Abstract
We previously reported that traumatic brain injuries (TBI) alter the cerebrovasculature near the injury site in rats, followed by revascularization over a 2-week period. Here, we tested our hypothesis that male and female adult mice have differential cerebrovascular responses following a moderate controlled cortical impact (CCI). Using in vivo magnetic resonance imaging (MRI), a new technique called vessel painting, and immunohistochemistry, we found no differences between males and females in lesion volume, neurodegeneration, blood–brain barrier (BBB) alteration, and microglia activation. However, females exhibited more astrocytic hypertrophy and heme-oxygenase-1 (HO-1) induction at 1 day post-injury (dpi), whereas males presented with increased endothelial activation and expression of β-catenin, shown to be involved in angiogenesis. At 7 dpi, we observed an increase in the number of vessels and an enhancement in vessel complexity in the injured cortex of males compared with females. Cerebrovasculature recovers differently after CCI, suggesting biological sex should be considered when designing new therapeutic agents.
Keywords: : controlled cortical impact, heme oxygenase-1, sex difference, vasculature, Wnt/β-catenin
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity, affecting 1.7 million people and representing a third of all injury-related deaths in the United States.1 TBI can lead to long-term disability including chronic suffering, as well as cognitive and behavioral decrements.2 Civilians, military personnel, and athletes all have reported long-term effects as a result of TBI, including concussions. However, most studies in neuroscience,3 or TBI research4 have been conducted in males, with little attention paid to differences between sex.
Biological sex differences in the epidemiology of TBI have been reported. For example, female athletes have a higher likelihood of getting a concussion than male athletes,5,6 and TBI resulted in 4 times more deaths among males than females in a 10-year study.7 However, little is known about the effect of biological sex on injury mechanisms, particularly in TBI. Neuroanatomical, neurochemical, and developmental differences between sexes have been described previously and strongly suggest that the response of the brain after an injury is under the influence of these differences.4,8,9 Sex differences in stroke have been more extensively studied and results show sex-steroid hormone dependent and independent differences in the pathophysiological mechanisms and in the response to treatment.10 Thus, there are sex-related differences in how the brain responds to injury, but lack of conclusive findings with regard to TBI prompted the current investigation.
A critical feature in the response and repair of brain injury is the cerebral vasculature, which is responsible for supporting tissue metabolism and edema resolution, as well as clearance of debris. We recently described alteration of the phenotype of middle cerebral artery smooth muscle cells in a model of intracerebral hemorrhage in rats.11 Emerging evidence is beginning to document that the cerebral vascular network is also modified after TBI, leading to functional deficits.12–15 The vast majority of studies that have reported cerebrovascular dysfunction after TBI have been in males with acute alterations in cerebral blood flow (CBF), autoregulatory impairment, blood–brain barrier (BBB) dysfunction in conjunction with potential edema formation, and hemorrhage.16–19 Clinical and experimental studies have shown acute morphological alterations of the vasculature in human autopsy20 and in rodent studies, decreased vessel density with gross morphological abnormalities in the vessels.21,22 However, our own recent work, as well as several other studies, have suggested that there is likely repair of the vasculature albeit at sub-acute time-points.15,23 In a rat acceleration impact model, Morgan and collaborators provided evidence of angio- and vasculogenesis 48 h after injury.24 Similarly, in a fluid percussion injury model in rats, Park and collaborators showed a recovery of the cortical microvascular coverage after 2 weeks, but which was remarkably altered.21 Hayward and colleagues also used a fluid percussion injury in rats and demonstrated early loss of peri-lesional capillaries, which was followed by repair at 2 weeks.25 In a recent rat study, we reported acute global changes in cerebral vessels after a controlled cortical impact (CCI) with dramatic loss of vessel density and complexity in the vascular network.14 Interestingly, in the same model we have now observed that vascular injury is followed by evidence of repair at 7 days with apparent return of vascular density at 2 weeks.15,23
However, questions remain on how sex modulates the vascular response to TBI and its consequences at a morphological level. In the current study, we tested our hypothesis that male and female adult mice have differential cerebrovascular responses following a moderate CCI. Using multiple outcome measures, including in vivo magnetic resonance imaging (MRI), vessel painting,14,26 and immunostaining we examined male/female differences in revascularization, inflammation, and neurodegeneration over the first 7 days post-injury (dpi).
Methods
Animals
All animal experiments and care complied with federal regulations and were approved by the Loma Linda University Animal Health and Safety Committee. Male (n = 38) and female (n = 37) C57BL6J mice (RRID:IMSR_JAX:000664, 6–7 months old, 20–30 g; Jackson Laboratories) were housed in a temperature-controlled animal facility on a 12-h light/dark cycle with free access to food and water. Animals were randomly assigned to two groups: Sham or TBI. All Sham animals were euthanized at 1 dpi (n = 14 Sham males, n = 16 Sham females). TBI animals were euthanized at either 1 dpi (n = 12 TBI 1d males, n = 11 TBI 1d females) or 7 dpi (n = 12 TBI 7d males, n = 10 TBI 7d females). Females were visually checked on surgery day to determine if they were in estrous. All animals were included for behavior, MRI analysis, and vessel painting procedures (∼12 per group). For immunohistochemistry, 4–5 animals per group were used.
Before surgery, female mice weighed 23.29 ± 0.23 g and males weighed 29.15 ± 0.22 g. Before perfusion, female mice weighed 22.45 ± 0.22 g, whereas male mice weighed 28.08 ± 0.24 g. On average, males and females lost the same percentage of body mass after surgery (3.6% for females and 3.8% for males).
Controlled cortical impact
Mice were anesthetized with isoflurane (3% induction, 1–2% maintenance) and placed in a stereotaxic frame to secure the head. Body temperature was maintained at 37 ± 1°C with a heating pad during the surgery. A midline incision of the skin was made to expose the skull. A 5-mm craniotomy was carefully performed on the right side between Bregma and lambda to expose the cortex. A moderate CCI was delivered using an electromagnetically driven piston (Leica Microsystems Company, Richmond, IL) with the following parameters: 1.5-mm depth, 3-mm diameter, 2.0 m/sec speed, 200 msec dwell time. The skin was sutured and buprenorphine (0.01 mg/kg, intramuscular) was administered after surgery to minimize pain. Sham animals underwent the entire procedure except for the impact. TBI animals were either sacrificed 1 or 7 dpi. Sham animals were sacrificed at 1 dpi.
Open field testing
General exploratory behavior, activity, and anxiety levels were evaluated using open field testing. Tests were carried out within the 12-h light cycle and experimenters were blinded to the treatment groups. Mice were placed in a 30 cm × 30 cm opaque open-top box and allowed to explore, unrestricted for 30 min. Movements for each mouse were recorded and tracked by EthoVision software (EthoVsion XT 11.0; Noldus Information Technology, Inc., Leesburg, VA). Total distance traveled, and time in each open field area (center vs. periphery) were assessed as a measure of overall activity and anxiety. Anxiety assessment is based on conflicting innate tendencies by mice to avoid bright lights and open spaces against that of exploring their environment. More exploration of the center area suggests less anxiety-like behavior, whereas more time in the periphery in the darker area suggests more anxiety-like behavior.
Magnetic resonance imaging and lesion analysis
All animals were imaged at 1 dpi (1 and 7 dpi for the 7-day groups). Mice were anesthetized with isoflurane (3% induction, 1% maintenance) and placed in the coil. In vivo T2-weighted images (T2WI; repetition time [TR]/echo time [TE] = 2395.5 msec/10 msec, 20 × 1-mm slices) and susceptibility-weighted images (SWI; TR/TE = 617.7 msec/7 msec, 40 × 0.5-mm slices) were collected with a 256 × 256 matrix on a 11.7T Bruker Avance Instrument (Bruker Biospin, Billerica, MA).
Lesion volume (hemorrhage and edema) were determined by personnel blinded to the groups. Edema volumes were identified by using the Hierarchical Regional Splitting (HRS) computational method (U.S. patent: 8731261; European patent: 11748009.5-1265), based on T2 relaxation times.27,28 T2 value ranges for hemorrhage, edema, and normal-appearing brain matter (NABM)-containing pixels were determined by taking the average T2 value of manually segregated NABM, hemorrhage, and edema pixels in 16 random animals (8 males and 8 females) where a T2 value of >57 msec was considered edema. Hemorrhage volumes were determined by using the Cheshire image processing software (version 4.3, Parexel, Waltham, MA). After confirming presence of hemorrhage with T2 relaxation times (range: 35–45 msec) and on corresponding SWI scans. The regions of interest (ROIs) were drawn on T2 scans. Hemorrhage and edema analysis was performed on brain slices between bregma 3 mm and bregma −4 mm.
Perfusion and vessel painting procedure
This technique is based on the ability of the fluorescent dye 1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate (DiI; Life Technologies, Carlsbad, CA) to bind to lipid membranes. The protocol used in our study has been modified from previous studies.14,26 At 1 or 7 dpi, immediately after MRI, mice were anesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). Vessel painting was performed by injecting a solution of DiI (0.3mg/mL in phosphate buffered saline [PBS] containing 4% dextrose, total volume 500 μL) in the left ventricle, followed by a 10 mL PBS flush and a 20 mL 4% paraformaldehyde (PFA) perfusion, using a peristaltic pump (8.4 mL/min). Brains were extracted and post-fixed in 4% PFA for 24 h. Brains were then washed and stored in PBS at 4°C until imaging. Successfully vessel painted brains were selected by an investigator blinded to sex and group and were selected if they showed uniform pink staining and excellent staining of large and small vessels on the cortical surface. In this study, 60% (45/75) of the brains were successfully stained and analyzed (n = 7 Sham males, n = 8 Sham females, n = 9 TBI 1d males, n = 7 TBI 1d females, n = 8 TBI 7d males, n = 6 TBI 7d females).
Imaging and analysis of vessel painted brains
Brains were imaged using a fluorescence microscope (Keyence BZ-X700; Keyence Corp., Osaka, Japan). Images of the entire brains (axial view) were acquired using the 2 × magnification and reconstructed using the XY-stitching and Z-stack features.
