Abstract
Subjected to countless daily injuries, the stomach still functions as a remarkably efficient digestive organ and microbial filter. Here, we follow the lead of the earliest gastroenterologists who were fascinated by the anti-septic and digestive power of gastric secretions. We propose that it is easiest to understand how the stomach responds to injury by stressing the central role of the most important gastric secretion, acid. The stomach follows two basic patterns of adaptation. The superficial response is a pattern whereby the surface epithelial cells migrate and rapidly proliferate to repair erosions induced by acid or other irritants. The stomach can also adapt through a glandular response when the source of acid is lost or compromised (i.e., the process of oxyntic atrophy). We primarily review the mechanisms governing the glandular response, which is characterized by a metaplastic change in cellular differentiation known as Spasmolytic Polypeptide-Expressing Metaplasia, or SPEM. We propose that the stomach, like other organs, exhibits marked cellular plasticity: the glandular response involves reprogramming mature cells to serve as auxiliary stem cells that replace lost cells. Unfortunately, such plasticity may mean that the gastric epithelium undergoes cycles of differentiation and de-differentiation that increase the risk for accumulating cancer-predisposing mutations.
INTRODUCTION
Historical Insights Into the Stomach
The human stomach is an exocrine and endocrine organ that initiates digestion. Some of the earliest scientific work on the digestive tract focused on the exocrine function of the stomach. This was likely because the live workings of most internal organs were mysteries; however, the secretions of the stomach were accessible with a little ingenuity. For example, in the early part of the 18th century, the pioneering French scientist Antoine Ferchault de Réaumur had animals swallow food in containers that allowed access to their digestive juices but resisted the stomach’s mechanical contractions (reviewed in 1). Réaumur’s work was expanded upon by the Italian Lazzaro Spallanzani in the late 1700s. Spallanzani showed that he could extract gastric juice and observe its digestive effects ex vivo over several days when these gastric secretions were mixed with food2. In so doing, he helped to prove that gastric secretions could turn food into an “impalpable mass” of chyme. By inducing injury in animal stomachs following the forced ingestion of various caustic (and sometimes sharp!) substances, he also was one of the first to learn of the stomach’s unique adaptive capacity.
Thus, from a historical perspective, it can be argued that the stomach first made gastroenterology a field worthy of careful scientific study. Most research in gastroenterology over the past few decades, however, has not focused on the stomach, and gastric cancer, though the third leading cause of cancer-related deaths worldwide3,4, remains the most poorly funded cancer of the gastrointestinal tract5. Moreover, we still have a rudimentary understanding of how gastric epithelial cells produce the secretions that so fascinated early physiologists. We are just beginning to understand how gastric epithelium develops, how it is maintained in homeostasis and in injury, and how unresolved injury can ultimately lead to disease. The stomach is subjected to countless chemical and microbial injuries on a daily basis while managing to maintain its epithelial integrity (as well as its digestive and anti-septic functions). As we will discuss, the stomach’s ability to withstand these insults is largely due to the interaction between its prodigious acid production and the plasticity of its epithelium.
We will focus on the epithelial cells in the stomach that both produce and protect against the powerful secretions that have intrigued scientists for centuries. How is the stomach organized at an anatomic and glandular level, and how does this organization change during disease? How is gastric epithelium replenished following different forms of injury? We propose a novel classification, based on known responses of the stomach to injury, comprising two distinct (though not mutually exclusive) types of repair mechanisms: 1) the superficial response, fueled by changes in the rapidly recycling surface epithelium lining the stomach lumen, and 2) the glandular response, involving adaptations by cells deeper in the gastric unit (acid-secreting parietal cells and digestive enzyme-secreting chief cells). In particular, we will highlight recent literature illustrating the remarkable plasticity of the chief cell lineage, demonstrating how various forms of injury can cause these cells to be recalled from their post-mitotic, differentiated state back into the cell cycle to initiate and fuel repair. This process of cellular reprogramming affords the stomach remarkable flexibility during repair but may also increase the risk of developing gastric cancer, further emphasizing the delicate balance between re-establishing homeostasis and progressing to gastric neoplasia.
Functional Organization of the Stomach and the Importance of Acid in Gastric Injury
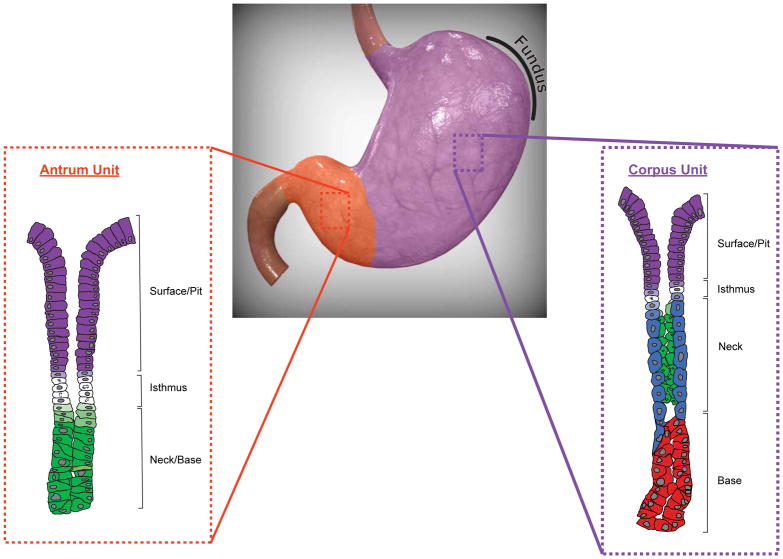
Generally speaking, the human stomach can be divided into two anatomic regions (Figure 1) that are developmentally and functionally distinct based on the organization of their glands6–10. The gastric corpus*, or body, makes up the majority of the stomach and is defined by oxyntic glands. Oxyntic glands are characterized by parietal cells that secrete hydrochloric acid and intrinsic factor (in humans) and chief cells that secrete digestive enzymes like pepsinogen. In contrast, the gastric units of the antrum, the distal portion of the stomach, are largely devoid of parietal cells and chief cells11. Antral units consist predominantly of mucous cells that express lower amounts of zymogenic proteins along with specific mucins like MUC612,13 and protective factors like TFF214. Gastric units in the corpus and antrum also contain scattered hormone-secreting endocrine cells15. For example, G cells, which are exclusive to the antrum, produce gastrin16, a hormone that stimulates acid secretion in the corpus. Tuft cells are rarer cells, whose functions are still being defined17,18, though they are known to expand during gastric injury19–21. Finally, the pit/foveolar cells line the surface of both the corpus and antrum and produce abundant mucus22,23. It is worth noting that the morphologic distinction between corpus and antral units is much sharper in common experimental animals like mice but less clear-cut in the human stomach, where few purely antral units and a significant proportion of mixed-type units, containing cells characteristic of both corpus (parietal cells) and antrum (G cells), exist24.
Figure 1. The anatomic and glandular organization of the human stomach.
The human stomach can be divided into two anatomic regions, the corpus (purple) and the antrum (orange). These regions are characterized by the cellular composition of their glands, with corpus units (purple inset) defined by acid-producing parietal cells (blue) and zymogenic chief cells (red). The antrum unit (orange inset) is largely devoid of parietal cells and chief cells and instead comprises mucous cells (green) that extend down to the gland base. Both corpus and antrum units contain an isthmus region, made up of proliferative stem cells (white), and a surface/pit region, with pit cells (purple) extending up from the isthmus to the luminal surface. Pre-parietal (light blue), pre-pit (light purple), and pre-mucous (light green) cells are also shown. Note that endocrine and tuft cells in both the corpus and antrum have been omitted. The gastric fundus has been outlined. Corpus and antrum units have been adapted from Willet and Mills9. The human stomach was modified from an original image obtained from turbosquid.com.
Our understanding of the mechanisms regulating the anatomic and cellular specification of the gastric corpus and antrum are still limited relative to what is known about organogenesis in most other organs. However, what is known has been recently summarized6,9 and will not be further discussed in this review. Instead, we will focus on how the stomach responds to injury. In a sense, the stomach is in a constant state of injury: large concentrations of ingested toxins, microbes, and physically damaging objects can spend hours in the stomach25,26. A rich network of capillaries27,28, with their inherent risk for hemorrhage, is separated from all gastric contents by as little as a single epithelial cell layer and the mucus elaborated by those cells. The potential insults to the stomach are largely neutralized by stomach acid, which can kill up to 10 billion microbes per hour29. Early physiologists like Spallanzani were fascinated by the “purifying” nature of stomach secretions and noted that these secretions could even degrade injurious objects like ingested needles2. However, gastric acid in itself is potentially harmful, so the stomach has evolved mechanisms to protect against its own principal weapon. As the stomach essentially uses the same mechanisms to respond to any potential irritant, we propose here that simply focusing on acid is an effective method for understanding patterns of injury and response in the stomach. We believe that this approach is easier than trying to categorize responses to any of the myriad potential environmental insults or even using the common pathological concepts of acute versus chronic inflammation30–33. We condense the response to gastric injury into two basic patterns, one in which the acid that usually protects the stomach inappropriately damages the stomach lining and one in which acid production is impaired.
Production of and Protection against Stomach Acid: the Superficial Response
Gastric acid secretion occurs through the acetylcholine-, histamine-, and gastrin-stimulated release of hydrochloric acid by parietal cells in the gastric corpus34. Gastric acid provides a highly effective, innate microbial filter that can regulate the microbiota of the entire gut35. Indeed, pathophysiologic or iatrogenic increases in gastric pH can increase susceptibility to certain enteric infections and alter gastric36 and enteric flora37–40. Gastric acid can be equally self-injurious, however, as it can breach gastric, esophageal, or duodenal epithelial integrity to cause bleeding and/or perforation41,42. In addition to this chemical injury, disruption of the mucosal barrier in the setting of sustained acid likely triggers a local inflammatory response, though this has been more definitively demonstrated in the esophagus in a gastroesophageal reflux disease model43. Regardless, the gastric mucosa must balance gastric acidity while protecting against acid-induced damage. These same defense mechanisms protect not only against the corrosive effects of gastric acid but also against endogenous (e.g., pepsin44,45, bile32,46,47) and exogenous (e.g., alcohol48,49, smoking50) agents. The gastric mucosa maintains its protective barrier against these insults as part of a pattern of adaptation that we refer to as the superficial response. The main mechanisms that constitute the superficial response are the secretion of topical defenses, the regulation of local blood flow, and the rapid regeneration of surface epithelium.
