Abstract
Introduction
Chronic periodontitis (CP) is an infectious disease resulting in inflammation of the supporting tissues of the teeth with progressive attachment loss and bone loss. This study aimed to evaluate the effect of 980-nm diode laser, as an adjunct to scaling and root planing (SRP) in the management of CP.
Methodology
A total of 40 systemically healthy subjects diagnosed with CP were randomly assigned into two groups G1 (SRP and sham application of laser) and G2 (SRP and laser irradiation) with equal numbers in each. The levels of Porphyromonas gingivalis (Pg) were estimated from plaque samples using real-time polymerase chain reaction. Clinical and microbiological parameters were assessed at baseline, 4–6, and 12–14 weeks posttreatment in both groups.
Results
A gradual reduction in the levels of Pg and improvement in clinical parameters were observed from baseline to 4–6 and 12–14 weeks in both groups. However, the comparison between groups, although clinically relevant, was not found to be statistically significant.
Conclusion
Although a 980-nm diode laser may not have any added benefit compared with SRP, it may emerge as an effective non-surgical treatment option in advanced periodontitis with complex inaccessible subgingival niches where comprehensive periodontal care may not be feasible.
Keywords: plaque samples, polymerase chain reaction, Porphyromonas gingivalis, scaling and root planing, chronic periodontitis, diode laser
Introduction
Chronic periodontitis (CP) is an infectious disease resulting in inflammation of the supporting tissues of the teeth with progressive attachment loss and bone loss associated with a complex subgingival ecosystem, predominantly the gram-negative bacterial species. Of these, the dark-pigmented microorganism, Porphyromonas gingivalis (Pg), is a major pathogen. Pg being a strict anaerobe occurs with greater frequency and higher levels in active sites of the disease. Periodontal health indicators are inversely correlated with the presence or levels of Pg. Low sensitivities of culturing techniques and sampling of a limited number of oral sites have caused an underestimation of the true prevalence of this organism in majority of the studies [1]. Polymerase chain reaction (PCR) assay has been shown to be a sensitive and rapid method for the detection and quantification of Pg [2]. Conventional mechanical debridement can achieve a temporary decrease in the subgingival levels of Pg along with other pathogens. However, mechanical therapy alone may fail to eliminate pathogenic bacterial niches in inaccessible areas, such as deep pockets, root concavities, furcation areas, etc. In search of more efficient and less-traumatic techniques to improve periodontal healing, researchers proposed the use of lasers in periodontal therapy [3].
The adjunctive use of lasers with conventional tools may facilitate treatment and have the potential to improve healing. The diode laser is highly absorbed in hemoglobin and other pigments and is excellent for use in soft tissue surgical procedures [3]. Laser application in improving clinical outcome in periodontal therapy needs to be further investigated with well-designed clinical trials. We attempted to evaluate the efficacy of diode laser as an adjunct to scaling and root planing (SRP) in the management of CP by evaluating clinical parameters coupled with quantitative estimation of Pg using real-time PCR (RT-PCR) assay.
Materials and Methods
A total of 40 systemically healthy subjects diagnosed with generalized moderate CP in the age group of 30–50 years with a minimum of 20 teeth having at least two non-adjacent sites per quadrant with probing pocket depth (PPD) of ≥5 mm and clinical attachment loss of 3–4 mm were recruited from the outpatient division of the Department of Periodontology, KLE Society’s Institute of Dental Sciences, Bangalore, India. Patients who had undergone periodontal therapy 6 months prior to the commencement of the study, subjects on antibiotics or immunosuppressants, chronic smokers, alcoholics, smokeless tobacco users, subjects with acute illnesses/acute intraoral lesions, pregnant women, and subjects who had undergone extensive restorative dental treatment were excluded from the study. A written informed consent was obtained from each subject recruited for the study. Ethical clearance for sample collection, standard non-surgical periodontal intervention, and diode laser therapy was obtained from the institutional ethics committee. The selected subjects were randomly assigned into two groups G1 and G2 with equal numbers in each.
