Abstract
Gene therapy approaches have been difficult to implement due to pre-existing immunity against the virus used for delivery. To circumvent this problem, a cell-based approach was developed that avoided the use of free virus within the animal. However, even cells transduced in vitro with E1- to E3-deleted adenovirus encoding bone morphogenetic protein 2 (AdBMP2) resulted in the production of virus-neutralizing antibodies in mice. Furthermore, when mice received an intramuscular injection of nonencoding adenovirus (AdEmpty)-transduced cells, AdBMP2-transduced cells were unable to launch bone formation when an intramuscular injection of these BMP2-producing cells was delivered 1 week later. This phenomenon was not observed in NOD/SCID mice, and could be overcome in C57BL/6 mice by encapsulating the adenovirus-transduced cells in a nondegradable hydrogel poly(ethylene glycol) diacrylate (PEGDA). Data collectively suggest that PEGDA hydrogel encapsulation of AdBMP2-transduced cells prevents pre-existing immunity from suppressing BMP2-induced bone formation.
Keywords: : bone formation, cell therapy, priming, in vivo delivery system
Introduction
Adenoviruses (Ad) have been extensively studied as vectors for cell-based gene therapy. The use of Ad vectors in cancer therapy and metabolic disorders has shown promising results in animal models.1–3 One advantage of using this approach is for delivery of growth factors.4 Because the adenovirus vector is nonintegrating, multiple copies of the virus can be delivered, leading to high-level expression and secretion of the growth factor at a target location. Furthermore, the stability of the vector, particularly when combined with agents designed for uptake of DNA, such as polyamine–lipid compounds,5 provides a reliable method for transduction, even in cells lacking adenovirus receptor that can readily be validated to ensure adequate growth factor expression. Because adenovirus has a tropism for liver and lung, the ex vivo transduction of cells avoids off-target effects. Furthermore, when virus transduction is performed in vitro, the dosage of virus required is much lower than when used in vivo.
One growth factor that has been widely studied for its ability to enhance de novo bone formation at target locations is bone morphogenetic protein 2 (BMP2). Gene therapy approaches for delivery of BMP2 are able to accommodate the protein's extensive posttranslational modification6 as well as its short half-life.7 Although this morphogen is capable of rapidly inducing de novo bone formation, harnessing this capacity has been a major challenge in the field of bone tissue engineering. Recombinant human BMP2 (rhBMP2) protein in combination with a collagen sponge carrier, which is thought to provide slower release and longer life span for the protein, is still one of the most used products in orthopedic surgery. However, recent studies suggest that the high doses of protein are required for its efficacy and that inflammation is associated with the collagen sponge, indicate a need for significant improvement.8–10 The unreliable nature of rhBMP2 for inducing robust bone formation further suggests that its optimal delivery has not yet been achieved.
Cell-based gene therapy approaches have shown promise for BMP2 delivery, release, and reliability in rodent models, especially in mice. However, these approaches have been slow to translate into larger animal models. In many cases, they do not result in bone formation after the delivery,11 which has led to much criticism. It has been suggested that pre-existing immunity against adenovirus, even in the cell-based systems, is responsible for the silencing in large animals. Because no adenovirus with similar structure to human viruses has a natural tropism toward rodents, these animals have no pre-existing immunity against the virus. However, in larger animals, such as dogs, sheep, and nonhuman primates, adenoviruses, either species-specific or human, have a tropism that could lead to immunity against the standard adenovirus type 5 vectors. In addition, much evidence has already shown that pre-existing immunity against adenovirus is common and a major obstacle for therapies.12
Ex vivo cell-based strategies are thought to circumvent this problem by removing the use of free adenovirus by providing cells that have been transduced with replication-defective vectors. However, little is known about whether the transduced cells possess enough adenovirus proteins and DNA components to launch an immune response. To test this, mice were given an intramuscular injection of cells transduced with an E1- to E3-deleted adenovirus type 5 vector possessing no transgene (AdEmpty). A week later, mice received a similar adenovirus with the BMP2 transgene through intramuscular injection. Surprisingly, de novo bone formation was completely ablated, suggesting that prior immunity to the adenovirus-transduced cells could silence the bone-forming potential of the therapy. Further studies to determine if this immunity was directed against adenovirus revealed neutralizing antibodies against the virus circulating in the mice.
Nondegradable hydrogel poly(ethylene glycol) diacrylate (PEGDA) has been used to encapsulate cells that secrete small proteins and growth factors used in a variety of therapies.13,14 In many of these studies, PEGDA has proven to be crosslinked in a fashion to facilitate the diffusion of small molecules such as proteins and growth factors, while preventing cell to cell contact and immune recognition.13 Not surprisingly, when cells transduced with an adenovirus containing the transgene for BMP2 were encapsulated in PEGDA microspheres, bone formation could be restored due to immunoprotection.11 Data collectively suggest that pre-existing immunity against adenovirus can completely ablate the bone formation launched by AdBMP2-transduced cells and that this immunity can be overcome by encapsulation of the transduced cells.