Classical vessel analysis was performed by using the AngioTool software developed by Zudaire and colleagues.29 It included measures of vessel density and number of junctions. Then, fractal geometry was used to describe the complexity of the cerebral vasculature. The ImageJ plug-in FracLac allowed for the fractal analysis of vascular complexity.14,30
Brain sectioning and immunohistochemistry
After imaging, brains (n = 4–5 per group) were embedded and freeze-sectioned at 30 μm in the coronal plane. All sections were collected in Antigen Preserve Solution (NeuroScience Associates, Knoxville, TN).
For immunostaining, tissue sections were first pre-treated with 1% sodium dodecyl sulfate for 10 min. They were then blocked with 2% bovine serum albumin (BSA; Sigma Aldrich, St. Louis, MO) solution for 1.5 h. Primary antibodies used are as follows: anti-β-catenin (1:100, Abcam Cat# ab6302 RRID:AB_305407, Cambridge, MA), anti-Von Willebrand Factor (vWF; 1:200, Abcam Cat# ab6994 RRID:AB_305689), anti-Ki67 (1:500, Abcam Cat# ab15580 RRID:AB_443209), anti-glial fibrillary acidic protein (GFAP; 1:500, Millipore Cat# MAB3402 RRID:AB_94844, Billerica, MA), anti-ionized calcium-binding adapter molecule 1 (IBA1; 1:400, Wako Cat# 019-19741 RRID:AB_839504, Richmond, VA), anti-heme-oxygenase 1 (HO-1; 1:200, Enzo Life Sciences Cat# ADI-SPA-895 RRID:AB_10618757, Farmingdale, NY), and fluorescein-labeled anti-mouse immunoglobulin G (IgG; 1:200, Sigma-Aldrich Cat# F0257 RRID:AB_259378). DyLight 594-labeled tomato-lectin (T-lectin; 1:200, Vector Laboratories Cat# FL-1171 RRID:AB_2307440, Burlingame, CA) was used to label the blood vessels. Primary antibodies or markers were incubated in antibody solution (0.5% BSA +0.5% Triton X) overnight. Appropriate secondary antibodies (all from Invitrogen, Eugene, OR) were incubated in antibody solution for 1.5 h. Slides were coverslipped with Vectashield mounting medium (Vector Laboratories Cat# H-1200 RRID:AB_2336790) containing DAPI.
Fluoro-Jade C (FJC) staining was performed on three slices 720 μm apart for each animal (n = 4–5 per group) to assess neuronal degeneration.31
IHC image analysis
All image analysis was performed by a blinded experimenter by using the ImageJ software (ImageJ, RRID:SCR_003070, National Institutes of Health, Bethesda, MD), n = 4–5 animals per group. For IgG, HO-1, and GFAP quantification, integrated density measures were performed on the top right quarter of the brain (containing the lesion site), on two brain slices, 720 μm apart. For IBA1, vWF, and β-catenin quantification, integrated density was measured for each animal in two images captured in the peri-lesional cortex at 20 × magnification. For FJC and Ki67, stained cells were counted in the ipsilateral cortex by a blinded experimenter (on three slices per animal for FJC and 1 slice for Ki67).
Statistical analysis
All measurements and analysis were performed without knowledge of the groups or the sex. All statistical analyses were performed using GraphPad Prism 6 software (Graphpad Prism, RRID:SCR_002798, San Diego, CA). For analyses among the same sex group, one-way analysis of variance (ANOVA) and post hoc Tukey tests were performed. For analyses between sex groups, two-way ANOVA and post hoc Sidak tests were performed. For analysis of the evolution of the lesion and for Ki67-positive cell counts at 7 dpi we used t tests. Outlier values were removed if they were above or below 1.5 times the interquartile range (IQR), which led to removal of data for: open field test (2 males Sham and 1 female TBI 7d); MRI analysis (for 3 females Sham, 3 males Sham, 2 females TBI 1d, 1 male and 1 female TBI 7d); AngioTool analysis (for 1 male Sham, 1 male TBI 1d and 1 male TBI 7d); fractal geometry analysis (for 1 female TBI 1d and 1 male TBI 7d); β-catenin analysis (for 1 male and 1 female TBI 1d); FJC analysis (for 1 female Sham, 1 male and 1 female TBI 7d); IgG analysis (for 1 female Sham, 1 male TBI 1d, 1 male and 1 female TBI 7d); GFAP analysis (for 1 female Sham and 1 male TBI 1d), HO-1 analysis (1 male and 1 female TBI 7d), vWF analysis (for 1 female Sham, 1 male and 1 female TBI 7d). All error bars are presented as standard error of the mean (SEM). Statistical significance was noted as *p < 0.05, **p < 0.01, ***p < 0.001, or ****p < 0.0001.
Results
Motor activity was increased after moderate TBI
Open field tests were used to assess global activity levels and anxiety-like behavior. No significant differences were found but mice showed a trend toward more anxiety and less exploration between males and females 1 day after CCI when compared with Shams (less time spent in the center, p = 0.120 for males, p = 0.357 for females). At 7 dpi, the animals spent more time in the center compared with the injured animals at 1 dpi (p = 0.034 for males, p = 0.380 for females). There were no sex differences among the treatment groups (data not shown).
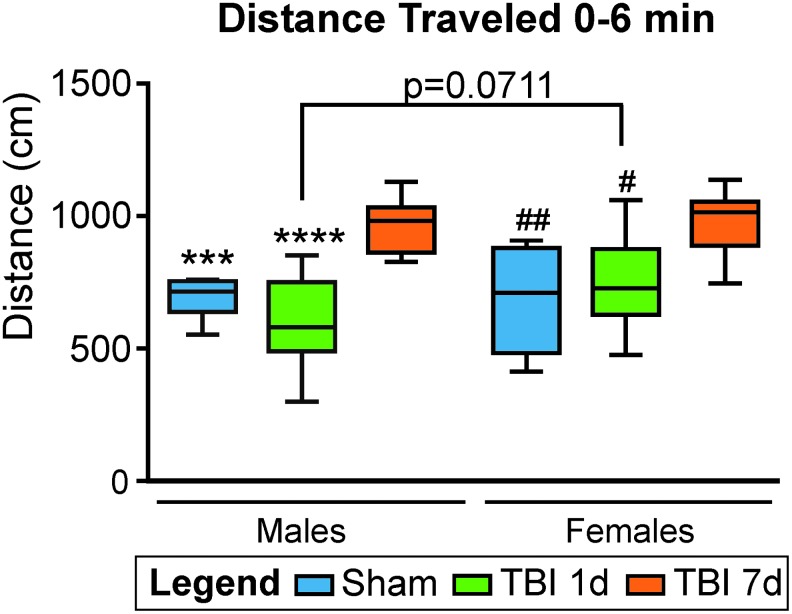
As we have previously described in males,32 male and female mice were hyperactive at 7 dpi, had increased travel distances during the first 6 min compared with the male and female Shams (p = 0.0006, t = 4.24 and p = 0.0032, t = 3.56, respectively, one-way ANOVA), and compared with the male and female mice at 1 dpi (p < 0.0001, t = 6.64 and p = 0.013, t = 2.99, respectively, one-way ANOVA). Interestingly, we observed a trend toward increased hyperactivity for the females compared with the males at 1 dpi where the females traveled 753.4 ± 54.8 cm compared with males traveling 600.8 ± 54.1 cm (p = 0.071, t = 2.33, two-way ANOVA; Fig. 1).
FIG. 1.
Open field assessment of motor activity in male and female mice after traumatic brain injury (TBI). During the first 6 min of the trial, injured male and female mice traveled more and were significantly more active at 7 days post-injury (dpi) compared with male and female Sham (***p < 0.001 and ##p < 0.01, respectively), and with male and female mice at 1 dpi (****p < 0.0001 and #p < 0.05, respectively). There is no statistical difference in the distance traveled between sexes for Sham and 7 dpi mice. However, females mice at 1 dpi exhibited a trend toward more hyperactivity compared with males at 1 dpi (p = 0.07) but not at 7 dpi (p = 0.99). *different compared with TBI males at 7 dpi, #different compared with TBI females at 7 dpi.
TBI lesion volumes were similar between sexes
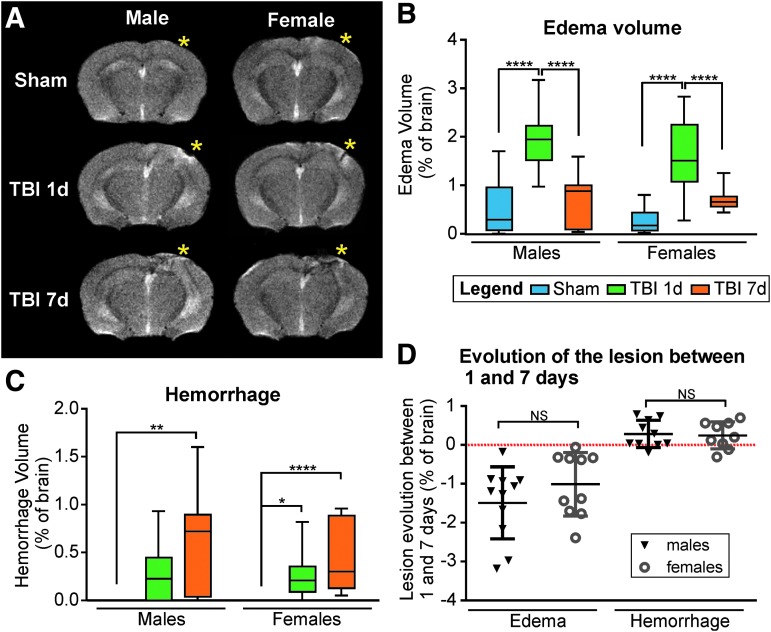
MRI in vivo using T2WI and SWI was utilized to assess total lesion volume, including edema and hemorrhage in all mice. Male and female mice exhibited edema and hemorrhage at 1 dpi (bregma -2 mm, *impact site, Fig. 2A). As previously shown in a rat model of mild TBI,33 by 7 dpi, the edema was principally resolved but increased hemorrhage was present. In Shams no hemorrhage was detected; however, a small but negligible amount of edema was present (Fig. 2A). The edema volumes did not differ significantly between males and females for Sham animals (0.53 ± 0.16% and 0.27 ± 0.06% of brain volume, respectively, p = 0.479, t = 1.31, two-way ANOVA), for 1 dpi animals (1.93 ± 0.13% and 1.64 ± 0.15%, respectively, p = 0.226, t = 1.76, two-way ANOVA), nor for 7 dpi animals (0.70 ± 0.15% and 0.70 ± 0.08%, respectively, p > 0.999, t = 0.02, two-way ANOVA; Fig. 2B). Similarly, the hemorrhage volumes did not differ significantly between males and females for 1 dpi animals (0.27 ± 0.06% and 0.25 ± 0.05% of brain volume, respectively, p = 0.994, t = 0.23, two-way ANOVA), nor for 7 dpi animals (0.65 ± 0.15% and 0.46 ± 0.12%, respectively, p = 0.347, t = 1.52, two-way ANOVA; Fig. 2C). Evolution of the lesion between 1 and 7 days after CCI showed a decrease in the edema volume for all the animals but no differences between males and females (p = 0.226, t = 1.25, unpaired t test; Fig. 2D). Hemorrhage volume was increased in most animals (8/10 males and 7/9 females) with time but no significant differences were detected between sexes (p = 0.823, t = 0.227, unpaired t test; Fig. 2D).