Gastric epithelium elaborates a variety of protective factors that act to topically neutralize or limit acid-induced damage (Figure 2). Gastric mucus provides a viscous gel matrix composed of water, mucin, electrolytes, and host and bacterial cellular components that serves to neutralize local acid production51. In addition to the bicarbonate and non-bicarbonate52 buffers that are retained in the mucus network53 and are primarily derived from the surface epithelium45, phospholipids within the mucus layer hinder the back diffusion of secreted protons54. Among the major constituents of the mucus layer, mucins, such as MUC5AC55, are glycoproteins that are predominantly secreted by surface/pit cells, and their production is regulated by acid secretagogues (acetylcholine, gastrin, histamine) as well as paracrine factors (NO, EGF, HGF) through distinct mechanisms51. Trefoil factor family proteins (TFFs) are co-secreted with mucins45 and work to enhance the viscoelastic properties of the mucus gel56. Mucin expression profiles also correlate with stages of mucosal regeneration following acid-induced injury57–59.
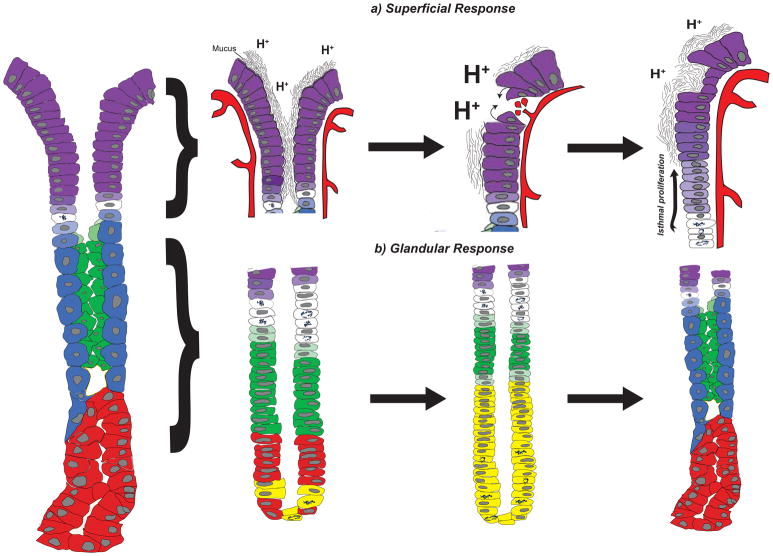
Figure 2. The superficial and glandular responses in the gastric corpus.
The corpus unit (left) responds to gastric injury through two main mechanisms, the superficial response (a) and the glandular response (b). (a) The surface epithelium (left) consists of pit cells (purple) that produce a viscous mucus barrier that protects against endogenous (e.g., acid, denoted by H+) and exogenous (not shown) injury. Breaches in the surface epithelium resulting from acid oversecretion, decreased mucus production, and/or ischemia can lead to ulcers and/or bleeding (middle). The superficial injury response restores the protective barrier of the surface epithelium by increasing mucus production, restoring local blood flow, and re-establishing epithelial integrity through restitution and cellular proliferation (right). (b) The glandular injury response correlates with the loss of acid-producing parietal cells (blue) and a replacement of the deeper glandular epithelium with metaplastic cells (yellow) that co-express markers of mucous neck cells (green) and chief cells (red). This metaplastic response (middle) is often termed Spasmolytic Polypeptide-Expressing Metaplasia (SPEM) and represents a transient response to re-establish homeostasis (right). Pre-pit (light purple), pre-parietal (light blue), and pre-mucous neck (light green) cells are also shown. The superficial and glandular responses are not mutually exclusive and can occur simultaneously within the same corpus unit.
Prostaglandins promote topical gastric protection by inhibiting acid production and stimulating mucus and bicarbonate secretion. Andre Robert first coined the term “adaptive cytoprotection,”60 referring to the protective effect of endogenous prostaglandins in response to mild gastric irritants that induced acid damage. These agents stimulate a cross-protective response, such that prostaglandins generated from mild gastric injury confer additional protection against subsequent, more severe injury61. For example, prostaglandin E2 (PGE2) is produced by the constitutively expressed enzymes cyclo-oxygenase 1 (COX-1 or PTGS) and cyclo-oxygenase 2 (COX-2 or PTGS2), whose expression is greatly increased following injury. PGE2 works through its EP3 receptors on parietal cells to directly inhibit acid secretion and indirectly on enterochromaffin-like (ECL) cells by blocking release of the acid secretagogue histamine62. It also stimulates mucus and bicarbonate secretion through EP1 and EP4 receptors63. The importance of constitutive and inducible production of PGE2 has been shown in numerous types of injury. The stomachs of cyclooxygenase-1-deficient (Ptgs1−/−) mice were more prone to two photon-induced gastric mucosal damage and less able to mount an epithelial protective response compared to Ptgs1+/− mice64. This defect could be corrected with exogenous dimethyl-PGE2 administration. Finally, non-steroidal anti-inflammatory drugs (NSAIDs) that inhibit constitutive prostaglandin production are one of the most common etiologies of peptic injury, and this has been extensively studied42,65–67.
An additional mechanism for protecting against the detrimental effects of acid is the regulation of mucosal blood flow to the surface epithelium68. Mucosal blood flow affects acid balance in various ways. For one, parietal cells secrete angiogenic factors, like vascular endothelial growth factor B69,70 (VEGFB), that help to maintain ample capillary networks to supply oxygen to their abundant mitochondria and provide the energy to efficiently pump acid. On the other hand, acute ischemia induces aberrant acid production and can cause focal erosions in the mucosal surface of corpus glands. In some cases, prolonged ischemia can lead to ulcers, as can be seen following central nervous system injuries (Cushing ulcers)71 or massive burns (Curling ulcers)72. Other angiogenic factors, such as basic fibroblast growth factor73,74 and vascular endothelial growth factor A75, typically help to limit acid-induced damage by promoting the re-establishment of the microvascular network. The restoration of blood flow also allows byproducts of acid production and other toxic metabolites to be carried away76. However, significant epithelial damage can occur during reperfusion of the gastric mucosa following an ischemic injury, as the acute restoration of oxygen and the infiltration of immune cells can result in the local production of reactive oxygen species77.
The vasodilator nitric oxide (NO) also plays a critical role in the superficial response. On the one hand, it maintains resting gastric blood flow to limit cellular damage78. On the other, NO provides additional protection through mechanisms that parallel those of prostaglandins79–81, including enhancing mucus production and limiting acid secretion. More importantly, the protective effects of NO and prostaglandins appear to be cooperative82, with NO providing a compensatory level of superficial protection in the setting of NSAID-induced gastric mucosal damage. Indeed, the injurious effects of inhibiting prostaglandin synthesis could be attenuated by exogenous administration of NO donors83,84. As a result, novel hybrid drug derivatives consisting of NSAIDs coupled to NO donor moieties have been developed as more gastro-protective therapeutic alternatives85,86, and some of these have shown promise in reducing the risk for peptic ulcer bleeding among chronic NSAID users87.
The final wing of the superficial response to damage is the rapid adaptation of the pit/surface cells themselves. In addition to limiting the extent and duration of peptic injury through alterations in the mucus layer, acid secretion, and local blood flow, the acute adaptation to acid-induced damage equally relies on reconstituting mucosal integrity by restoring cellular junctions (restitution) and through the rapid regeneration of surface epithelial cells (proliferation). Restitution, which occurs within minutes, involves the rapid migration of pit cells to cover exposed basal lamina following acute ethanol injury88, for example. In addition to rapid cellular migration, pit cells can adapt to acid-induced injury by increasing the rate of proliferation of their progenitors. The ultimate progenitors for pit/surface mucous cells are thought to be stem cells within the isthmus, the region between the gastric pits and the deeper gastric glands (Figure 1). We note, however, that it has not been formally proven that pit cells come from a multi-potent stem cell as opposed to from a long-lived progenitor that is dedicated to making only pit cells. We have discussed the uncertainty in the field in a recent review9. Regardless, what is clear is that pit cell precursors emerge from their progenitors in the isthmus and subsequently differentiate during their upward migration to the luminal surface, a process that occurs within days89. Surface mucous cells then undergo cell death and are either phagocytosed by a neighboring cell or extruded to the surface, a shedding process that may serve to prevent gastric micro-organisms from gaining a foothold within the gastric gland90.
The cellular regeneration and proliferation of surface epithelium are also enhanced by topical and paracrine factors secreted by gastric epithelium. For example, prostaglandins exert proliferative effects in addition to their locally protective topical properties. Early studies in acutely injured rat stomachs demonstrated that gastric epithelial regeneration occurred more rapidly and efficiently in rats pretreated with prostaglandins91. Growth factors derived from mesenchymal components, though not addressed in this review, have also been shown to play a role in pit cell differentiation in vitro23. Mitogenic factors of the epidermal growth factor family (e.g., EGF92,93, TGF-α93, amphiregulin93,94, HB-EGF95) promote the proliferation of pit progenitor cells and the regeneration of surface epithelium. Some of the earliest studies on TGF-α, for example, identified its enrichment in mucous neck cells and parietal cells93, and subsequent evidence has illustrated its paracrine effects on the gastric gland, including inhibiting acid secretion, increasing gastric mucin levels, and stimulating epithelial restitution96. Its expression is upregulated following acute mucosal injury in mice97,98 and promotes cell migration and cellular proliferation during ulcer healing99–101. When transgenically expressed under the regulation of the metallothionein promoter, TGF-α induced severe hypertrophic gastropathy and achlorhydria in mouse stomachs102, highly reminiscent of Ménétrier’s disease, a rare, human gastric premalignant condition that can be treated with anti-EGF/TGF-α therapy103.