Materials and Methods
Cell culture and transduction
Murine skin fibroblasts (C57BL/6) were propagated in modified essential medium (α-MEM) supplemented with 10% FBS (Hyclone Logan, UT), 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (Life Technologies, Inc., Gaithersburg, MD). Cells were grown at 37°C and 5% CO2 in humidified air. Replication defective, E1-E3-deleted first generation human type 5 adenovirus possessing cDNA for BMP2 (AdBMP2) or no transgene (AdEmpty) were constructed as previously described.15 The vector lacking a transgene cassette in the E1 region was used as a control (AdEmpty). The resultant purified viruses, Ad5BMP2 and Ad5Empty, had viral particle (VP)-to-plaque-forming unit (PFU) ratios of 21 and 36, respectively.
All viruses were confirmed to be negative for replication-competent adenovirus. Cells were transduced with AdBMP2 or the AdEmpty control virus at 5000 VP/cell with 0.75% GeneJammer (Agilent Technologies, Santa Clara, CA)5 with transduction efficiency of greater than 90%. Where indicated, transduced cells were microencapsulated in poly(ethylene glycol) diacrylate (PEGDA).16,17
PEGDA hydrogel microspheres
PEGDA was synthesized by reacting 10 kDa PEG with a twofold molar excess of acryloyl chloride as previously described.16,17 PEGDA was analyzed by gel permeation chromatography using an evaporative light scattering detector (Polymer Laboratories, Amherst, MA) and by 1H NMR (400 MHz; Varian, Palo Alto, CA). Hydrogel precursor solutions were prepared by combining 0.1 g/mL 10 kDa PEGDA (10% w/v) with 1.5% (v/v) triethanolamine/HEPES-buffered saline (pH 7.4), 37 mM 1-vinyl-2-pyrrolidinone, 0.1 mM eosin Y, 0.1% pluronic F68, and transduced cells for a final concentration of 5 × 106 cells/mL. A hydrophobic photoinitiator solution (2,2-dimethoxy-2-phenyl acetophenone in 1-vinyl-2-pyrrolidinone; 300 mg/mL) was combined in mineral oil (3 μL/mL, embryo tested, sterile filtered; Sigma-Aldrich; St. Louis, MO). The microspheres were formed after adding the hydrogel precursor solution into the mineral oil, emulsifying by vortexing for 2 s while exposing to white light for an additional 20 s. Microspheres were isolated through centrifugation at 330 g for 5 min.
Pre-exposure of mice
All animal studies were performed with Baylor College of Medicine Institutional Animal Care and Use Committee approval in accordance with the Office of Laboratory Animal Welfare guidelines. Animals, male and female, were randomly selected based on age and health and placed in experimental groups. Each animal was given a number that was linked only to its group in the medical record. Therefore, investigators involved in data collection and analysis were blinded. Animal sample sizes were based on historical power analysis data; however, all power analyses were repeated after data collection to confirm group sizes were adequate.
AdEmpty-transduced cells or cells that were not transduced were suspended at a concentration of 5 × 106 cells/100 μL of sterile pharmaceutical grade saline and delivered through intramuscular injection into the hindlimb hamstring muscles of C57BL/6 or NOD/SCID mice. A control group received 100 μL of sterile pharmaceutical grade saline. One week later, mice in all groups received a second injection of 5 × 106 AdBMP2-transduced cells/100 μL sterile pharmaceutical grade saline in the contralateral limb. Two weeks later, animals were euthanized and tissues were isolated and placed in formalin for microcomputed tomography (microCT) and histological analyses. Blood was also drawn at the time of euthanasia through cardiac puncture for serum marker studies.
Luciferase quantification of cell clearance
Mice, four per group, received an intramuscular injection of AdEmpty-transduced cells or 300 μL of sterile pharmaceutical grade saline, followed 1 week later by an injection of adenovirus encoding luciferase (AdLuc)-transduced cells in the opposite leg. Mice were then imaged for luciferase daily starting 1–3 days after the injection of the AdLuc-transduced cells. Mice were given an intraperitoneal injection of D-luciferin (150 mg/kg), 10 min before their imaging using the IVIS 100 (Xenogen, Alameda, CA). Mice were imaged in three positions (face down, face up, and side) and all images quantified. Fluorescence intensity (radiance measured as p/sec/cm2/sr) of each 2 min exposure was computed for each image using Living Image software (Xenogen) and summed to determine the total fluorescence intensity for each mouse. Statistical significance was determined using a student's t-test.
Microcomputed tomography
MicroCT of whole hindlimb specimens was performed at 7 μm resolution (SkyScan 1174; Micro Photonics, Inc., Allentown, PA). A pair of hydroxyapatite phantoms with mineral densities of 0.25 and 0.75 g/cm3 was scanned to convert scan data from arbitrary units to units of equivalent bone density. A three-dimensional (3D) region of interest (ROI) was defined for each specimen. Specialized software (CTAn; Micro Photonics, Inc.) was used to analyze microCT images to determine bone volume and mineral density. Thresholds excluded tissue with a density less than 0.1 g/cm3. The tissue volume within ROIs was calculated as a measure of the total bone volume. Tissue mineral content or the total mineral in the region, and tissue density were calculated to quantify density of the mineralized tissue. The 3D microCT reconstructions were created using Mimics and Magics software (Materialise, Leuven, Belgium). All quantitative data were analyzed using an analysis of variance (ANOVA) with Tukey's post hoc test for multiple comparisons with alpha set at 0.05 (SPSS 20; IBM, Armonk, NY).
