD, McHugh KP, Ross FP, Ramos DM. Differential expression of αv integrins in K1735 melanoma cells. Invasion Metastasis. 1998;18:1–14. doi: 10.1159/000024494. [DOI] [PubMed] [Google Scholar]
- Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000;113:3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Nesbitt SA, Helfrich MH, Horton MA, Polakova K, Hart IR. Integrin expression in human melanoma cell lines: heterogeneity of vitronectin receptor composition and function. Int J Cancer. 1991;49:924–931. doi: 10.1002/ijc.2910490621. [DOI] [PubMed] [Google Scholar]
- McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora MM, Teitelbaum S, Chappel J, Alvarez J, Mimura H, Ross FP, Hruska K. 1 α,25-dihydroxyvitamin D3 up-regulates expression of the osteoclast integrin αvβ3. J Biol Chem. 1993;268:1456–1461. [PubMed] [Google Scholar]
- Montgomery AM, Reisfeld RA, Cheresh DA. Integrin αvβ3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci USA. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff M, Liu S, Erle DJ, Ginsberg MH. Integrin β cytoplasmic domains differentially bind to cytoskeletal proteins. J Biol Chem. 1998;273:6104–6109. doi: 10.1074/jbc.273.11.6104. [DOI] [PubMed] [Google Scholar]
- Porter JC, Hogg N. Intern cross talk: activation of lymphocyte function-associated antigen-1 on human T cells alters α4β1- and α5β1-mediated function. J Cell Biol. 1997;138:1437–1447. doi: 10.1083/jcb.138.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retta SF, Balzac F, Ferraris P, Belkin AM, Fassler R, Humphries MJ, De Leo G, Silengo L, Tarone G. β1-Integrin cytoplasmic subdomains involved in dominant negative function. Mol Biol Cell. 1998;9:715–731. doi: 10.1091/mbc.9.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retta SF, Barry ST, Critchley DR, Defilippi P, Silengo L, Tarone G. Focal adhesion and stress fiber formation is regulated by tyrosine phosphatase activity. Exp Cell Res. 1996;229:307–317. doi: 10.1006/excr.1996.0376. [DOI] [PubMed] [Google Scholar]
- Retta SF, Ferraris P, Tarone G. Purification of fibronectin from human plasma. Methods Mol Biol. 1999;96:119–124. doi: 10.1385/1-59259-258-9:119. [DOI] [PubMed] [Google Scholar]
- Sachs AB. Messenger RNA degradation in eukaryotes. Cell. 1993;74:413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Lakonishok M, Thomas DA, Muschler J, Horwitz AF. Integrin α subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J Cell Biol. 1996;133:169–184. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Sepp NT, Li LJ, Lee KH, Brown EJ, Caughman SW, Lawley TJ, Swerlick RA. Basic fibroblast growth factor increases expression of the αvβ3 integrin complex on human microvascular endothelial cells. J Invest Dermatol. 1994;103:295–299. doi: 10.1111/1523-1747.ep12394617. [DOI] [PubMed] [Google Scholar]
- Simon KO, Nutt EM, Abraham DG, Rodan GA, Duong LT. The αvβ3 integrin regulates α5β1-mediated cell migration toward fibronectin. J Biol Chem. 1997;272:29380–29389. doi: 10.1074/jbc.272.46.29380. [DOI] [PubMed] [Google Scholar]
- Swerlick RA, Brown EJ, Xu Y, Lee KH, Manos S, Lawley TJ. Expression and modulation of the vitronectin receptor on human dermal microvascular endothelial cells. J Invest Dermatol. 1992;99:715–722. doi: 10.1111/1523-1747.ep12614207. [DOI] [PubMed] [Google Scholar]
- Tarone G, Hirsch E, Brancaccio M, De Acetis M, Barberis L, Balzac F, Retta F, Botta C, Altruda F, Silengo L. Integrin function and regulation in development. Int J Dev Biol. 2000;44:725–731. [PubMed] [Google Scholar]
- Tomatis D, Echtermayer F, Schober S, Balzac F, Retta SF, Silengo L, Tarone G. The muscle-specific laminin receptor α7β1 integrin negatively regulates α5β1 fibronectin receptor function. Exp Cell Res. 1999;246:421–432. doi: 10.1006/excr.1998.4315. [DOI] [PubMed] [Google Scholar]
- Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- Wayner EA, Orlando RA, Cheresh DA. Integrins αvβ3 and αvβ5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991;113:919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fassler R. β1 Integrin-dependent and -independent polymerization of fibronectin. J Cell Biol. 1996;132:227–238. doi: 10.1083/jcb.132.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Hughes PE, Ginsberg MH, McDonald JA. Identification of a new biological function for the integrin αvβ3: initiation of fibronectin matrix assembly. Cell Adhes Commun. 1996;4:149–158. doi: 10.3109/15419069609014219. [DOI] [PubMed] [Google Scholar]
- Xu J, Clark RA. Extracellular matrix alters PDGF regulation of fibroblast integrins. J Cell Biol. 1996;132:239–249. doi: 10.1083/jcb.132.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Brown KE, Yamada KM. Differential mRNA regulation of integrin subunits αV, β1, β3, and β5 during mouse embryonic organogenesis. Cell Adhes Commun. 1995;3:311–325. doi: 10.3109/15419069509081016. [DOI] [PubMed] [Google Scholar]
- Yang JT, Bader BL, Kreidberg JA, Ullman-Cullere M, Trevithick JE, Hynes RO. Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev Biol. 1999;215:264–277. doi: 10.1006/dbio.1999.9451. [DOI] [PubMed] [Google Scholar]
- Yang JT, Hynes RO. Fibronectin receptor functions in embryonic cells deficient in α5β1 integrin can be replaced by αV integrins. Mol Biol Cell. 1996;7:1737–1748. doi: 10.1091/mbc.7.11.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Z, Menter DG, Nicolson GL. Involvement of integrin αvβ3 in cell adhesion, motility, and liver metastasis of murine RAW117 large cell lymphoma. Cancer Res. 1996;56:3103–3111. [PubMed] [Google Scholar]