Taken together, the stomach’s superficial response entails a rapid adaptation to protect against acid-induced breaches in epithelial integrity (Figure 2a). This response relies on the secretion of topical protective factors to neutralize acid’s corrosive effects, the regulation of mucosal blood flow to limit the duration and extent of injury, and the regeneration of surface epithelium through restitution and proliferation.
Defining the Glandular Response: the Initiation of SPEM
Under certain types of gastric injury, the gastric mucosa can undergo a second type of adaptation, which we will refer to as the glandular response. In contrast to the superficial response, which aims at protecting against the corrosive effects of endogenous acid, the glandular response occurs when acid production is compromised or lost. Specifically, this injury response is characterized by the loss (atrophy) of acid-producing (oxyntic) parietal cells. In extreme cases of oxyntic atrophy, all parietal cells and chief cells within a gastric unit simply die104, resulting in gland dropout with only surface/pit cells remaining. The principal histological pattern seen in response to oxyntic atrophy, however, is a repopulation of the gland, depleted of mature parietal cells and chief cells, with metaplastic cells105. The metaplastic cells constitute a hybrid phenotype, co-expressing proteins like TFF2, normally expressed by the chief cell progenitor mucous neck cells, and pepsinogen, a digestive enzyme normally expressed by mature chief cells (Figure 2b). This pattern of corpus glandular differentiation has been termed Spasmolytic Polypeptide-Expressing Metaplasia (SPEM)106–109, as it is defined by cells deep in the gland (where chief cells normally reside) that express spasmolytic polypeptide (also known as TFF2). SPEM represents a metaplastic response inherent to the gastric corpus, as the gastric antrum is largely devoid of parietal cells and chief cells110.
Though oxyntic atrophy technically means the loss of acid-secreting cells, both parietal cells and mature chief cells seem to be invariably absent. Accordingly, in multiple murine injury models107,111, SPEM has been assumed to be specifically triggered by parietal cell death. Indeed, two of these acute injury models (DMP-777112 and high-dose tamoxifen113) likely act through the H+/K+-ATPase, and their effects can be mitigated by pre-treatment with the proton pump inhibitor, omeprazole. However, a recent study in mice showed that targeted ablation of parietal cells failed to initiate SPEM114. Thus, the initiation of SPEM may require parietal cell loss as well as additional injury along the glandular axis and/or specific immune or mesenchymal cell changes. Various epithelial (e.g., NOTCH1/2115, Shh116,117, gastrin112,118,119), mesenchymal (e.g., BMP-4120, noggin121), and immune factors (e.g., IFNγ122, IL-1β123,124, IL-33125, IL-13126) contribute to the establishment of a metaplastic milieu and may play a role either in loss of parietal cells or induction of SPEM. Regardless, the glandular response during oxyntic atrophy represents a unique mechanism for adaptation and repair that highlights epithelial plasticity in the gastric corpus.
Superficial Versus Glandular Injury: Implications for Gastric Disease
The utility of distinguishing superficial from glandular injury is not academic. Previous methods for categorizing injury in the stomach have relied on the duration of injury (acute versus chronic) in the presence (gastritis) or absence (gastropathy) of mucosal inflammation31,127. Acute gastritis, to a pathologist, is identified by local infiltration of polymorphonuclear leukocytes (neutrophils) to the area of injury, whereas a chronic gastritis is characterized by a mononuclear infiltrate128. These distinctions are based on histopathology but are confounded by the fact that the same injurious agents can produce overlapping effects. Chronic conditions, like infection with the stomach-adapted bacterium Helicobacter pylori (Hp), can cause repeated bouts of acute inflammation129. Acute foci of inflammation from regional biopsies can be superimposed on a background of chronic inflammatory cell infiltrate. Agents that typically induce gastropathy (e.g., refluxed bile, alcohol, non-steroidal anti-inflammatory drugs) can also lead to gastritis130. In the case of chronic inactive gastritis secondary to portal hypertension, for example, the resulting injury is characterized by mucosal damage both with and without inflammation131. Inflammatory responses to chronic gastropathic diseases, like Ménétrier’s disease103 and Zollinger-Ellison syndrome132 (gastropathy secondary to a gastrin-producing endocrine tumor), can be variable.
As a result, we would argue that a clearer method for categorizing gastric injuries is to group them as problems of either excessive or insufficient acid. The stomach’s response to injury is best understood, as discussed above, as either superficial or glandular adaptations. This system cleaves closer to the underlying etiologies and observed responses than do other systems that force often overlapping disease patterns into categories based on temporal (acute versus chronic) or inflammatory (gastropathy versus gastritis) properties. The superficial response, as previously described, describes a mucosal adaptation to a pathophysiologic increase in acid. It enhances acid-protective mechanisms predominantly in the superficial cells to protect the epithelium from the corrosive effects of exogenous (e.g., ingested acids133) or endogenous (e.g., acid, bile47) substances, mechanical trauma (e.g., nasogastric tube placement134), and/or microbial infections135,136.
The glandular response, on the other hand, is an adaptation of epithelial lineages to an injury affecting the gastric gland, the portion of the gastric unit extending from the isthmus down to the base. Though it is possible that the stomach can undergo a glandular adaptation following substantial injury to any of the glandular cell lineages, current data have shown that parietal cell loss correlates with the glandular response105. In animal models, glandular adaptation is not as rapid as the superficial injury response, though it can still occur on the order of days107. It has been hypothesized that focal glandular responses might occur sporadically throughout the human stomach137, for example, but these have only been seen in cases of sustained damage to glandular cells. The types of damage that can induce a glandular response can be broadly classified into infectious and non-infectious, with chronic Hp infection accounting for the majority of infectious cases. The most representative example of a non-infectious, purely glandular injury is autoimmune gastritis, an autoimmune disease that targets parietal cells, ultimately leading to hypo/achlorhydria and pernicious anemia138. Interestingly, some have regarded autoimmune gastritis as an autoimmune manifestation of Hp-induced chronic gastritis139–142.
Distinguishing superficial from glandular adaptation also helps us to understand the etiologies and mechanisms underlying the responses to the disparate insults that the stomach faces. One key example is the pathophysiology of Hp infection. Approximately half of the world is infected with Hp143, though the majority of those infected will remain asymptomatic and histologically manifest as focal acute gastritis with superficial injuries. Approximately 10–15% will at one point develop clinically significant peptic ulcer disease144,145: Hp infection causes more than half of gastric and essentially all duodenal ulcers146,147. In the case of a gastric ulcer, the balance between endogenous acid production and mucosal protection is disrupted148. For an ulcer to develop, this disruption must be relatively chronic, yet the injury response largely involves superficial mechanisms with little effect on cells deeper in the gland. Indeed, a gastric ulcer would be far less likely to occur if it induced a large-scale glandular adaptation in the stomach, as the glandular response is characterized by the loss of parietal cells, the source of acid. Note that focal glandular adaptations in the few gastric units bordering a chronic ulcer may occur, but the glandular adaptation during peptic ulcer disease is not extensive149. In contrast, a minority (~1%) of patients infected with Hp will develop gastric adenocarcinoma150. In these cases, the tumors nearly invariably arise in patients who have developed an extensive, glandular adaptation of the corpus with oxyntic atrophy and metaplastic chief cell differentiation105,151. Interestingly, epidemiological studies have shown that those patients who develop ulcers from Hp, specifically duodenal ulcers secondary to acid oversecretion, may have a lower incidence of gastric cancer compared to the general population152, though some have argued against this153. The oncogenic risk therefore correlates with the type of injury response to Hp infection, regardless of the histologic presence of acute or chronic inflammation. Patients who respond to chronic Hp infection with exclusively superficial mechanisms are less likely to progress to gastric cancer than those whose stomachs undergo glandular adaptations154. The reasons behind the inherent oncogenic potential of these glandular/metaplastic changes will be discussed later.
In summary, the adaptive mechanisms that underlie the superficial and glandular injury responses are fundamentally different at the cellular level and confer distinct oncogenic risks. Our overall understanding of the superficial injury response is more comprehensive, perhaps because this response is mechanistically less complex. Simplistically, the superficial injury response relies on replenishing the pit cell lineage through cellular death, restitution, and proliferation. While the surface epithelium can respond to glandular injury signals (e.g., EGF92,93, TGF-α93, gastrin112), this usually results in pit cell proliferation and an expansion of the pit region, a process known as foveolar hyperplasia96. Glandular injury, on the other hand, induces a deeper epithelial response involving multiple lineages along the gastric unit. The glandular response can be transient or prolonged, and the mechanisms for its regulation are currently unclear. Our understanding of the initiation, expansion, and pathologic significance of the glandular response is therefore still evolving. It is worth noting that, because glandular adaptation is a response to parietal cell loss and the metaplastic differentiation of chief cells105,108, it is a process that is best understood in the gastric corpus, whereas the superficial response can occur throughout the stomach. Though it has not been formally studied, it is possible that, because human antral units can harbor parietal and chief cells, some aspects of the glandular response might also occur in these antral units155.
The remainder of the review will illustrate our current understanding of the glandular response. As we are concerned with the cellular and molecular mechanisms underlying the glandular adaptation to injury, we will refer from here on out to this pattern of injury as SPEM. Which cells are important for repopulating the gland during SPEM? How might SPEM progress through the stomach, and why might this be pathologically relevant? More importantly, what is unique about the establishment and expansion of SPEM that harbors its oncogenic potential?
