Abstract
The article reveals patterns of changes in the parameters of oxidative modification of proteins for different periods of the inflammatory process in periodontal tissues during periodontitis. Biochemical researches of phenylhydrazones, aldehyde-, and ketone derivatives of neutral and basic proteins were determined in the blood of white rats on the 7th, 14th, and 30th days of the experimental periodontitis development, as well as in intact animals. The method for determination of the oxidative modification of proteins was based on the interaction of oxidized amino acid residues with 2,4-dinitrophenylhydrazine to form 2,4-dinitrophenylhydrazones. At the early stage of experimental periodontitis development, i.e., on the 7th day, an increase in the products of oxidative modification of proteins of basic and neutral nature was observed in the blood serum, but later, on the 14th day, this index changed in opposite direction, i.e., it began to decrease; however, it was higher relative to the intact group of animals. The obtained factual data evidence that under the conditions of experimental periodontitis formation, there is an intensive increase in the level of oxidative modification of proteins especially in the early period of the inflammatory process development.
Keywords: periodontitis, oxidative modification of proteins, inflammation, processes of lipid peroxidation, oxidative stress
Background
The development of inflammatory-destructive changes in periodontal tissues is associated with impaired microcirculation and transcapillary exchange in the presence of pronounced hypoxia. Among all the consequences and complications of hypoxia, the most serious is the intensification of free radical oxidation and the suppression of antioxidant protection of biological tissues [1]. The activation of lipid peroxidation (LPO) occurs in mechanisms of inflammatory process development in the periodontal complex and is one of the trigger mechanisms of stress damage with a disruption in the metabolism of cells, which are primarily associated with the damage of cellular and subcellular membranes [2–5]. The activation of LPO and a decrease in antioxidant activity occurred due to the accumulation of free modificated cholesterol, esterified cholesterol, lysophosphatides, cardiolipin, phosphatidylcholine, a decrease in non-esterified fatty acids, etc. [6, 7]. The elucidation of the mechanisms of their arise at the level of metabolic processes disorder that lead to structural damage in the periodontal tissues and lead to different degree heavy of an inflammatory process [8]. It is known fact about the role of oxidative stress in the pathogenesis of periodontitis that allow us to consider the activity of LPO in saliva and its antioxidant potential as potential predictors of the inflammatory periodontal lesion progression [9, 10]. The aim of this investigation is to elucidate the pathogenetic role of aldehyde- and ketone derivatives of neutral and basic character in the dynamics of experimental periodontitis (EP) development.
Materials and Methods
The experimental studies were performed with the use of clinically healthy white rats weighing 150–200 g in the conditions of vivarium, kept under a standard diet balanced for the basic elements. The investigation was conducted in conformity with the general rules and regulations of the “European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes” (Strasbourg, 1986) and the “General Ethical Principles of Animal Experimentation” (Kyiv, 2001).
The animals were divided into four groups: I – intact animal, controls (n = 10); II – animals with EP on the 7th day of the experiment (n = 8); III – animals with EP on the 14th day of the experiment (n = 8); IV – animals with EP on the 30th day of the experiment (n = 8). EP was produced in the experimental animals by introducing complex mixtures of microorganisms diluted in egg protein into periodontal tissues [11]. Simultaneously with the injections of the pathogen, a complete Freund’s adjuvant was injected in the rat’s paw to enhance the immune response. When conducting studies with animals of group IV, on the 14th day, repeated entry of pathogenic and injection of adjuvant was carried out. On the 7th and 14th day of the experiment, animals were killed by bleeding using thiopental anesthesia. For further testing, serum was selected. In serum was determined the level of oxidative modificated proteins (OMP) by neutral and basic characters. The method for determining the oxidative modification of proteins was based on the interaction of oxidized amino acid residues with 2,4-dinitrophenylhydrazine (2,4-DNPH) to form 2,4-dinitrophenylhydrazones. The optical density of the experimental sample of aldehyde- and ketone derivatives of a neutral character was recorded at 370 nm (OMP370), and the main sample at 430 nm (OMP430) relative to the control sample on a SF-46 spectrophotometer [12]. The results were statistically analyzed using non-parametric indexes in the Excel software (Microsoft, USA) and STATISTICA 10.0 (StatSoft, USA). The reliability of the differences in values between independent quantitative values was determined with a normal distribution according to the Mann–Whitney U criterion [13].
