Abstract
BACKGROUND
It has been suggested that beta-blockers (BB) may increase mortality in patients with cirrhosis and refractory ascites but the effect of BB discontinuation or re-initiation has not been examined.
AIMS
To compare, in hospitalized patients with cirrhosis and ascites, the effect of BB on survival and to examine the effect/predictors of BB discontinuation and re-initiation.
METHODS
Sub-analysis of NACSELD (North American Consortium for the Study of End-Stage Liver Disease, database containing prospective data on hospitalized patients with cirrhosis) data from 7 centers enrolling >100 patients with ascites. Data on BB discontinuation and re-initiation was collected by chart review.
RESULTS
716 patients: 307 (43%) on BB at admission and 366 (51%) with refractory ascites were followed to death or hospital discharge. BB use was associated with a lower white blood cell count at admission. BB use in hospitalized patients with ascites was not associated with a higher mortality, even in those with refractory ascites. No significant changes in mean arterial pressure (MAP) were observed between groups. Discontinuation of BB (49%) was driven by low MAP, infection and acute kidney injury at time of discontinuation but was not associated with a higher mortality. BB re-initiation occurred in 40% prior to discharge and was mainly driven by an increase in MAP.
CONCLUSIONS
BB use is safe in patients with cirrhosis and ascites (including those with refractory ascites) provided BB are discontinued in the presence of a low MAP and reinitiated once MAP re-increases. A potentially beneficial anti-inflammatory effect of BB is suggested.
Keywords: cirrhosis, ascites, beta-blockers, discontinuation, mortality
Non-selective beta-adrenergic blockers (NSBB) constitute the backbone of the management of portal hypertension in patients with cirrhosis, both in the prevention of first variceal hemorrhage and its recurrence(1). NSBB reduce portal pressure by decreasing portal venous inflow through a decrease in cardiac output (β-1 adrenergic blockade) and, more importantly, through splanchnic vasoconstriction (β-2 adrenergic blockade that allows an unopposed vasoconstrictive alpha-adrenergic effect on the splanchnic vasculature). Because NSBB act on one of the main pathophysiological mechanisms that maintain portal hypertension, their use has been associated not only to a decreased incidence of variceal hemorrhage but also to a reduction in the development of other complications of cirrhosis (ascites, encephalopathy) and an improved survival (2, 3).
However, a single-center observational study by Sersté et al suggested that, in patients with cirrhosis and refractory ascites, those on propranolol had a higher mortality, even after adjustment for severity of liver disease (4). A subsequent retrospective study showed an increased mortality in patients with spontaneous bacterial peritonitis but not in those with refractory ascites (5).
Subsequent studies performed in large cohorts of outpatients with cirrhosis and ascites found either no difference (6) or even an improved survival in patients treated with NSBB (7, 8), including those with refractory ascites. A third study based on a European consortium of hospitalized patients with acute-on-chronic liver failure showed an improved survival for those admitted on beta-blockers, and suggested an anti-inflammatory effect (9). The determinants of BB reinitiation after discontinuation are however, unclear.
The aim of our study was to compare, in a North American cohort of hospitalized patients with decompensated cirrhosis and ascites (refractory or not), the effect of beta-blockers (BB) on survival. The study also aimed at investigating predictors of beta-blocker discontinuation during hospitalization and its effect, if any, on mortality, as well as factors that led to BB re-initiation.
METHODS
This is a multicenter, observational study of hospitalized patients with cirrhosis enrolled between January 2014 and September 2015 in the NACSELD (North American Consortium for the Study of End-Stage Liver Disease) cohort (10, 11). NACSELD is a consortium of 16 tertiary-care hepatology centers that prospectively enrolls non-electively admitted patients with cirrhosis and follows them through hospitalization and 12-months post discharge. The study protocol has been approved by the Institutional Review Boards in all participating centers and all patients or their family members provided written informed consent for inclusion into the study.
A diagnosis of cirrhosis was established in this cohort by endoscopic or radiological evidence of portal hypertension or cirrhosis, compatible biopsy findings, and/or signs of cirrhosis decompensation including hepatic encephalopathy, jaundice, variceal bleeding or ascites.
For this study, consecutive patients with cirrhosis and ascites who gave informed consent were included from 7 NACSELD sites that had included more than 100 patients with ascites in the study period. Data collected prospectively include site, patient demographics, etiology of cirrhosis, co-morbid conditions including diabetes mellitus, complications of cirrhosis, use of medications such as BB and the type of BB, reason for admission, physical exam findings, routine labs, development of infection or acute kidney injury during hospitalization, transfer to intensive care unit (ICU) and outcome of admission (death, discharge to home, hospice care or nursing home, or liver transplant).
Because the potentially deleterious effect of BB in patients with ascites is due to their effect in lowering cardiac output and blood pressure, the use of any type of BB (selective or non-selective) was recorded. Specific data on type of BB, BB dose, date and reason for discontinuation during hospitalization, whether BB were reinitiated during hospitalization and BB on discharge were obtained through chart review. Vital signs and routine labs at time of BB discontinuation and re-initiation were also collected.
