Abstract
On binding to its receptor, transforming growth factor β (TGFβ) induces apoptosis in a variety of cells, including human B lymphocytes. We have previously reported that TGFβ-mediated apoptosis is caspase-dependent and associated with activation of caspase-3. We show here that caspase-8 inhibitors strongly decrease TGFβ-mediated apoptosis in BL41 Burkitt's lymphoma cells. These inhibitors act upstream of the mitochondria because they inhibited the loss of mitochondrial membrane potential observed in TGFβ-treated cells. TGFβ induced caspase-8 activation in these cells as shown by the cleavage of specific substrates, including Bid, and the appearance of cleaved fragments of caspase-8. Our data show that TGFβ induces an apoptotic pathway involving sequential caspase-8 activation, loss of mitochondrial membrane potential, and caspase-9 and -3 activation. Caspase-8 activation was Fas-associated death domain protein (FADD)-independent because cells expressing a dominant negative mutant of FADD were still sensitive to TGFβ-induced caspase-8 activation and apoptosis. This FADD-independent pathway of caspase-8 activation is regulated by p38. Indeed, TGFβ-induced activation of p38 and two different inhibitors specific for this mitogen-activated protein kinase pathway (SB203580 and PD169316) prevented TGFβ-mediated caspase-8 activation as well as the loss of mitochondrial membrane potential and apoptosis. Overall, our data show that p38 activation by TGFβ induced an apoptotic pathway via FADD-independent activation of caspase-8.
INTRODUCTION
Apoptosis is a highly regulated process involving various intracellular signaling pathways and a large number of molecules. Among these molecules, the proteases of the caspase family play a crucial role in triggering and controlling the execution of apoptosis (Cohen, 1997). These caspases are cysteine-related proteases that are synthesized as inactive proenzymes and are activated by most apoptotic stimuli. The proenzymes are activated by proteolysis at specific aspartate sites. The cleavage products form dimers, which are the active enzymes (Alnemri, 1997). There are 14 known caspases, of which caspase-8 and caspase-3 play key roles in control of the various steps of apoptosis. In recent years, an increasing number of investigations has contributed to elucidate the mechanisms underlying the activation of these two caspases (Kumar, 1999). Thus, caspase-3 may be activated via mitochondria-dependent or -independent pathways (Porter and Janicke, 1999). One of these pathways is dependent on the release by mitochondria of cytochrome c, which, in the presence of ATP, associates with the cytoplasmic Apaf1 and inactive proforms of caspase-9 to form a complex called apoptosome (Li et al., 1997; Qin et al., 1999). Autocleavage and activation of caspase-9 occur in this complex. In turn, caspase-9 then directly cleaves and activates caspase-3 proforms.
An alternative pathway, observed in type I Jurkat T cells in response to Fas ligation, is independent of mitochondrial activation and requires the direct cleavage of caspase-3 proforms by activated caspase-8 (Stennicke et al., 1998; Scaffidi et al., 1999). Caspase-8 activation has been extensively studied in apoptosis mediated by members of the tumor necrosis factor-receptor (TNF-R) family such as Fas (CD95) and TNF-R itself (Ashkenazi and Dixit, 1998). Indeed, the activation of CD95 by its natural ligand or agonist antibody ligands results in Fas-associated death domain protein (FADD) recruitment through their respective death domain (Boldin et al., 1996; Muzio et al., 1996). Interactions between Fas and FADD via their COOH-terminal death domains expose the NH2-terminal death effector domain (DED) of FADD, which can interact with DED domains in the caspase-8 proform, resulting in the oligomerization of this protease and its subsequent autocleavage and activation (Kischkel et al., 1995; Medema et al., 1997). Caspase-8 by cleaving the proapoptotic member of the Bcl-2 family protein, Bid, is then responsible for changes in the mitochondria, including opening of the permeability transition pore, a decrease in mitochondrial membrane potential, and the release of cytochrome c into the cytoplasm (Li et al., 1998; Luo et al., 1998; Schendel et al., 1999). Whereas Fas binds directly to FADD, it is generally believed that other members of the TNF-R family bind to FADD via the adaptor molecule TNF receptor-associated death domain (Hsu et al., 1996). Thus, FADD is the final common link between the death domain-containing receptors and caspase-8. Activation of caspase-8 by caspase-3 has also been reported, and Wesselborg and colleagues recently reported that anticancer drug-mediated caspase-8 activation is FADD-independent (Slee et al., 1999; Wesselborg et al., 1999). However, the nature and regulation of these FADD-independent pathways of caspase-8 activation remain unknown.
The serine/threonine kinases of the mitogen-activated protein kinase (MAPK) family are also key modulators of cell activation, including apoptosis. To date, three major MAPKs have been identified: the extracellular signal-regulated kinases (ERK1/2), the c-Jun NH2-terminal protein kinase (JNK), and the p38 mitogen-activated protein kinase (p38). These kinases differ in their involvement in the control of apoptosis (Ip and Davis, 1998; Tibbles and Woodgett, 1999). ERK1/2 are mainly activated by growth factors and are involved in the regulation of cell proliferation (Hartsough and Mulder, 1995; Seger and Krebs, 1995; Taieb et al., 1995; Blanchard et al., 2000). On the other hand, JNK and p38 are stress-associated protein kinases that may regulate apoptosis positively or negatively depending on the cell type and stimulus (Raingeaud et al., 1995; Xia et al., 1995; Yamaguchi et al., 1995; Graves et al., 1996; Ichijo et al., 1997; Juo et al., 1997; Kummer et al., 1997; Seimiya et al., 1997; Wang et al., 1998; Franklin et al., 1998; Callsen and Brune, 1999; Kimura et al., 2000). Although the involvement of p38 in apoptosis has been reported in various systems, the mechanism by which p38 regulates apoptosis is still unclear. Hsu et al. (1999) reported that p38 activates the expression of Fas-L, thereby mediating apoptosis by regulating Fas signaling. More recently, Zhuang et al. (2000) reported that, during singlet oxygen-induced apoptosis, p38 may regulate the cleavage of Bid in a caspase-8-independent manner. To date, the role of p38 in caspase activation has not been clearly assessed.
We previously reported that TGFβ mediates the apoptosis of human B lymphocytes (Chaouchi et al., 1995). This apoptosis is caspase-dependent and associated with caspase-3 activation (Schrantz et al., 1999). However, the pathways responsible for caspase-3 activation are still poorly defined and, in particular, the role of caspase-8 in this response has not been clearly defined during TGFβ-mediated B cell apoptosis. On the other hand, in addition to ERK activation, TGFβ can stimulate both MKK4-JNK and MKK3-p38 pathways by activating TGFβ-activated kinase 1 (Hartsough and Mulder, 1995; Yamaguchi et al., 1995; Atfi et al., 1997; Frey and Mulder, 1997; Hanafusa et al., 1999). This led us to investigate the roles of caspase-8 and p38 in the activation of caspase-3 observed during the TGFβ-mediated apoptosis of B lymphocytes. We report here that TGFβ induces caspase-8 activation, which in turn regulates both the loss of mitochondrial membrane potential and caspase-3 activation. We also found that TGFβ-mediated p38 phosphorylation controlled this caspase-8 activation in an FADD-independent manner.
Materials and Methods
Reagents
zAEVD-fmk, zIETD-fmk, zAEVD-pNA, and zIETD-pNA were purchased from R & D Systems (Wiesbaden, Germany), and zVAD-fmk was obtained from Bachem Biochimie SARL (Voisin le Bretonneux, France). Stock solutions of zAEVD-fmk, zIETD-fmk, and zVAD-fmk were prepared in dimethyl sulfoxide and stored at −20°C. The working dilutions were prepared immediately before use. 3,3′dihexylocarbocyanine iodide [DiOC6(3)] was purchased from Molecular Probes (Leiden, The Netherlands), purified porcine TGFβ was obtained from R&D Systems, the cell-permeable fluorigenic substrate PhiPhilux G2D2 from OncoImmunin (Kensington, MD), and ionomycin from Sigma (St. Louis, MO). The CH11 and the ZB4 monoclonal antibodies (mAbs) were from Immunotech (Marseille, France). SB203580, PD169316, PD98059, and U0126 were from Calbiochem (San Diego, CA).