Results
Introduction of complex mixtures of microorganisms diluted in egg protein into periodontal tissues had caused hyperergic inflammatory process with expressed changes in the soft tissue of the lower jaw, accompanied by edema and hyperemia of the mucous membrane and the characteristics of the symptoms were the same as the changes in humans [11, 14]. Significant quantitative changes were found in prooxidant–antioxidant system [15].
These results of LPO activity changes showed that irrespective of the conditions of their study in the process of periodontitis development proceed the formation and accumulation of intermediate toxic products of lipoperoxidation in serum that occur at different stages of its chain branching.
An important index of free radical processes is the oxidative modification of proteins, in a result of which the proteolysis in proteosomes is activated and alterative changes in the inflammatory area are intensified. Oxidation of amino acids in proteins causes structural changes in them, which are manifested by aggregation, fragmentation, and also increased sensitivity to proteolysis. OMP products in comparison with lipid peroxides are stable and have the ability to be quickly metabolized by low molecular antioxidants and peroxidases [15].
The results of these experiments are presented in Table I.
Table I.
The content of OMP370 and OMP430 in the rats serum for different periods of experimental periodontitis formation (M ± m)
| The form of experiment | Control, intact animals | Animals with periodontitis | ||
|---|---|---|---|---|
| Experiment duration (days) | – | 7 | 14 | 30 |
| Number of animals | 10 | 8 | 8 | 8 |
| OMP370, mol/L | 0.48 ± 0.02 | 0.86 ± 0.01 | 0.65 ± 0.01 | 0.79 ± 0.03 |
| p1 < 0.01 | p1 < 0.01, p2 < 0.01 | p1 < 0.01, p2 < 0.01, p3 < 0.01 | ||
| OMP430, mol/L | 0.61 ± 0.01 | 1.07 ± 0.01 | 0.86 ± 0.01 | 0.89 ± 0.03 |
| p1 < 0.01 | p1 < 0.01, p2 < 0.01 | p1 < 0.01, p2 < 0.01, p3 > 0.05 | ||
p1 – significance of differences in relation to intact animals
p2 – significance of differences in relation to the animals with periodontitis on the 7th day of the experiment
p3 – significance of differences in relation to the animals with periodontitis on the 14th day of the experiment
One can see from the date of the table, the concentration products of oxidative modification of proteins neutral characters (OMP370) increased by 7.79 times (p < 0.01) on the 7th day of the experiment, and by 1.35 times on the 14th day (p < 0.01) as compared with the intact group. It should be noted that on the 14th day OMP370 products decreased in serum as compared with 7th day by 1.32 times (p < 0.01) (Fig. 1).
Fig. 1.
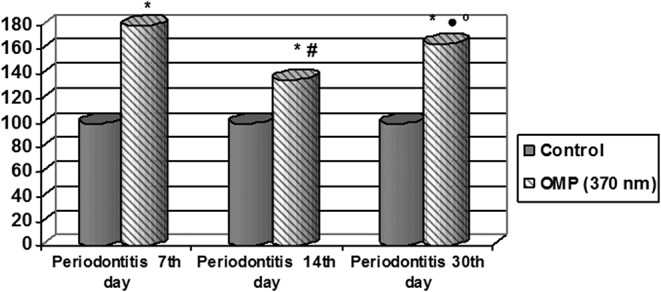
Changes in the indices of OMP370 in the rats serum in the dynamics of experimental periodontitis (% of control). * – Significance of differences in relation to intact animals (p < 0.01); # – significance of differences in relation to the animals with periodontitis on the 7th day of the experiment (p < 0.01); • – significance of differences in relation to the animals with periodontitis on the 7th day of the experiment (p > 0.05); º – significance of differences in relation to the animals with periodontitis on the 14th day of the experiment (p < 0.01)
In comparison of the above meant, aldehyde- and ketone derivatives quantity to the 30th day of the development of EP was established an increase in these parameters as compared with the 14th day (by 1.22 times, p < 0.01), but were less as compared with 7th day of the experiment, but these changes proved to be insignificant. However, this index was also significantly higher relative to the control group of animals (by 1.65 times, p < 0.01).