Microbial sampling [4]
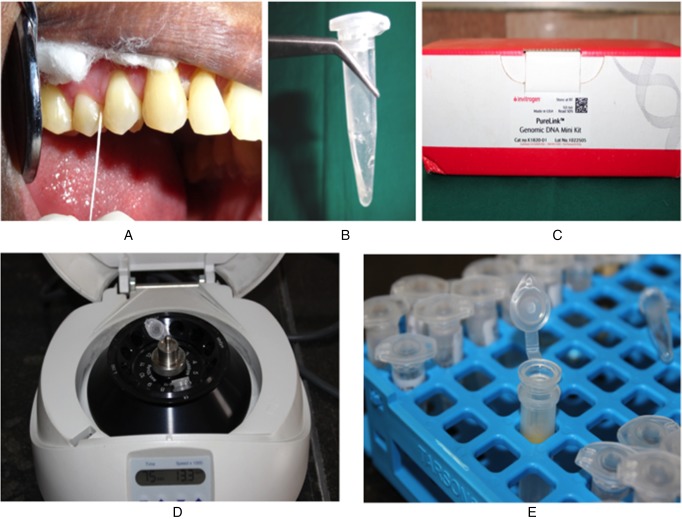
The site with the deepest PPD was selected for microbial sampling. After careful isolation, supragingival plaque was gently removed with a sterile curette. A sterile paper point was inserted at the selected site and left undisturbed for 15 s. The paper points containing pooled subgingival plaque samples were transferred to the laboratory in a selective transport media for microbiological evaluation of Pg by RT-PCR. Samples were stored at −70 °C until processing in the laboratory (New Brunswick Scientific Innova ultra-low temperature freezer) (Fig. 1).
Fig. 1.
Microbial sampling and processing. (A) Plaque sample collection using a sterile paper point. (B) Sample placed in vial containing 10× TE (proteinase, DNase, and RNase transport medium). (C) DNA isolation kit. (D) Centrifugation. (E) Purified genomic DNA
Clinical examination
For all subjects enrolled in the study, the plaque index (PI; Silness and Loe 1964) [5] and gingival index (GI; Loe and Silness 1963) [5] were recorded. The pocket depth was recorded using a William’s graduated periodontal probe to the nearest millimeter as the distance from the crest of the gingival margin to the base of the pocket [6]. The clinical attachment level (CAL) was recorded using customized acrylic stents with guiding grooves. The average percentage of total sites with bleeding on probing (BOP) was recorded by moving the periodontal probe around the gingival sulcus and waiting for 30–60 s to elicit bleeding from the gingival sulcus [6, 7].
The assessment of clinical and microbiological parameters was carried out at baseline, 4–6, and 12–14 weeks’ posttreatment in both the groups [8].
In G1, following SRP, sham application of laser (directing the laser device without turning on the light beam) was performed. In G2, following SRP, laser irradiation was performed with 980-nm diode laser using a 320-μm optical fiber moved in the corono-apical direction of the pocket in parallel paths with an inclination of approximately 20° for 30 s twice, with a 60-s interval (Fig. 2). The procedure of sham laser and laser irradiation was repeated after 1 week in G1 and G2, respectively [4]. All the safety protocols for class 4 lasers were followed. Patients of both the groups were put on a meticulous oral hygiene regimen and periodically monitored throughout the study period.
Fig. 2.