Cell Lines
The Burkitt's lymphoma cell line BL41, kindly provided by Drs. Alan Calender and Gilbert Lenoir (Edward Herriot Hospital, Lyon, France), does not contain the EBV genome. The Jurkat cell line was obtained from American Type Culture Collection (Rockville, MD). Both cell lines were cultured in RPMI 1640 Glutamax culture medium (Seromed; Biochrom, Berlin, Germany) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (Invitrogen, Carlsbad, CA).
Cell Transfection
The pcDNA-3.0/FADD-DN vector (kindly provided by Dr. V. Dixit) carries a truncated FADD cDNA (aa 80/208) lacking the DED region (Chinnaiyan et al., 1995, 1996). The dominant active human pcDNA-MKK3(b)E and pcDNA-MKK6(b)E vectors were kindly provided by Dr. J. Han and have been previously described (Han et al., 1996). pIRES.hrGFP was from Stratagene (San Diego, CA). BL41 cells were transfected by electroporation (960 μF, 240 V in a Bio-Rad apparatus). Stable transfectants, expressing FADD-DN were selected by incubating the cells with 1 mg/ml G418 for ∼3 wk. Stable clonal transfectants were isolated from resistant G418 cells with the use of the limiting dilution technique, and the expression of FADD-DN protein in the various clones was analyzed by Western blotting with the use of a rabbit anti-FADD antibody (StressGen Biotechnologies, British Columbia, Victoria, Canada). Cells were transiently transfected with green fluorescent protein (GFP) and either MKK3 or MKK6 vectors. Eighteen hours after transfection, dead cells, due to the electroporation shock, were removed from the cultures by centrifugation through Ficoll gradient. Viable cells were then cultured at 37°C for 24 h.
Detection of Apoptotic Cells
Cells were washed in phosphate-buffered saline, pelleted, and resuspended in phosphate-buffered saline. Their dot-blot light scatter profiles were analyzed by flow cytometry with the use of an FACScan flow cytometer (BD Biosciences, San Jose, CA). Shrunken cells with relatively high side-scatter and low forward-scatter properties were considered to be apoptotic and enumerated as a percentage of the total population. For both MKK3- and MKK6-expressing cells, the GFP-positive cells were analyzed for apoptosis by determining cell shrinkage.
Analysis of Mitochondrial Transmembrane Potential (ΔΨm)
ΔΨm was evaluated by staining cells (106) with DiOC6(3) at a final concentration of 40 nM (stock solution 1 μM in ethanol) for 15 min at 37°C. The fluorescence emitted by cells was analyzed with an FACScan flow cytometer (BD Biosciences) with the use of the FL1 channel.
Concomitant Analysis of ΔΨm and Caspase-3 Activity in a Single Cell
The cell-permeable fluorogenic substrate (Phiphilux-G2D2) and DiOC6(3) were used to monitor both caspase-3 activity and ΔΨm in a single cell. Cells (106) were stimulated by incubation with TGFβ (1 ng/ml) for 48 h. They were collected by centrifugation and resuspended in 50 μl of Phiphilux-G2D2 substrate solution supplemented with 5% fetal calf serum. Cells were incubated in a 5% CO2 incubator at 37°C for 45 min. They were then incubated with DiOC6(3), at a final concentration of 40 nM, in a 5% CO2 incubator at 37°C for another 15 min. The cells were pelleted and resuspended in 500 μl of Phiphilux dilution buffer (OncoImmunin), and fluorescence emission was immediately determined with the use of the FL-1 (ΔΨm) and FL-2 (caspase-3 activity) channels in an FACScan flow cytometer (BD Biosciences).
Assay of Caspase-8 Activity
The caspase-8 activity was determined with the use of a colorimetric caspase assay (R & D Systems). Briefly, cells treated with 1 ng/ml TGFβ for various periods of time were collected and lysed according to the manufacturer's instructions. Caspase 8 colorimetric substrates (IETD-pNA or AEVD-pNA) were added to the cell lysate and assays were performed in a 100-μl volume in 96-well flat-bottomed plates. Absorbance was measured on a microplate reader at a wavelength of 405 nm after 1 h of incubation at 37°C and was standardized with the use of free colorimetric substrate. The results are expressed as fold-increase in the caspase activity in stimulated cells with the use of unstimulated cells as the reference.
Western Blot Analysis
Cells were lysed by incubation in modified Laemnli buffer (60 mM Tris, pH 6.8, 10% glycerol, and 2% SDS, without β-mercaptoethanol and bromophenol blue) and sonication for 30 s on ice. The samples were centrifuged for 5 min at 15,000 × g. The supernatants were boiled for 5 min and frozen at −80°C or used immediately. Aliquots of the supernatants were assayed for protein concentration (microBCA protein assay; Pierce, Rockford, IL). β-Mercaptoethanol and bromophenol blue were added and cell lysate proteins (20 μg/lane) were resolved by SDS-PAGE. Proteins were then electroblotted onto 0.45-μm pore-size nitrocellulose filters, and the filters were blocked by incubation for 1 h with 5% nonfat milk in Tris-buffered saline, 0.1% Tween 20. The filters were then incubated for 1 h at room temperature or overnight at 4°C with anticaspase-8 mAb (clone 5F7; Upstate Biotechnology, Lake Placid, NY), FADD mAb (StressGen Biotechnologies), Bid antibody (R & D Systems) or anticleaved caspase-9 antibody (New England Biolabs, Beverly, MA), anticaspase-3 antibody (polyclonal rabbit anti-caspase-3 antiserum; PharMingen, San Diego, CA), phospho-p38 mAb (New England Biolabs) or p38 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Blots were washed three times for 10 min, in Tris-buffered saline, 0.1% Tween 20 and incubated for 1 h with peroxidase-labeled anti-mouse or anti-rabbit immunoglobulins. Blots were developed with the use of the enhanced chemiluminescence detection system (Pierce).
RESULTS
TGFβ-induced ΔΨm Loss Is Caspase-dependent
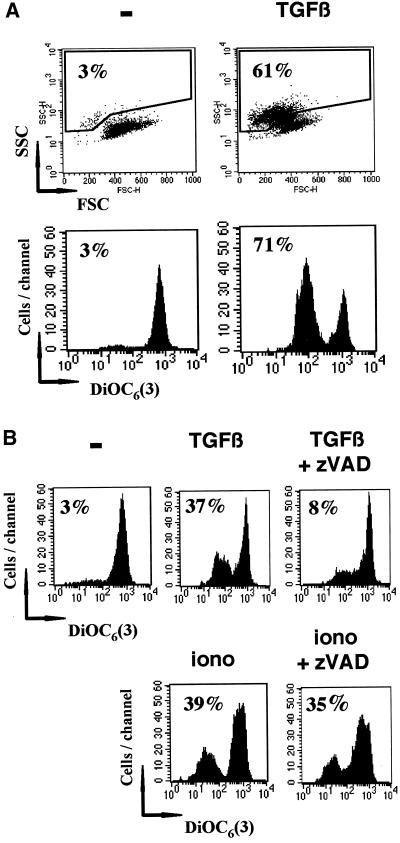
We previously reported that TGFβ-mediated apoptosis in the Burkitt's lymphoma cell line (BL41) was dependent on caspase-3 activation. To characterize upstream events involved in both caspase-3 activation and apoptosis induced by TGFβ, we first studied mitochondrial transmembrane potential, which is an important marker of mitochondria involvement during the apoptotic process. TGFβ-induced cell shrinkage (61 vs. 3% in control cells after 48 h of stimulation), as assessed by cell dot-blot light scatter profiles and flow cytometry, was associated with a loss of ΔΨm, as quantified by staining with DiOC6(3) (71 vs. 3% in control cells) (Figure 1A). Similar results were obtained when BL41 cells were activated with either recombinant TGFβ or porcine TGFβ in the presence or the absence of fetal calf serum during the first 60 min of the stimulation. In addition, the loss of ΔΨm observed in the presence of TGFβ, was considerably reduced in the presence of the broad-spectrum caspase inhibitor zVAD-fmk (50 μM), whereas loss of ΔΨm mediated by ionomycin, which is known to be caspase-independent, was not affected by zVAD-fmk (Figure 1B). These observations suggest that TGFβ causes a reduction in mitochondrial transmembrane potential via a caspase-dependent pathway.