FIG. 2.
(A) Representative T2 images showing location of the lesion (bregma level: −2 mm, yellow asterisks represent the impact site). Increased edema is apparent at 1 day post-injury (dpi) compared with 7 dpi and increased hemorrhage at 7 dpi compared with 1 dpi. (B) For both males and females, edema volume was significantly increased at 1 dpi (****p < 0.0001) compared with Shams, but resolved by 7 dpi. (C) For both males and females, hemorrhage was present at 1 and 7 dpi, but absent in Shams. (D) The evolution of the edema and hemorrhage volumes between 1 and 7 dpi revealed no significant differences between males and females. Note the decreased edema in all animals, and the increased hemorrhage in most of the animals at 7 dpi.
The cortical vasculature was equally altered at 1 dpi but was more complex in males at 7 dpi
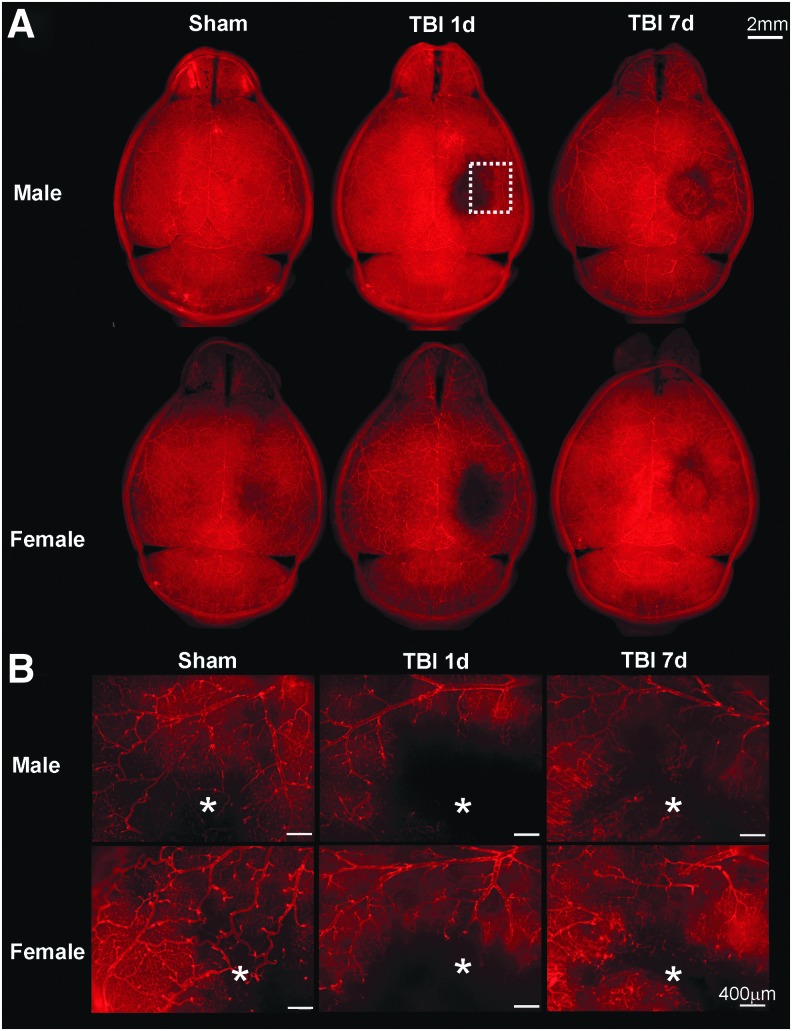
All animals underwent vessel painting procedure after MRI at either 1 or 7 dpi. The entire cortical vasculature was visible on the axial view of Sham animal brains (Fig. 3A). In TBI animals the vascular loss was clearly visible at the lesion site on the right cortex at 1 dpi. As described in previous studies in males, we found that revascularization occurs at 7 dpi in male as well as in female mice.21,23,25 Figure 3B illustrates the vascular network at the lesion site (*) revealing the irregular aspect of the “newly formed” vessels at 7 dpi. Overall, visual inspection of the vasculature did not reveal any differences between males and females.
FIG. 3.
(A) Representative images of vessel painted brains in axial view (scale bar: 2 mm) illustrate equal loss of vasculature between males and females. (B) Higher magnification at the lesion and peri-lesion region (from dotted line region in A, white asterisks represent the impact sites, scale bar: 400 μm) illustrates that at 1 dpi, there was a dramatic loss of the cortical vasculature in the impact site for both males and females. At 7 dpi, apparent revascularization was observed in male and female mice.
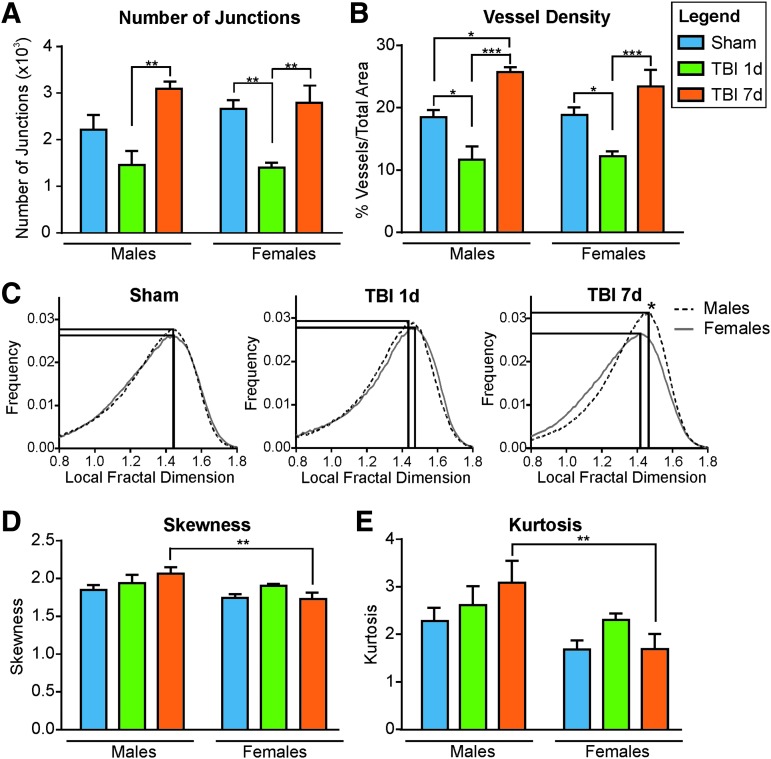
To quantitate vascular characteristics between males and females we assessed classical vessel features by using the AngioTool software.29 The number of vessel junctions in the ipsilateral cortex was decreased between Sham and 1 dpi mice (from 2213.40 ± 316.14 to 1440.25 ± 319.92 for males, p = 0.1758, F(2,10) = 7.85, one-way ANOVA; from 2659.00 ± 193.57 to 1398.00 ± 110.45 for females, p = 0.007, F(2,15) = 10.53, one-way ANOVA). At 7 dpi the number of junctions increased compared with 1 dpi in males (from 1440.25 ± 319.92 to 3096.50 ± 150.85, p = 0.007, F(2,10) = 7.85, one-way ANOVA), and in females (from 1398.00 ± 110.45 to 2787.50 ± 372.76, p = 0.002, F(2,15) = 10.53), consistent with a return to baseline (Fig. 4A). Vessel density followed the same pattern, with a decrease between Sham and 1 dpi mice (from 18.47 ± 1.15% to 11.69 ± 2.08% for males, p = 0.017, F(2,10) = 21.73, one-way ANOVA; from 18.84 ± 1.21% to 12.19 ± 0.82% for females, p = 0.043, F(2,15) = 11.45, one-way ANOVA) followed by an increase at 7 dpi relative to 1 dpi animals (25.59 ± 0.93% for males, p = 0.0002, F(2,10) = 21.73, one-way ANOVA; and 23.39 ± 2.66% for females, p = 0.0007, F(2,15) = 11.45, one-way ANOVA). Although vessel density was significantly increased in males at 7 dpi compared with Shams (from 18.47 ± 1.15% to 25.59 ± 0.93, p = 0.013, F(2,10) = 11.45, one-way ANOVA; Fig. 4B) we did not find a significant difference between males and females among Shams (p = 0.998, t = 0.15), 1 dpi (p = 0.995, t = 0.21) or 7 dpi (p = 0.761, t = 0.90) groups (two-way ANOVA; Fig. 4B).
FIG. 4.
Analysis of vessel painted brains in the ipsilateral cortex. (A) The number of junctions in the ipsilateral cortex was decreased between Sham and 1 day post-injury (dpi) mice (not significant for males, p = 0.18; **p < 0.01 for females). In contrast, the number of junctions is increased at 7 dpi compared with 1 dpi in males and females (**p < 0.01). No significant differences were observed between 7 dpi and Sham animals (p = 0.11 for males, and p = 0.93 for females). (B) Vessel density in the ipsilateral cortex was decreased between Sham and 1 dpi mice (*p < 0.05) but was increased at 7 dpi compared with 1 dpi (***p < 0.001). For the males the vessel density was significantly increased at 7 dpi compared with Shams (*p < 0.05). No difference in vessel density between males and females among Shams, 1 dpi, or 7 dpi groups was observed. (C) Distribution of the local fractal dimension (LFD) in the ipsilateral cortex for males and females in Sham, 1 dpi, and 7dpi. At 7 dpi, there was an increased complexity of the vascular network in males as evidenced by a rightward shift LFD shift, as well as an increased number of vessels in males, evidenced by a higher amplitude of the peak. (D,E) Analysis of the LFD distribution from the ipsilateral cortex shows a significant reduced skewness (D, **p < 0.01) and kurtosis (E, **p < 0.01) between males and females at 7 dpi. These results demonstrate an increased complexity of the vasculature (higher skewness) and an increased number of vessels (higher kurtosis) for the 7 dpi males.