Histology
Mouse hindlimbs were formalin fixed and decalcified before processing. Paraffin-embedded serial sections were then stained with Hematoxylin and Eosin for the presence of bone.15
Statistical analysis
All data were reported as mean ± standard deviation. Statistical analysis included a one-way ANOVA with Tukey–Kramer's post hoc test at a significance level of p ≤ 0.05.
Results
In vivo assessment of BMP2-induced bone formation after inoculation with AdEmpty-transduced cells or cells alone
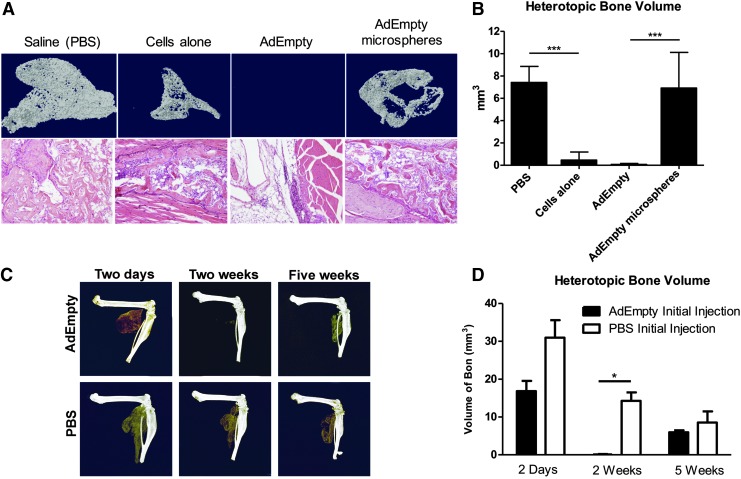
To test whether pre-exposure to adenovirus-transduced cells could impact bone formation, mice were first given an intramuscular injection of AdEmpty-transduced cells, cells alone, or vehicle (saline) into the hindlimb muscle. One week later, the mice were given an intramuscular injection of AdBMP2-transduced cells in the contralateral limb. Bone formation progressed as expected15 in the group receiving saline, but was absent in the group that had previously been exposed to the adenovirus-transduced cells (Fig. 1A, B). Representative histological sections were analyzed to confirm that no bone was present in the group receiving AdEmpty-transduced cells (Fig. 1A). Additionally, mice receiving an injection of the cells alone 1 week before the AdBMP2-transduced cell delivery also exhibited significant suppression of BMP2-induced bone formation; however, upon histological analysis and quantification by microCT, there was significant bone formation present in the limb (Fig. 1B, D).
FIG. 1.
Radiographical analysis of BMP2-induced bone formation quantified 2 weeks after delivery of AdBMP2-transduced cells in C57BL/6 mice that have been previously exposed to AdEmpty-transduced cells, cells alone, or vehicle (saline). (A) Three-dimensional reconstructions of the microCT images of heterotopic bone in mouse hindlimb with corresponding representative Hematoxylin and Eosin-stained photomicrographs. (B) Quantification of bone volume from microCT analysis. The graph depicts sample (n = 3) averages and error bars of SEM. ANOVA with Bonferroni post hoc test was performed. *** indicates statistically significant difference (p ≤ 0.0015). (C) Three-dimensional microCT reconstruction of heterotopic bone formed 2 weeks after delivery of AdBMP2-transduced cells in C57BL/6 mice that have been exposed to AdEmpty-transduced cells or vehicle (saline) 2 days, 2 weeks, and 5 weeks prior. (D) Quantification of bone volume from microCT analysis. The graph depicts sample (n = 3) averages and SEM. * indicates statistically significant difference (p ≤ 0.01). ANOVA, analysis of variance; microCT, microcomputed tomography; SEM, standard error of the mean.
Previous studies demonstrated the ability to encapsulate the adenovirus-transduced cells in a nondegradable PEGDA hydrogel photopolymerized into injectable microspheres.17 These hydrogels prevent direct contact of immune cells in the external cellular environment with the adenovirus-transduced cells, but permit free diffusion of growth factors and cytokines. To determine if such blocking would prevent the suppression of bone formation, AdEmpty-transduced cells were encapsulated in the PEGDA hydrogel microspheres and injected into the mouse hindlimb. One week later, mice received an injection of AdBMP2-transduced cells in the contralateral limb. Surprisingly, bone formation progressed similarly to the animal receiving saline, and no statistically significant suppression was observed between these two groups (Fig. 1A, B).
To determine if the silencing of heterotopic ossification would persist long term, mice received the intramuscular injection of the AdEmpty-transduced cells, and then 2 days, 2 weeks, or 5 weeks later, mice received a second injection of AdBMP2-transduced cells in the contralateral limb. The resultant bone formation was analyzed 2 weeks after delivery of the AdBMP2-transduced cells (Fig. 1C). The group that received adenovirus-transduced cells 2 days before the second injection made a significant amount of bone, similar to what is observed when animals are not preexposed (Fig. 1C); whereas, the samples at 2 weeks consistently showed no bone formation (Fig. 1C). The samples at 5 weeks, however, appeared to have similar amounts of bone formation as the ones taken at 2 days (Fig. 1C, D).