Figure 1.
TGFβ induces the caspase-dependent loss of ΔΨm. (A) BL41 cells were cultured for 48 h without (−) or with TGFβ (1 ng/ml). Apoptotic cells were selected as shrunken cells with high side-scatter (SSC) and low forward-scatter (FSC) properties as assessed by flow cytometry. Apoptotic cells were counted and their number expressed as a percentage of the total cells. After staining with DiOC6(3), ΔΨm was assessed by flow cytometry, and cells with low ΔΨm were counted and their number expressed as a percentage of the total population. (B) BL41 cells were cultured for 48 h without (−) or with TGFβ (1 ng/ml) alone (TGFβ), or in combination with 100 μM zVAD-fmk (TGFβ + zVAD), or in the presence of ionomycin (10 μg/ml) alone (iono), or in combination with 100 μM zVAD-fmk (iono + zVAD). After staining with DiOC6(3) cells with a low ΔΨm were counted as described above. The results are representative of at least three independent experiments.
TGFβ Promotes Caspase-8 Activation and Bid Cleavage
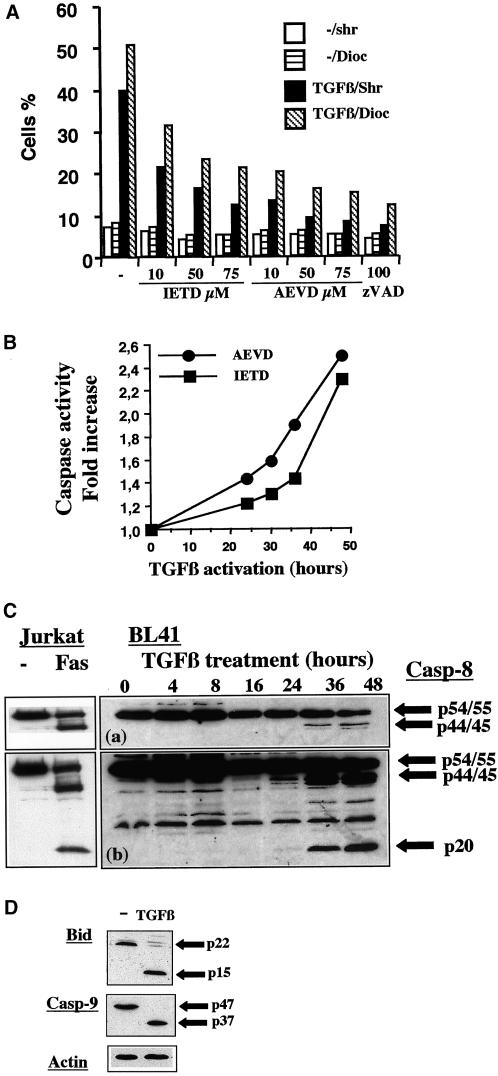
Caspase-8 has been reported to regulate mitochondria activation in various models (Muzio et al., 1996; Li et al., 1998). We then investigated whether various specific inhibitors (IETD-fmk and AEVD-fmk) of caspase-8 modulated apoptosis and the loss of ΔΨm induced by TGFβ (Figure 2A). These two inhibitors caused dose-dependent inhibition of both cell shrinkage and loss of the ΔΨm. Inhibition was observed at an inhibitor concentration of 10 μM and was more marked at a concentration of 75 μM. In our experimental conditions, inhibition was stronger with AEVD-fmk than with IETD-fmk and was similar for concentration of 75 μM AEVD-fmk and 100 μM zVAD-fmk. The increase in apoptosis induced by TGFβ was similarly inhibited: the increase of shrinkage and loss of ΔΨm mediated by TGFβ were 5.7- and 6.2-fold, respectively, but only 2.4- and 3.6-fold in the presence of IETD-fmk (75 μM), 1.6 and 1.7-fold in the presence of AEVD-fmk (75 μM), and 1.4- and 1.5-fold in the presence of zVAD-fmk (75 μM), respectively (Figure 2A). The effects of these inhibitors were specific because the carrier dimethyl sulfoxide, used at the same concentration, had not effect on the loss of ΔΨm and cell shrinkage induced by TGFβ. To assess further the involvement of caspases sensitive to IETD-fmk and AEVD-fmk during TGFβ-mediated activation, we tested whether cell lysates from TGFβ-treated BL41 cells cleaved these two substrates. Indeed, we showed by enzyme-linked immunosorbent assay that both AEVD-pNA and IETD-pNA substrates were cleaved in vitro by lysates from TGFβ-treated cells (Figure 2B). Caspase activity was already apparent at 24 h but was maximal after 48 h of TGFβ activation, showing kinetics similar to that for the decrease in ΔΨm (Figure 2B). The activation of caspase-8 upon stimulation with TGFβ was directly demonstrated by the detection of cleaved p44/45 and p20 kilodalton fragments in Western blots of cell extracts obtained at various times after TGFβ treatment. The cleaved fragments were comparable with control cleaved caspase-8 forms observed in Jurkat cells stimulated with anti-Fas antibody (Figure 2C). The kinetics of appearance of the cleaved forms of caspase-8 was similar to that of caspase activity with the use of IETD-pNA and AEVD-pNA as substrates (Figure 2, B and C).
Figure 2.
TGFβ promotes the caspase-8 dependent loss of ΔΨm and apoptosis. (A) BL41 cells were cultured for 48 h without (−) or with TGFβ (1 ng/ml) and various concentrations of IETD-fmk (10, 50, 75 μM), AEVD-fmk (10, 50, 75 μM), or ZVAD-fmk (100 μM). Cell shrinkage (Shr) and the loss ΔΨm (Dioc) were assessed by flow cytometry, as described in Figure 1. (B) Cells were cultured with TGFβ (1 ng/ml) and caspase activity was determined at various times after TGFβ treatment, with the use of IETD-pNA and AEVD-pNA as substrates. Results are expressed as the ratio between caspase activity in TGFβ-treated cells and that in control cells. (C) Cells were cultured with TGFβ (1 ng/ml) for various periods of time. Whole cell extracts were separated by SDS-PAGE and the various forms of caspase-8 were detected by immunoblotting with the use of anticaspase-8 antibodies. A short (a) and a longer (b) exposure of the films is shown. As control for caspase-8 specificity, Jurkat cells were cultured for 24 h in the absence (−) or the presence of anti-Fas antibody (CH11 antibody, 1 μg/ml). (D) Cells were cultured without (−) or with TGFβ (1 ng/ml) for 48 h. Whole cell extracts were separated by SDS-PAGE and the levels of the various forms of Bid and caspase-9 were determined by immunoblotting, with the use of anti-Bid or anti-caspase-9 antibodies. Results are representative of three independent experiments. The amount of protein loaded in each lane was assessed by stripping the filter and reprobing it with an antibody specific for human actin.
Caspase-8 activation may be monitored in the cell by following the cleavage of its natural substrate, Bid, which in turn mediates cytochrome c release and caspase-9 activation. Western blot analysis showed that TGFβ treatment of BL41 cells mediated the cleavage of Bid, as shown by the disappearance of the p22 uncleaved form of Bid and the production of p15 fragment (Figure 2D). We also observed caspase-9 activation, as shown by the disappearance of the p47 proform and the production of the cleaved fragment p37 (Figure 2D). We could not observe cleavage of caspase-10, but due to the lack of a reliable antibody specific for the cleaved fragments of caspase-10, we were unable to rule out the possibility that TGFβ also mediates caspase-10 activation.