At the early stage of EP development, that is, on the 7th day, an increase in the products of oxidative modification of proteins of the basic character (OMP430) in blood serum (by 1.75 times, p < 0.01), but later, on 14th day, this index changed in the opposite direction, that is, it began to decrease (by 1.24 times, p < 0.01) as compared with the animals on the 7th day of the experiment; however, its level was higher relative to the intact group of animals (by 1.46 times, p < 0.01) (Fig. 2).
Fig. 2.
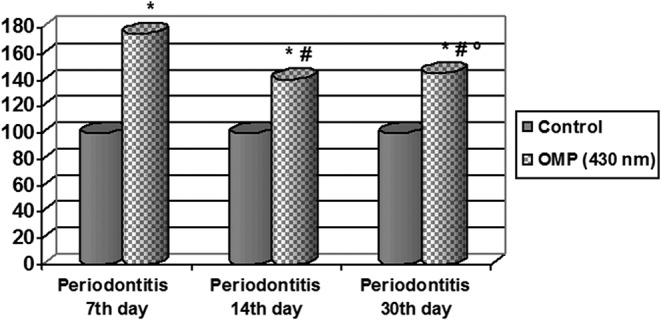
Changes in the indices of OMP430 in the rats serum in the dynamics of experimental periodontitis (% of control). * – Significance of differences in relation to intact animals (p < 0.01); # – significance of differences in relation to the animals with periodontitis on the 7th day of the experiment (p < 0.01); º – significance of differences in relation to the animals with periodontitis on the 14th day of the experiment (p > 0.05)
Then, on the 30th day of EP development, the concentration of aldehyde- and ketone derivatives of the basic character was increased insignificantly in comparison with the 14th day, but these changes were insignificant (p > 0.05). As compared with the 7th day of the experiment this index was decreased (by 1.20 times, p < 0.01). However, its level was higher relative to the control group of animals (by 1.46 times, p < 0.01).
By calculating the ratio of the neutral and basic aldehyde- and ketone derivatives (Table II) in serum (OMP370/OMP430), it was recorded an increase in the rats on the 7th and 30th days after modeling of EP, and a decrease on the 14th day as compared with the control; however, these changes were also insignificant (p > 0.05). Nevertheless, comparing this ratio, on the 7th and 14th days of the experiment, in a later period, the index decreased (by 1.05 times, p < 0.05). At the same time on the 30th day, it was increased as compared with the 14th day (by 1.17 times, p < 0.05).
Table II.
Correlation of OMP370/OMP430 in the serum of the rats for different periods of experimental periodontitis (M ± m)
| The form of experiment | Control, intact animals | Animals with periodontitis | ||
|---|---|---|---|---|
| Experiment duration (days) | – | 7 | 14 | 30 |
| Number of animals | 10 | 8 | 8 | 8 |
| OMP370/OMP430 | 0.79 ± 0.03 | 0.80 ± 0.01 | 0.76 ± 0.01 | 0.89 ± 0.01 |
| p1 > 0.05 | p1 > 0.05, p2 < 0.05 | p1 > 0.05, p2 > 0.05, p3 < 0.05 | ||
p1 – significance of differences in relation to intact animals
p2 – significance of differences in relation to the animals with periodontitis on the 7th day of the experiment
p3 – significance of differences in relation to the animals with periodontitis on the 14th day of the experiment
The results of these experiments are presented in Table II.