Ablation of bone formation does not result from rapid removal of the adenotransduced cells
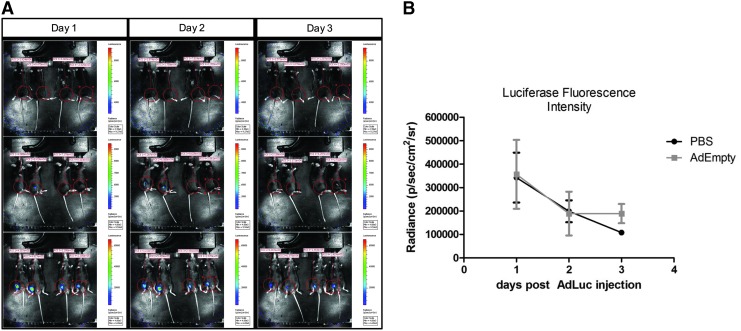
To determine if the prior exposure of the mice to adenotransduced cells was leading to a more rapid clearance of the cells, mice received either AdEmpty-transduced cells, cells alone, or saline; the mice were then injected with AdLuc-transduced cells in the contralateral limb 1 week later. These mice were then imaged daily starting 24 h after the delivery of the AdLuc-transduced cells and continuing daily for a total of 3 days, at a point when the luciferase signal was disappearing from both groups. To ensure that the total signal was collected in all mice, they were imaged on three planes (Fig. 2A), and the positive signal from each mouse totaled for comparisons between groups (Fig. 2B). Comparison of luciferase expression in mice receiving the AdEmpty-transduced cells versus vehicle showed no statistical difference (Fig. 2B).
FIG. 2.
(A) Representative photos of luciferase expression in mice in three planes. Mice received an initial intramuscular injection of AdEmpty-transduced cells or vehicle (saline) in one hindlimb and an intramuscular injection of AdLuc-transduced cells in the contralateral limb 1 week later. Expression was quantified and summed to provide a total of luciferase expression for each animal. (B) The graph depicts sample (n = 4) averages and SEM. Comparisons were made between groups using an ANOVA with Bonferroni post hoc test, and found that data were not significantly different (p ≤ 0.05).
The time frame was selected based on previous experiments with this model that showed the adenovirus-transduced cells were rapidly cleared within 4–5 days.18,19 Data suggest that clearance of the cells follows similar kinetics regardless of whether the mice are pre-exposed to the adenovirus-transduced cells.
B and T cells are responsible for the ablation of bone formation
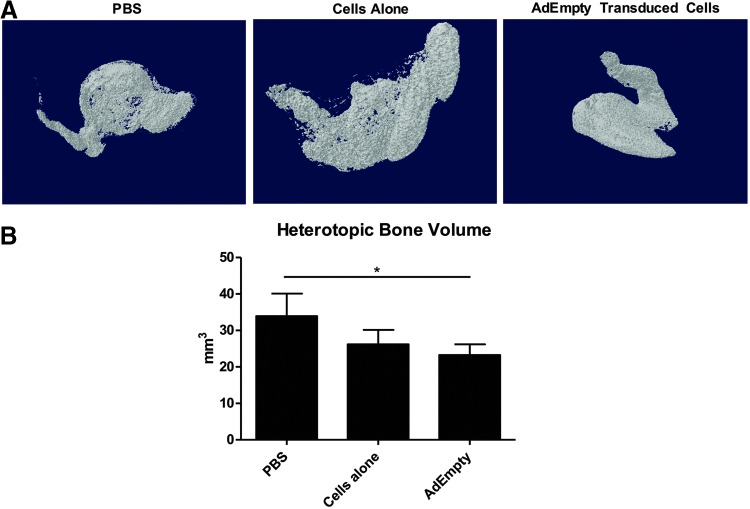
To determine if immunological priming contributes to the suppression of BMP2-induced bone formation, experiments were repeated in NOD/SCID mice, which lack mature B and T cells, but still possess NK, dendritic, and other myeloid cells. Accordingly, NOD-SCID mice were initially injected with AdEmpty-transduced cells, cells alone, or vehicle, and then 1 week later subjected to a second intramuscular injection of AdBMP2-transduced cells in the contralateral limb. The results show that mice pre-exposed to AdEmpty-transduced cells formed bone, which was unlike the ablation of bone formation in wild-type mice (Fig. 3A, B).
FIG. 3.
(A) Three-dimensional microCT reconstructions of heterotopic bone formation measured 2 weeks after delivery of AdBMP2-transduced cells in NOD/SCID mice that had been pre-exposed 1 week before AdEmpty-transduced cells, cells alone, or vehicle (saline). (B) Graph depicts sample (n = 4) averages and SEM. Comparisons were made between groups using an ANOVA with Bonferroni post hoc test, and * indicates statistically significant difference (p ≤ 0.05).