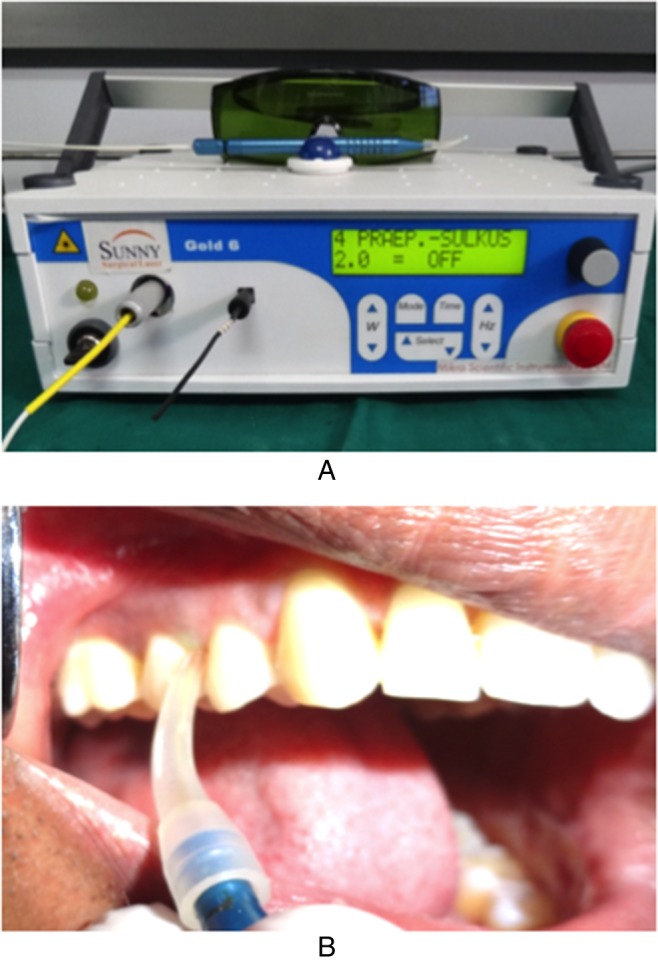
LASER irradiation. (A) Diode laser (980 nm, 2W, continuous mode). (B) Irradiation with 980-nm diode laser by 320-μm optic fiber
PCR: DNA extraction [4]
DNA extraction from the plaque samples was done using highly purified invitrogen DNA isolation kit (PureLink™ DNA extraction kit).The standard proteinase K method was followed for DNA isolation. The water bath was set at 55 °C. 20 μl of proteinase K was added to a sterile microcentrifuge tube. 200 μl of sample was transferred to the tube containing proteinase K and incubated at 55 °C for 30 min. 20 μl of RNAse A was added to the lysate and mixed well by briefly vortexing and incubated at room temperature for 2 min. 200 μl of PureLink genomic lysis/binding buffer was added and mixed well by vortexing to obtain a homogenous solution. 200 μl of 96%–100% ethanol was mixed well by vortexing for 5 s to obtain a homogeneous solution to proceed for the purification protocol immediately.
Purification method [4]
The purification procedure was designed for purifying genomic DNA using a spin column-based centrifugation procedure for a duration of 10–15 min. PureLink™ spin column was removed in a collection tube from the package. The entire lysate prepared with PureLink™ genomic lysis/binding buffer and ethanol was added to the spin column. The column was centrifuged at 10,000 rpm for 1 min at room temperature. The collection tube was placed into a clean PureLink™ collection tube supplied with the kit. An amount of 500 μl of wash buffer prepared with ethanol was added to the column. The column was centrifuged at maximum speed for 3 min at room temperature. The collection tube was discarded. The spin column was placed in a sterile 1.5-ml microcentrifuge tube. An amount of 25–200 μl of PureLink™ genomic elution buffer was added to the column and incubated at room temperature for 1 min. The column was then centrifuged at the maximum speed for 1 min at room temperature, thus obtaining purified genomic DNA in the tube. Purified DNA was stored at −20 °C until further processing.
DNA quantification [4]
Custom SYBR Green assay reagents for Pg (Applied Biosystems, India) were used in this study. The primer sequence specific to Pg selected for the study was as follows: Pg forward –3′-TGCAACTTGCCTTACAGAGGG-5′ and Pg reverse –5′-ACTCGTATCGCCCGTTATTC-3′. A reaction solution was composed of SYBR Green Universal PCR Master Mix (10 μl), forward primer (1 μl), and reverse primer (1 μl) for Pg, extracted DNA of unknown sample (1 μl) and nucleus-free water to make a complete reaction volume of 20 μl. The conditions for RT-PCR were as follows: Holding stage at 95 °C for 10 s followed by 40 cycles of shuttle heating at 95 °C for 15 s and at 60 °C for 1 min. The melt curve stage was at 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s. Relative quantity for Pg was based on the cycle threshold (the number of PCR cycles necessary to obtain the threshold signal of fluorescence) values. All the calculations were done using Applied Biosystems Software.
The statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS version 10.5) software. The normality of data was assessed using Shapiro–Wilk test. The p value for Pg levels measured in percentage was <0.001. Thereby, non-parametric test, Kruskal–Wallis, and Mann–Whitney U tests were employed. The p value was >0.05 for clinical parameters. Thereby, a non-parametric test like one-way analysis of variance was used to test the difference between groups.