Vessel complexity within the ipsilateral cortex was analyzed using fractal geometry measures.30 The fractal features we used were: the local fractal dimension (LFD), skewness, and kurtosis. Distribution of the LFD in the ipsilateral cortex showed no significant difference between males and females in Shams nor TBI 1 dpi groups (Fig. 4C). However, at 7 dpi the LFD revealed higher complexity of the vascular network for the males compared with the females (LFD peak shift to the right). Moreover, the peak frequency value was also increased in the males, suggesting an increased number of vascular components for the 7 dpi males compared with the females (Fig. 4C). These data are in line with the vessel density, which showed a significant increase between Sham and 7 dpi in the males but not in the females (Fig. 4B). Finally, the skewness and kurtosis measures are another way to present the complexity (shift of the peak to right or left, corresponding to an increased or decreased skewness, respectively) and the number of vascular components (higher/lower peak corresponding to a higher/lower kurtosis). Our results showed a significantly increased skewness (p = 0.008, t = 3.29, two-way ANOVA; Fig. 4D) and kurtosis (p = 0.009, t = 3.27, two-way ANOVA; Fig. 4E) in the males compared with the females at 7 dpi, confirming a higher complexity and a higher number of vascular components in the males. This could mean that males and females were at different stages of their vascular repair process.
Analysis of BBB integrity and neurodegeneration showed no effect of biological sex
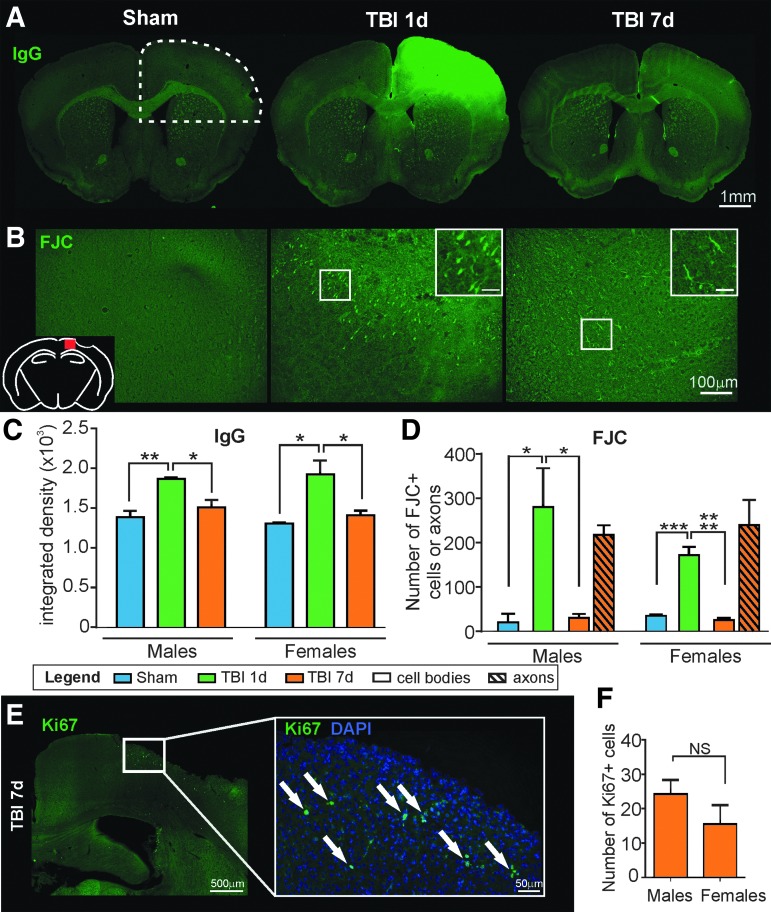
The influence of altered BBB between males and females was analyzed using detection of IgG extravasation (top right ipsilateral quarter, see dotted line in Fig. 5A) and we found an increased staining density at 1 dpi compared with Shams in both males (from 1384.44 ± 81.24 to 1864.71 ± 20.86, p = 0.009, F(2,8) = 8.76, one-way ANOVA) and females (from 1304.90 ± 15.64 to 1932.50 ± 166.91, p = 0.020, F(2,7) = 7.98, one-way ANOVA; Fig. 5A,C). At 7 dpi, IgG staining significantly decreased and returned to Sham levels for males (1508.19 ± 94.27, p = 0.038, F(2,8) = 8.76, one-way ANOVA) and for females (1409.53 ± 57.45, p = 0.044, F(2,7) = 7.98). IgG extravasation was present in the ipsilateral cortex and white matter, as well as in the sub-cortical structures (corpus callosum, as shown in Fig. 5A, and h ippocampus, not shown). There was no difference in IgG extravasation between males and females among the Sham (p = 0.932, t = 0.55), 1 dpi (p = 0.956, t = 0.47), or 7 dpi (p = 881, t = 0.678) groups (two-way ANOVA; Fig. 5C).
FIG. 5.
(A) Representative images of immunoglobulin G (IgG) staining demonstrates a large area of IgG extravasation at the injury site of the 1 days post-injury (dpi) animals that resolves by 7 dpi (scale bar: 1 mm). (B) Representative images of FJC staining in the ipsilateral cortex (see red square on schematic) of Sham, 1 dpi, and 7 dpi mice. Fluoro-Jade C (FJC)-positive cell bodies were present at the lesion site at 1 dpi but at 7 dpi the FJC-positive staining was mainly present in axons (scale bar: 100 μm, inset: 25 μm). (C) The quantification of IgG extravasation in the hemisphere containing the lesion (see dotted line in A) demonstrates that in both males and females there is increased staining between Sham and 1 dpi animals (**p < 0.01 and *p < 0.05, respectively). In both males and females, IgG staining decreases from 1 to 7 dpi, returning to Sham levels (*p < 0.05). There was no difference in IgG extravasation between males and females among Shams, 1 dpi, or 7 dpi groups. (D) Very few FJC-positive cells were present in the ipsilateral cortex of the Sham animals. At 1 dpi, there was a significant increase in the number of degenerative neurons (*p < 0.05 for males; ***p < 0.001 for females). In the 7 dpi animals, there was a decrease in FJC-positive cell bodies and appearance of FJC-positive axons. There was no significant difference in neurodegenerative cells between males and female mice among Sham, 1 dpi, or 7 dpi groups. (E) Representative image illustrating Ki67 staining on the ipsilateral side at 7 dpi. White arrows identify Ki67-positive cells (scale bar: 500 μm, 50 μm in the enlarged image). (F) There was no significant difference between males and females in the number of Ki6- positive cells at 7 dpi. No Ki67-positive cells were found in Sham or 1 dpi mice.
We also analyzed cortical neurodegeneration by using FJC staining in the ipsilateral cortex. Few neurodegenerative cell bodies were found in Sham animals (20.25 ± 19.26 for males and 35.00 ± 3.00 for females), possibly due to the craniotomy (Fig. 5B). As shown in Figure 5B,D, FJC staining was present in cell bodies at 1 dpi in the cortical lesion area. At 7 dpi, the FJC-positive staining was greatly reduced in cell bodies but was present in axons, suggesting the ongoing process of neuronal death at 7 dpi. Quantification revealed no significant difference between males and females among groups for cell staining (Shams: p = 0.993, t = 0.25; 1 dpi: p = 0.182, t = 1.98; 7 dpi: p = 0.999, t = 0.10, two-way ANOVA) or filament staining (7 dpi: p = 0.836, t = 0.77, two-way ANOVA).
We assessed repair and proliferation at 7 days using Ki67 immunostaining but were unable to observe proliferative cells in the cortex in Sham or 1 dpi animals. At 7 dpi, no Ki67 staining was found in endothelial cells (no co-localization with t-lectin, data not shown). The number of Ki67-positive cells in the ipsilateral cortex (presumptive neurons) found no significant differences (p = 0.237, t = 1.29, unpaired t test) between males and females at 7 dpi (Fig. 5E–F), suggesting no differences in the repair process related to sex.