However, there was a small but significant decrease in bone formation, suggesting that the immune mechanisms remaining in these immunodeficient mice may also play a role (Fig. 3B). Furthermore, resultant bone formation in mice previously exposed to the cells alone appeared to be slightly suppressed, although not statistically significant. Data suggest that B and T cells play a functional role in the ablation of bone formation in pre-exposed mice.
Detection of neutralizing antibodies against adenovirus after intramuscular injection of AdEmpty-transduced cells
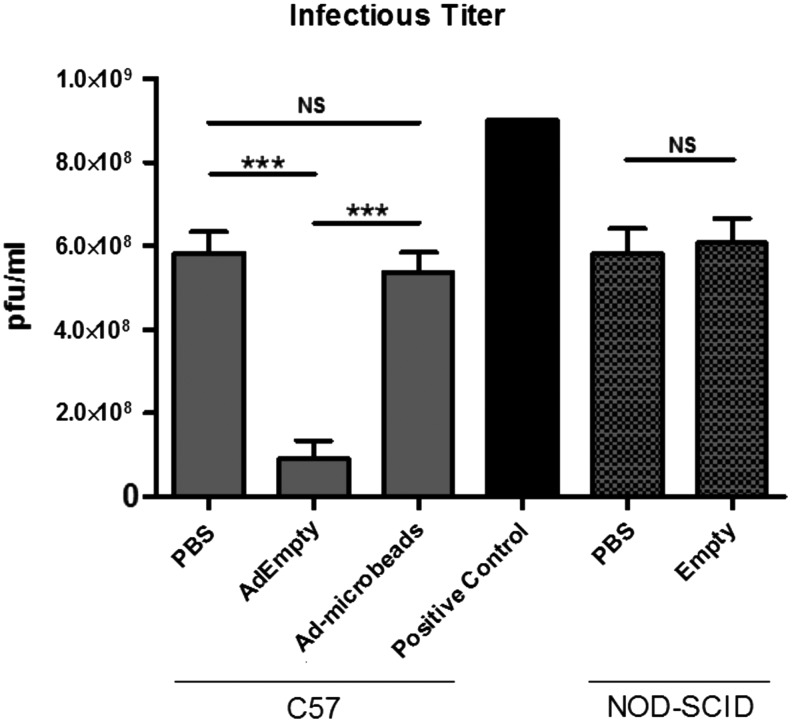
It is well documented that adenovirus infection leads to long-term expression of neutralizing antibodies against the virus.20 To determine if the mice are generating neutralizing antibodies against adenovirus through exposure to adenovirus-transduced cells, mice were injected with AdEmpty-transduced cells or vehicle, and 1 week later serum was isolated for detection of antibodies that could block adenovirus infection. In these experiments, serum was mixed with a known concentration of virus prior adsorption of the mixture to cells to allow time for antibodies in the serum to bind the virus and block or neutralize the ability of the virus to infect 293 cells. Virus PFUs were then measured, calculated, and compared (Fig. 4). Titers were significantly lower in all cases where mouse serum was added to the virus as compared with the addition of saline.
FIG. 4.
Quantification of neutralizing antibodies against adenovirus in serum of mice pre-exposed to adenovirus-transduced cells, cells alone, or vehicle (saline). Mouse serum was collected and tested for the presence of neutralizing antibodies against adenovirus by exposure of the adenovirus to the serum before determining the titer. Reduction in the titer correlates with increased level of antibodies in the serum. Sample (n = 3) averages and SEM are depicted. *** indicates statistically significant difference (p ≤ 0.0005). NS indicates no statistical significance.
Furthermore, virus titers in the presence of the serum of mice receiving an intramuscular injection of AdEmpty-transduced cells were significantly lower than the other two groups (Fig. 4). Encapsulation of the Ad-transduced cells in PEGDA hydrogel was able to block the production of neutralizing antibodies. The serum isolated from NOD/SCID mice, whether previously exposed to the AdEmpty-transduced cells or vehicle, did not reduce the adenovirus titer, supporting the conclusion that these mice were not able to generate antibodies against adenovirus.
To confirm that the PEGDA hydrogel encapsulation of the AdEmpty-transduced cells prevents activation of adaptive immunity, serum was isolated from these animals and compared with animals who had received saline. The results show no significant difference in virus titer (Fig. 4). Data collectively suggest that PEGDA hydrogel can prevent activation of adaptive immunity, either in naive animals or those immunologically primed to react to the adenovirus-transduced cells.
PEGDA hydrogel can prevent activation of adaptive immunity and secondary response against the adenovirus-transduced cells
Previous studies have shown that encapsulation of the AdBMP2-transduced cells does not affect the free diffusion of BMP2 into the local environment.17 To determine whether the hydrogel could rescue the bone formation in mice previously exposed to the adenovirus-transduced cells, mice received an intramuscular injection of AdEmpty-transduced cells and 1 week later received a second injection in the contralateral limb of AdBMP2-tranduced cells or similar cells encapsulated in the PEGDA hydrogel.