Stem Cell Dynamics and the Cell of Origin for Metaplasia
The cellular origin(s) of SPEM remains an area of debate, in part because a resident stem cell population has not been definitively identified in the adult gastric corpus the way other specific stem cell populations have been confirmed in organs like skin and intestine9. If it is not clear which cells are actually performing this quotidian progenitor role, how can there be consensus on the cell of origin in a pathophysiologic pattern of differentiation? Here, we highlight various independent lines of evidence supporting a model whereby the cellular origins of SPEM may be fluid, illustrating that the stomach may be plastic with regard to the source of cells that respond to injury and fuel metaplasia. Such plasticity has become recognized as an established feature of other organs like skin156,157 and intestine156,158. Hypotheses related to the roles of stem cells in gastric corpus and antrum have been recently reviewed6,9,159,160. There is certainly consensus supporting the conclusions from the landmark pulse-chase labeling experiments of Karam and Leblond, which identified that the most actively proliferating and ultra-structurally least differentiated cells (granule-free) reside within the presumptive isthmus zone between the surface/pit and the gland161. The isthmus has the most morphologically undifferentiated, mitotically active cells in both the corpus and antrum, though its location along the gastric unit varies, as it is found about a third of the way down from the lumen in corpus units and about a third of the way up from the base in antral units (Figure 1). In continuous labeled nucleotide infusion experiments, labeled cells arise in the isthmus and eventually spread to all cell lineages along the gland unit89,162,163. Based on these studies, it has been the canon that the isthmus harbored a constitutively active multi-potent stem cell that could replenish all of the mature lineages within the gland on a daily basis. While there is broad consensus that labeled nucleotides do indeed spread bi-directionally at varying rates from the isthmus, some have questioned the interpretation of certain aspects of these studies9,164, and further experiments are warranted.
There is more definitive information about the behavior of stem cells in the antrum. Lgr5 was initially identified as a stem cell marker in the gastric antrum165, and subsequent lineage tracing experiments identified Cck2r as a marker of an antral stem cell with the potential to give rise to Lgr5+ antral cells166. We discuss the advantages and caveats with using lineage tracing to characterize stem cell dynamics in the BOX. Glandular injury following Hp colonization was recently shown to activate and expand Lgr5+ cells in the antrum167. However, Lgr5+ antral cells are located at the gland base (and not in the isthmus region). Most of the cells in the antral gland base are differentiated, deep mucus cells that happen to be the most differentiated cells within the gastric unit, lacking the “granule-free,” primitive morphological characteristics described by Karam and Leblond in the corpus161 and by Lee and Leblond in the antrum168. It is possible that Lgr5+ undifferentiated stem cells hide out in this niche, but this has not been definitively shown; interpretation may be compounded by the fact that the original tool used to identify Lgr5+ cells (Lgr5CreERT2-iRes-EGFP) shows mosaic expression169. Unlike in the corpus, turnover of all antral cells, except endocrine cells, has long been known to be rapid170. In addition, multiple promoters that drive expression of reporter genes can show “stem cell” activity upon lineage tracing171–173. For example, in addition to promoters for Lgr5 and Cck2r, it has been shown that even promoter elements for the intestine-specific gene Villin174, or for a gene that is expressed in many adult gastric cell lineages and critical to stomach development (Sox2115,175,176), can lineage trace in the antrum.
BOX.
Lineage tracing: utility and pitfalls
Lineage tracing occurs when a single, index progenitor cell is identified and its progeny traced172. The most common lineage tracing system uses genetic marking, wherein Cre recombinase expression is induced in an index cell also harboring a reporter gene, whose expression is induced when Cre recombines loxP sites in its promoter. Cre expression leads to an irreversible induction of the reporter (e.g., a fluorescent gene like dsTomato), which will thereafter be expressed in all progeny of the index cell. The inducible Cre-loxP system has become indispensable for the in vivo study of tissue development in homeostasis and in injury, though results must be interpreted with possible confounders in mind. One should consider: 1) how the mouse pedigree with a stem cell-specific gene promoter that governs Cre expression is generated (e.g., via traditional transgenic vs. BAC transgenic vs. recombination of Cre into the endogenous stem cell gene locus241); 2) the system used to activate Cre in the index cell, for example by using a Cre construct designed to travel to the nucleus only when it binds an exogenous agent like the estrogen mimetic tamoxifen242. In this case, tamoxifen is possibly confounding because it can also cause dose-dependent toxicity, especially in the stomach113; 3) that stem cells often transcribe low levels of differentiation-specific genes that can be sufficient to induce reporter expression even if the cells do not normally express detectable levels of the gene whose promoter was used to drive Cre expression243; 4) the efficiency of reporter gene induction; 5) that cells can migrate between the administration of the Cre-activating agent and when the reporter gene is transcribed and translated244; and 6) that there is likely inherent cellular plasticity, with differentiated cells being able to revert to a progenitor cell fate and subsequently lineage trace158. Taken together, these limitations in lineage tracing experiments that rely on inducible Cre recombinase should be considered and appropriately controlled.
The literature, our own unpublished work, and anecdotal evidence in the field all support the conclusion that it is rare for a cell population in the antrum to not have stem cell potential in a lineage tracing experiment; to our knowledge, only some of the endocrine cell-specific promoters do not trace readily into other lineages9. Also, it has become clear that other organs, like intestine, are remarkably plastic, with fluid cell identities that are readily adaptable to different environmental conditions156. Thus, we conclude that the antrum harbors cells with varying degrees of stochastic stem cell potential. Perhaps the isthmal cells, the cells showing the least differentiated morphology and the most propensity for proliferation, are the most likely to serve as stem cells on a daily basis, with other populations having varying likelihood of being recruited for that function. As in the intestine, injury can promote the recruitment of more differentiated cells back into the cell cycle156,158. Recent work from Meyer and coworkers supports this assertion177, as they demonstrate that the undifferentiated, isthmal antral cells are highly Wnt responsive and express the Wnt-responsive co-receptor Lgr4 at much higher levels than Lgr5. The Lgr5-high expressing cells are Lgr4-negative and located at the base of the gland. Both isthmal Lgr4+ and basal Lgr5+ populations can serve as stem cells, but the activity of Lgr4+ isthmal cells is more rapid at baseline. To fully understand stem cells and differentiation in the antrum, we will have to examine, as this group did, the relative potential for various cell populations to serve as stem cells under homeostasis and injury.
One method that could be used to quantitatively assess stem cell potential is to isolate individual cells and use them to grow gastric organoids178–180 (“gastroids”) ex vivo, though this method has its caveats181. Single CCK2R+166, LGR5+165, and LGR4+177 cells have all been shown to have the capacity to serve as stem cells for gastroid formation. We reason that the raison d’être for stem cell activity and plasticity in the antrum is to support the superficial response to injury described above, as there is currently only scant evidence to suggest that the antrum undergoes a glandular-like response akin to that of the corpus (i.e., a metaplastic response involving a change in differentiation state of the glandular cells)155. Applying antral stem cell dynamics as a guide for understanding stem cell behavior during the glandular response in the corpus may therefore not be accurate. However, there is considerable evidence that the corpus exhibits plasticity, specifically in the setting of glandular adaptation to injury. Indeed, metaplastic glands have been clearly observed in settings of corpus glandular injury, including Hp infection182, autoimmune gastritis183,184, and various murine models for acutely inducing metaplasia107. As a result, some cell within the corpus gastric unit must be able to exhibit plasticity by changing its identity, a process termed “trans-differentiation156” by some. Though one of two candidate cells - either the undifferentiated cells in the isthmus (the presumptive stem cell discussed above) or the differentiated chief cell in the base of the unit - has been proposed, it is equally possible that both isthmal and chief cells can serve as the cell of origin for SPEM to varying degrees under different injury conditions.
Evidence of isthmal cell regenerative capacity has been implied in ex vivo gastric organoid models180. In addition, acute injury models that rapidly and reversibly induce SPEM show increased proliferation in the isthmus of corpus glands114,171,185. If the isthmal stem cell is the exclusive cell of origin for SPEM, as has been recently proposed160,186, the model would imply several features of how metaplasia must unfold. The aspects of an isthmal cell serving as the cell of origin for SPEM have been schematized in Figure 3a. One aspect of this model depends on the fact that nearly all of the SPEM glands in mice and in humans are depleted of parietal cells, lack normal chief cells, and are instead populated nearly exclusively with metaplastic cells. This would assume that, for the stem cell to populate the gastric unit with metaplastic cells, the parietal cells, chief cells, and (likely) mucous neck cells must die and be replaced by metaplastic cells derived from a trans-differentiated stem cell. If any mucous neck cells remained, the cell fueling the more basal SPEM cells would have to migrate from their origin in the isthmus through this neck cell region. In murine models for acutely inducing SPEM, where SPEM peaks by 3 days111,113,187, an isthmal cell of origin model would mean that the isthmal cell would have to produce SPEM progeny to replenish the entire neck and base of the unit at a remarkable rate of cell division. Moreover, a focus of proliferation can be seen at the gland base within one to two days after SPEM induction113,169, implying that the isthmal cell would need to migrate from the isthmus to the gland base within this time frame.