Statistical analyses
Categorical variables were described as number and percentage while continuous variables were summarized by means and standard deviation. Associations between patients using BB on admission (BB group) and those not on BB at admission (non-BB group) were first compared by univariate analysis using Student’s t test for comparisons of continuous variables and chi-square test or Fisher’s exact test for categorical variables. Primary outcome was death defined as patients who died during hospitalization or who were discharged to hospice care (date of death in the latter was considered as the date of discharge from the hospital). Survival curves were estimated using the Kaplan-Meier method and compared using the log rank test. In an attempt to avoid type II error, and as a sensitivity analysis, the Wilcoxon test, which gives greater weight to earlier time points in the observation period when the number at risk is larger, was also performed to compare survival curves. Patients transplanted during hospitalization were censored on the date of liver transplant.
The initial analysis included all patients with ascites (refractory or not). A secondary analysis included patients with refractory ascites either as determined in the NACSELD database and/or if the patient had history of repeated large-volume paracenteses. Patients who discontinued BB during admission were compared to those in whom BB were not discontinued.
Multivariate Cox regression analysis was performed to identify independent predictors of death and BB discontinuation. For predictors of death, variables significantly (p<0.05) different between alive vs. dead patients on univariate Cox analysis and the type of BB (selective or non-selective) were entered in different models, avoiding collinearity. For predictors of BB discontinuation, variables that should lead to discontinuation as determined by consensus, were selected for the multivariable model.
RESULTS
After exclusion of 28 patients with terminal hepatocellular carcinoma admitted for palliative care or prior to transfer to hospice, the cohort was composed of 718 hospitalized patients with cirrhosis and ascites, of which 307 (43%) were on BB at admission and 366 (51%) had refractory ascites. Mean age of the entire cohort was 57 years, 80% were Caucasian and 65% were male. The etiologies of cirrhosis in over two-thirds of the patients were alcohol, hepatitis C or a combination of both with about half of the cases being alcohol-related (Table 1).
Table 1.
Characteristics at admission of all patients with ascites
| All patients with ascites (n=718) |
Not on Beta-Blockers (n=411) |
On Beta-Blockers (n=307) |
p value (BB vs. no BB) |
|
|---|---|---|---|---|
|
| ||||
| Age (years) | 57 ± 10 | 56 ± 10 | 58 ± 10 | 0.06 |
|
| ||||
| Gender (% male) | 465 (65%) | 256 (62%) | 209 (68%) | 0.11 |
|
| ||||
| Etiology | ||||
| - Alcohol alone | 236 (33%) | 140 (34%) | 96 (31%) | 0.10 |
| - HCV alone | 150 (21%) | 80 (19.5%) | 70 (23%) | |
| - Alcohol + HCV | 107 (15%) | 63 (15.5%) | 44 (14.5%) | |
| - NASH/Cryptogenic | 140 (19%) | 71 (17%) | 69 (22.5%) | |
| - Miscellaneous | 84 (12%) (n=717) | 57 (14%) (n-411) | 27 (9%) (n=306) | |
|
| ||||
| Diabetes (%) | 225 (32%) (n=708) | 112 (28%) (n=404) | 113 (37%) (n=304) | 0.008 |
|
| ||||
| On statins (%) | 64 (9%) (n=710) | 23 (6%) (n=404) | 41 (13%) (n=306) | <0.001 |
|
| ||||
| History of variceal hemorrhage (%) | 166 (23%) (n=709) | 67 (16%) (n=406) | 99 (33%) (n=303) | <0.001 |
|
| ||||
| Refractory ascites (%) | 366 (51%) | 199 (48%) | 167 (54%) | 0.11 |
|
| ||||
| HCC (%) | 67 (9%) | 28 (7%) | 39 (13%) | 0.007 |
|
| ||||
| Infected (%) | 202 (28%) | 122 (30%) | 80 (26%) | 0.29 |
|
| ||||
| BMI | 29 ± 7 (n=592) | 28 ± 7 (n=335) | 30 ± 8 (n=257) | 0.003 |
|
| ||||
| Temperature (°F) | 98.1 ± 1 (n=712) | 98.1 ± 1.1 (n=406) | 98.1 ± 0.9 (n=306) | 0.92 |
|
| ||||
| Heart rate (bpm) | 86 ± 17 (n=716) | 90 ± 16 (n=409) | 80 ± 17 (n=307) | <0.001 |
|
| ||||