Interactions between Caspase-8 and Caspase-3 Activation
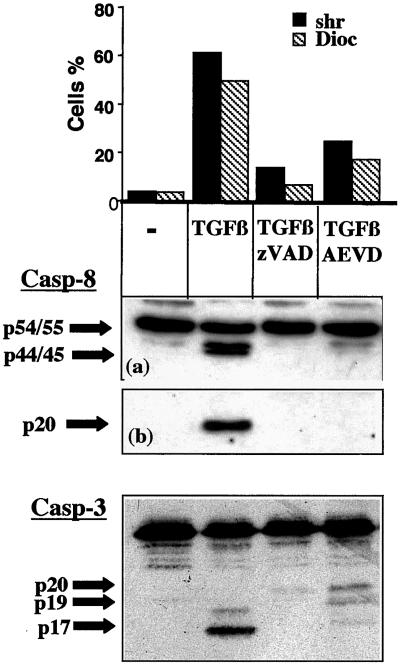
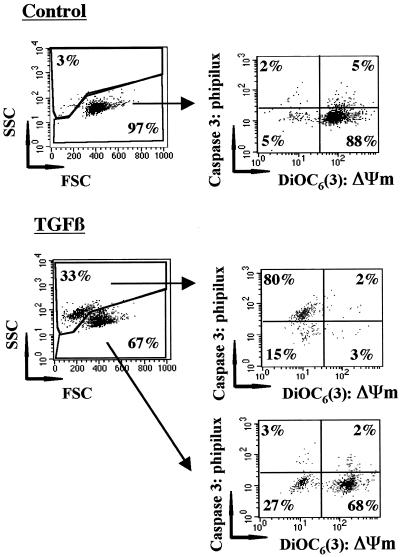
We next determined the sequence of events between caspase-8 and caspase-3 activation and the loss of ΔΨm. For this, we first investigated whether AEVD-fmk, which inhibited the loss of ΔΨm induced by TGFβ in BL41 cells more strongly than did IETD-fmk, also modulated caspase-3 activation. In the presence of TGFβ, both shrinkage and the loss of ΔΨm were associated with the appearance of the active fragments p44/45 and p20 of caspase-8 and the p19 and p17 fragments of caspase-3 (Figure 3). Morphological changes and the activation of caspase-8 and caspase-3 were completely prevented by zVAD-fmk. Inhibition of the loss of ΔΨm in the presence of AEVD-fmk correlated with a decrease in the amount of the active cleaved fragments p44/45 and p20 of caspase-8 detected. Under these conditions, the activation of caspase-3 was also prevented. In particular, the cleavage of the caspase-3 proform into p20 and p19 was greatly reduced in the presence of AEVD-fmk. These observations are consistent with a pathway of caspase-3 activation involving upstream activation of caspase-8. We then investigated the possible relationship between the loss of ΔΨm and caspase-3 activation. For this, BL41 cells, unactivated or activated by incubation for 48 h with TGFβ, were stained with both DiOC6(3), to measure loss of ΔΨm, and a cell-permeable fluorogenic substrate containing the specific caspase-3 sequence GDEVDG (PhiPhilux-G2D2), to detect caspase-3 activity in a single cell by flow cytometry (Figure 4). As expected, 97% of control cells were not shrunken, displaying intense DiOC6(3) labeling and no cleavage of PhiPhilux-G2D2. After TGFβ treatment, in the viable cell compartment (67% of total cells), most cells displayed a high ΔΨm with no caspase-3 activity (68%), with a smaller proportion of cells having low ΔΨm with no caspase-3 activity (27%). This strongly suggests that caspase-3 activation and shrinkage occur in cells that have decreased ΔΨm. In contrast, only 15% of TGFβ-induced shrunken cells displayed a low ΔΨm with no caspase-3 activity, with most cells (80%) displaying low ΔΨm and caspase-3 activity. This is consistent with the finding that caspase-3 is activated only when ΔΨm falls. Thus, our results show that 1) activation of caspase-8 and caspase-3 and loss of ΔΨm are prevented by a caspase-8 inhibitor, and 2) caspase-3 is activated in cells exhibiting loss of ΔΨm. Thus, these data (Figures 3 and 4) are consistent with a sequence of events involving caspase-8 activation, associated with loss of ΔΨm and caspase-3 activation.
Figure 3.
AEVD inhibits TGFβ-mediated caspase-8 and caspase-3 activation. BL41 cells were cultured for 48 h without (−) or with TGFβ (1 ng/ml) in association with ZVAD-fmk (50 μM), IETD-fmk (75 μM), or AEVD-fmk (75 μM). Cell shrinkage and loss of ΔΨm were assessed by flow cytometry, as described in Figure 1. The cleavage of caspase-8 and of caspase-3 was assessed by immunoblotting. A short (a) and a longer (b) exposure of the films is shown for caspase-8. Results are representative of three independent experiments.
Figure 4.
TGFβ mediates ΔΨm loss and caspase-3 activation in the same cells. BL41 cells were cultured for 48 h without (−) or with TGFβ (1 ng/ml) and were stained with DiOC6(3) and PhiPhilux G2D2. They were analyzed for FSC/SSC and gated on the basis of their FSC/SSC characteristics. The gated populations were analyzed for Phiphilux and DiOC6(3) staining. Results are representative of four independent experiments.
TGFβ-mediated Caspase-8 Activation Is FADD-independent
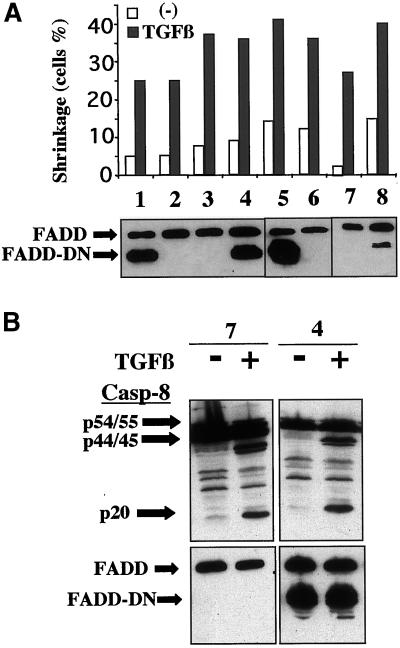
Many studies have demonstrated that caspase-8 activation is dependent on the oligomerization of caspase-8 by its association with the adaptor protein FADD, via the DEDs of the two molecules. This requires the interaction of FADD either directly or indirectly with surface receptors such as Fas or TNF-R, which possess death domains. Because no such death domains have been described in TGFβ receptors and because some Burkitt cell lines are sensitive to Fas-mediated apoptosis (Lens et al., 1996), we investigated the possible involvement of death receptors in TGFβ-mediated apoptosis. In our experimental conditions, the BL41 cells were insensitive to Fas-mediated apoptosis, as assessed with the use of the agonist antibody ligand CH11 antibody (up to 10 μg/ml)) in the presence or absence of TGFβ. In addition, the antagonist ZB4 antibody, which inhibited CH11-mediated Jurkat apoptosis, did not affect the extent of TGFβ-apoptosis in BL41 cells. Thus, the apoptosis and caspase-8 activation triggered by TGFβ are independent of the Fas pathway. Similarly, the TNF-R pathway was not involved in our experimental conditions because 1) the addition of TNF-α in the presence or absence of TGFβ did not affect the level of apoptosis, which was similar to that observed with TGFβ alone; and 2) we detected no TNF-α production upon TGFβ-stimulation. Because receptors known to recruit FADD do not seem to be involved in TGFβ-mediated apoptosis, we investigated more directly the possible involvement or requirement of the FADD molecule in our system. For this, we established stable transfectant clones of BL41 cells expressing a dominant negative mutant of FADD lacking the DED domain, which were therefore unable to associate with caspase-8 and as a consequence TNF-mediated apoptosis is blocked (Chinnaiyan et al., 1995, 1996). Various clones of BL41, expressing either only endogenous FADD (Figure 5A, lanes 2, 3, 6, and 7) or various levels of the truncated FADD, which was detected as a band that migrated faster than the wild-type band (Figure 5A, lanes 1, 4, 5, and 8) were selected. We observed that TGFβ induced apoptosis in all clones, independently of the production of dominant negative FADD molecule, as assessed by cell shrinkage (Figure 5A) or decreases in DiOC6(3) staining. We also observed that TGFβ-induced caspase-8 cleavage into the active fragments p44/45 and p20 occurred to a similar extent in cells producing or not producing FADD-DN (Figure 5B). Thus, TGFβ-mediated caspase-8 activation and apoptosis are independent of the presence of the DED of FADD.
Figure 5.