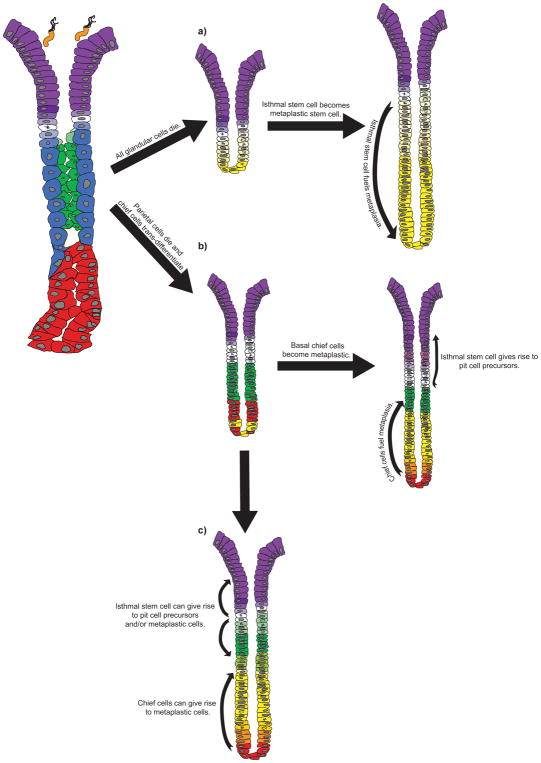
Figure 3. Distinguishing the possible cellular origins of SPEM.
Three possible mechanisms for the initiation of SPEM and the replacement of depleted glandular epithelium are presented. For the isthmal stem cell (white) to serve as the unique cell of origin for SPEM (a)186, all of the glandular cells - parietal cells (blue), chief cells (red), and mucous neck cells (green) - must die and be replaced with cells derived from a proliferative, isthmal stem cell (yellow) that has trans-differentiated to become a stem cell for metaplastic cells. (b) Others108,195 have hypothesized that SPEM arises from the trans-differentiation of chief cells into metaplastic cells following the loss of parietal cells. These basal metaplastic cells (yellow and orange) repopulate the corpus unit from the base up, while isthmal proliferation results only in an expansion of pit cells (purple), a process known as foveolar hyperplasia. Another hybrid model can also be envisioned wherein both the isthmal stem cells and chief cells can contribute to SPEM (c). In this scenario, two foci of proliferation may be dedicated to generating different cells along the gland axis, with the isthmal stem cell giving rise to pit cell precursors (light purple) and/or mucous neck cell precursors (light green), and the chief cells giving rise to metaplastic cells (yellow and orange). Bacteria represent Helicobacter pylori.
The other extreme for the cellular origin of SPEM argues that all metaplastic cells are derived from chief cells that undergo cellular reprogramming (Figure 3b). This model has been proposed and substantiated by Goldenring and colleagues over the last decade169,188–190. Along with the Goldenring group, we and others have also independently observed that chief cells can reprogram to serve as proliferating cells114,190–192. In particular, the evidence for chief cell plasticity has been demonstrated by lineage tracing using CreERT2 driven by the differentiated chief cell marker Mist1171, elements of the Runx1 promoter (known as eR1)190, Troy (Tnfrsf19)191, and most recently Lgr5169. Using an expression cassette that better matches endogenous expression of the Lgr5 gene, Barker and colleagues discovered that LGR5 labels chief cells deep within the gland169,193. Additionally, Mist1, eR1, and Lgr5 have all been shown to cause metaplasia and even dysplasia if these promoters drive expression of an oncogenic K-Ras. In those studies, SPEM was clearly characterized by both an increase in proliferation of the isthmal cells and an induction in proliferation of chief cells at the base. We have also noted that, in human pathology sections, cells with phenotypes that are transitional between chief and SPEM cells tend to occur in the base194. Because the chief cells reside at the gland base, we interpret those observations as indicating that the transition in differentiation between normal and SPEM occurs in chief cells. Finally, in unpublished studies, we find that treatment with 5-fluorouracil, an inhibitor of DNA synthesis, has little effect on the induction of SPEM, indicating that SPEM can occur in the absence of isthmal cell proliferation (Radyk and Mills, unpublished).
The preponderance of evidence therefore indicates that chief cells serve as the predominant cell of origin for SPEM195. That the post-mitotic, differentiated chief cell has the ability to reprogram and behave as a proliferative, metaplastic cell is not unique to stomach, given the emerging parallel literature of cellular reprogramming in other gastrointestinal tissues156,158,196. For example, in the pancreas, which does not have a resident stem cell, the zymogenic acinar cell exhibits plasticity in response to injury by co-expressing ductal markers and becoming proliferative, a process known as acinar-to-ductal metaplasia197–199. Recent data suggest that shared cellular mechanisms may exist to allow post-mitotic cells, like chief cells and acinar cells, to be recruited back into the cell cycle. This process is initiated by an auto-degradative phase in which existing secretory architecture is recycled, followed by the induction of metaplastic genes, and concludes with the proliferation of metaplastic cells158,192,200.
The magnitude and extent of gastric injury likely dictate the glandular response, and a single focus of proliferation and metaplasia may not represent the most efficient method to fuel repair. Most of the murine models for inducing SPEM result in a rapid, simultaneous, pan-gastric injury that results in the appearance of two distinct foci of proliferative activity in each gastric unit107. In addition to a focus of proliferation at the gland base, proliferation at the isthmus is clearly seen, and these isthmal cells could contribute to metaplasia migrating down from the isthmus (Figure 3c). Two foci of epithelial regeneration within the gland would allow for a more rapid and efficient reconstitution of injured epithelium. In the setting of a chronic injury which gradually injures parietal cells and chief cells, it is possible that two regenerative foci would not be required. Another possibility is that the two zones of proliferation during the glandular response are devoted to generating different cells. The isthmus will continue to generate pit cells and may also generate new parietal cells and/or mucous neck cells, whereas proliferating cells from deeper in the gland may be dedicated to regenerating chief cells. It should also be mentioned that, while the chief cell has been identified as a cell capable of reprogramming and answering the call for repair, other cell types along the gland axis (i.e., the mucous neck cell) may be capable of serving a similar role, though this has never been demonstrated. This would imply that multiple lineages of cells along the corpus glandular unit, similar to the previously described antral unit, exhibit an inherent stemness that allows the gland to efficiently respond to injury.
Why Hp Might Benefit from SPEM
As we continue to explore the cellular mechanisms behind the initiation of SPEM, we must equally consider the significance of the expansion of SPEM. In human patients, SPEM appears to slowly expand gland-by-gland along a front extending proximally from the corpus/antrum transition. The leading edge of metaplasia has sometimes been referred to as the “atrophic front,”153 a progressing zone of inflammation that moves proximally from the antrum to the corpus along the lesser curvature. The atrophic front blurs the normal, sharp, histological transition between corpus and antrum. As a result, this transition becomes dynamic following chronic glandular adaptation. While the corpus and antrum are functionally and developmentally distinct7, glandular adaptation in the corpus (SPEM) causes the corpus to histologically resemble the antrum, a process that has been termed “antralization” of the corpus201–204. Glandular adaptation to injury in the corpus has taken on various names (atrophic gastritis30,205,206, oxyntic atrophy105, pseudopyloric metaplasia207,208, SPEM109) which, in our opinion, represent the same cellular and molecular processes. Antralization of the corpus does not mean that the corpus is converted into antrum209, but morphologic and molecular similarities can be seen between corpus units, that have lost their parietal cells and chief cells, and antral units.
How and why might chronic Hp infection lead to an antralization of the corpus? In chronically infected patients that eventually develop extensive atrophy and metaplasia, the natural course of chronic Hp infection mimics the pattern of extension of the atrophic front. It has been proposed that the corpus/antrum transition is initially colonized by Hp and serves as a critical niche for the establishment of a chronic infection210. Why Hp may target or hone in on this region is unclear, but, teleogically, this relatively short span of hybrid glands211 may represent the first hospitable micro-environment that this bacterium encounters in terms of favorable intra-gastric pH. In the setting of Hp infection and its associated inflammation, we propose that SPEM first arises from this corpus/antrum transition and progresses towards the corpus as more and more glands undergo antralization (Figure 4a). It is also possible, though less likely, that several areas of Hp colonization distributed throughout the corpus can each give rise to foci of SPEM expansion. Over decades, Hp can expand its niche along this atrophic front, progressing into the corpus and resulting in a pan-gastritis, a risk factor for the development of gastric adenocarcinoma212. The topographic spread of Hp may represent the bacterium’s unique adaptation to a changing environment.
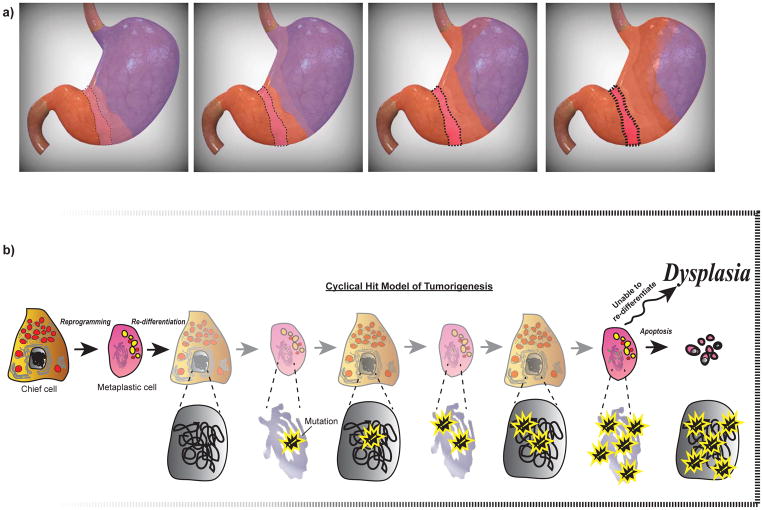
Figure 4. The expansion of SPEM and the Cyclical Hit model: a possible mechanism for dysplasia.