Results
The levels of Pg showed a statistically significant reduction from baseline to 4–6 weeks in both G1 and G2 (p value < 0.001). Although the reduction in levels of Pg in G2 from baseline (44.25%) to 4–6 weeks (21.2%) was more significant in comparison with G1 from baseline (41.1%) to 4–6 weeks (30.2%), it was not found to be statistically significant. There was an increase in the levels of Pg at 12–14 weeks in both G1 (34.7%) and G2 (36.25%), which was also not found to be statistically significant (p value = 0.063) (Table I, Fig. 3).
Table I.
Comparison of levels of Pg between G1 and G2 in percentage at baseline, 4–6, and 12–14 weeks
| Visit | Group | N | Mean | SD | Median | Min | Max | Mann–Whitney U | p value |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | G1 | 20 | 41.10 | 3.582 | 40.00 | 35 | 52 | 77.000 | 0.001 |
| G2 | 20 | 44.25 | 2.731 | 45.00 | 38 | 52 | |||
| 4–6 weeks | G1 | 20 | 30.20 | 4.663 | 29.00 | 25 | 40 | 40.000 | <0.001 |
| G2 | 20 | 21.20 | 5.764 | 20.00 | 15 | 38 | |||
| 12–14 weeks | G1 | 20 | 34.70 | 3.181 | 35.00 | 30 | 45 | 131.500 | 0.063 |
| G2 | 20 | 36.25 | 3.210 | 35.00 | 30 | 42 |
Fig. 3.
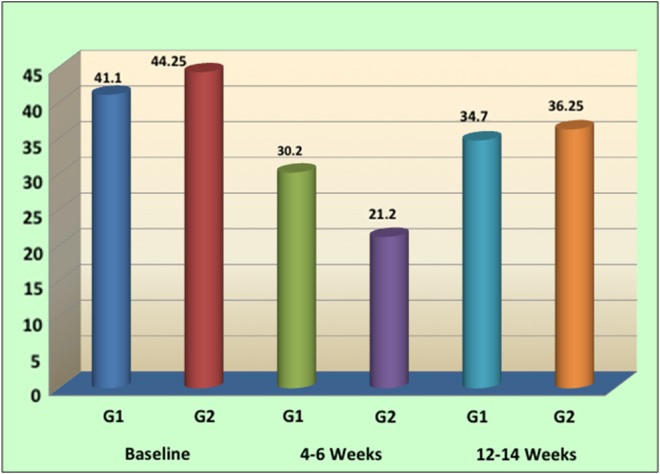
Comparison of levels of Pg in percentage between G1 and G2
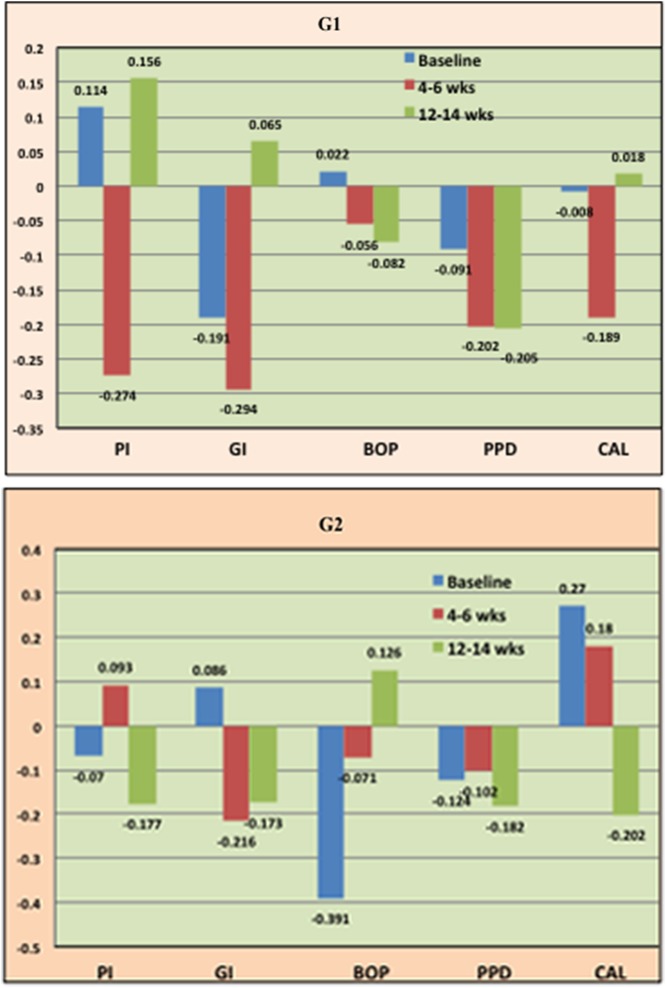
In G1, between the levels of Pg and PI, a positive correlation at baseline (0.114), a negative correlation at 4–6 weeks (−0.274), and a positive correlation at 12–14 weeks (0.156) was observed. There was a negative correlation between the levels of Pg and GI, at baseline (−0.191) and 4–6 weeks (−0.294), respectively and positive correlation at 12–14 