TGFβ-mediated caspase-8 activation is FADD-independent. (A) Independent clones of BL41 cells producing either endogenous FADD only or various amounts of the truncated form of FADD were cultured for 48 h without (−) or with TGFβ (1 ng/ml). Shrunken cells were counted by flow cytometry, as described in Figure 1. The production of FADD and FADD-DN was assessed by immunoblotting of the cell lysates obtained from each of the unstimulated clones. (B) After 48 h of incubation with or without TGFβ, the cleavage of caspase-8 was determined by immunoblotting the cell lysates obtained from clones 7 and 4. The blot was reprobed with anti-FADD antibodies to determine FADD and FADD-DN levels.
TGFβ-mediated p38 Activation Regulates Caspase-8 Cleavage and Apoptosis
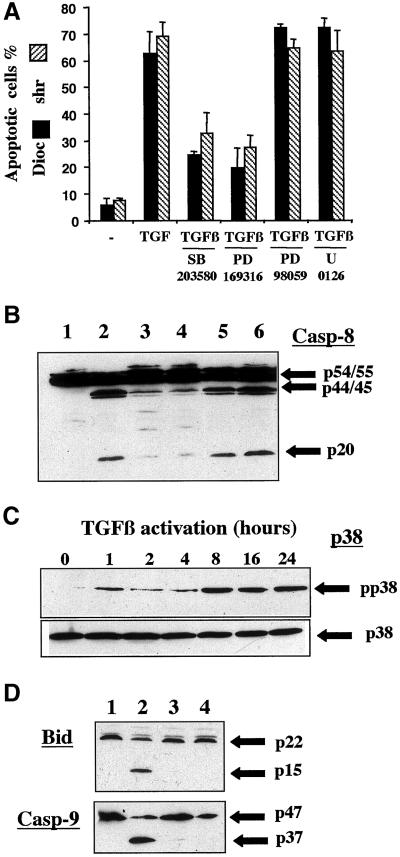
To identify the pathway leading to caspase-8 activation upon TGFβ-treatment of BL41 cells, we investigated regulation of the activation of members of the MAPK family. Indeed, various members of this serine/threonine kinase family have been shown to regulate both cell cycle progression and apoptosis and to be activated by TGFβ (Hartsough and Mulder, 1995; Yamaguchi et al., 1995; Atfi et al., 1997; Frey and Mulder, 1997; Hanafusa et al., 1999; Tibbles and Woodgett, 1999). We previously reported that TGFβ induced both cell cycle arrest and apoptosis in BL41 cells (Schrantz et al., 1999). We therefore investigated the possible role of these kinases in TGFβ-mediated apoptosis in these cells. The involvement of ERK or p38 in the TGFβ-mediated induction of apoptotic features was investigated in the presence of various inhibitors specific for the activation of p38 (SB203580 or PD169316) or ERK (PD98059 or U0126). In the presence of SB203580 (20 μM) or PD169316 (2 μM), both cell shrinkage and the decreasing of DiOC6(3) labeling were significantly inhibited (Figure 6A), whereas cell cycle arrest (assessed by quantification of cells arrested in G1) was not prevented. In addition, the ERK-specific inhibitors U0126 and PD98059 did not interfere with TGFβ-mediated apoptosis (Figure 6A). Similar conclusions were reached when we quantified the inhibition of the fold-increase of apoptosis by TGFβ in the presence of various doses of inhibitors. Because the loss of ΔΨm was sensitive to p38 inhibitors, we investigated whether p38 also affected TGFβ-induced caspase-8 activation. Indeed, the production of the cleaved fragments p44/45 and p20, observed upon TGFβ-activation (Figure 6B, lane 2), was much reduced in the presence of the p38 inhibitors SB203580 (Figure 6B, lane 3) and PD169316 (Figure 6B, lane 4), whereas U0126 (Figure 6B, lane 5) and PD98059 (Figure 6B, lane 6) did not prevent the production of these active cleaved fragments. We demonstrated that TGFβ activated the p38 pathway by Western blot analysis with an antibody recognizing specifically the phosphorylated form of the protein. The active phosphorylated form (pp38) was detected after 1 h of stimulation, was present in a larger amount at 8 h, and remained present in a large amount for up to 24 h of treatment with TGFβ (Figure 6C). In this cell line, we detected no activation of the JNK pathway by TGFβ, with the use of antibodies against the phosphorylated form of JNK or by in vitro JNK kinase assay. The importance of p38 in control of the early steps of TGFβ-induced apoptosis was also demonstrated by the ability of the 2 p38 inhibitors SB203580 and PD169316 (Figure 6D, lanes 3 and 4, respectively) to prevent the cleavage of both Bid and caspase-9 observed in TGFβ-treated cells (Figure 6D, lane 2 vs. control lane 1).
Figure 6.
TGFβ-mediated apoptosis and caspase-8 activation are p38-dependent. (A) BL41 cells were cultured for 48 h without (−) or with TGFβ (1 ng/ml) in the absence or presence of SB203580 (20 μM) or with PD169316 (2 μM), PD98059 (20 μM), or U0126 (10 μM). Shrunken cells and cells with low ΔΨm were counted by flow cytometry, as described in Figure 1. (B) BL41 cells were cultured for 48 h without (lane 1) or with TGFβ (1 ng/ml) (lane 2), or with TGFβ and SB203580 (lane 3), TGFβ and PD169316 (lane 4), TGFβ and U0126 (lane 5), or TGFβ and PD98059 (50 μM) (lane 6). The cleavage of caspase-8 was determined by immunoblotting. (C) BL41 cells were cultured for the times indicated with TGFβ (1 ng/ml), and the p38 and phosphorylated forms of p38 (pp38) were determined by immunoblotting with the use of specific antibodies. (D) Cells were cultured for 48 h without (lane 1) or with TGFβ (1 ng/ml) (lane 2), or with TGFβ and SB203580 (lane 3), or TGFβ and PD169316 (lane 4). Whole cell extracts were separated by SDS-PAGE and the various forms of Bid and caspase-9 were detected by immunoblotting with the use of anti-Bid or anti-caspase-9 antibodies.
TGFβ-mediated Apoptosis Is Dependent on Durable p38 Activation
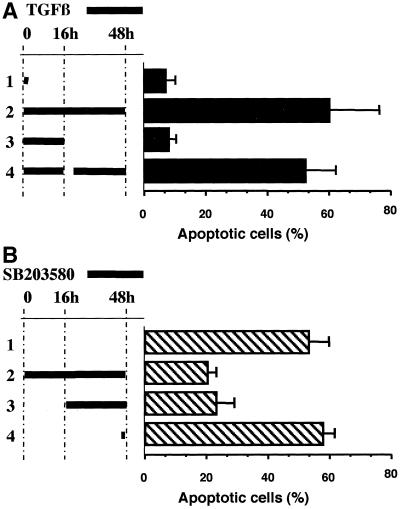
Because both TGFβ-mediated caspase-8 activation and apoptosis were observed only after 24 h and were maximum after 48 h of stimulation, we used kinetic experiments to investigate the mechanisms underlying the TGFβ-mediated apoptosis. First, we determined whether a brief activation by TGFβ was sufficient to promote the apoptosis in BL41 cells. Activation of 1 h (Figure 7A, lane 1) or for 16 h (Figure 7A, lane 3) was not sufficient to promote an apoptotic response 48 h later: <10% of cells were apoptotic compared with 58% when TGFβ was present during all the entire culture period (Figure 7A, lane 2). Addition of TGFβ for the remaining time of culture (16–48 h) to cells activated with TGFβ for 16 h (Figure 7A, lane 4) resulted in a level of apoptosis comparable with that in the cultures activated with TGFβ throughout the culture period. Therefore, the apoptotic response was dependent on the continuous presence of TGFβ. These data, associated with the observation that TGFβ promotes prolonged activation of p38 (Figure 6C), prompted us to investigate whether this long-lasting activation of p38 was necessary to mediate the TGFβ-induced apoptosis observed in BL41 cells. The presence of the p38 inhibitor SB203580 during the last 32 h of a 48-h culture inhibited the TGFβ-mediated apoptosis to a similar extent as the presence of the inhibitor SB203580 throughout the culture (Figure 7B). These data support the conclusion that TGFβ-mediated apoptosis in BL41 cells is dependent on the continuous presence of TGFβ and on a prolonged activation of p38.
Figure 7.