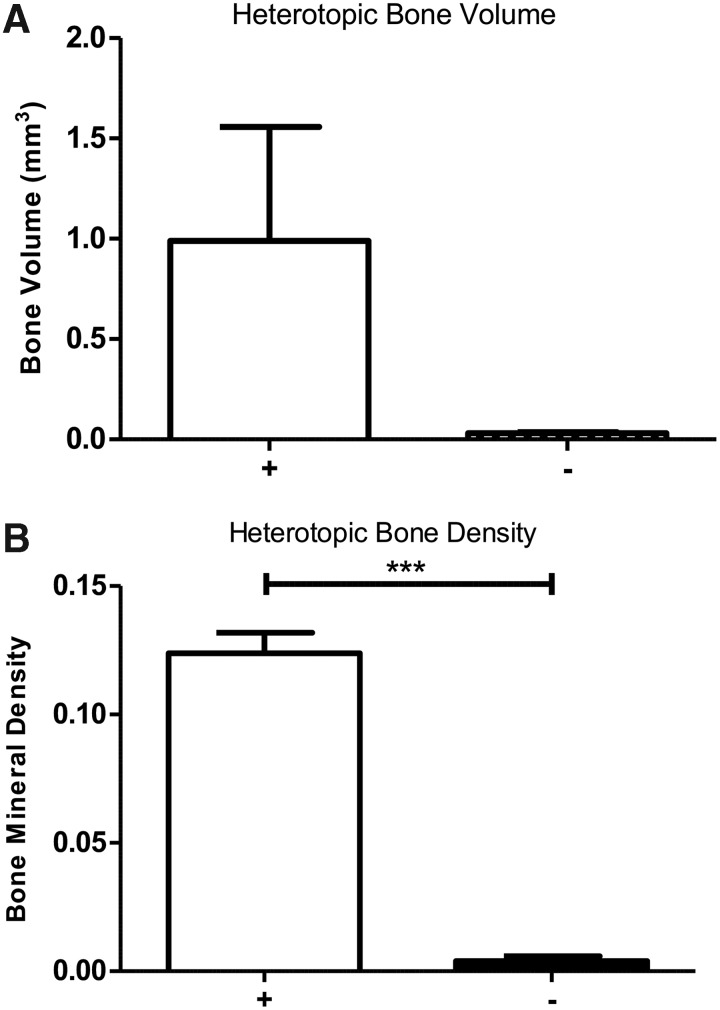
The results show that bone formation was rescued by encapsulation of the cells (Fig. 5A, B). In all cases PEGDA hydrogel encapsulation of the cells led to bone formation (+), whereas no bone was observed in the group that received the cells without encapsulation (−) (Fig. 5A, B).
FIG. 5.
Radiographical analysis of BMP2-induced bone formation through delivery of AdBMP2-transduced cells either encapsulated in PEGDA hydrogel microspheres (+) or directly injected (−) in wild-type mice that have been previously exposed to AdEmpty-transduced cells. (A) Bone volume was quantified and sample (n = 3) averages and SEM depicted in the graph. (B) Bone density was quantified and sample (n = 3) averages and SEM depicted in the graph. *** indicates statistically significant difference (p ≤ 0.00005).
Discussion
The use of growth factors, such as BMP2s, is essential in tissue engineering and/or regeneration. However, delivery of such growth factors through cells transduced with adenoviral vectors has proven to be inefficient to obtain maximum or optimal delivery of the growth factors. When cells transduced with BMP2 are injected into larger animals, no bone formed, suggesting that the adenovirus-transduced cells can lead to a significant inflammatory response, which then blocks the ability of BMP2 to launch bone formation.21 The goal of this study was to characterize the immune response against the transduced cells after immunological priming through pre-exposure of the mice to AdEmpty-transduced cells and to determine its effects on BMP2-induced bone formation. A further goal was to determine if the immunological response can be suppressed by encapsulating the cells in a PEGDA hydrogel.
This study encompassed several features of the adaptive immune response to cells transduced with BMP2 1 week after injection of cells transduced with AdEmpty. The studies showed that a circulating factor could suppress bone formation if the mouse was exposed to the adenovirus-transduced cells at least 1 week before the delivery of AdBMP2-transduced cells. Studies tracking the cells using a luciferase reporter showed that the cells were cleared similarly in the animals, regardless of pre-exposure to the adenovirus-transduced cells. Data suggest that the mechanism may be more complex than simple induction of factors (e.g., cytotoxic cells) leading to clearance of the cells and early termination of BMP2 secretion.
Additionally, the suppression of BMP2-induced bone formation required time after pre-exposure to launch the effect. Alternatively, the effect waned after 2 weeks, and bone formation progressed normally at 5 weeks. Finally, experiments in NOD/SCID mice that lack B and T cells, but possess NK and other cells involved in innate immunity, showed a small but significant drop in bone formation through pre-exposure. However, unlike the wild-type mice, pre-exposure to the adenovirus-transduced cells still resulted in significant bone formation, suggesting that the activation of adaptive immunity alone could lead to ablation of BMP2-induced bone formation.