| MAP (mmHg) | 86 ± 14 (n=717) | 86 ± 14 (n=410) | 86 ± 14 (n=307) | 0.72 |
|
| ||||
| WBC | 8.2 ± 5.2 (n=710) | 8.7 ± 5.8 (n=404) | 7.4 ± 4.4 (n=306) | <0.001 |
|
| ||||
| Platelet count | 112 ± 73 (n=708) | 119 ± 76 (n=402) | 104 ± 66 (n=306) | 0.004 |
|
| ||||
| Albumin (g/dL) | 2.8 ± 0.6 (n=681) | 2.8 ± 0.7 (n=387) | 2.8 ± 0.6 (n=294) | 0.28 |
|
| ||||
| Bilirubin (mg/dL) | 5.7 ± 6.7 (n=708) | 5.9 ± 6.8 (n=404) | 5.4 ± 6.4 (n=304) | 0.24 |
|
| ||||
| INR | 1.7 ± 0.5 (n=699) | 1.7 ± 0.5 (n=399) | 1.7 ± 0.6 (n=300) | 0.65 |
|
| ||||
| Creatinine (mg/dL) | 1.6 ± 1.4 (n=715) | 1.6 ± 1.4 (n=408) | 1.6 ± 1.3 (n=307) | 0.62 |
|
| ||||
| Serum Na (mEq/L) | 133 ± 6 (n=713) | 133 ± 6 (n=406) | 134 ± 6 (n=307) | 0.030 |
|
| ||||
| Child score | 10 ± 2 (n=631) | 10 ± 2 (n=358) | 10 ± 2 (n=273) | 0.67 |
|
| ||||
| MELD | 20 ± 8 (n=694) | 20 ± 8 (n=395) | 20 ± 8 (n=299) | 0.11 |
|
| ||||
| Esophageal varices | ||||
| - No or small | 355 (66%) | 210 (74%) | 145 (58%) | <0.001 |
| - Medium/large | 179 (34%) (n=534) | 73 (26%) (n=283) | 106 (42%) (n=251) | |
n in parentheses represents the number of patients for which data for that particular variable was available
BMI: Body mass index; HCV: hepatitis C virus infection; INR: International normalized ratio; MAP: mean arterial pressure; MELD: model for end-stage liver disease; Na: sodium; NASH= non-alcoholic steatohepatitis; WBC: white cell count;
It is interesting to note that patients on NSBB had exactly the same MAP as those not on NSBB
Of 307 patients on BB at admission, 245 (80%) were on a non-selective BB (157 nadolol, 65 propranolol, and 23 carvedilol). The indication for non-selective BB was primary or secondary prophylaxis of variceal hemorrhage; the median daily dose for propranolol was 40 mg, 20 mg for nadolol and 12.5 mg for carvedilol. Patients on selective BB (20%) were on them for a cardiac indication. BB discontinuation during hospitalization occurred in 151/307 (49%) patients and were reinitiated in 61 (40%) of them.
Comparison between patients with ascites on or off BB at the time of admission
As shown in Table 1, patients in the BB group were more likely to have medium/large varices, a history of variceal hemorrhage, diabetes and to be on statins. Patients on BB had a lower heart rate without any differences in mean arterial pressure (86±14 mmHg in both groups) or MELD score (20 in both groups) and a lower platelet count. Notably, patients on BB had a lower admission WBC (7.4± 4.4 vs. 8.7± 5.8 in the BB vs. the non-BB group, respectively, p<0.001).
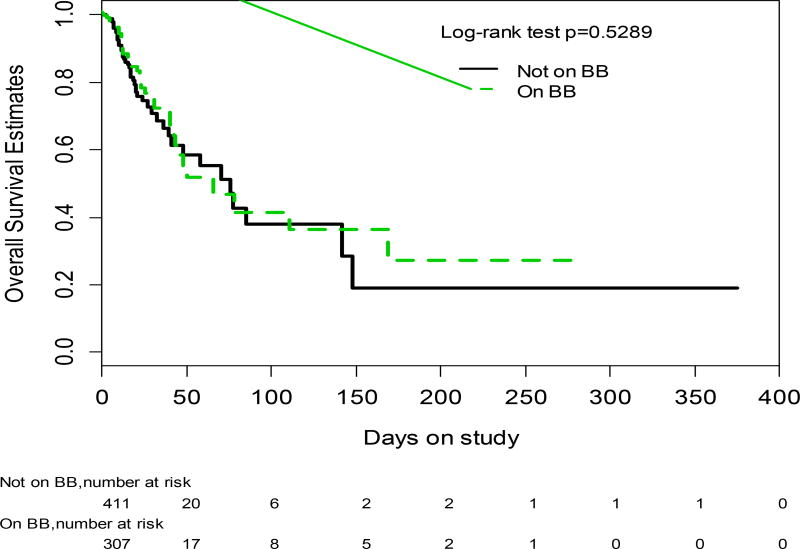
In-hospital mortality was not different between groups, with a death rate of 13% (39/307) in the BB group and of 14% (56/411) in the non-BB group (p=0.719) in a mean follow-up time of 15 ± 28 days (0 to 375). The probability of survival was not different between study groups (log rank p=0.5289; Wilcoxon p=0.6392)(Figure 1). On univariate Cox analysis, patients who died had a significantly lower MAP and serum sodium, higher temperature, MELD (and its components) and Child-Turcotte-Pugh (CTP) scores on admission than those who did not die (Supplementary Table 1). On Cox regression analysis that included MAP, temperature, Na and MELD (or CTP score in another model) plus BB on admission (vs. not on BB at admission), the only variables that were significant were temperature and the MELD score (HR 1.060 [95% CI 1.033–1.089] in the model including MELD), or the CTP score (HR 1.187 [95%CI 1.058–1.332] in the model including CTP score) (Supplementary Table 2). Being on BB at admission was not a predictor of mortality in either the MELD model (HR 1.121 [CI 0.731–1.718]) or in the model using CTP score (HR 1.142 [95% CI 0.738–1.769] or CTP score]). Additionally, analysis by type of BB (selective vs. nonselective) did not show any differences in mortality (univariate analysis, log-rank) nor was it significant on Cox analysis (data not shown). Only 25 patients were transplanted, of which 11 were on BB.