TGFβ-mediated apoptosis is dependent on prolonged p38 activation. (A) BL41 cells were cultured for 48 h in the presence of TGFβ (1 ng/ml) for various times. Lane 1, for the first hour of culture; lane 2, during the whole culture. For lanes 3 and 4, cells were activated with TGFβ for the first 16 h of culture, harvested, washed, and cultured for an additional 30 h in the absence (lane 3) or the presence of TGFβ (lane 4). (B) BL41 cells were activated for 48 h with TGFβ (1 ng/ml) in the absence (lane 1) or the presence of SB203580 (20 μM) during the whole culture (lane 2), from 16 to 48 h (lane 3), or for the last hour of culture (lane 4). Shrunken cells were counted by flow cytometry, as described in the legend to Figure 1.
p38 Activation Can Promote Apoptosis in BL41 Cells
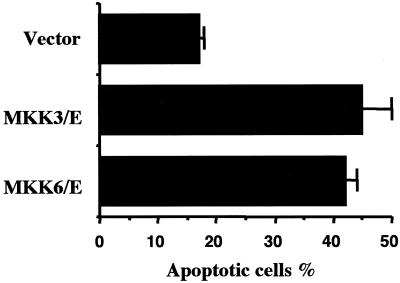
We therefore investigated whether p38 activation was sufficient to induce apoptosis in BL41 cells. For this, we overexpressed, by transient transfection, two dominant active mutants of upstream activators of p38, MKK3(b)E or MKK6(b)E (Han et al., 1996; Raingeaud et al., 1996) together with a plasmid encoding GFP to allow the identification of transfected cells. Eighteen hours after transfection, dead cells were removed by centrifugation through Ficoll and viable cells were cultured for a further 24 h. Apoptotic cells (characterized as shrunken cells) were then quantified in the GFP-positive cells populations. Fewer than 18% of cells transfected with the empty vector were apoptotic, whereas 42 and 45% of those transfected with MKK3(b)E and MKK6(b)E, respectively, were apoptotic (Figure 8). Thus, activation of p38 can promote an apoptotic death of these cells and the involvement of p38 activation in TGFβ-mediated apoptosis in BL41 cells is confirmed.
Figure 8.
Overexpression of MKK3 and MKK6 can promote apoptosis in BL41 cells. BL41 cells were transiently transfected with the pcDNA-MKK3(b)E (MKK3/E) or pcDNA-MKK(b)/E (MKK6/E) or the corresponding empty vector (vector) and the pIRES.hrGFP vector. Eighteen hours after transfection, dead cells, due to the electroporation shock, were removed from the cultures by centrifugation through a Ficoll gradient. Viable cells were then cultured at 37°C for 24 h. and the positive FL1-gated cells, that is, the cells transfected with GFP and either MKK3 and MKK6, were tested for apoptosis by assessing cell shrinkage.
DISCUSSION
TGFβ-mediated apoptosis is a complex process associated with cell cycle arrest. We previously reported that, in contrast to TGFβ-mediated cell cycle arrest, TGFβ-induced apoptosis in Burkitt lymphoma B cells was dependent on caspase activation (Schrantz et al., 1999). We showed that caspase-3 was activated and responsible for the control of various apoptotic features and Rb cleavage. Several studies have shown that caspase-3 may be activated by mitochondrial-dependent or -independent pathways (Porter and Janicke, 1999). Indeed, in type I Jurkat cells, Fas-activated caspase-8 may directly activate caspase-3, whereas in type II Jurkat cells, caspase-3 activation is dependent on a mitochondrial pathway associated with the caspase-8-dependent cleavage of Bid (Scaffidi et al., 1999). In turn, cleaved Bid induces opening of the permeability transition pore and the release of cytochrome c into the cytoplasm, which controls the activation of caspase-9 (Schendel et al., 1999). These different pathways leading to caspase-3 activation have been extensively explored with other stimuli, but the TGFβ-induced events involved in this apoptotic process upstream from caspase-3 remain poorly defined. We found that TGFβ induced caspase-8 activation, as shown by the appearance of the cleaved fragments p44/45 and p20, and the ability of cell lysates from TGFβ-activated BL41 cells to cleave in vitro colorimetric substrates specific for caspase-8 (IETD-pNA and AEVD-pNA). This caspase-8 activation was correlated with Bid cleavage. TGFβ also induced a decrease in mitochondrial membrane potential and the activation of caspase-9. All these events were inhibited by AEVD and IETD inhibitors, strongly suggesting that TGFβ-induced mitochondrial depolarization processes were mediated by Bid after its cleavage by caspase-8. In addition, flow cytometry analysis of ΔΨm and caspase-3 activity in the same cell indicated that caspase-3 activation did not occur in the absence of a loss of ΔΨm. This correlates well with the reported pathway of caspase-3 activation, which is dependent on the mitochondria release of cytochrome c. The association of cytochrome c and ATP with cytoplasmic Apaf1 then leads to autocleavage and the activation of caspase-9, which is directly responsible for the cleavage of caspase-3 (Li et al., 1997; Qin et al., 1999). Indeed, in BL41 cells, this pathway is probably responsible for caspase-3 activation because TGFβ mediates the activation of both caspase-9 and caspase-3. Our data are consistent with the notion that, in Burkitt's lymphoma cells, the TGFβ-induced apoptotic response is based on the sequential activation of caspase-8, cleavage of Bid, and loss of ΔΨm associated with caspase-9 and caspase-3 activation.
It has been suggested that caspase-10, which is very similar to caspase-8, is recruited along with caspase-8 into apoptosis signaling complexes associated with the death receptor and that caspase-10 plays a functional role in death receptor-mediated apoptosis (Fernandes-Alnemri et al., 1996; Vincenz and Dixit, 1997). However, the cleavage of this caspase was not demonstrated due to a lack of reliable antibodies. AEVD and IETD both inhibit caspase-10, although to a lesser extent than caspase-8 (Thornberry et al., 1997; Garcia-Calvo et al., 1998). Thus, the involvement of caspase-10 in TGFβ-activated pathways cannot be ruled out.
Recently, TGFβ has also been shown to activate caspase-8 in hepatoma cells as well as B cells, but the mechanisms involved in this activation are not well understood (Inman and Allday, 2000; Shima et al., 1999). Caspase-8 activation is mainly associated with apoptosis mediated by members of the TNF-R family, which possess a death domain in their cytoplasmic region (Ashkenazi and Dixit, 1998). On ligand binding, these receptors may directly or indirectly recruit the FADD adaptor protein. Caspase-8 activation results in autocleavage of the oligomerized proforms after association with FADD via interactions between the DEDs of the two molecules (Boldin et al., 1996; Muzio et al., 1996). The mechanism leading to caspase-8 activation by TGFβ was indirect because TGFβ receptors lack death domains. Interestingly, TGFβ-induced loss of ΔΨm and apoptosis show a late kinetics and were maximal after 48 h of stimulation. This suggests that TGFβ-induced caspase-8 activation and apoptosis may be mediated by a biphasic process resulting from the induction by TGFβ of ligands able to recruit FADD molecules via their death domains directly or indirectly. In agreement with Inman and Allday (2000), we found no evidence for the involvement of Fas/FasL or TNF/TNF-R interactions in our experimental conditions. This is consistent with the reported inhibition by TGFβ of CD95L-induced neutrophil apoptosis (Chen et al., 1998; Genestier et al., 1999). We therefore investigate directly the possible involvement of FADD in the TGFβ-induced activation of caspase-8, with the use of a dominant negative FADD molecule lacking the DED domain. Various clones producing only the endogenous FADD molecule or various amounts of the DED-truncated FADD-DN displayed similar patterns of apoptotic response and caspase-8 cleavage after TGFβ treatment. This suggests that TGFβ-mediated caspase-8 activation and apoptosis are independent of FADD. It is not clear whether TGFβ-induced caspase-8 activation is completely independent of FADD molecule or whether it could be mediated by FADD molecules devoid of DED domains. It has recently been reported that anticancer drugs induce apoptosis and caspase-8 cleavage in a FADD-independent manner, suggesting that death receptor activation is not a prerequisite for drug-induced caspase-8 activation (Wesselborg et al., 1999). The nature of the adaptor molecules capable of mediating caspase-8 oligomerization and cleavage remains to be determined. Nevertheless, our data are consistent with the emerging hypothesis that caspase-8 activation is not restricted to death receptors.