Although data suggest a role for adaptive immunity, changes in innate immunity could still be directly responsible for the effect. Cytotoxic T lymphocytes express many factors that can regulate innate immunity. For instance, expression of Wnts that are key factors in bone metabolism22 has recently been shown to negatively regulate innate immune response.23
A similar suppression of bone formation was observed when mice were exposed to the cells only. Surprisingly, cells, which were isolated from the specific strain of genetically inbred mice, led to a suppression of bone formation, although less severe since some bone formation was able to occur in these animals. This suggests that even slight modifications in immunogenicity will launch an adaptive immune response sufficient to abrogate bone formation
Humoral immunity is a component of adaptive immunity. The humoral immune response carried out by induction of antibodies by B cells has long been known to produce adenovirus-neutralizing antibodies. This is the basis of viral vaccines, including a vaccine for adenovirus.24 Although, it is well known that viruses induce such immunity possessed by individuals vaccinated with either live-attenuated virus, killed virus, or certain viral components can block both infection and disease through this process, the ability of virus-infected cells to generate such immunity is less well studied, especially if virus replication in the infected cells is limited.
In these studies, the process of priming the animal immune system was confirmed by directly measuring neutralizing antibody titers against adenovirus in the circulation (i.e., serum). The laboratory strains of mice do not possess antibodies against the human adenovirus type 5; however, upon exposure to virus-transduced cells, they raised antibodies that could directly neutralize the virus. Encapsulation of the AdEmpty-transduced cells in the PEGDA hydrogel did not result in the production of neutralizing antibodies when injected into the mouse, supporting the idea that encapsulation blocks activation of humoral immunity. These findings are interesting because the adenovirus vector used was replication-defective, and even though it is a first-generation adenoviral vector, its ability to replicate inside the cell was limited. Since the transduction is done ex vivo, and the cells are extensively washed, there should be no virus particles delivered to the animals.
Data suggest that encapsulation of the adenovirus-transduced cells in PEGDA hydrogel can prevent an adaptive immune response. Furthermore, data support that in the presence of this response, inclusion of the PEGDA hydrogel was able to rescue the bone formation. The crosslinked PEGDA provides relatively unimpeded diffusion of smaller proteins, such as growth factors, but is impermeable to cells and has very low permeability to large proteins like antibodies.17,25 Previous studies have shown that hydrogels enhance therapeutic delivery by allowing for a controlled release of small molecules to a localized target area.26 Various types of hydrogels allowing for increased localization to areas of inflammation or injury are being tested in models of colitis, nerve regeneration in facial tissue, and many other medical applications.27,28
However, another key function of the hydrogel used in these studies is preventing the immune system from recognizing and targeting a cell being used in a therapy for delivery of an important protein. Previous studies have developed a therapy for type one diabetes mellitus that incorporates PEGDA hydrogel to act as a barrier to autoimmunity by encapsulating insulin-producing beta cells. These studies have shown the success of PEGDA to immunoprotect the pancreatic beta islet cells from autoimmunity while also allowing for insulin produced by the cells to diffuse into the circulation.29
The studies presented here show the ability of this biomaterial to both prevent immunological detection of the foreign cells and virus and also block immunological priming. Surprisingly, even in animals that are immunologically primed, the inability of the external environment to access the cells allows them to continue to express the growth factors and induce bone formation. This finding is critical to the success of these cellular therapies since humans normally possess antibodies to adenovirus, and data suggest that even cells genetically matched to the recipient resulted in launching adaptive immunity. The results demonstrating the ability of PEGDA hydrogel to prevent immune reactivity and clearance or premature termination of the cells/growth factor are a significant finding and could potentially be the first step toward being able to implement many cell and gene therapy approaches.
Acknowledgments
This research was funded by the Department of Defense contracts DAMD W81XWH-12-1-0475 and W81XWH-13-1-0286. The authors would also like to thank Rita Nistal for her assistance in generating all the tissue sections required for histological analysis.
Disclosure Statement
No competing financial interests exist.