weeks (0.065).There was a positive correlation between the levels of Pg and BOP at baseline (0.022) and a negative correlation at 4–6 weeks (−0.056) and 12–14 weeks (−0.082), respectively. There was a negative correlation between the levels of Pg and PPD at baseline (−0.091), 4–6 weeks (−0.202), and 12–14 weeks (−0.205), respectively. There was a negative correlation between the level of Pg and CAL at baseline (−0.294) and 4–6 weeks (−0.189), respectively and a positive correlation at 12–14 weeks (0.018) (Fig. 4).
Fig. 4.
Correlation coefficient between levels of Pg and clinical parameters in G1 and G2
In G2, between the levels of Pg and PI, a negative correlation at baseline (−0.070) and 4–6 weeks (−0.177) and a positive correlation at 12–14 weeks (0.093) was observed. There was a positive correlation between the levels of Pg and GI, at baseline (0.086) and negative correlation at 4–6 weeks (−0.216) and at 12–14 weeks (−0.173), respectively. There was a negative correlation between the levels of Pg and BOP at baseline (−0.391) and 4–6 weeks (−0.071), respectively and a positive correlation at 12–14 weeks (0.126). There was a negative correlation between the levels of Pg and PPD at baseline (−0.124), 4–6, (−0.102) and 12–14 weeks (−0.182), respectively. There was a positive correlation between the levels of Pg and CAL at baseline (0.270) and 4–6 weeks (0.180), respectively and a negative correlation at 12–14 weeks (–0.202) (Fig. 4).
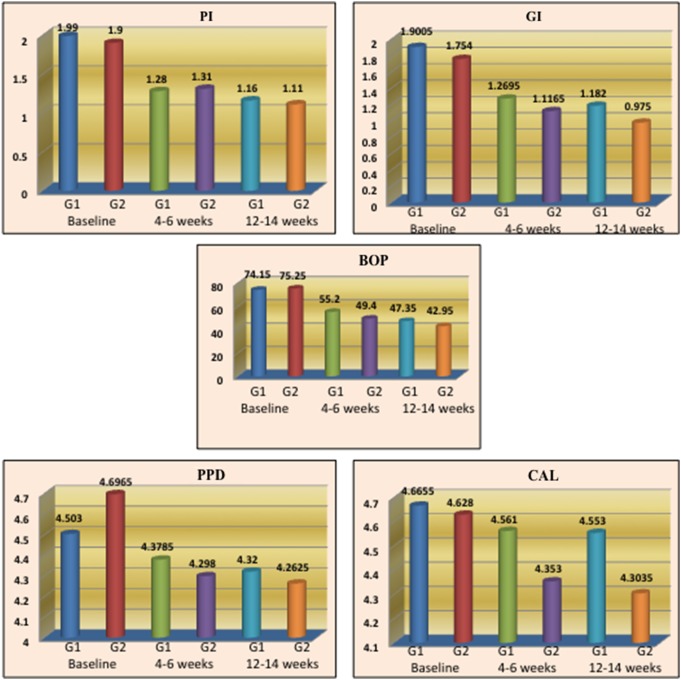
The comparison of clinical parameters (PI, GI, BOP, PPD, and CAL) between G1 and G2 did not show any statistically significant difference (Fig. 5).