Figure. 1.
Probability of survival in patients with ascites on BB at admission (discontinuous line) vs. those not on BB on admission (continuous line)
Comparison between patients with refractory ascites on or off BB at the time of admission
As shown in Table 2, of 366 patients with refractory ascites, 167 (46%) were on BB on admission while 199 (54%) were not. Similar to results from the overall group, patients taking a BB group were more likely to have large varices and to be on statins, they had a lower heart rate without a significant difference in mean arterial pressure (85±14 in both groups, p=0.91) and a lower platelet count. Notably, admission WBC was lower in patients on BB (7.6±4.6 v 8.9±5.5, p=0.0104). Unlike the overall group, patients in the BB group were more likely to be male (71% vs. 59%) and to have a lower MELD score (19±2 vs. 21±7, p=0.024).
Table 2.
Characteristics at admission of patients with refractory ascites
| All patients with refractory ascites (n=366) |
Not on Beta-Blockers (n=199) |
On Beta-Blockers (n=167) |
p value | |
|---|---|---|---|---|
|
| ||||
| Age (years) | 58 ± 10 | 57 ± 10 | 58 ± 10 | 0.50 |
|
| ||||
| Gender (% male) | 236 (64%) | 118 (59%) | 118 (71%) | 0.024 |
|
| ||||
| Etiology | ||||
| - Alcohol alone | 125 (34%) | 69 (34%) | 56 (33%) | 0.34 |
| - HCV alone | 74 (20%) | 36 (18%) | 38 (23%) | |
| - Alcohol + HCV | 49 (14%) | 28 (14%) | 21 (13%) | |
| - NASH/Cryptogenic | 78 (21%) | 39 (20%) | 39 (23%) | |
| - Miscellaneous | 40 (11%) | 27 (14%) | 13 (8%) | |
|
| ||||
| Diabetes (%) | 120 (34%) (n=357) | 59 (31%) (n=193) | 61 (37%) (n=164) | 0.19 |
|
| ||||
| On statins (%) | 43 (12%) (n=360) | 14 (7%) (n=194) | 29 (18%) (n=166) | 0.003 |
|
| ||||
| History of variceal hemorrhage (%) | 105 (29%) (n=360) | 45 (23%) (n=197) | 60 (37%) (n=163) | 0.004 |
|
| ||||
| HCC (%) | 28 (8%) | 9 (4%) | 19 (11%) | 0.014 |
|
| ||||
| Infected (%) (? In the past) | 102 (28%) | 61 (31%) | 41 (24%) | 0.19 |
|
| ||||
| BMI | 29 ± 8 (n=310) | 28 ± 7 (n=170) | 30 ± 8 (n=140) | 0.023 |
|
| ||||
| Temperature (°F) | 98.1 ± 0.9 (n=361) | 98.1 ± 1 (n=195) | 98 ± 0.9 (n=161) | 0.85 |
|
| ||||
| Heart rate (bpm) | 86 ± 17 (n=364) | 90 ± 16 (n=197) | 81 ± 17 (n=167) | <0.001 |
|
| ||||
| MAP (mmHg) | 85 ± 14 (n=365) | 85 ± 14 (n=198) | 85 ± 14 (n=167) | 0.91 |
|
| ||||
| WBC | 8.3 ± 5.1 (n=362) | 8.9 ± 5.5 (n=195) | 7.6 ± 4.6 (n=167) | 0.0104 |
|
| ||||
| Platelet count | 114 ± 73 (n=361) | 121 ± 80 (n=194) | 104 ± 63 (n=167) | 0.025 |
|
| ||||
| Albumin (g/dL) | 2.9 ± 0.6 (n=342) | 2.9 ± 0.7 (n=185) | 2.8 ± 0.6 (n=157) | 0.005 |
|
| ||||
| Bilirubin (mg/dL) | 5.1 ± 6 (n=362) | 5.1 ± 5.6 (n=197) | 5.0 ± 6.2 (n=165) | 0.83 |
|
| ||||
| INR | 1.6 ± 0.5 (n=358) | 1.6 ± 0.4 (n=195) | 1.6 ± 0.5 (n=163) | 0.88 |
|
| ||||
| Creatinine (mg/dL) | 1.7 ± 1.4 (n=365) | 1.8 ± 1.5 (n=198) | 1.5 ± 1.2 (n=167) | 0.025 |
|
| ||||
| Serum Na (mEq/L) | 132 ± 6 (n=364) | 132 ± 6 (n=197) | 133 ± 6 (n=167) | 0.06 |
|
| ||||
| Child score | 10 ± 2 (n=310) | 10 ± 2 (n=166) | 10 ± 2 (n=144) | 0.85 |