References
- 1.Miest T.S., and Cattaneo R. New viruses for cancer therapy: meeting clinical needs. Nat Rev Microbiol 12, 23, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., and Gao G. State-of-the-art human gene therapy: part I. Gene delivery technologies. Discov Med 18, 67, 2014 [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., and Gao G. State-of-the-art human gene therapy: part II. Gene therapy strategies and clinical applications. Discov Med 18, 151, 2014 [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K., Silva E.A., and Mooney D.J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 8, 153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouletier-Dilling C.M., Bosch P., Davis A.R., Shafer J.A., Stice S.L., Gugala Z., Gannon F.H., and Olmsted-Davis E.A. Novel compound enables high-level adenovirus transduction in the absence of an adenovirus-specific receptor. Hum Gene Ther 16, 1287, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Zhao B., Katagiri T., Toyoda H., Takada T., Yanai T., Fukuda T., Chung U.I., Koike T., Takaoka K., and Kamijo R. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem 281, 23246, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Shimmi O., and Newfeld S.J. New insights into extracellular and post-translational regulation of TGF-beta family signalling pathways. J Biochem 154, 11, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salisbury E.A., Olmsted-Davis E.A., and Davis A.R. Adverse events and bone morphogenetic protein-2. Spine J 11, 802, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Carragee E.J., Hurwitz E.L., and Weiner B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 11, 471, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Carragee E.J., Chu G., Rohatgi R., Hurwitz E.L., Weiner B.K., Yoon S.T., Comer G., and Kopjar B. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am 95, 1537, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Davis E.L., Sonnet C., Lazard Z.W., Henslee G., Gugala Z., Salisbury E.A., Strecker E.V., Davis T.A., Forsberg J.A., Davis A.R., and Olmsted-Davis E.A. Location-dependent heterotopic ossification in the rat model: the role of activated matrix metalloproteinase 9. J Orthop Res 34, 1894, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Gordo E., Podgorski I.I., Downes N., and Alemany R. Circumventing antivector immunity: potential use of nonhuman adenoviral vectors. Hum Gene Ther 25, 285, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCall J.D., Lin C.C., and Anseth K.S. Affinity peptides protect transforming growth factor beta during encapsulation in poly(ethylene glycol) hydrogels. Biomacromolecules 12, 1051, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruise G.M., Hegre O.D., Lamberti F.V., Hager S.R., Hill R., Scharp D.S., and Hubbell J.A. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant 8, 293, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Olmsted-Davis E.A., Gugala Z., Gannon F.H., Yotnda P., McAlhany R.E., Lindsey R.W., and Davis A.R. Use of a chimeric adenovirus vector enhances BMP2 production and bone formation. Hum Gene Ther 13, 1337, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Olabisi R.M., Lazard Z., Heggeness M.H., Moran K.M., Hipp J.A., Dewan A.K., Davis A.R., West J.L., and Olmsted-Davis E.A. An injectable method for noninvasive spine fusion. Spine J 11, 545, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olabisi R.M., Lazard Z.W., Franco C.L., Hall M.A., Kwon S.K., Sevick-Muraca E.M., Hipp J.A., Davis A.R., Olmsted-Davis E.A., and West J.L. Hydrogel microsphere encapsulation of a cell-based gene therapy system increases cell survival of injected cells, transgene expression, and bone volume in a model of heterotopic ossification. Tissue Eng Part A 16, 3727, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dilling C.F., Wada A.M., Lazard Z.W., Salisbury E.A., Gannon F.H., Vadakkan T.J., Gao L., Hirschi K., Dickinson M.E., Davis A.R., and Olmsted-Davis E.A. Vessel formation is induced prior to the appearance of cartilage in BMP-2-mediated heterotopic ossification. J Bone Miner Res 25, 1147, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gugala Z., Olmsted-Davis E.A., Gannon F.H., Lindsey R.W., and Davis A.R. Osteoinduction by ex vivo adenovirus-mediated BMP2 delivery is independent of cell type. Gene Ther 10, 1289, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol 62, 2321, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen J., James A.W., Zara J.N., Asatrian G., Khadarian K., Zhang J.B., Ho S., Kim H.J., Ting K., and Soo C. BMP2-induced inflammation can be suppressed by the osteoinductive growth factor NELL-1. Tissue Eng Part A 19, 2390, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan S.H., Senarath-Yapa K., Chung M.T., Longaker M.T., Wu J.Y., and Nusse R. Wnts produced by Osterix-expressing osteolineage cells regulate their proliferation and differentiation. Proc Natl Acad Sci U S A 111, E5262, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baril M., Es-Saad S., Chatel-Chaix L., Fink K., Pham T., Raymond V.A., Audette K., Guenier A.S., Duchaine J., Servant M., Bilodeau M., Cohen E., Grandvaux N., and Lamarre D. Genome-wide RNAi screen reveals a new role of a WNT/CTNNB1 signaling pathway as negative regulator of virus-induced innate immune responses. PLoS Pathog 9, e1003416, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paris R., Kuschner R.A., Binn L., Thomas S.J., Colloca S., Nicosia A., Cortese R., Bailer R.T., Sullivan N., and Koup R.A. Adenovirus type 4 and 7 vaccination or adenovirus type 4 respiratory infection elicits minimal cross-reactive antibody responses to nonhuman adenovirus vaccine vectors. Clin Vaccine Immunol 21, 783, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweller R.M., and West J.L. Encoding hydrogel mechanics via network cross-linking structure. ACS Biomater Sci Eng 1, 335, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao W., Zhang Y., Zhang Q., and Zhang L. Nanoparticle-hydrogel: a hybrid biomaterial system for localized drug delivery. Ann Biomed Eng 44, 2049, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S., Ermann J., Succi M.D., Zhou A., Hamilton M.J., Cao B., Korzenik J.R., Glickman J.N., Vemula P.K., Glimcher L.H., Traverso G., Langer R., and Karp J.M. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci Transl Med 7, 300ra128, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao X., Xu L., Li J., Han Y., Li X., Mao Y., Shang H., Fan Z., and Wang H. Facilitation of facial nerve regeneration using chitosan-beta-glycerophosphate-nerve growth factor hydrogel. Acta Otolaryngol 136, 585, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Lin C.C., and Anseth K.S. Glucagon-like peptide-1 functionalized PEG hydrogels promote survival and function of encapsulated pancreatic beta-cells. Biomacromolecules 10, 2460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]