(a) The topographic expansion of SPEM during sustained glandular injury results in the “antralization” of the corpus, such that corpus units become metaplastic and morphologically resemble antral units. The antralization of the corpus likely emerges from an initial focus of metaplasia (boxed red area) at the transition between corpus (purple) and antrum (orange) and expands proximally along the lesser curvature before spreading to the greater curvature. The transition zone (light purple) represents a dynamic, hybrid region that progresses along the leading edge of antralization. The earliest sites of antralization will have the longest history of metaplasia with associated dedifferentiation-redifferentiation cycles, increased risk of accumulation of mutations, and an increased likelihood that those mutations will seed dysplasia/neoplasia: this increased risk is denoted by an increasing color saturation of the boxed region at the corpus-antrum border. (b) The Cyclical Hit Model for the development of gastric dysplasia is presented. As post-mitotic chief cells become metaplastic and re-enter the cell cycle to proliferate and fuel metaplasia, they can accumulate genetic mutations (shown with yellow outlined symbol) through replicative stress. Chief cells within the initial focus of metaplasia (boxed area in a), a region that would have sustained the longest duration of glandular injury, harbor genetic mutations that can become unmasked and prevent re-differentiation, either leading to apoptosis or potentially serving as a cell of origin for dysplasia.
The host and microbial factors that determine these patterns of Hp colonization and spread remain largely unexplored, however. In the setting of chronic Hp infection, inflammation likely indirectly promotes gastric cellular reprogramming, but Hp colonization may also actively drive the expansion of SPEM through the elaboration of virulence factors like CagA213,214 and VacA215 that promote inflammation and glandular reorganization. From the point of view of Hp, the glandular response of the corpus (i.e., SPEM) may be a method for the bacterium to expand its niche. The reorganization of the corpus glands renders them more like antrum, for which Hp has an affinity, at least early in its pathogenesis. The metaplastic cells in SPEM are also proliferative far deeper into the gland than the normal isthmus216, and Hp has been shown to actively interact with this proliferative zone217. In short, Hp may exhibit a tropism for SPEM glands and hijack the very alterations in the gastric landscape that it has induced.
The Oncogenic Potential of SPEM
The initiation and expansion of SPEM likely have clinical significance in terms of explaining the distribution of gastric cancer. A conundrum in the gastric cancer field has been the disconnect between the topographic distribution of glandular injury and the anatomic location of gastric tumors. Given the strong epidemiological link between pan-gastritis and extensive metaplasia in the corpus, it has been assumed that loss of parietal cells and chief cells in the corpus is a prerequisite for the development of gastric adenocarcinoma218. What has been confounding, however, is that the majority of human gastric adenocarcinomas seem to arise within the antrum or at the corpus/antrum transition219–221, suggesting that parietal/chief cell loss and metaplasia may simply be a surrogate marker for the overall state of chronic inflammation in the stomach. Perhaps this inflammatory state promotes tumors in the antrum, but this does not necessarily mean that the metaplastic/atrophic tissue is itself the origin of gastric cancer.
Rather than being epiphenomenological, we propose that these metaplastic, transitional areas represent regions where many gastric cancers likely arise. Hp primarily colonizes the antrum during the initial establishment of infection. As it expands its niche and promotes the progression of an atrophic front, the first glands undergoing metaplasia would be those with parietal cells and chief cells along the border of the corpus and antrum (Fig. 4a). What makes metaplastic tissue particularly prone to becoming neoplastic? We must first acknowledge that SPEM is a normal, transient, glandular response to injury for re-establishing homeostasis. We believe that SPEM is fueled by differentiated cells re-entering the cell cycle, proliferating, and re-differentiating. One could imagine that repeated rounds of proliferation/differentiation cycles increase the chances of acquiring mutations via replicative stress (Figure 4b). Studies in pancreas and other models of tumorigenesis indicate that certain oncogenic mutations, like constitutively active K-Ras, do not have an effect in differentiated cells but can be unmasked when they are expressed in proliferating (i.e., metaplastic) cells222–224. In the stomach, if these mutations do not block re-differentiation as the gland recovers from injury, these mutations can be harbored in quiescent, seemingly normal, differentiated chief cells. Over time, glands that have sustained the longest duration of injury will have accumulated substantial mutational burdens (Figures 4a and 4b). The corpus/antrum transition exemplifies one such area, as it likely represents the initial focus of Hp colonization and hence metaplasia. We also know that this region shifts as the corpus gradually becomes antralized during chronic Hp infection201. Endoscopic mapping studies looking at the patterns of metaplasia that predicted progression to gastric adenocarcinoma found that the extension of metaplasia along the lesser curvature, known as the “magenstrasse” (German for “narrow street”) pattern, carried a significantly greater cancer risk than other observed patterns225. This high-risk pattern would correlate with the progression of the atrophic front and expansion of SPEM, arising from the corpus/antrum transition, the initial focus of metaplasia.
A parallel argument could be made for cancers at the gastro-esophageal junction, where a similar transition zone between squamous mucosa of the esophagus and glandular mucosa of the stomach may represent an area of increased metaplastic (and oncogenic) potential226–228. Indeed, gastro-esophageal cancers are increasingly common229 and appear to be more similar to gastric cancer at the molecular level230. One could similarly imagine the distal expansion (i.e., toward stomach, away from esophagus) of this gastro-esophageal transition zone, with a focus of metaplasia at the gastro-esophageal transition (referred to as the cardia in humans)231 serving as a possible starting point for the expansion of metaplasia. Unlike metaplasia at the corpus/antrum transition, metaplasia in the gastro-esophageal transition zone is not usually caused by Hp but rather correlates with acid and/or bile exposure that can be linked to metaplasia of the distal esophagus (i.e., Barrett’s metaplasia)232,233. The cellular origins of Barrett’s metaplasia234–236 and the possible expansion of metaplastic glands derived from the stomach into the esophagus have been proposed237,238. As in the stomach, the focus of metaplasia at this transition zone and the subsequent spread of atrophy and metaplasia into the surrounding tissue may carry a similar risk for cancer, in this case proximal gastric adenocarcinomas.
Conclusions and Remaining Questions
In addition to serving as a highly efficient immune barrier for the gut, the stomach is a versatile organ that is capable of recovering from various forms of injury by relying on mechanisms for superficial and glandular adaptation. The superficial response, which largely centers on how the stomach protects itself from its own acid, has been relatively well studied. Here, we have focused on the less understood glandular response, which is of particular import because it epitomizes the delicate balance between re-establishing homeostasis and progressing to neoplasia, a theme common to multiple organs. We have presented evidence for the mechanisms and cellular origins of SPEM, an evolutionarily conserved mechanism for responding to glandular injury, though we still do not fully understand the cellular signals and mechanisms that regulate metaplasia in the stomach. While acute injury models for inducing SPEM suggest that this is a reversible process107,113, how does reversion to homeostasis occur? Is there a point at which metaplasia is irreversible?
More importantly, what is the clinical significance of SPEM as a pre-neoplastic lesion? Does gastric dysplasia arise from SPEM? While this review has predominantly focused on the initial injury response and speculated on mechanisms for the expansion of SPEM, it should be noted that most of the pathology literature related to gastric cancer focuses on gastric intestinal metaplasia, a precursor lesion to gastric adenocarcinoma that, like SPEM, emerges after the development of oxyntic atrophy218. Intestinal metaplasia involving the gastric corpus carries a significant oncogenic risk that has formed the basis for endoscopic surveillance guidelines239. Compared to SPEM, however, the cellular origin of intestinal metaplasia is less understood, largely due to a lack of adequate animal models. It remains to be seen whether SPEM gives rise to intestinal metaplasia240 or whether the two precursor lesions can independently give rise to gastric adenocarcinoma137. Regardless, it is becoming more evident that the zymogenic chief cell plays a crucial role in the initiation of SPEM and in repairing glandular injury169, though the precise mechanisms by which the chief cell undergoes cellular reprogramming to fuel metaplasia warrant further investigation. This reprogramming likely constitutes a sequence of intra-cellular mechanisms that are shared across other exocrine organs as part of a conserved response to glandular injury. As we begin to uncover the molecular players involved in this reprogramming sequence, we can begin to identify how specific mutations contribute to the development and progression of gastric cancer for millions of patients at risk.
Key points.
The stomach is a versatile organ that protects against countless forms of endogenous and exogenous injury, mainly through the production of acid.
The stomach’s injury response can be classified into two main patterns, one that protects against endogenous acid (superficial response) and one that adapts when the source of acid is lost or compromised (glandular response).
The glandular response is a process that is best understood in the gastric corpus and involves a replacement of injured epithelium with metaplastic cells, a process known as Spasmolytic Polypeptide-Expressing Metaplasia, or SPEM.
Recent studies highlight the epithelial plasticity of the gastric corpus, in particular the ability of post-mitotic zymogenic chief cells to re-enter the cell cycle and fuel repair of injured epithelium.
Acknowledgments
The authors are supported by NIDDK awards DK094989, DK105129, DK110406, by the Alvin J. Siteman Cancer Center/Barnes Jewish Hospital Foundation Cancer Frontier Fund, NIH NCI P30 CA091842, and The Barnard Trust to JCM. JBS holds a Postdoctoral Enrichment Program Award from the Burroughs Wellcome Fund and is also supported by the AGA Gastric Cancer Foundation Research Scholar Award.
Glossary
- Surface Epithelium
mucus-secreting cells that line the surface of the stomach; also referred to as surface, foveolar, or pit cells.
- Gastric Pit
The surface epithelium invaginates into gastric units that are funnel-shaped and dive downward towards the gastric muscular wall. The mouth-like opening of each gastric unit represents the gastric pit. The zone where the pit narrows into the gland harbors actively dividing stem cells and is called the isthmus.
- Gastric Gland
We use here the human pathology definition of the gastric gland as being separate from the gastric pit. The glandular portion of a gastric unit is located between the base (i.e., nearest the stomach muscular wall) and extends up to the isthmus. In the corpus, the gastric gland comprises parietal, chief, mucous neck, and endocrine cells. In the antrum, the gastric gland contains mucous and endocrine cells.
- Superficial Response
how the stomach (both corpus and antrum) repairs erosive injury (most commonly due to acid) on the epithelial surface.
- Glandular Response
how the stomach adapts to injury involving loss of acid-secreting parietal cells and digestive enzyme-secreting chief cells from gastric glands in the corpus.