Analysis of the inflammation process revealed more astrocyte reactivity and HO-1 induction in females at 1 dpi
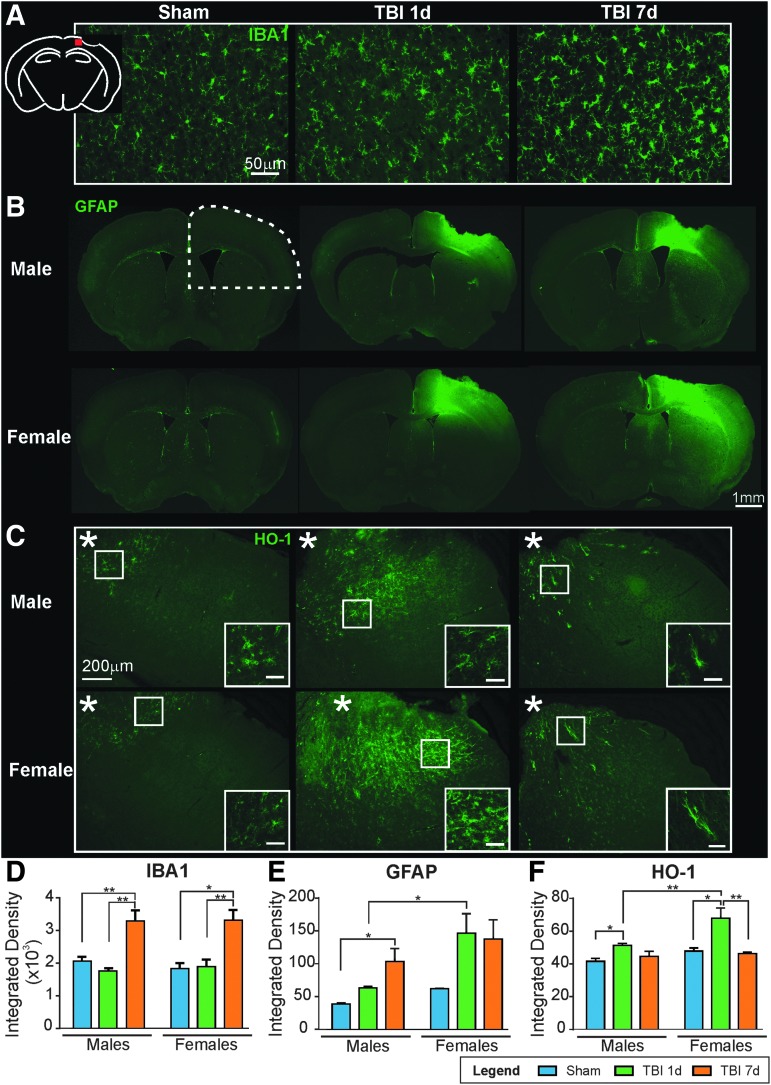
The role of inflammation between males and females in the ipsilateral cortex was quantified for microglial recruitment using IBA1 staining, and astrocytic reaction using GFAP staining. We also assessed the expression of HO-1, a known anti-oxidant molecule activated by stressors such as heme, hypoxia, and heat.34
In males and females, IBA1 immunoreactivity was not significantly different between Sham and 1 dpi animals (2071.80 ± 127.85 and 1772.04 ± 74.13 for males, p = 0.671, F(2,10) = 12.49; 1830.50 ± 167.67 and 1894.45 ± 220.03 for females, p = 0.980, F(2,9) = 12.52). At 7 dpi, both males and females show a significant increase in IBA1 staining density compared with Sham (males: p = 0.010, F(2,10) = 12.49; females: p = 0.004, F(2,9) = 12.52, one-way ANOVA) and TBI 1 dpi (males: p = 0.002, F(2,10) = 12.49; females: p = 0.006, F(2,9) = 12.52, one-way ANOVA) animals (Fig. 6A). There were no differences between sexes among the groups (Shams: p = 0.866, t = 0.71; 1 dpi: p = 0.979, t = 0.36; 7 dpi: p = 0.999, t = 0.10, two-way ANOVA).
FIG. 6.
(A) Representative images of anti-ionized calcium-binding adapter molecule 1 (IBA1) staining in the ipsilateral cortex (see red square in schematic) illustrating microglial activation at 7 days post-injury (dpi) (scale bar: 50 μm). (B) Representative images of anti-glial fibrillary acidic protein (GFAP) staining with astrocytic reactivity at 1 and 7 dpi in both males and females. Females exhibited increased GFAP staining compared with males at 1 and 7 dpi (scale bar: 1 mm). (C) Representative images of heme-oxygenase-1 (HO-1) staining in ipsilateral cortex confirmed few HO-1-positive astrocytes in Sham animals but at 1 dpi, females exhibited higher astrocytic HO-1 staining compared with males. At 7dpi, HO-1 staining remained in fewer astrocytes and exhibited a protoplasmic phenotype (scale bar: 200μm, inset: 50 μm). (D) Quantification of IBA1 staining in the ipsilateral cortex found no difference between Sham and 1 dpi animals. Both males and females at 7 dpi revealed a significant increase in IBA1 staining compared with Sham and 1 dpi animals. (E) GFAP quantification within the lesion quarter (see dotted line in B) showed astrogliosis, with an increase between Sham and 7 dpi animals (*p < 0.05 for males, and p = 0.19 for females). Interestingly, females had significantly higher GFAP staining at 1 dpi compared with males (*p < 0.05). (F) The quantification of HO-1 staining in the lesion quarter demonstrated a significant increase between Sham and 1 dpi animals (*p < 0.05). At 1 dpi, females had a significant increase in HO-1 staining compared with males (**p < 0.05). At 7 dpi, the staining density was comparable to Sham animals.
GFAP immunohistochemistry revealed increased staining indicative of reactive astrocytes between Sham and 7 dpi animals (from 38.53 ± 1.57 to 103.38 ± 19.69 for males, p = 0.023, F(2,9) = 5.55; and from 61.67 ± 0.97 to 137.37 ± 29.34 for females, p = 0.189, F(2,8) = 2.73, one-way ANOVA; Fig. 6B). Interestingly, and as shown in Figure 6E, females had significantly greater astrocyte reactivity within the cortex adjacent to the lesion at 1 dpi compared with males (integrated density: 146.55 ± 29.62 vs. 62.91 ± 2.32, p = 0.048, t = 2.67, two-way ANOVA; Fig. 6B).
Finally, HO-1 expression was significantly increased between Sham and 1 dpi animals (from 41.57 ± 1.73 to 51.38 ± 1.08 for males, p = 0.027, F(2,9) = 5.321; and from 47.85 ± 1.84 to 67.85 ± 6.23 for females, p = 0.010, F(2,7) = 11.35, one-way ANOVA; Fig. 6C). Interestingly, 1 dpi females showed a significantly larger increase in HO-1 staining compared with males (51.38 ± 1.08 vs. 67.85 ± 6.23, p = 0.003, t = 4.07, two-way ANOVA). No significant sex difference was found between Sham (p = 0.302, t = 1.68) and 7 dpi (p = 0.967, t = 0.42, two-way ANOVA) mice (Fig. 6C). As illustrated in Figure 6C, HO-1 staining was found in cortical astrocytes/microglial cells adjacent to the lesion (data not shown for astrocytic and microglial co-staining). A few HO-1-positive cells were present in Sham animals. At 1 dpi, HO-1 expression was 32% higher in the females compared with the males (**p = 0.003). Finally, at 7 dpi, a reduced number of cells still expressed HO-1 but their phenotype was more protoplasmic compared with the Sham or 1 dpi animals, which showed fibrous HO-1 positive cells (see insets, Fig. 6C).
These results would suggest a different inflammatory response in males compared with females after our TBI model.
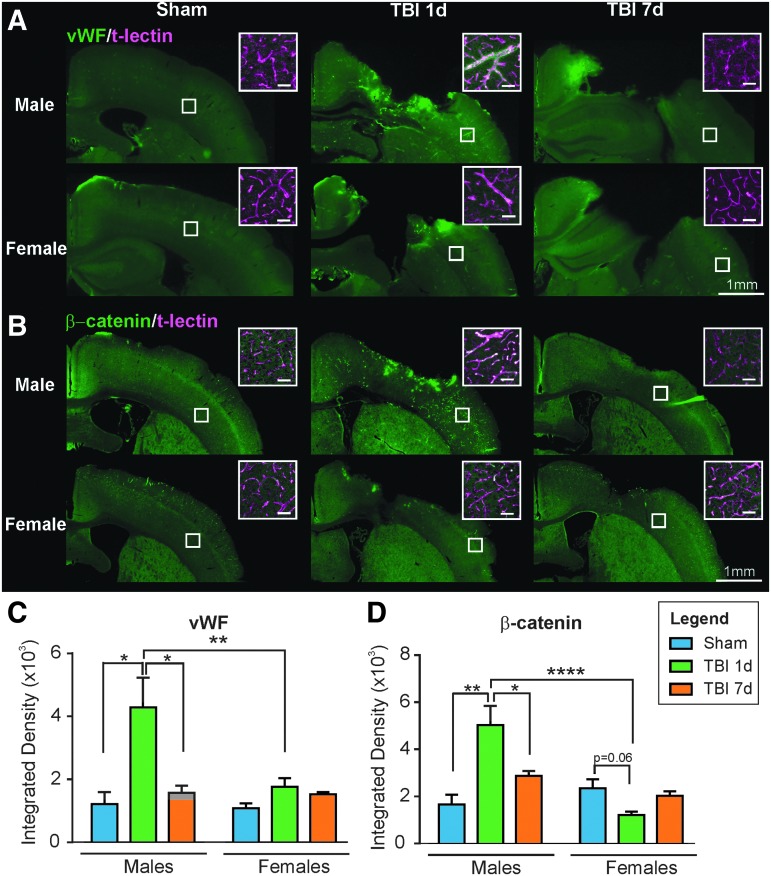
Endothelium activation and β-catenin were increased in males at 1 dpi
vWF is a glycoprotein known for its role in hemostasis but is also implicated in other biological processes such as endothelial activation and regulation of angiogenesis.35 Here, we used vWF staining and found a 3.5-fold increase in vWF integrated density in the ipsilateral cortex of 1 dpi males compared with Sham males (from 1215.77 ± 376.73 to 4283.77 ± 945.02, p = 0.014, F(2,9) = 7.77, one-way ANOVA; Fig. 7A). Surprisingly, females did not exhibit this large rise at 1 dpi (p = 0.118, F(2,7) = 2.71, one-way ANOVA) and by 7 dpi, vWF expression had returned to Sham levels in males without any change in females (Fig. 7A). As shown in Figure 7A, vWF staining was highly increased in the vessels (co-staining with t-lectin, see inset) of the males 1 day after TBI. We did not detect vWF in the Sham animals. A few cortical vessels exhibited staining in the females 1 dpi and in both males and females 7 dpi (Fig. 7C).
FIG. 7.
(A) Representative photomicrographs illustrating anti-Von Willebrand Factor (vWF) staining in the ipsilateral cortex in males and females. vWF was present in vessels as confirmed with t-lectin co-labeling (see insets). There was more vWF staining in the vessels of the 1 day post-injury (dpi) males compared with Sham and 7 dpi males, and compared with the 1 dpi females (scale bar: 1 mm, inset: 50 μm). (B) β-catenin staining in the ipsilateral cortex in males and females was localized to vessels, confirmed by t-lectin co-labeling (see insets). There was more β-catenin staining in the vessels of 1 dpi males compared with Sham and 7 dpi males, and compared with the 1 dpi females (scale bar: 1 mm, inset: 50 μm). (C) Quantification of vWF staining in ipsilateral cortex revealed increased staining in males at 1dpi compared with Sham and 7 dpi (*p < 0.05) but not in females between groups. There was significantly higher vWF staining in the males compared with the females at 1 dpi (**p < 0.01). (D) Quantification of β-catenin staining in ipsilateral cortex exhibited the same profile as vWF. There was increased staining in males at 1 dpi compared with Sham and 7 dpi (**p < 0.01). In females, there was a trend toward a decrease between Sham and 1 dpi mice (p = 0.06). There was significantly higher β-catenin staining in the males compared with the females at 1 dpi (****p < 0.0001).