|
| ||||
| MELD | 20 ± 8 (n=358) | 21 ± 7 (n=195) | 19 ± 8 (n=163) | 0.024 |
|
| ||||
| Esophageal varices | ||||
| - No or small | 174(64%) | 102 (72%) | 72 (54%) | 0.003 |
| - Medium/large | 100 (36%) (n=274) | 40 (28%) (n=142) | 60 (46%) (n=132) | |
N in parentheses represents the number of patients for which data for that particular variable was available
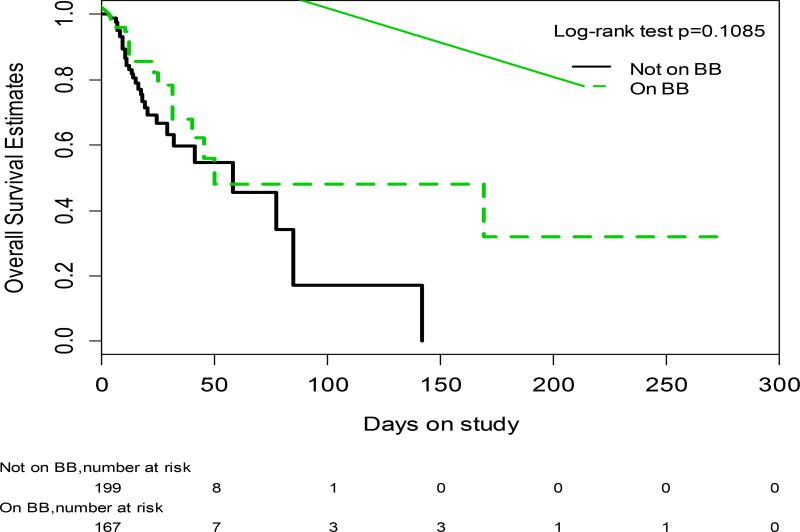
Mortality was not different between study groups. In a mean follow-up period of 14 ± 24 days (range 0 to 286, 54/366 (15%) died, 19/167(12%) in the BB group and 35/199 (17%) in the non-BB group (p=0.4315). The probability of survival was not statistically significantly different between study groups (log rank p=0.1085;Wilcoxon p=0.2946)(Figure 2).
Fig. 2.
Probability of survival in patients with refractory ascites on BB at admission (discontinuous line) vs. those not on BB at admission (continuous line)
On univariate Cox analysis (Supplementary Table 3), only MELD and Child score were significantly different between groups. On Cox regression analysis that included BB on admission (vs. no BB at admission) and MELD (in one model) or Child score (in another model), the only variable that was independently predictive of death in patients with refractory ascites was the admission Child score (HR 1.191; CI 1.031–1.376) in the model including Child. In the model including MELD and BB, no factor independently predicted death, although MELD was of borderline significance (p=0.0586; HR 1.033 [CI 0.999–1.068])(Supplementary Table 4). Being on BB at admission was not a predictor of increased mortality in either of these models (HR 1.576 [CI 0.859–2.891] in the model using Child score and HR 1.480 [CI 0.819–2.675] in the model using MELD score). Additionally, analysis by type of BB (selective vs. nonselective) did not show any differences between them neither in mortality nor in their lack of effect in predicting death (data not shown).
Comparison of patients on BB at admission who did vs. did not discontinue BB during hospitalization
Of the 307 patients on BB at admission, BBs were discontinued in 151 patients (49%) during hospitalization. The mean time between admission and BB discontinuation was 18 ± 24 days (range 1 to 169 days). In patients with refractory ascites, BB were discontinued in 44% (74/167) compared to 55% (77/140) BB discontinuation in patients with non-refractory ascites (p=0.07).
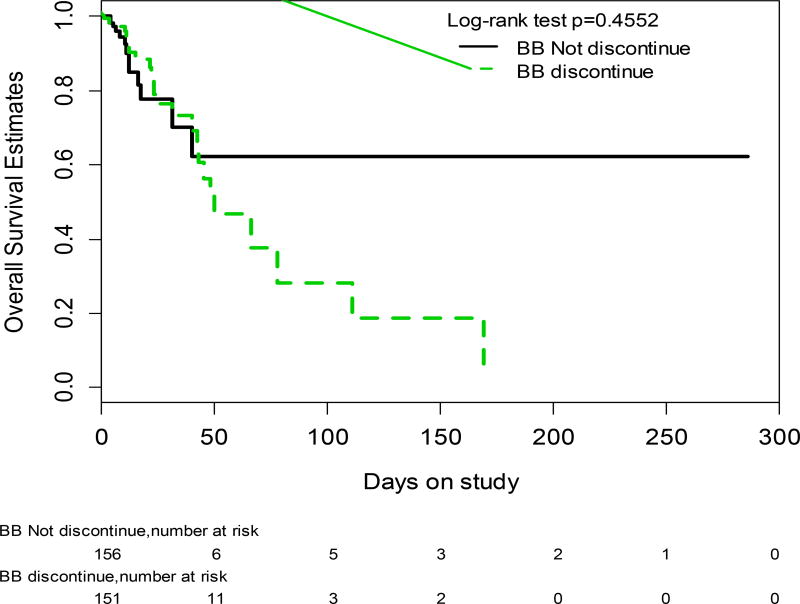
As shown in Table 3, patients who discontinued BB had, on admission, a lower MAP and a higher MELD and were more likely to have been admitted with an infection, have developed an infection during admission, or developed AKI, compared to patients who did not discontinue BB. Despite being significantly sicker, those who discontinued BB had a similar probability of survival compared to those who did not discontinue BB (log rank p=0.4552; Wilcoxon p=0.7106) (Figure 3) even when only those with refractory ascites were considered (log rank p=0.9741; Wilcoxon p=0.3160).