Fig. 5.
Comparison of clinical parameters between G1 and G2 at baseline, 4–6, and 12–14 weeks
Discussion
Emerging evidence indicates that Pg has a more complex relationship with the host. It is not only considered as the most virulent periopathogen but rather a “key-stone pathogen” possessing an array of virulence factors and the potential to cause dysbiosis of the subgingival biofilm environment. The intracellular invasion of Pg could constitute a reservoir of bacteria for repopulation of treated subgingival sites. Pg isolated from sites with the disease has greater invasion capabilities than from healthy sites. By moving deeper into the epithelial layers, Pg can ensure access to viable non-shedding epithelial cells. These pathogens are also known to penetrate the basement membrane and gain access to the connective tissue [9]. In addition, in the disease states, intracellular bacteria are less likely to be physically removed by SRP and are more resistant to antibiotics [10, 11]. To overcome these barriers and limitations of conventional periodontal therapy, laser irradiation may possibly emerge as an effective adjunct to SRP.
The use of the diode laser in periodontal treatment has not been the subject of much investigation. Several authors have reported the use of the Nd:YAG for this purpose. The Nd:YAG laser emits radiation in the infrared spectrum and can be used in the destruction of periodontal pathogens, presumably through its thermal effect. However, changes in the adjacent cells may be brought about by virtue of these thermal effects. The diode laser belongs to the spectrum ranging from 655 to 980 nm and could represent a safer alternative [12]. Diode laser does not ablate calculus on the root surface [4]. Pg being an asaccharolytic obligatory anaerobe produces black pigments in agar–blood Brucella media. It is possible to affirm that hemoglobin in the soft tissue of periodontal pockets is an absorber chromophore for the high-power diode laser and acts as an endogenous dye, enhancing the laser’s effect at this site [13]. Therefore, it may be useful as an adjunct to SRP due to its bactericidal and detoxification effects [14]. Several studies have shown that multiple adjunctive applications of a 980-nm diode laser with SRP improve clinical parameters only in sites with moderate periodontal pockets of depth 4–6 mm [15].
In this study, the reduction in levels of Pg in G1 and G2 was statistically significant and similar results were found in several other studies that showed a reduction in levels of Pg from baseline to 3 months’ post-SRP. The clinical improvement noted after SRP could be associated with qualitative changes in the subgingival microbiota [8, 16]. However, treated sites tend to get recolonized by bacteria after several weeks or months. It is probable that bacteria remaining in the pocket after debridement play an important role in repopulation and reestablishment of subgingival microbiota. There was an increase in levels of Pg at 12–14 weeks compared with the levels at 4–6 weeks. However, this increase was not statistically significant. A similar observation was made in another study that attributed this to bacterial recolonization [16]. Another study found a favorable bacterial reduction at 2 weeks’ post-laser therapy for all the bacterial species tested, although all species were significantly reduced or eliminated at 12 weeks when compared with the pretreatment levels. This favorable effect might be due to the ability of laser to eliminate bacteria in the dentinal tubules that can act as reservoirs for recolonization and reinfection of the periodontal pocket [8]. Several other studies have showed that the diode laser has an effective antibacterial effect [17]. However, some studies have shown that gallium–aluminum–arsenide diode laser has no additional microbiological and clinical benefits over conventional mechanical debridement [4, 7].
In this study, G1 and G2 showed improvement in all clinical variables measured from baseline to 4–6 weeks and baseline to 12–14 weeks. This was concurrent to the results of other studies employing a similar methodology [8, 13, 18–22].
A positive correlation as suggested by several studies was observed between the levels of Pg and clinical parameters evaluated at baseline, 4–6, and 12–14 weeks in both the study groups. This suggested that the levels of Pg play an integral role in the initiation and progression of CP [7, 23–27].
The reduction in levels of Pg and improvement in clinical parameters were not found to be statistically significant between the two groups. This could suggest that laser may not have any added benefit compared with the gold standard of periodontal therapy in the management of mild to moderate CP but hypothetically emerge as an effective non-surgical treatment modality in patients with advanced periodontitis with multiple complex, inaccessible subgingival niches who prefer non-surgical treatment and in medically compromised patients, in whom standard, comprehensive periodontal care may not be feasible. Probably, a longer follow-up and a larger sample size would have contributed more to the data. Only Pg was included in this study as a microbiological marker. However, periodontal diseases are known to be polymicrobial in nature involving complex interactions between various microbial species. In addition, due to ethical considerations, histomorphometric analysis could not be performed to assess the depth of penetration of laser.