One clue for the characterization of this FADD-independent caspase-8 activation pathway is related to the TGFβ-mediated activation of p38. Indeed, this member of the serine/threonine MAPK family has been implicated in the regulation of apoptosis mediated by various stimuli. Several groups have reported that TGFβ promotes p38 activation dependent on upstream activation of MKK6 and TGFβ-activated kinase 1, but the role of p38 in TGFβ-mediated apoptosis is still unclear (Yamaguchi et al., 1995; Ichijo et al., 1997). TGFβ also promotes activation of the JNK pathway in various cell types (Atfi et al., 1997; Frey and Mulder, 1997). Although p38 was activated in our experimental conditions, we observed no activation of JNK, as assessed both by JNK phosphorylation and in vitro kinase assays, in TGFβ-treated BL41 cells. Although they differ between cell types and stimuli, p38 pathways are more frequently involved in the induction of the apoptotic response through different mechanisms. For instance, p38 has been reported to be involved in the induction of Fas-L, suggesting that one possible role for p38 is to activate, via the regulation of transcription factors such as activating transcription factor 2, the production of ligands of various members of the TNF-R family (Hsu et al., 1999). Fas has also been reported to promote activation of p38, suggesting that p38 may play a more direct role in the triggering of the apoptotic response (Juo et al., 1997). Zhuang et al. (2000) have recently reported that singlet oxygen-induced mitochondrial dysfunction, caspase-3 activation, and apoptosis are dependent on p38-mediated Bid cleavage. They reported that although singlet oxygen promotes both caspase-8 and Bid cleavage, the inhibition of p38 prevents the cleavage of Bid but has no effect on caspase-8 cleavage, suggesting that in their experimental conditions, p38-dependent Bid cleavage and mitochondrial activation were mediated by a caspase-8-independent pathway. p38 has also recently been shown to be involved in early events of cadmium-induced apoptosis upstream from the mitochondria (Galan et al., 2000). We used two different inhibitors of the p38 pathway to show that the TGFβ-mediated caspase-8 activation and loss of ΔΨm were p38-dependent. Together with the observation that inhibition of the loss of ΔΨm was also prevented by caspase-8 inhibitors, our data are consistent with the notion that, on TGFβ stimulation, p38 controls the activation of caspase-8, which is responsible for mitochondrial activation. Further evidence that p38 acts upstream from the caspase cascade is provided by the observation that p38 phosphorylation was not prevented by zVAD-fmk. Thus, p38-induced caspase-8 activation has previously been shown to be mediated by death domain receptor signaling, but our results provide evidence for another p38-dependent pathway independent of these death receptors.
The implication of p38 in the apoptotic process triggered by TGFβ in BL41 cells was strengthened by the observation that transient overexpression of active forms of MKK3 or MKK6, which lead to p38 activation, promotes a significant amount of apoptosis in these cells. In addition, when BL41 cells were transfected with MKK3 or MKK6 only transfected cells (GFP-positive cells) were apoptotic, whereas nontransfected cells (GFP-negative cells) also present in the same culture were not apoptotic, which is in favor of the hypothesis that p38 regulates TGFβ-mediated apoptosis of BL41 through an intracellular rather than an autocrine pathway. Nevertheless, the exact contribution of p38 activation during more physiological stimuli, like the presence of TGFβ, remains to be elucidated. Indeed, although p38 activation is necessary to promote apoptosis in BL41 cells in the presence of TGFβ, as demonstrated by the effect of various p38 inhibitors, it is possible that the regulation of the full apoptotic process required cooperation between p38 and the Smads-mediated pathway. This type of cooperation between the Smads and transcription factors activated by members of the MAPK family (including ERK and p38) has been observed (Hanafusa et al., 1999; Yue and Mulder, 2000), and our preliminary data also suggested cooperation between p38 and Smads during TGFβ-mediated BL41 activation. This raises the question of whether p38 directly modulates the phosphorylation states of adaptor molecules responsible for caspase-8 activation or could contribute to regulation by acting on the transcription of the genes encoding the regulatory molecules. Our preliminary data showing that the p38 inhibitor SB203580 inhibited a reporter gene that contains a TGFβ-inducible promotor are compatible with the hypothesis that TGFβ, through p38 activation, and thus probably activation of transcription factors, regulates the expression of a novel adaptor molecule, distinct from FADD. The further characterization of this (or these molecules) would then allow a better understanding of the precise role of p38 during TGFβ-mediated caspase-8 activation.
The activation of p38 by TGFβ appears to be biphasic or long lasting. Indeed, although p38 activation was detected as early as 1 h after TGFβ stimulation, maximum p38 phosphorylation was observed after 8 h and phosphorylation levels remained high until 24 h. Because caspase-8 activation and the loss of ΔΨm occurred only after 24 h of activation, the kinetics of p38 activation suggest that the apoptotic signaling induced by TGFβ was associated with this late p38 activation. Indeed, we observed that the presence of TGFβ during the first 24 h of stimulation was sufficient to promote cell cycle arrest, but only low levels of apoptosis. Maximum apoptosis at 48 h was observed only if cells were cultured continuously in the presence of TGFβ. This suggests that a first round of signaling, which may involve the regulation of cyclin-dependent kinase inhibitors or other pathways, occurs early during the first 24 h of incubation with TGFβ. Although, p38 was activated during this period, this pathway did not seem be involved in cell cycle control because p38 inhibitors SB203580 and PD169316 were not able to counteract the G1 accumulation of TGFβ-treated BL41 cells. In contrast, a late cell activation by TGFβ, involving p38 activation, seems to be required for caspase-8 activation and subsequent apoptosis because addition of SB203580 after 16 h of TGFβ stimulation prevented apoptosis measured after 48 h of stimulation. A similar biphasic pattern of activation of JNK by TGFβ human fibrosarcoma cells as well as activation of p38 and JNK mediated by TNF in hepatocyte cells has been reported previously but was not directly associated with caspase-8 activation (Hocevar et al., 1999; Talarmin et al., 1999), In addition, the requirement for long-lasting activation of JNK during apoptosis of Fas-activated human neuroblastoma cells and TNF-stimulated rat mesangial cells has also been reported (Goillot et al., 1997; Guo et al., 1998). Thus, the delayed activation of various members of the MAPK family plays a crucial role in determining their ability to regulate various biological activities. Indeed, biphasic activation has also been reported for ERK and the control of G1 progression and G1/S transition are directly regulated by late activation of ERK (Talarmin et al., 1999). Our data are consistent with a TGFβ-mediated apoptosis pathway dependent on the late activation of p38. Although the exact mechanism by which p38 activates caspase-8 is unknown, the finding that this MAPK pathway is involved in caspase activation provides new insight into the cascade of events leading to apoptosis mediated by TGFβ.
In conclusion, our data link TGFβ-activated signal transduction pathways to the caspase cascade by providing evidence that the cleavage of caspase-8 by TGFβ is controlled by upstream activation of the MAPK p38. This p38-mediated activation of caspase-8 is mediated by a novel pathway that appears to be independent of FADD.
ACKNOWLEDGMENTS
We thank F. Petit and J. Estaquier for their kindly collaboration and Drs V. Dixit and J. Han for the FADD-DN, MKK3(b) and MKK6(b) expression plasmids used in this study. This work was supported by INSERM and grants from the Association pour la Recherche sur le Cancer (ARC, Villejuif, France) and Fondation de France. N. Schrantz receives a fellowship from Foundation pour la Recherche Médicale.