Finally, we evaluated the expression of β-catenin, which we found to be associated with vascular repair after TBI.15,23 As described in our recent study,23 we found an increase in β-catenin staining in the ipsilateral cortex of the males at 1 dpi compared with Shams (Fig. 7B,D; from 1655.38 ± 412.65 to 5021 ± 823.32, p = 0.005, F(2,9) = 9.71, one-way ANOVA). For the females, we noted a trend toward a decrease in β-catenin staining from Sham to 1 dpi (from 2338.11 ± 383.29 to 1209.15 ± 143.03, p = 0.060, F(2,8) = 3.87, one-way ANOVA). At 7 dpi, β-catenin levels decreased for the males compared with 1 dpi (from 5021 ± 823.32 to 2860.21 ± 223.86, p = 0.050, F(2,9) = 9.71, one-way ANOVA), whereas it did not significantly change for the females (p = 0.180, F(2,8) = 3.87, one-way ANOVA). As shown in Figure 7B, the staining was present in a few vessels (co-stained with t-lectin, see insets) in Sham and 7 dpi animals. In the 1 dpi males, β-catenin staining was highly increased in vessels in the ipsilateral side of the brain. These results suggest that the activation of the Wnt/β-catenin signaling pathway in response to TBI only occurred in males. Further, these results concur with the vWF findings in males described above.
Discussion
TBI is known to elicit cerebrovascular dysfunction,12–15 however little is known about the effect of biological sex on the response to TBI-induced vascular impairment. We tested the hypothesis that male and female mice have differential cerebrovascular responses to a moderate TBI using multiple outcome measures from behavior to vascular assessments. We observed the following novel sex-related differences where, a) female mice exhibited increased (25%) motor activity at 1 dpi compared with males, b) there was increased numbers of reactive astrocytes and HO-1 induction in females at 1 dpi, c) there was a more complex vasculature in the ipsilateral cortex at 7 dpi for the males, and d) there was increased vascular vWF as well as increased β-catenin expression within vessels in males at 1 dpi. However, there was no difference in lesion volume, neurodegeneration, or BBB alteration between sexes.
Our open field results showing hyperactivity in males and females mice 7 days after CCI are in line with what we and others have observed in male mice.32,36–41 However, our results also show a trend toward more hyperactivity at 1 day for the females compared with the males (p = 0.07). Exacerbated activity in females has also been shown in mice after a CCI42 and in rats after an impact-acceleration model of diffuse TBI.43 Interestingly, in these studies, female animals tended to be more active than males, independently of the injury status. Moreover, the study by O'Connor and colleagues reported that the behavioral effect was not only dependent on the biological sex, but also on the anesthetic used during surgery. Female rats exposed to isoflurane were the most active.43 Our current experimental paradigm also used isoflurane but we did not see an increased activity in female Shams compared with male Shams. Even though not significant (males 1 dpi vs. females 1 dpi, p = 0.06, t test), our results suggest a trend toward a sex-injury interaction at 1 dpi. Increasing the number of replicates may support this interaction.
Lesion volumes were not different between sexes nor were neurodegeneration or altered BBB measures. Our lack of differences in lesion volume findings were consistent with what has been reported in studies for pediatric (at 2 months post-injury44), adolescent (at 30 dpi45), and adult (at 1 and 7 dpi,46 at 30 dpi,42 and at 35 dpi47) models of CCI in male and female mice. However, in different models of TBI that have a more diffuse injury pattern, several groups have shown sex differences that were attributed to female steroid hormones: Roof and associates as well as O'Connor and co-workers found that female rats exhibited less edema compared with males after a severe bilateral cortical contusion48 or after an impact acceleration injury.49 Moreover, Bramlett and Dietrich showed a smaller contusion area in intact females compared with males and ovariectomized female rats 3 days after a parasagittal fluid percussion injury.50 Thus, the reported sex differences in TBI lesions appear to reflect model and mode of injury. Similar to our lesion volume findings, we did not observe sex differences in neurodegeneration or BBB alteration, confirming previous results showing an equivalent extent of neurodegeneration between males and females after a CCI46 or a focal penetrating brain injury.51 In the case of a diffuse injury (impact-acceleration), Kupina and colleagues found significantly more neurodegeneration in males compared with females in the cortex, striatum, and hippocampus.52 It has been suggested that the lack of differences in neurodegeneration between males and females in focal injuries could be due to the rapid evolution of the secondary injury, leaving insufficient time for female sex steroids to exert their neuroprotective effects.46 BBB alteration has been shown to be influenced by female steroid hormones where 5 h after a diffuse TBI, females presented reduced Evans blue extravasation compared with males and ovariectomized females.49,53 We do not observe any differences in IgG extravasation between sexes; these differences could be due to the injury type. Evaluation of BBB alteration at earlier time-points may have potentially identified sex-dependent differences. The fact that we did not observe neuroprotection in the females in our study could be due to the non-estrous stage at the time of the injury. As previously shown in rats, females in pro-estrous are less vulnerable to injury compared with females in non-pro-estrous.54 In our CCI model, the lesion, neurodegeneration, and BBB alteration were found to be comparable between males and females.
Inflammation is an important part of the secondary injury cascade that could influence vessel repair/angiogenesis after a TBI. As reviewed by Caplan and collaborators, the literature on the effect of sex on microglial response after a TBI is complicated and incomplete.55 Conflicting results exist in the literature: for example, in a model of cortical stab injury, Acaz-Fonceca and colleagues found increased IBA1-positive cells in the injured cortex of males compared with females.56 In a CCI model in mice, Villapol and colleagues showed a stronger microglial response in the cortex of males at 1 dpi compared with females. At 7 dpi, the level of microglial activation was not significantly different between sexes, suggesting a more delayed activation in females.57 Barreto and co-workers suggested that the modulation of estrogen receptors could reduce the activation of microglia in female rats (aged and young ovariectomized) and provide a neuroprotective effect.58 However, in our study we observed no differences in microglial activation in the ipsilateral cortex between males and females at 1 and 7 dpi. Our data align with others at 1 day after a model of penetrating brain injury in mice51 or 7 days after a CCI.59 Our results show no effect of sex on microglial activation.
Whereas others found no differences in astrogliosis between males and females after TBI,51 we observed a more robust astrocytic response in females compared with males at 1 dpi in the ipsilateral cortex and hippocampus. Interestingly, Villapol's CCI study showed a more robust astrogliosis in males compared with females at 1 and 7 dpi (in cortex, hippocampus, and thalamus).57 This discrepancy could be due to the age difference (2–3 months vs. 6–7 months60) or to the estrous cycle difference (random cycling vs. out of estrous61), which are known to influence astrocyte response. Our results are, however, consistent with a study from Cordeau and colleagues reporting a significantly higher GFAP induction in female mice out of estrous compared with males after an ischemic injury.62 Similarly, after an excitotoxic insult in mice, Zhang and collaborators observed an enhanced astrocytic proliferation in the hippocampus of aging females compared with aging males.63 Higher GFAP expression in females at 1 dpi was accompanied by a higher HO-1 expression in astrocytes. To our knowledge, the effect of biological sex on the expression of HO-1 has never been investigated after TBI. In other models of brain injury (iron-induced) in rats, males exhibited a higher induction of HO-1, but this could be due to the fact that the injury severity in these studies is greater in males compared with females.64,65 In our study the lesion volumes were not significantly different between males and females, thus suggesting a specific biological sex effect on astrocyte activation and HO-1 expression in astrocytes and microglia in response to a CCI. In the periphery using a model of hemorrhagic shock in rats, Toth and associates found an increased HO-1 induction in the liver of females compared with males, also suggesting an influence of sex on the induction and activity of HO-1.66 Our results suggest an effect of sex on astrogliosis and HO-1 induction after CCI.
Revascularization after a TBI has previously been shown in males in different models.21,24,25 Our own results using vessel painting clearly demonstrated a lack of vessels at the injury site at 1 dpi followed by revascularization at 7 dpi.14,15,23 To our knowledge, the revascularization after a TBI has never been investigated with regard to biological sex. Both males and females exhibited a similar loss of vessels at 1 dpi with revascularization at 7 dpi in both sexes. However, quantitative analysis of the vascular network revealed that male revascularization resulted in more complexity in the ipsilateral cortex compared with females. One putative mechanism is the differential effects of sex steroid hormones. Sieveking and collaborators demonstrated that androgens could increase angiogenic events in vitro, but only in male endothelial cells.67 Another study by Chenu and colleagues showed that testosterone protects male mice against skin necrosis by acting on revascularization through its estrogenic and androgenic derivatives.68 There is a clear paucity of literature on male/female revascularization after acquired brain injury, but our results suggest more vessels and a more complex network in the ipsilateral side in males at 7 dpi.
vWF is used as a marker for endothelial activation, which is a pro-inflammatory and pro-coagulant state of the endothelial cells. vWF is stored in the Weibel-Palade bodies of the endothelial cells, which, when activated, release the protein into the plasma and near the basement membrane.69 Increased vWF has previously been shown in the serum of TBI patients.70 In our study, we observed a 2.4-fold increase in vWF staining in cortical blood vessels in the 1 dpi males compared with females, suggesting more endothelial activation in males. Outside of greater activity levels in plasma from women, no sex differences have been described regarding vWF protein levels in endothelial cells.71,72 Our data show increased endothelial activation in the cortex of males 1 day after CCI.
β-catenin is part of the canonical Wnt signaling pathway. Little is known about the effects of sex on this pathway, but it has been postulated that 17β-estradiol could activate the Wnt/β-catenin pathways by attenuating the ischemic-induced elevation of Dickkopf-1 (Dkk1), an antagonist of the canonical Wnt pathway.73,74 We found robust activation of the pathway with increased β-catenin expression in males at 1 dpi but we observed a decrease in β-catenin expression at 1 dpi in females compared with the Shams.
The increased vessel numbers and enhanced complexity in the male cortical vasculature may be due to a different revascularization process after injury. We postulate that the time-course of the Wnt/β-catenin pathway activation is different between sexes or an alternate process in females by HO-1 induction, which is known to promote angiogenesis.75
Vascular differences have been reported in humans where women's cerebral arteries show a decreased sensitivity to angiotensin II and endothelin-1 compared with men.76 Moreover, we know that estrogens protect vascular cells by inhibiting smooth muscle cell proliferation,77 and by accelerating endothelial cell recovery after vascular injury.78,79 Our study suggests vascular repair mechanisms between males and females after TBI are likely different, highlighting the fact that consideration of sex is a critical step toward precision medicine.