In the correlation of contents OMP370/OMP430 in blood of the second and fourth experimental animals, group changes were insignificant (p > 0.05).
Conclusions
-
1.
The accumulation of aldehyde and ketone derivatives of a neutral and basic character in serum blood is observed in EP of bacterial-immune genesis.
-
2.
The indices of oxidative modification of neutral and basic proteins in the dynamics of EP in the acute inflammatory reaction especially due to intensification of LPO.
Funding Statement
Funding sources: No financial support was received for this study.
Authors’ contribution
AD and YB made the critical review and prepared figures; PAH and YB prepared the article and manuscript; and PAH did literature review. All authors read and approved the final form of this manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Omarov IA, Bolevych SB, Saveteeva-Lyubimova TN, Silina EV, Sivak KV: Oxidative stress and antioxidant energy-correction complex in the treatment of periodontitis. Stomatologia 90, 10–17 (2011) [PubMed] [Google Scholar]
- 2.Butyugin IA, Kornilov NV, Abramov OV: Comparative analysis of the effectiveness of topical application of antioxidants in the treatment of chronic generalized periodontitis. Stomatologia 92, 31–34 (2013) [PubMed] [Google Scholar]
- 3.Kolisnyk MI, Kolisnyk GV, Vlizlo VV: Reactive oxygen species and their role in the metabolism of cells. Biologiya 11, 58–69 (2009) [Google Scholar]
- 4.Melnichuk GM, Kostyuk IR: Dynamics of indices of lipid peroxidation and antioxidant protection in the blood serum of children with permanent teeth granulating periodontitis and chronic heightened course influenced treatment. Sovremennaya Stomatologiya 3, 25–28 (2012) [Google Scholar]
- 5.Nesterov YV, Turchenko NV: The structural features of the air-blood barrier in conditions of acute lung hypo- and hyperoxic stress. Estestvennye Nauki 3, 112–116 (2010) [Google Scholar]
- 6.Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci O: Oxidative stress in asthma. World Allergy Organ 4, 151–158 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, Aziz N, Sekhon L, Agarwal R, Mansour G, Li J, Agarwal A: Lipid peroxidation and antioxidant status in preeclampsia: A systematic review. Obstet Gynecol Surv 64, 750–759 (2009) [DOI] [PubMed] [Google Scholar]
- 8.Demkovych A: Pathogenetic factors in the mechanisms of development and course of periodontal inflammatory processes. Med Chem 62, 107–113 (2015) [Google Scholar]
- 9.Butyugin IA, Volchehorskyy IA: System status of lipid peroxidation – Antioxidant protection in mixed saliva of patients with chronic periodontitis. Clin Lab Diagn 2, 44–47 (2014) [Google Scholar]
- 10.Dahiya P, Kamal R, Gupta R, Bhardwaj R, Chaudhary K, Kaur S: Reactive oxygen species in periodontitis. J Indian Soc Periodontol 17, 411–416 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demkovych A, Bondarenko YI: Pathogenetic basis periodontitis modeling in rats. Achieve Clin Exp Med 1, 54–57 (2015) [Google Scholar]
- 12.Meschishen IF: The method of determining the oxidative modification of plasma proteins. Bukovynsky Med J 2, 156–158 (1998) [Google Scholar]
- 13.Berger RL, Casella G. (2001): Statistical Inference, 2nd ed. Duxbury Press, Pacific Grove, CA, 374 p [Google Scholar]
- 14.Demkovych A: The peculiarities of microbiocoenosis formation in development of inflammatory periodontal diseases. Infect Dis 1, 87–92 (2015) [Google Scholar]
- 15.Medynska KO, Shelyuk OV, Lityuha VV, Omelyanyuk VS: The study of structural characteristics and determine the extent of damage of oxidation-modified rabbit skeletal muscle actomyosin under the influence of ultrasound. Phys Living 18, 164–167 (2010) [Google Scholar]