REFERENCES
- Alnemri ES. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem. 1997;64:33–42. doi: 10.1002/(sici)1097-4644(199701)64:1<33::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor beta-mediated signaling. J Biol Chem. 1997;272:1429–1432. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- Blanchard DA, Mouhamad S, Auffredou MT, Pesty A, Bertoglio J, Leca G, Vazquez A. Cdk2 associates with MAP kinase in vivo and its nuclear translocation is dependent on MAP kinase activation in IL-2-dependent Kit 225 T lymphocytes. Oncogene. 2000;19:4184–4189. doi: 10.1038/sj.onc.1203761. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Callsen D, Brune B. Role of mitogen-activated protein kinases in S-nitrosoglutathione-induced macrophage apoptosis. Biochemistry. 1999;38:2279–2286. doi: 10.1021/bi982292a. [DOI] [PubMed] [Google Scholar]
- Chaouchi N, Arvanitakis L, Auffredou MT, Blanchard DA, Vazquez A, Sharma S. Characterization of transforming growth factor-beta 1 induced apoptosis in normal human B cells and lymphoma B cell lines. Oncogene. 1995;11:1615–1622. [PubMed] [Google Scholar]
- Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–1717. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, Tepper CG, Seldin MF, O'Rourke K, Kischkel FC, Hellbardt S, Krammer PH, Peter ME, Dixit VM. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Armstrong RC, Krebs J, Srinivasula SM, Wang L, Bullrich F, Fritz LC, Trapani JA, Tomaselli KJ, Litwack G, Alnemri ES. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CC, Srikanth S, Kraft AS. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc Natl Acad Sci USA. 1998;95:3014–3019. doi: 10.1073/pnas.95.6.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey RS, Mulder KM. Involvement of extracellular signal-regulated kinase 2 and stress-activated protein kinase/Jun N-terminal kinase activation by transforming growth factor beta in the negative growth control of breast cancer cells. Cancer Res. 1997;57:628–633. [PubMed] [Google Scholar]
- Galan A, Garcia-Bermejo ML, Troyano A, Vilaboa NE, de Blas E, Kazanietz MG, Aller P. Stimulation of p38 mitogen-activated protein kinase is an early regulatory event for the cadmium-induced apoptosis in human promonocytic cells. J Biol Chem. 2000;275:11418–11424. doi: 10.1074/jbc.275.15.11418. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- Genestier L, Kasibhatla S, Brunner T, Green DR. Transforming growth factor beta1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc. J Exp Med. 1999;189:231–239. doi: 10.1084/jem.189.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goillot E, Raingeaud J, Ranger A, Tepper RI, Davis RJ, Harlow E, Sanchez I. Mitogen-activated protein kinase-mediated Fas apoptotic signaling pathway. Proc Natl Acad Sci USA. 1997;94:3302–3307. doi: 10.1073/pnas.94.7.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves JD, Draves KE, Craxton A, Saklatvala J, Krebs EG, Clark EA. Involvement of stress-activated protein kinase and p38 mitogen-activated protein kinase in mIgM-induced apoptosis of human B lymphocytes. Proc Natl Acad Sci USA. 1996;93:13814–13818. doi: 10.1073/pnas.93.24.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YL, Kang B, Williamson JR. Inhibition of the expression of mitogen-activated protein phosphatase-1 potentiates apoptosis induced by tumor necrosis factor-alpha in rat mesangial cells. J Biol Chem. 1998;273:10362–10366. doi: 10.1074/jbc.273.17.10362. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Jiang Y, Li Z, Feng L, Ulevitch RJ. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- Hartsough MT, Mulder KM. Transforming growth factor beta activation of p44mapk in proliferating cultures of epithelial cells. J Biol Chem. 1995;270:7117–7124. doi: 10.1074/jbc.270.13.7117. [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Gavrilin MA, Tsai MH, Han J, Lai MZ. p38 mitogen-activated protein kinase is involved in Fas ligand expression. J Biol Chem. 1999;274:25769–25776. doi: 10.1074/jbc.274.36.25769. [DOI] [PubMed] [Google Scholar]
- Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates S.A.P.K/J.N.K. and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Allday MJ. Apoptosis induced by TGF-beta 1 in Burkitt's lymphoma cells is caspase 8 dependent but is death receptor independent. J Immunol. 2000;165:2500–2510. doi: 10.4049/jimmunol.165.5.2500. [DOI] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Juo P, Kuo CJ, Reynolds SE, Konz RF, Raingeaud J, Davis RJ, Biemann HP, Blenis J. Fas activation of the p38 mitogen-activated protein kinase signaling pathway requires ICE/CED-3 family proteases [published erratum appears in Mol. Cell. Biol. (1997) 17, 1757] Mol Cell Biol. 1997;17:24–35. doi: 10.1128/mcb.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. BMP2-induced apoptosis is mediated by activation of the TAK1–p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem. 2000;275:17647–17652. doi: 10.1074/jbc.M908622199. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Mechanisms mediating caspase activation in cell death. Cell Death Differ. 1999;6:1060–1066. doi: 10.1038/sj.cdd.4400600. [DOI] [PubMed] [Google Scholar]
- Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- Lens SM, Tesselaar K, den Drijver BF, van Oers MH, van Lier RA. A dual role for both CD40-ligand and TNF-alpha in controlling human B cell death. J Immunol. 1996;156:507–514. [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Qin H, Srinivasula SM, Wu G, Fernandes-Alnemri T, Alnemri ES, Shi Y. Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature. 1999;399:549–557. doi: 10.1038/21124. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- Schendel SL, Azimov R, Pawlowski K, Godzik A, Kagan BL, Reed JC. Ion channel activity of the BH3 only Bcl-2 family member, BID. J Biol Chem. 1999;274:21932–21936. doi: 10.1074/jbc.274.31.21932. [DOI] [PubMed] [Google Scholar]
- Schrantz N, Blanchard DA, Auffredou MT, Sharma S, Leca G, Vazquez A. Role of caspases and possible involvement of retinoblastoma protein during TGFβ-mediated apoptosis of human B lymphocytes. Oncogene. 1999;18:3511–3519. doi: 10.1038/sj.onc.1202718. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Seimiya H, Mashima T, Toho M, Tsuruo T. c-Jun NH2-terminal kinase-mediated activation of interleukin-1beta converting enzyme/CED-3-like protease during anticancer drug-induced apoptosis. J Biol Chem. 1997;272:4631–4636. doi: 10.1074/jbc.272.7.4631. [DOI] [PubMed] [Google Scholar]
- Shima Y, Nakao K, Nakashima T, Kawakami A, Nakata K, Hamasaki K, Kato Y, Eguchi K, Ishii N. Activation of caspase-8 in transforming growth factor-beta-induced apoptosis of human hepatoma cells. Hepatology. 1999;30:1215–1222. doi: 10.1002/hep.510300503. [DOI] [PubMed] [Google Scholar]
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennicke HR, Jurgensmeier JM, Shin H, Deveraux Q, Wolf BB, Yang X, Zhou Q, Ellerby HM, Ellerby LM, Bredesen D, Green DR, Reed JC, Froelich CJ, Salvesen GS. Pro-caspase-3 is a major physiologic target of caspase-8. J Biol Chem. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- Taieb J, Blanchard DA, Auffredou MT, Chaouchi N, Vazquez A. In vivo association between p56lck and MAP kinase during IL-2-mediated lymphocyte proliferation. J Immunol. 1995;155:5623–5630. [PubMed] [Google Scholar]
- Talarmin H, Rescan C, Cariou S, Glaise D, Zanninelli G, Bilodeau M, Loyer P, Guguen-Guillouzo C, Baffet G. The mitogen-activated protein kinase kinase/extracellular signal-regulated kinase cascade activation is a key signaling pathway involved in the regulation of G(1) phase progression in proliferating hepatocytes. Mol Cell Biol. 1999;19:6003–6011. doi: 10.1128/mcb.19.9.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenz C, Dixit VM. Fas-associated death domain protein interleukin-1beta-converting enzyme 2 (FLICE2), an ICE/Ced-3 homologue, is proximally involved in CD95- and p55-mediated death signaling. J Biol Chem. 1997;272:6578–6583. doi: 10.1074/jbc.272.10.6578. [DOI] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signaling pathways on cell survival. Biochem J. 1998;333:291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselborg S, Engels IH, Rossmann E, Los M, Schulze-Osthoff K. Anticancer drugs induce caspase-8/FLICE activation and apoptosis in the absence of CD95 receptor/ligand interaction. Blood. 1999;93:3053–3063. [PubMed] [Google Scholar]
- Xia X, Deckens M, Raingeaud J, Davis RJ, Greenberg ME. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Requirement of Ras/MAPK pathway activation by transforming growth factor beta for transforming growth factor beta 1 production in a smad-dependent pathway. J Biol Chem. 2000;275:35656. [PubMed] [Google Scholar]
- Zhuang S, Demirs JT, Kochevar IE. p38 Mitogen-activated protein kinase mediates Bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen, but not by hydrogen peroxide. J Biol Chem. 2000;275:25939–25948. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]