Table 3.
Characteristics at admission of patients with ascites who discontinued vs. those who did not discontinue BB during hospitalization
| Did not discontinue beta- blockers (n=156) |
Discontinued Beta-Blockers (n=151) |
p value p ve |
|
|---|---|---|---|
|
| |||
| Age (years) | 59 ± 9.8 | 56.6 ± 10.5 | 0.05 |
|
| |||
| Gender (% male) | 105 (67.3%) | 104 (68.8%) | 0.77 |
|
| |||
| Etiology | 68 (44%) | 72 (48%) | |
| -Related to alcohol | 87 (56%) (n=156) | 79 (52%) (n=151) | 0.50 |
| -Not related to alcohol | |||
|
| |||
| Diabetes (%) | 60 (39%) (n=154) | 53 (35%) (n=150) | 0.51 |
|
| |||
| On statins (%) | 23 (15%), n=156 | 18 (12%), n=150 | 0.48 |
|
| |||
| History of variceal hemorrhage (%) | 54 (35%) (n=155) | 45 (30%) (n=148) | 0.41 |
|
| |||
| Refractory ascites (%) | 93 (60%) | 74 (49%) | 0.06 |
|
| |||
| HCC (%) | 23 (15%) | 16 (11%) | 0.28 |
|
| |||
| Infected (%) | 33 (21%) | 47 (31%) | 0.047 |
|
| |||
| BMI | 30 ± 8 (n=135) | 30 ± 7 (n=122) | 0.97 |
|
| |||
| Temperature (°F) | 98.1 ± 0.8 (n=156) | 98 ± 1 (n=150) | 0.62 |
|
| |||
| Heart rate (bpm) | 77.9 ± 16.5 | 82.3 ± 17.9 | 0.028 |
|
| |||
| MAP (mmHg) | 88.2 ± 13.8 | 82.6 ± 13.8 | <0.001 |
|
| |||
| WBC | 7.07 ± 4.4 (n=156) | 7.8 ± 4.4 (n=150) | 0.15 |
|
| |||
| Platelet count | 103.3 ± 70.5 (n=156) | 104.4 ± 61.7 (n=150) | 0.88 |
|
| |||
| Albumin (mg/dL) | 2.76 ± 0.5 (n=146) | 2.76 ± 0.6 (n=148) | 0.95 |
|
| |||
| Bilirubin (mg/dL) | 4.8 ± 5.9 (n=153) | 5.9 ± 6.8 (n=151) | 0.11 |
|
| |||
| INR | 1.6 ± 0.5 (n=150) | 1.7 ± 0.6 (n=150) | 0.039 |
|
| |||
| Creatinine (mg/dL) | 1.4 ± 1.1 | 1.8 ± 1.5 | 0.006 |
|
| |||
| Serum Na (mEq/L) | 134 ± 5.4 | 133 ± 6.6 | 0.07 |
|
| |||
| Child score | 9.6 ± 1.9 (n=135) | 10.5 ± 1.5 (n=138) | <0.001 |
|
| |||
| MELD | 17.8 ± 6.5 (n=149) | 21.3 ± 8 (n=150) | <0.001 |
|
| |||
| Esophageal varices | |||
| -No or small | 74 (59%) | 71 (56%) | 0.65 |
| - Medium/large | 51 (41%) (n=125) | 55 (44%) (n=126) | |
N in parentheses represent the number of patients for which data for that particular variable was available
Fig. 3.
Probability of survival in patients who discontinued BB during hospitalization (discontinuous line) vs. those who did not discontinue BB (continuous line).
Compared to admission values, patients who discontinued BB had significantly lower MAP and Child score at time of BB discontinuation (the latter perhaps as a result of albumin infusions and increased serum albumin). Serum albumin increased and serum creatinine and MELD score were unchanged (Supplementary Table 5). On Cox regression analysis that included the three variables (systolic blood pressure, serum sodium and creatinine) that have been recommended by the Baveno consensus to consider BB discontinuation (12), admission systolic blood pressure (HR 0.977; 95%CI 0.65–0.989) and admission serum creatinine (HR 1.284 (95% CI 1.040–1.586), but not serum sodium, were independent predictors of BB discontinuation. When looking at the specific criteria recommended by the Baveno consensus (12), i.e., systolic blood pressure <90 mmHg, serum sodium <130 mEq/L or presence of acute kidney injury, patients meeting any of these criteria were significantly more likely to discontinue BB (65% of patients with ascites, 69% of patients with refractory ascites) than those not meeting such criteria (p<0.0001).
Analysis of patients who discontinued BB and then reinitiated them during hospitalization
Of 151 patients in whom BB were discontinued, they were reinitiated in 61 (40%) during hospitalization. When comparing characteristics at discontinuation to those at reinitiation, the main factor that determined reinitiation was blood pressure (systolic, diastolic and mean arterial pressure) (Supplementary Table 6).