- Oxyntic Atrophy
a process characterized by the loss of acid-producing, or oxyntic, glands from the corpus.
- Atrophic Front
The stomach-adapted bacterium Helicobacter pylori is known to cause atrophy and metaplasia of the corpus in a subset of chronically infected patients. This atrophy spreads along a “front” from the antrum into the corpus along the lesser curvature.
- Cyclical Hit Model of Tumorigenesis
a proposal that mutations may accumulate and be stored in differentiated cells. Following injury, differentiated can re-enter the cell cycle and proliferate. During their proliferative phase, these mutations can be acquired. As the cells re-differentiate, the acquired mutations are stored. These stored mutations may accumulate with little effect until the cells either undergo apoptosis or become trapped in a (proliferative) dysplastic state.
Biography
Jason C. Mills is an expert in epithelial stem cells, metaplasia, and the cells of origin in cancer. He is director of research and co-director of the Digestive Disease Center in the Division of Gastroenterology at Washington University. He is also a Professor in the Departments of Medicine, Pathology and Immunology, and Developmental Biology at the Washington University School of Medicine. José B. Sáenz is an Instructor of Medicine in the Division of Gastroenterology at the Washington University School of Medicine and is particularly interested in how Helicobacter pylori adapts to alterations in the gastric landscape.
Footnotes
The human stomach is morphologically different from the murine stomach, a common model for studying gastric pathophysiology. One anatomic difference is the gastric fundus, the deepest, pouch-like portion of the human stomach that is composed of relatively long oxyntic glands (Figure 1). No equivalent region exists in the mouse, as the equivalent anatomic region of the mouse stomach, referred to as the forestomach, is lined with squamous epithelium. In this review, we will eschew the term “fundus” and refer to the proximal, non-antral stomach as the “corpus,” a term that is equivalent for mouse models and humans.
Competing interests
The authors declare no competing interests.
References
- 1.De Fourcroy AF. Élémens d’Histoire Naturelle et de Chimie. Cuchet; Paris: 1791. pp. 357–62. [Google Scholar]
- 2.Spallanzani L. Dissertations Relative to the Natural History of Animals and Vegetables. Vol. 1. J. Murray; London: 1789. This was one of the first treatises focusing on the stomach and its role in digestion. [Google Scholar]
- 3.Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Colquhoun A, et al. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64:1881–1888. doi: 10.1136/gutjnl-2014-308915. [DOI] [PubMed] [Google Scholar]
- 5.Carter AJ, Nguyen CN. A comparison of cancer burden and research spending reveals discrepancies in the distribution of research funding. BMC Public Health. 2012;12:526. doi: 10.1186/1471-2458-12-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim TH, Shivdasani RA. Stomach development, stem cells and disease. Development. 2016;143:554–565. doi: 10.1242/dev.124891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCracken KW, et al. Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature. 2017;541:182–187. doi: 10.1038/nature21021. This was the first paper to produce mature lineages of gastric corpus epithelium from human embryonic stem cells and demonstrated how Wnt plays a role in gastric corpus development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCracken KW, Wells JM. Mechanisms of embryonic stomach development. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willet SG, Mills JC. Stomach Organ and Cell Lineage Differentiation: from Embryogenesis to Adult Homeostasis. Cell Mol Gastroenterol Hepatol. 2016;2:546–559. doi: 10.1016/j.jcmgh.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor A, O’Morain C. Digestive function of the stomach. Dig Dis. 2014;32:186–191. doi: 10.1159/000357848. [DOI] [PubMed] [Google Scholar]
- 11.Ban S. In: Morson and Dawson’s Gastrointestinal Pathology. Shepherd NA, Warren BF, Williams GT, Greenson JK, Lauwers GY, Novelli MR, editors. Ch 9. Wiley-Blackwell; Hoboken: 2013. [Google Scholar]
- 12.De Bolos C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995;109:723–734. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- 13.Longman RJ, et al. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut. 2000;47:792–800. doi: 10.1136/gut.47.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanby AM, et al. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993;105:1110–1116. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- 15.Li HJ, et al. Distinct cellular origins for serotonin-expressing and enterochromaffin-like cells in the gastric corpus. Gastroenterology. 2014;146:754–764. doi: 10.1053/j.gastro.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson SA, Grabowska AM, El-Zaatari M, Takhar A. Gastrin - active participant or bystander in gastric carcinogenesis? Nat Rev Cancer. 2006;6:936–946. doi: 10.1038/nrc2014. [DOI] [PubMed] [Google Scholar]
- 17.Sato A. Tuft cells. Anat Sci Int. 2007;82:187–199. doi: 10.1111/j.1447-073X.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 18.O’Neil A, Petersen CP, Choi E, Engevik AC, Goldenring JR. Unique Cellular Lineage Composition of the First Gland of the Mouse Gastric Corpus. J Histochem Cytochem. 2017;65:47–58. doi: 10.1369/0022155416678182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam KT, et al. Gastric tumor development in Smad3-deficient mice initiates from forestomach/glandular transition zone along the lesser curvature. Lab Invest. 2012;92:883–895. doi: 10.1038/labinvest.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saqui-Salces M, et al. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol. 2011;136:191–204. doi: 10.1007/s00418-011-0831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Huang X. Investigation of doublecortin and calcium/calmodulin-dependent protein kinase-like-1-expressing cells in the mouse stomach. J Gastroenterol Hepatol. 2010;25:576–582. doi: 10.1111/j.1440-1746.2009.06114.x. [DOI] [PubMed] [Google Scholar]
- 22.Prasanna LC. Analysis of the Distribution of Mucins in Adult Human Gastric Mucosa and Its Functional Significance. J Clin Diagn Res. 2016;10:AC01–04. doi: 10.7860/JCDR/2016/12323.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ootani A, Toda S, Fujimoto K, Sugihara H. Foveolar differentiation of mouse gastric mucosa in vitro. Am J Pathol. 2003;162:1905–1912. doi: 10.1016/S0002-9440(10)64324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi E, et al. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut. 2014;63:1711–1720. doi: 10.1136/gutjnl-2013-305964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellmig S, et al. Gastric emptying time of fluids and solids in healthy subjects determined by 13C breath tests: influence of age, sex and body mass index. J Gastroenterol Hepatol. 2006;21:1832–1838. doi: 10.1111/j.1440-1746.2006.04449.x. [DOI] [PubMed] [Google Scholar]
- 26.Leontiadis GI, et al. Effects of Helicobacter pylori infection on gastric emptying rate in patients with non-ulcer dyspepsia. World J Gastroenterol. 2004;10:1750–1754. doi: 10.3748/wjg.v10.i12.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gannon B, Browning J, O’Brien P, Rogers P. Mucosal microvascular architecture of the fundus and body of human stomach. Gastroenterology. 1984;86:866–875. [PubMed] [Google Scholar]
- 28.Kvietys PR. In: The Gastrointestinal Circulation Integrated Systems Physiology: From Molecule to Function. Granger J, Granger DN, editors. Chapter 9. Morgan & Claypool Life Sciences; San Rafael: 2010. [PubMed] [Google Scholar]
- 29.Giannella RA, Broitman SA, Zamcheck N. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut. 1972;13:251–256. doi: 10.1136/gut.13.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rugge M, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008;40:650–658. doi: 10.1016/j.dld.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 31.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Correa P. Chronic gastritis: a clinico-pathological classification. Am J Gastroenterol. 1988;83:504–509. [PubMed] [Google Scholar]
- 33.Rugge M, Genta RM. Staging and grading of chronic gastritis. Hum Pathol. 2005;36:228–233. doi: 10.1016/j.humpath.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Schubert ML. Functional anatomy and physiology of gastric secretion. Curr Opin Gastroenterol. 2015;31:479–485. doi: 10.1097/MOG.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 35.Imhann F, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanduleanu S, Jonkers D, de Bruine A, Hameeteman W, Stockbrugger RW. Changes in gastric mucosa and luminal environment during acid-suppressive therapy: a review in depth. Dig Liver Dis. 2001;33:707–719. doi: 10.1016/s1590-8658(01)80050-5. [DOI] [PubMed] [Google Scholar]
- 37.McDonald EG, Milligan J, Frenette C, Lee TC. Continuous Proton Pump Inhibitor Therapy and the Associated Risk of Recurrent Clostridium difficile Infection. JAMA Intern Med. 2015;175:784–791. doi: 10.1001/jamainternmed.2015.42. [DOI] [PubMed] [Google Scholar]
- 38.Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102:2047–2056. doi: 10.1111/j.1572-0241.2007.01275.x. [DOI] [PubMed] [Google Scholar]
- 39.Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107:1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 40.Freedberg DE, Lebwohl B, Abrams JA. The impact of proton pump inhibitors on the human gastrointestinal microbiome. Clin Lab Med. 2014;34:771–785. doi: 10.1016/j.cll.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tielleman T, Bujanda D, Cryer B. Epidemiology and Risk Factors for Upper Gastrointestinal Bleeding. Gastrointest Endosc Clin N Am. 2015;25:415–428. doi: 10.1016/j.giec.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Lanas A, Chan FK. Peptic ulcer disease. Lancet. 2017 doi: 10.1016/S0140-6736(16)32404-7. [DOI] [PubMed] [Google Scholar]