In conclusion, our results suggest important differences between male and female mice in terms of the cerebrovascular response to a moderate TBI. Females exhibited a higher astrocytic reaction and HO-1 induction at 1 dpi. Males, however, showed an increased vascular vWF and increased β-catenin at 1 dpi that is accompanied with higher complexity of the cortical vasculature and higher number of vessels in response to injury at 7 dpi. Our results highlight the importance of inclusion of both males and females in TBI studies, as well as in other cerebrovascular disorders. We caution extrapolation of previous studies examining a single sex as equivalent in the other sex. As we have noted above, sex differences in inflammatory response and in activation of molecular pathways have been reported. Moreover, as the recovery of the cerebrovasculature appears to be sex dependent after TBI, we strongly believe that sex as a biological factor should be considered when designing new therapeutic agents.
Acknowledgments
We would like to thank Saburi Eliamani for acquiring the MRI data. This study was supported by a NIH Program Project grant from NINDS (POINS082184, Project 3).
AJ, AS, and AO designed the research; AJ, AS, BA, EH, AA, and MH performed the research; AJ, AS, BA, MB, EH, AA, MW, IE, SB, JT, JHZ, WJP, and AO analyzed the data; AJ, AS, and AO wrote the article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Xu L., Wald M.M., and Coronado V. (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths, 2002–2006. National Center for Injury Prevention and Control. CDC: Atlanta, GA [Google Scholar]
- 2.Obenaus A. (2015). Traumatic brain injury, in: Encyclopedia of Mental Health. Friedman H. (ed). Academic Press.: Waltham, MA, pps. 329–340 [Google Scholar]
- 3.Shansky R.M., and Woolley C.S. (2016). Considering sex as a biological variable will be valuable for neuroscience research. J. Neurosci. 36, 11817–11822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirschberg R., Weiss D., and Zafonte R. (2008). Traumatic brain injury and gender: what is known and what is not. Future Neurol. 3, 483–489 [Google Scholar]
- 5.Covassin T., Swanik C.B., and Sachs M.L. (2003). Sex differences and the incidence of concussions among collegiate athletes. J. Athl. Train. 38, 238–244 [PMC free article] [PubMed] [Google Scholar]
- 6.Hootman J.M., Dick R., and Agel J. (2007). Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J. Athl. Train. 42, 311–319 [PMC free article] [PubMed] [Google Scholar]
- 7.Coronado V.G., Xu L., Basavaraju S.V., McGuire L.C., Wald M.M., Faul M.D., Guzman B.R., Hemphill J.D., Centers for Disease Control and Prevention. (2011). Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. MMWR Surveill. Summ. 60, 1–32 [PubMed] [Google Scholar]
- 8.Cosgrove K.P., Mazure C.M., and Staley J.K. (2007). Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry 62, 847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy M.M., Arnold A.P., Ball G.F., Blaustein J.D., and De Vries G.J. (2012). Sex differences in the brain: the not so inconvenient truth. J. Neurosci. 32, 2241–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haast R.A., Gustafson D.R., and Kiliaan A.J. (2012). Sex differences in stroke. J. Cereb. Blood Flow Metab. 32, 2100–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearce W.J., Doan C., Carreon D., Kim D., Durrant L.M., Manaenko A., McCoy L., Obenaus A., Zhang J.H., and Tang J. (2016). Imatinib attenuates cerebrovascular injury and phenotypic transformation after intracerebral hemorrhage in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R1093–R1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jullienne A., Obenaus A., Ichkova A., Savona-Baron C., Pearce W.J., and Badaut J. (2016). Chronic cerebrovascular dysfunction after traumatic brain injury. J. Neurosci. Res. 94, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenney K., Amyot F., Haber M., Pronger A., Bogoslovsky T., Moore C., and Diaz-Arrastia R. (2016). Cerebral vascular injury in traumatic brain injury. Exp. Neurol. 275 Pt. 3, 353–366 [DOI] [PubMed] [Google Scholar]
- 14.Obenaus A., Ng M., Orantes A.M., Kinney-Lang E., Rashid F., Hamer M., DeFazio R.A., Tang J., Zhang J.H., and Pearce W.J. (2017). Traumatic brain injury results in acute rarefication of the vascular network. Sci. Rep. 7, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salehi A., Zhang J.H., and Obenaus A. (2017). Response of the cerebral vasculature following traumatic brain injury. J. Cereb. Blood Flow Metab. 37, 2320–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alford P.W., Dabiri B.E., Goss J.A., Hemphill M.A., Brigham M.D., and Parker K.K. (2011). Blast-induced phenotypic switching in cerebral vasospasm. Proc. Natl. Acad. Sci. U S A 108, 12705–12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryan R.M., Jr, Cherian L., and Robertson C. (1995). Regional cerebral blood flow after controlled cortical impact injury in rats. Anesth. Analg. 80, 687–695 [DOI] [PubMed] [Google Scholar]
- 18.Immonen R., Heikkinen T., Tahtivaara L., Nurmi A., Stenius T.K., Puolivali J., Tuinstra T., Phinney A.L., Van Vliet B., Yrjanheikki J., and Grohn O. (2010). Cerebral blood volume alterations in the perilesional areas in the rat brain after traumatic brain injury–comparison with behavioral outcome. J. Cereb. Blood Flow Metab. 30, 1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nag S., Venugopalan R., and Stewart D.J. (2007). Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood-brain barrier breakdown. Acta Neuropathol. 14, 459–469 [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Baeza A., Reina-de la Torre F., Poca A., Marti M., and Garnacho A. (2003). Morphological features in human cortical brain microvessels after head injury: a three-dimensional and immunocytochemical study. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 273, 583–593 [DOI] [PubMed] [Google Scholar]
- 21.Park E., Bell J.D., Siddiq I.P., and Baker A.J. (2009). An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: a role for hypoxia-inducible factors in traumatic brain injury. J. Cereb. Blood Flow Metab. 29, 575–584 [DOI] [PubMed] [Google Scholar]
- 22.Perez-Barcena J., Romay E., Llompart-Pou J.A., Ibanez J., Brell M., Llinas P., Gonzalez E., Merenda A., Ince C., and Bullock R. (2015). Direct observation during surgery shows preservation of cerebral microcirculation in patients with traumatic brain injury. J. Neurol. Sci. 353, 38–43 [DOI] [PubMed] [Google Scholar]
- 23.Salehi A., Jullienne A., Baghchechi M., Hamer M., Walsworth M., Donovan V., Tang J., Zhang J.H., Pearce W.J., and Obenaus A. (2017). Up-regulation of Wnt/b-catenin expression is accompanied with vascular repair after traumatic brain injury. J. Cereb. Blood Flow Metab. 38, 274–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan R., Kreipke C.W., Roberts G., Bagchi M., and Rafols J.A. (2007). Neovascularization following traumatic brain injury: possible evidence for both angiogenesis and vasculogenesis. Neurol. Res. 29, 375–381 [DOI] [PubMed] [Google Scholar]
- 25.Hayward N.M., Tuunanen P.I., Immonen R., Ndode-Ekane X.E., Pitkanen A., and Grohn O. (2011). Magnetic resonance imaging of regional hemodynamic and cerebrovascular recovery after lateral fluid-percussion brain injury in rats. J. Cereb. Blood Flow Metab. 31, 166–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes S., Dashkin O., and Defazio R.A. (2014). Vessel painting technique for visualizing the cerebral vascular architecture of the mouse. Methods Mol. Biol. 1135, 127–138 [DOI] [PubMed] [Google Scholar]
- 27.Ghosh N., Recker R., Shah A., Bhanu B., Ashwal S., and Obenaus A. (2011). Automated ischemic lesion detection in a neonatal model of hypoxic ischemic injury. J. MRI 33, 772–781 [DOI] [PubMed] [Google Scholar]
- 28.Ghosh N., Sun Y., Bhanu B., Ashwal S., and Obenaus A. (2014). Automated detection of brain abnormalities in neonatal hypoxia ischemic injury from MR images. Med. Image Anal. 18, 1059–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zudaire E., Gambardella L., Kurcz C., and Vermeren S. (2011). A computational tool for quantitative analysis of vascular networks. PloS One 6, e27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karperien A., Ahammer H., and Jelinek H.F. (2013). Quantitating the subtleties of microglial morphology with fractal analysis. Front. Cell. Neurosci. 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmued L.C., Stowers C.C., Scallet A.C., and Xu L. (2005). Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 1035, 24–31 [DOI] [PubMed] [Google Scholar]
- 32.Bajwa N.M., Halavi S., Hamer M., Semple B.D., Noble-Haeusslein L.J., Baghchechi M., Hiroto A., Hartman R.E., and Obenaus A. (2016). Mild concussion, but not moderate traumatic brain injury, is associated with long-term depression-like phenotype in mice. PloS One 11, e0146886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donovan V., Bianchi A., Hartman R., Bhanu B., Carson M.J., and Obenaus A. (2012). Computational analysis reveals increased blood deposition following repeated mild traumatic brain injury. Neuroimage. Clin. 1, 18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp F.R., Zhan X., and Liu D.Z. (2013). Heat shock proteins in the brain: role of Hsp70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential. Transl. Stroke Res. 4, 685–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randi A.M., and Laffan M.A. (2017). Von Willebrand factor and angiogenesis: basic and applied issues. J. Thromb. Haemost. 15, 13–20 [DOI] [PubMed] [Google Scholar]
- 36.Amenta P.S., Jallo J.I., Tuma R.F., and Elliott M.B. (2012). A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 90, 2293–2305 [DOI] [PubMed] [Google Scholar]
- 37.Budinich C.S., Tucker L.B., Lowe D., Rosenberger J.G., and McCabe J.T. (2013). Short and long-term motor and behavioral effects of diazoxide and dimethyl sulfoxide administration in the mouse after traumatic brain injury. Pharmacol, Biochem. Behav. 108, 66–73 [DOI] [PubMed] [Google Scholar]