Discussion
NSBB have been shown to prevent first and recurrent variceal hemorrhage in patients with cirrhosis and, in hemodynamic responders, BB have also been shown to prevent decompensation and death (3). The effect appears to be independent of the presence or absence of ascites as demonstrated in two meta-analyses: one including 11 RCTs of BB for primary prophylaxis of variceal hemorrhage (VH) that demonstrated NSBB lowered first bleeding rates in patients with and without ascites (13); the other was a meta-analysis of 12 RCTs on secondary prophylaxis of VH, that showed a significant reduction in both re-bleeding and death in NSBB-treated patients with “severe” liver disease (14).
The main pathophysiologic mechanism in patients with cirrhosis and ascites is splanchnic and systemic vasodilatation that leads to activation of neuro-humoral systems, sodium and fluid retention, resulting in increased cardiac output, and a hyperdynamic circulatory state (15). In patients with refractory ascites, these abnormalities are maximal and a relative decrease in cardiac output can lead to a decrease in renal perfusion and to hepatorenal syndrome (16). Beta-blockers could, at least theoretically, precipitate this decrease in cardiac output and lead to renal dysfunction and death. Therefore, it is plausible that BB use could lead to a higher mortality in patients with refractory ascites as suggested by an observational study performed in 151 patients (4). Another retrospective study in 182 patients on BB failed to show a greater mortality in patients with refractory ascites but showed a higher mortality in patients with spontaneous bacterial peritonitis (5).
Subsequent retrospective studies including larger number of patients with ascites and/or refractory ascites (a collective of over 2,000 patients) have shown that BB use is either unrelated to an increased mortality (6), or is actually associated with an improved survival (7, 8). In fact, a recent meta-analysis including these observational studies and randomized studies of BB in the prevention of VH, shows that BB use was not associated with increased all-cause mortality in patients with ascites, non-refractory ascites alone or refractory ascites alone (17).
A survival benefit of BB was demonstrated in a study performed as part of a European consortium (CANONIC) in hospitalized patients with acute-on-chronic liver failure (9), the stage of cirrhosis associated with the highest short-term mortality and in which the main pathogenic mechanism is systemic inflammation leading to worsening vasodilatation and multi-organ failure (18). Because patients on NSBB had a lower WBC count on admission compared to those not on BB, an anti-inflammatory effect of NSBB was suggested in that study (19). .
Our study was performed in patients with cirrhosis and ascites recruited in 7 of the 16 centers that compose a North American consortium (NACSELD) that prospectively collects data of non-electively admitted patients to centers in the United States and Canada. We show that BB use in hospitalized cirrhotic patients with ascites is not associated with an increased mortality compared to non-BB users, even in those with refractory ascites. The probability of survival was the same in BB users vs. non-users both in patients with ascites overall and in patients with refractory ascites. Importantly, as demonstrated by the European consortium, we found that patients on BB had a lower WBC count on admission.
One novel aspect of our study is the analysis of discontinuation and re-initiation of BB in this patient population. BB were discontinued during hospitalization in about half the patients. This was primarily driven by a decrease in blood pressure (systolic, diastolic and mean arterial pressure) mostly in the setting of an infection and an acute kidney injury. Notably, even though patients who discontinued BB were sicker (higher MELD, lower MAP) their probability of survival was not different compared to those in whom BB were not discontinued, demonstrating that the strategy of discontinuing BB under these circumstances does not have a deleterious effect on outcome. In fact, when evaluating published studies on BB use in cirrhosis, those that show a deleterious effect of BB on survival are those that show a significantly lower MAP in the BB group (20). In the most recent propensity score-matched study, there was a relationship between the dose of propranolol and survival, with doses lower than 160 mg/day associated with a lower mortality (8). Although the dose-effect relationship with MAP was not examined in our study, it could be assumed that larger doses would be associated with larger decreases in MAP and worse outcomes.
In the recent Baveno portal hypertension workshop, consensus among experts concluded that the dose of BB should be reduced or BB discontinued in the setting of a systolic blood pressure <90 mmHg, hyponatremia (serum sodium <130 mEq/L) or the development of acute kidney injury. Our data largely validate these consensus recommendations, with around two thirds of patients with ascites who met any of these criteria having BB discontinued, however this discontinuation was driven mostly by a decrease in blood pressure. Notably, BB could be reinitiated in 40% of patients prior to discharge and, not surprisingly, the main driver of BB re-initiation was once again an increase in blood pressure. This relatively large proportion of inpatients who had their BB reinstated despite going through infections, AKI and hypotensive episodes, suggests that clinical practice in North America is cognizant of the potential benefits of BB therapy despite the mixed literature surrounding their discontinuation.
Even though data collection at admission was prospective, data on discontinuation and reinitiation of BB during hospitalization was retrospective and therefore there is potential for selection bias. There is no common protocol among centers for discontinuation of BB but, because all centers are academic centers they are more likely to follow guidelines regarding discontinuation of BB and this is in fact reflected in our results. However, our conclusions may not apply to those admitted to non-specialist centers.