Conclusions
Non-surgical periodontal therapy in CP resulted in overall improvement in clinical parameters as well as reduction in the levels of Pg at the end of 12–14 weeks. The 980-nm diode laser used as an adjunct to SRP led to clinically significant improvement in clinical parameters and microbial profile compared with SRP alone. However, the comparison between groups, though clinically relevant, was not found to be statistically significant. Increase in the levels of Pg at 12–14 weeks compared with the 4- to 6-week interval in both the groups could be attributed to bacterial recolonization. RT-PCR served as a reliable diagnostic tool for detection and quantification of Pg, which has a pivotal role to play in the initiation and progression of CP. Longitudinal studies with a longer follow-up can bring about a better understanding of the added benefit of 980-nm diode laser as an adjunct to SRP for more effective management of CP.
Acknowledgement
The work was carried out at the Department of Periodontology, KLE Society’s Institute of Dental Sciences, Bangalore and Department of Microbiology, Rajarajeshwari Dental College and Hospital, Bangalore.
Funding Statement
Funding sources: No financial support was received for this study.
Authors’ contribution
All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
None.
References
- 1.Komerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M: In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother 47, 932–940 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutaga K, Winkelhoff AJ, Vandenbroucke-Grauls CM, Savelkoul PH: Comparison of real-time PCR and culture for detection of Porphyromonas gingivalis in subgingival plaque samples. J Clin Microbiol 41, 4950–4954 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki A, Sasaki MK, Watanabe H, Ishikawa I: Lasers in nonsurgical periodontal therapy. Periodontol 2000 36, 59–97 (2004) [DOI] [PubMed] [Google Scholar]
- 4.Caruso U, Nastri L, Piccolomini R, d’Ercole S, Mazza C, Guida L: Use of diode laser 980 nm as adjunctive therapy in the treatment of chronic periodontitis. A randomized controlled clinical trial. New Microbiol 31, 513–518 (2008) [PubMed] [Google Scholar]
- 5.Loe H: The Gingival Index, the Plaque Index and the Retention Index System. J Periodontol 38, 610–616 (1967) [DOI] [PubMed] [Google Scholar]
- 6.Isidor F, Karring T, Attstrom R: Reproducibility of pocket depth and attachment level measurements when using a flexible splint. J Clin Periodontol 11, 662–668 (1994) [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz S, Kuru B, Kuru L: Effect of gallium arsenide diode laser on human periodontal disease: A microbiological and clinical study. J Clin Laser Med Surg 30, 60–66 (2002) [DOI] [PubMed] [Google Scholar]
- 8.Kamma JJ, Vasdekis VG, Romanos GE: The effect of diode laser (980 nm) treatment on aggressive periodontitis: Evaluation of microbial and clinical parameters. Photomed Laser Surg 27, 11–19 (2009) [DOI] [PubMed] [Google Scholar]
- 9.Grenier D, Cazalis J, Gagnon G: Response of periodontitis and healthy patients in a Porphyromonas gingivalis stimulated whole blood model. J Investig Clin Dent 2, 38–42 (2011) [DOI] [PubMed] [Google Scholar]
- 10.Giannopoulou C, Cappuyns I, Cancela J, Cionca N, Mombelli A: Effect of photodynamic therapy, diode laser, and deep scaling on cytokine and acute-phase protein levels in gingival crevicular fluid of residual periodontal pockets. J Periodontol 83, 1018–1027 (2012) [DOI] [PubMed] [Google Scholar]
- 11.Karlsson MR, Lofgren CD, Jansson HM: The effect of laser therapy as an adjunct to non-surgical periodontal treatment in subjects with chronic periodontitis: A systematic review. J Periodontol 79, 2021–2028 (2008) [DOI] [PubMed] [Google Scholar]