- 38.Hsieh C.L., Niemi E.C., Wang S.H., Lee C.C., Bingham D., Zhang J., Cozen M.L., Charo I., Huang E.J., Liu J., and Nakamura M.C. (2014). CCR2 deficiency impairs macrophage infiltration and improves cognitive function after traumatic brain injury. J. Neurotrauma 31, 1677–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimbler D.E., Shields J., Yanasak N., Vender J.R., and Dhandapani K.M. (2012). Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PloS One 7, e41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakade C., Sukumari-Ramesh S., Laird M.D., Dhandapani K.M., and Vender J.R. (2010). Delayed reduction in hippocampal postsynaptic density protein-95 expression temporally correlates with cognitive dysfunction following controlled cortical impact in mice. J. Neurosurg. 113, 1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu F., Wang Z., Tchantchou F., Chiu C.T., Zhang Y., and Chuang D.M. (2012). Lithium ameliorates neurodegeneration, suppresses neuroinflammation, and improves behavioral performance in a mouse model of traumatic brain injury. J. Neurotrauma 29, 362–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tucker L.B., Fu A.H., and McCabe J.T. (2016). Performance of male and female C57BL/6J mice on motor and cognitive tasks commonly used in pre-clinical traumatic brain injury research. J. Neurotrauma 33, 880–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connor C.A., Cernak I., and Vink R. (2003). Interaction between anesthesia, gender, and functional outcome task following diffuse traumatic brain injury in rats. J. Neurotrauma 20, 533–541 [DOI] [PubMed] [Google Scholar]
- 44.Semple B.D., Dixit S., Shultz S.R., Boon W.C., and O'Brien T.J. (2017). Sex-dependent changes in neuronal morphology and psychosocial behaviors after pediatric brain injury. Behav. Brain Res. 319, 48–62 [DOI] [PubMed] [Google Scholar]
- 45.Mannix R., Berglass J., Berkner J., Moleus P., Qiu J., Jantzie L.L., Meehan W.P., 3rd, Stanley R.M., and Robinson S. (2014). Sex differences in the effect of progesterone after controlled cortical impact in adolescent mice: a preliminary study. J. Neurosurg. 121, 1337–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall E.D., Gibson T.R., and Pavel K.M. (2005). Lack of a gender difference in post-traumatic neurodegeneration in the mouse controlled cortical impact injury model. J. Neurotrauma 22, 669–679 [DOI] [PubMed] [Google Scholar]
- 47.Xiong Y., Mahmood A., Lu D., Qu C., Goussev A., Schallert T., and Chopp M. (2007). Role of gender in outcome after traumatic brain injury and therapeutic effect of erythropoietin in mice. Brain Res. 1185, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roof R.L., Duvdevani R., and Stein D.G. (1993). Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 607, 333–336 [DOI] [PubMed] [Google Scholar]
- 49.O'Connor C.A., Cernak I., and Vink R. (2006). The temporal profile of edema formation differs between male and female rats following diffuse traumatic brain injury. Acta Neurochir. Suppl. 96, 121–124 [DOI] [PubMed] [Google Scholar]
- 50.Bramlett H.M., and Dietrich W.D. (2001). Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J. Neurotrauma 18, 891–900 [DOI] [PubMed] [Google Scholar]
- 51.Gunther M., Plantman S., Davidsson J., Angeria M., Mathiesen T., and Risling M. (2015). COX-2 regulation and TUNEL-positive cell death differ between genders in the secondary inflammatory response following experimental penetrating focal brain injury in rats. Acta Neurochir. 157, 649–659 [DOI] [PubMed] [Google Scholar]
- 52.Kupina N.C., Detloff M.R., Bobrowski W.F., Snyder B.J., and Hall E.D. (2003). Cytoskeletal protein degradation and neurodegeneration evolves differently in males and females following experimental head injury. Exp. Neurol. 180, 55–73 [DOI] [PubMed] [Google Scholar]
- 53.O'Connor C.A., Cernak I., and Vink R. (2005). Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 1062, 171–174 [DOI] [PubMed] [Google Scholar]
- 54.Maghool F., Khaksari M., and Siahposht Khachki A. (2013). Differences in brain edema and intracranial pressure following traumatic brain injury across the estrous cycle: involvement of female sex steroid hormones. Brain Res. 1497, 61–72 [DOI] [PubMed] [Google Scholar]
- 55.Caplan H.W., Cox C.S., and Bedi S.S. (2017). Do microglia play a role in sex differences in TBI? J. Neurosci. Res. 95, 509–517 [DOI] [PubMed] [Google Scholar]
- 56.Acaz-Fonseca E., Duran J.C., Carrero P., Garcia-Segura L.M., and Arevalo M.A. (2015). Sex differences in glia reactivity after cortical brain injury. Glia [Epub ahead of print] [DOI] [PubMed]
- 57.Villapol S., Loane D.J., and Burns M.P. (2017). Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 65, 1423–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barreto G.E., Santos-Galindo M., and Garcia-Segura L.M. (2014). Selective estrogen receptor modulators regulate reactive microglia after penetrating brain injury. Front. Aging Neurosci. 6, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruce-Keller A.J., Dimayuga F.O., Reed J.L., Wang C., Angers R., Wilson M.E., Dimayuga V.M., and Scheff S.W. (2007). Gender and estrogen manipulation do not affect traumatic brain injury in mice. J. Neurotrauma 24, 203–215 [DOI] [PubMed] [Google Scholar]
- 60.Chisholm N.C., and Sohrabji F. (2016). Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging. Neurobiol. Dis. 85, 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arias C., Zepeda A., Hernandez-Ortega K., Leal-Galicia P., Lojero C., and Camacho-Arroyo I. (2009). Sex and estrous cycle-dependent differences in glial fibrillary acidic protein immunoreactivity in the adult rat hippocampus. Horm. Behav. 55, 257–263 [DOI] [PubMed] [Google Scholar]
- 62.Cordeau P., Jr., Lalancette-Hebert M., Weng Y.C., and Kriz J. (2008). Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke 39, 935–942 [DOI] [PubMed] [Google Scholar]
- 63.Zhang X.M., Zhu S.W., Duan R.S., Mohammed A.H., Winblad B., and Zhu J. (2008). Gender differences in susceptibility to kainic acid-induced neurodegeneration in aged C57BL/6 mice. Neurotoxicology 29, 406–412 [DOI] [PubMed] [Google Scholar]
- 64.Wang L.F., Yokoyama K.K., Lin C.L., Chen T.Y., Hsiao H.W., Chiang P.C., and Hsu C. (2016). Knockout of ho-1 protects the striatum from ferrous iron-induced injury in a male-specific manner in mice. Sci. Rep. 6, 26358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng M., Du H., Gao F., Koch L.G., Britton S.L., Keep R.F., Xi G., and Hua Y. (2016). Effect of gender on iron-induced brain injury in low aerobic capacity rats. Acta Neurochir. Suppl. 121, 367–371 [DOI] [PubMed] [Google Scholar]
- 66.Toth B., Yokoyama Y., Kuebler J.F., Schwacha M.G., Rue L.W., 3rd, Bland K.I., and Chaudry I.H. (2003). Sex differences in hepatic heme oxygenase expression and activity following trauma and hemorrhagic shock. Arch. Surg. 138, 1375–1382 [DOI] [PubMed] [Google Scholar]
- 67.Sieveking D.P., Lim P., Chow R.W., Dunn L.L., Bao S., McGrath K.C., Heather A.K., Handelsman D.J., Celermajer D.S., and Ng M.K. (2010). A sex-specific role for androgens in angiogenesis. J. Exp. Med. 207, 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chenu C., Adlanmerini M., Boudou F., Chantalat E., Guihot A.L., Toutain C., Raymond-Letron I., Vicendo P., Gadeau A.P., Henrion D., Arnal J.F., and Lenfant F. (2017). Testosterone prevents cutaneous ischemia and necrosis in males through complementary estrogenic and androgenic actions. Arterioscler. Thromb. Vasc. Biol. 37, 909–919 [DOI] [PubMed] [Google Scholar]
- 69.Sporn L.A., Marder V.J., and Wagner D.D. (1989). Differing polarity of the constitutive and regulated secretory pathways for von Willebrand factor in endothelial cells. J. Cell Biol. 108, 1283–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yokota H., Naoe Y., Nakabayashi M., Unemoto K., Kushimoto S., Kurokawa A., Node Y., and Yamamoto Y. (2002). Cerebral endothelial injury in severe head injury: the significance of measurements of serum thrombomodulin and the von Willebrand factor. J. Neurotrauma 19, 1007–1015 [DOI] [PubMed] [Google Scholar]
- 71.Ossei-Gerning N., Wilson I.J., and Grant P.J. (1998). Sex differences in coagulation and fibrinolysis in subjects with coronary artery disease. Thromb, Haemost, 79, 736–740 [PubMed] [Google Scholar]
- 72.Sarji K.E., Graves J.M., and Colwell J.A. (1975). Von Willebrand factor activity in normal subjects: sex difference and variability. Thromb. Res. 7, 885–895 [DOI] [PubMed] [Google Scholar]
- 73.Scott E.L., and Brann D.W. (2013). Estrogen regulation of Dkk1 and Wnt/beta-Catenin signaling in neurodegenerative disease. Brain Res. 1514, 63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Q.G., Wang R., Khan M., Mahesh V., and Brann D.W. (2008). Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J. Neurosci. 28, 8430–8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dulak J., Loboda A., Zagorska A., and Jozkowicz A. (2004). Complex role of heme oxygenase-1 in angiogenesis. Antioxid. Redox Signal. 6, 858–866 [DOI] [PubMed] [Google Scholar]
- 76.Ahnstedt H., Cao L., Krause D.N., Warfvinge K., Saveland H., Nilsson O.G., and Edvinsson L. (2013). Male-female differences in upregulation of vasoconstrictor responses in human cerebral arteries. PloS One 8, e62698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhalla R.C., Toth K.F., Bhatty R.A., Thompson L.P., and Sharma R.V. (1997). Estrogen reduces proliferation and agonist-induced calcium increase in coronary artery smooth muscle cells. Am. J Physiol. 272, H1996–H2003 [DOI] [PubMed] [Google Scholar]
- 78.Krasinski K., Spyridopoulos I., Asahara T., van der Zee R., Isner J.M., and Losordo D.W. (1997). Estradiol accelerates functional endothelial recovery after arterial injury. Circulation 95, 1768–1772 [DOI] [PubMed] [Google Scholar]
- 79.Meyer M.R., Haas E., and Barton M. (2006). Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension 47, 1019–1026 [DOI] [PubMed] [Google Scholar]