In conclusion, this large cohort of North American patients with cirrhosis and ascites in whom data was collected prospectively at admission, demonstrates that BB use is safe in hospitalized patients with ascites and, specifically, in those with refractory ascites. We also show that BB discontinuation is mostly driven by a low mean arterial pressure in these patients and that, in this setting, BB discontinuation has no effect on survival and would constitute a best practice. Importantly, we demonstrate that BB can be reinitiated in 40% of the patients prior to discharge and this practice is driven by an increase in blood pressure. Our study also supports a potential anti-inflammatory effect of BB observed in a cohort of patients with acute-on-chronic liver failure (9) and indicates that, in general, unless there is a decrease in mean arterial pressure, BB should not be discontinued in patients with ascites/refractory ascites.
Supplementary Material
Acknowledgments
Study was supported from an unrestricted grant from Grifols
Financial Support:
Investigator-Initiated Grant by Grifols Pharmaceuticals
Yale Liver Center NIH P30 DK34989
List of abbreviations
- BB
beta-blockers
- CTP
Child-Turcotte-Pugh
- HR
hazard ratio
- MAP
mean arterial pressure
- MELD
Model of End-stage Liver Disease
- NACSELD
North-American Consortium for the Study of End Stage Liver Disease
- NSBB
non-selective beta-blocker
- RCT
randomized controlled trial
- VH
variceal hemorrhage
- WBC
white blood cells
References
- 1.Garcia-Tsao G, Abraldes J, Berzigotti A, Bosch J. Portal Hypertensive Bleeding in Cirrhosis: Risk Stratification, Diagnosis and Management - 2016 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2017 Jan 1;65(1):310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 2.Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodes J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003 Apr;37(4):902–908. doi: 10.1053/jhep.2003.50133. [DOI] [PubMed] [Google Scholar]
- 3.D'Amico G, Garcia-Pagan JC, Luca A, Bosch J. HVPG reduction and prevention of variceal bleeding in cirrhosis. A systematic review. Gastroenterology. 2006;131:1611–1624. doi: 10.1053/j.gastro.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Serste T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010 Sep;52(3):1017–1022. doi: 10.1002/hep.23775. [DOI] [PubMed] [Google Scholar]
- 5.Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, et al. Nonselective beta blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014 Jun;146(7):1680–1690. doi: 10.1053/j.gastro.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Bossen L, Krag A, Vilstrup H, Watson H, Jepsen P. Non-selective beta-blockers do not affect mortality in cirrhosis patients with ascites: Post hoc analysis of three RCTs with 1198 patients. Hepatology. 2016;63:1968–1976. doi: 10.1002/hep.28352. [DOI] [PubMed] [Google Scholar]
- 7.Leithead JA, Rajoriya N, Tehami N, Hodson J, Gunson BK, Tripathi D, et al. Non-selective beta-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut. 2015 Oct 3;64(7):1111–1119. doi: 10.1136/gutjnl-2013-306502. [DOI] [PubMed] [Google Scholar]
- 8.Bang UC, Benfield T, Hyldstrup L, Jensen JB, Bendtsen F. Effect of propranolol on survival in patients with decompensated cirrhosis: A nationwide study based Danish patient registers. Liver Int. 2016 Mar 16;36(9):1304–1312. doi: 10.1111/liv.13119. [DOI] [PubMed] [Google Scholar]
- 9.Mookerjee RP, Pavesi M, Thomsen KL, Mehta G, MacNaughtan J, Bendtsen F, et al. Treatment with non-selective beta-blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol. 2016;64:574–582. doi: 10.1016/j.jhep.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Bajaj JS, O'Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012 Dec;56(6):2328–2335. doi: 10.1002/hep.25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajaj JS, O'Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250–6. doi: 10.1002/hep.27077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Franchis R. Expanding consensus in portal hypertension. Report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Poynard T, Cales P, Pasta L, Ideo G, Pascal JP, Pagliaro L, et al. Beta-adrenergic antagonists in the prevention of first gastrointestinal bleeding in patients with cirrhosis and oesophageal varices. An analysis of data and prognostic factors in 598 patients from four randomized clinical trials. N Engl J Med. 1991;324:1532–1538. doi: 10.1056/NEJM199105303242202. [DOI] [PubMed] [Google Scholar]
- 14.Bernard B, Lebrec D, Mathurin P, Opolon P, Poynard T. Beta-adrenergic antagonists in the prevention of gastrointestinal rebleeding in patients with cirrhosis: A meta-analysis. Hepatology. 1997;25:63–70. doi: 10.1053/jhep.1997.v25.pm0008985266. [DOI] [PubMed] [Google Scholar]
- 15.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodes J. Peripheral arterial vasodilation hypothesis - A proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Gines P, Moreira V, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005 Aug;42(2):439–447. doi: 10.1002/hep.20766. [DOI] [PubMed] [Google Scholar]
- 17.Chirapongsathorn S, Valentin N, Alahdab F, Krittanawong C, Erwin PJ, Murad MH, et al. Nonselective beta-Blockers and Survival in Patients With Cirrhosis and Ascites: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016 Aug;14(8):1096–1104. doi: 10.1016/j.cgh.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013 Jun;144(7):1426–37. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 19.Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013 May;58(5):911–921. doi: 10.1016/j.jhep.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Tsao G. Beta blockers in cirrhosis: The window re-opens. J Hepatol. 2016 Mar;64:532–534. doi: 10.1016/j.jhep.2015.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.