- 12.Klein MI, Gonçalves RB: Detection of Tannerella forsythensis (Bacteroides forsythus) and Porphyromonas gingivalis by polymerase chain reaction in subjects with different periodontal status. J Periodontol 74, 798–802 (2003) [DOI] [PubMed] [Google Scholar]
- 13.De Micheli G, de Andrade AK, Alves VT, Seto M, Pannuti CM, Cai S: Efficacy of high intensity diode laser as an adjunct to non-surgical periodontal treatment: A randomized controlled trial. Lasers Med Sci 26, 43–48 (2011) [DOI] [PubMed] [Google Scholar]
- 14.Rossmann JA, Cobb CM: Lasers in periodontal therapy. Periodontol 2000 9, 150–164 (1995) [DOI] [PubMed] [Google Scholar]
- 15.Dukic W, Bago I, Aurer A, Roguljic M: Clinical effectiveness of diode laser therapy as an adjunct to non-surgical periodontal treatment: A randomized clinical study. J Periodontol 84, 1111–1117 (2013) [DOI] [PubMed] [Google Scholar]
- 16.Cobb CM, Blue MS, Beaini NE, Umaki MR, Satheesh KM: Diode laser offers minimal benefit for periodontal therapy. Compend Contin Educ Dent 33, 67–73 (2012) [PubMed] [Google Scholar]
- 17.Euzebio Alves VT, de Andrade AK, Toaliar JM, Conde MC, Zezell DM, Cai S, Pannuti CM, De Micheli G: Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: A 6-month clinical trial. Clin Oral Investig 17, 87–95 (2013) [DOI] [PubMed] [Google Scholar]
- 18.Philstrom BL, McHugh RB, Oliphant TH, Ortiz-Campos C: Comparison of surgical and nonsurgical treatment of periodontal disease. A review of current studies and additional results after 6 1/2 years. J Clin Periodontol 10, 524–541 (1983) [DOI] [PubMed] [Google Scholar]
- 19.Lindhe J, Westfelt E, Nyman S, Socransky SS, Haffajee AD: Long-term effect of surgical/non-surgical treatment of periodontal disease. J Clin Periodontol 11, 448–458 (1984) [DOI] [PubMed] [Google Scholar]
- 20.Badersten A, Nilveus R, Egelberg J: Effect of non-surgical periodontal therapy. II. Severely advanced periodontitis. J Clin Periodontol 11, 63–76 (1984) [DOI] [PubMed] [Google Scholar]
- 21.Ramfjord SP, Caffesse RG, Morridon EC, Hill RW, Kerry GJ, Appleberry EA, Nissle RR, Stults DL: Four modalities of periodontal treatment compared over 5 years. J Clin Periodontol 14, 445–452 (1978) [DOI] [PubMed] [Google Scholar]
- 22.Cugini MA, Haffajee AD, Smith C, Kent RL, Socransky SS: The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J Clin Periodontol 27, 30–36 (2000) [DOI] [PubMed] [Google Scholar]
- 23.Doungudomdacha S, Rawlinson A, Walsch TF, Douglas CW: Effect of non-surgical periodontal treatment on clinical parameters and the numbers of Porphyromonas gingivalis, Prevotella intermedia and Actinobacillus actinomycetemcomitans at adult periodontitis sites. J Clin Periodontol 28, 437–445 (2001) [DOI] [PubMed] [Google Scholar]
- 24.Wu YM, Yan J, Chen L, Zhi GY: Association between infection of different strains of Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans in subgingival plaque and clinical parameters in chronic periodontitis. J Zhejiang Univ Sci B 8, 121–131 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vajawat M, Kumar V, Rajeshwari KG, Deepika PG: Clinical, microbiological and molecular study of Porphyromonas gingivalis in patients with chronic periodontitis. Int J Basic Appl Med Sci 3, 56–61 (2013) [Google Scholar]
- 26.Griffen AL, Becker MR, Lyons SR, Moeschberger ML, Leys EJ: Prevalence of Porphyromonas gingivalis and periodontal health status. J Clin Microbiol 36, 3239–3242 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang HW, Huang YF, Chou MY: Occurrence of Porphyromonas gingivalis and Tannerella forsythensis in periodontally diseased and healthy subjects. J Periodontol 75, 1077–1083 (2004) [DOI] [PubMed] [Google Scholar]