- 43.Souza RF, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. 2009;137:1776–1784. doi: 10.1053/j.gastro.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 44.Taylor WH. Pepsins of patients with peptic ulcer. Nature. 1970;227:76–77. doi: 10.1038/227076a0. [DOI] [PubMed] [Google Scholar]
- 45.Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288:C1–19. doi: 10.1152/ajpcell.00102.2004. [DOI] [PubMed] [Google Scholar]
- 46.Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2008;88:1547–1565. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 47.Dixon MF, O’Connor HJ, Axon AT, King RF, Johnston D. Reflux gastritis: distinct histopathological entity? J Clin Pathol. 1986;39:524–530. doi: 10.1136/jcp.39.5.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singer MV, Leffmann C, Eysselein VE, Calden H, Goebell H. Action of ethanol and some alcoholic beverages on gastric acid secretion and release of gastrin in humans. Gastroenterology. 1987;93:1247–1254. doi: 10.1016/0016-5085(87)90252-6. [DOI] [PubMed] [Google Scholar]
- 49.Boltin D, Niv Y. Pharmacological and alimentary alteration of the gastric barrier. Best Pract Res Clin Gastroenterol. 2014;28:981–994. doi: 10.1016/j.bpg.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Ma L, Chow JY, Cho CH. Effects of cigarette smoking on gastric ulcer formation and healing: possible mechanisms of action. J Clin Gastroenterol. 1998;27(Suppl 1):S80–86. doi: 10.1097/00004836-199800001-00013. [DOI] [PubMed] [Google Scholar]
- 51.Ichikawa TIK. In: Protective Effects of Gastric Mucus, Gastritis, and Gastric Cancer - New Insights in Gastroprotection, Diagnosis, and Treatments. Tonino P, editor. Chapter 1. InTech; Rijeka: 2011. [Google Scholar]
- 52.Allen A, Flemstrom G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev. 1993;73:823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- 53.McColl KE. The elegance of the gastric mucosal barrier: designed by nature for nature. Gut. 2012;61:787–788. doi: 10.1136/gutjnl-2011-301612. [DOI] [PubMed] [Google Scholar]
- 54.Yandrapu H, Sarosiek J. Protective Factors of the Gastric and Duodenal Mucosa: An Overview. Curr Gastroenterol Rep. 2015;17:24. doi: 10.1007/s11894-015-0452-2. [DOI] [PubMed] [Google Scholar]
- 55.Ho SB, et al. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig Dis Sci. 2004;49:1598–1606. doi: 10.1023/b:ddas.0000043371.12671.98. [DOI] [PubMed] [Google Scholar]
- 56.Thim L, Madsen F, Poulsen SS. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur J Clin Invest. 2002;32:519–527. doi: 10.1046/j.1365-2362.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 57.Ikezawa T, et al. Appearance of specific mucins recognized by monoclonal antibodies in rat gastric mucosa healing from HCl-induced gastric mucosal damage. J Gastroenterol. 2004;39 doi: 10.1007/s00535-003-1261-1. [DOI] [PubMed] [Google Scholar]
- 58.Hayashida H, et al. Expression of a specific mucin type recognized by monoclonal antibodies in the rat gastric mucosa regenerating from acetic acid-induced ulcer. Scand J Gastroenterol. 2001;36:467–473. doi: 10.1080/003655201750153214. [DOI] [PubMed] [Google Scholar]
- 59.Boltin D, et al. Gastric mucin expression in Helicobacter pylori-related, nonsteroidal anti-inflammatory drug-related and idiopathic ulcers. World J Gastroenterol. 2012;18:4597–4603. doi: 10.3748/wjg.v18.i33.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robert A, et al. Mild irritants prevent gastric necrosis through “adaptive cytoprotection” mediated by prostaglandins. Am J Physiol. 1983;245:G113–121. doi: 10.1152/ajpgi.1983.245.1.G113. This was one of the first studies to elucidate the protective roles of prostaglandins in the superficial response to gastric irritants. [DOI] [PubMed] [Google Scholar]
- 61.Konturek SJ, et al. Role of locally generated prostaglandins in adaptive gastric cytoprotection. Dig Dis Sci. 1982;27:967–971. doi: 10.1007/BF01391740. [DOI] [PubMed] [Google Scholar]
- 62.Ham M, Akiba Y, Takeuchi K, Montrose MH, Kaunitz JD. In: Physiology of the Gastrointestinal Tract. Johnson LR, editor. Academic Press; Oxford: 2012. pp. 1169–1208. [Google Scholar]
- 63.Ohnishi A, et al. EP1 and EP4 receptors mediate exocytosis evoked by prostaglandin E(2) in guinea-pig antral mucous cells. Exp Physiol. 2001;86:451–460. doi: 10.1113/eph8602160. [DOI] [PubMed] [Google Scholar]
- 64.Starodub OT, Demitrack ES, Baumgartner HK, Montrose MH. Disruption of the Cox-1 gene slows repair of microscopic lesions in the mouse gastric epithelium. Am J Physiol Cell Physiol. 2008;294:C223–232. doi: 10.1152/ajpcell.00395.2006. [DOI] [PubMed] [Google Scholar]
- 65.Melcarne L, Garcia-Iglesias P, Calvet X. Management of NSAID-associated peptic ulcer disease. Expert Rev Gastroenterol Hepatol. 2016;10:723–733. doi: 10.1586/17474124.2016.1142872. [DOI] [PubMed] [Google Scholar]
- 66.Scarpignato C, Pelosini I. Prevention and treatment of non-steroidal anti-inflammatory drug-induced gastro-duodenal damage: rationale for the use of antisecretory compounds. Ital J Gastroenterol Hepatol. 1999;31(Suppl 1):S63–72. [PubMed] [Google Scholar]
- 67.Takeuchi K. Pathogenesis of NSAID-induced gastric damage: importance of cyclooxygenase inhibition and gastric hypermotility. World J Gastroenterol. 2012;18:2147–2160. doi: 10.3748/wjg.v18.i18.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tarnawski AS, Ahluwalia A, Jones MK. The mechanisms of gastric mucosal injury: focus on microvascular endothelium as a key target. Curr Med Chem. 2012;19:4–15. doi: 10.2174/092986712803414079. [DOI] [PubMed] [Google Scholar]
- 69.Capoccia BJ, Huh WJ, Mills JC. How form follows functional genomics: gene expression profiling gastric epithelial cells with a particular discourse on the parietal cell. Physiol Genomics. 2009;37:67–78. doi: 10.1152/physiolgenomics.90408.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lo HG, et al. A single transcription factor is sufficient to induce and maintain secretory cell architecture. Genes Dev. 2017;31:154–171. doi: 10.1101/gad.285684.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spirt MJ. Stress-related Mucosal Disease. Curr Treat Options Gastroenterol. 2003;6:135–145. doi: 10.1007/s11938-003-0014-9. [DOI] [PubMed] [Google Scholar]
- 72.Pilkington KB, Wagstaff MJ, Greenwood JE. Prevention of gastrointestinal bleeding due to stress ulceration: a review of current literature. Anaesth Intensive Care. 2012;40:253–259. doi: 10.1177/0310057X1204000207. [DOI] [PubMed] [Google Scholar]
- 73.Paimela H, et al. Restitution of frog gastric mucosa in vitro: effect of basic fibroblast growth factor. Gastroenterology. 1993;104:1337–1345. doi: 10.1016/0016-5085(93)90342-a. [DOI] [PubMed] [Google Scholar]
- 74.Kawano S, Tsuji S. Role of mucosal blood flow: a conceptional review in gastric mucosal injury and protection. J Gastroenterol Hepatol. 2000;15(Suppl):D1–6. doi: 10.1046/j.1440-1746.2000.02142.x. [DOI] [PubMed] [Google Scholar]
- 75.Jones MK, et al. Activation of VEGF and Ras genes in gastric mucosa during angiogenic response to ethanol injury. Am J Physiol. 1999;276:G1345–1355. doi: 10.1152/ajpgi.1999.276.6.G1345. [DOI] [PubMed] [Google Scholar]
- 76.Szabo S. “Gastric cytoprotection” is still relevant. J Gastroenterol Hepatol. 2014;29(Suppl 4):124–132. doi: 10.1111/jgh.12735. [DOI] [PubMed] [Google Scholar]
- 77.Hernandez LA, et al. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699–703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 78.Pique JM, Whittle BJ, Esplugues JV. The vasodilator role of endogenous nitric oxide in the rat gastric microcirculation. Eur J Pharmacol. 1989;174:293–296. doi: 10.1016/0014-2999(89)90324-5. [DOI] [PubMed] [Google Scholar]
- 79.Brown JF, Keates AC, Hanson PJ, Whittle BJ. Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am J Physiol. 1993;265:G418–422. doi: 10.1152/ajpgi.1993.265.3.G418. [DOI] [PubMed] [Google Scholar]
- 80.Brown JF, Hanson PJ, Whittle BJ. Nitric oxide donors increase mucus gel thickness in rat stomach. Eur J Pharmacol. 1992;223:103–104. doi: 10.1016/0014-2999(92)90824-n. [DOI] [PubMed] [Google Scholar]
- 81.Kato S, Kitamura M, Korolkiewicz RP, Takeuchi K. Role of nitric oxide in regulation of gastric acid secretion in rats: effects of NO donors and NO synthase inhibitor. Br J Pharmacol. 1998;123:839–846. doi: 10.1038/sj.bjp.0701691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–520. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- 83.Takeuchi K, Yasuhiro T, Asada Y, Sugawa Y. Role of nitric oxide in pathogenesis of aspirin-induced gastric mucosal damage in rats. Digestion. 1998;59:298–307. doi: 10.1159/000007506. [DOI] [PubMed] [Google Scholar]
- 84.Jimenez D, et al. Mechanisms involved in protection afforded by L-arginine in ibuprofen-induced gastric damage: role of nitric oxide and prostaglandins. Dig Dis Sci. 2002;47:44–53. doi: 10.1023/a:1013203217788. [DOI] [PubMed] [Google Scholar]
- 85.Wallace JL, et al. Novel nonsteroidal anti-inflammatory drug derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology. 1994;107:173–179. doi: 10.1016/0016-5085(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 86.Kodela R, Chattopadhyay M, Velazquez-Martinez CA, Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications. Biochem Pharmacol. 2015;98:564–572. doi: 10.1016/j.bcp.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lanas A, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507–515. doi: 10.1111/j.1572-0241.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 88.Lacy ER, Ito S. Rapid epithelial restitution of the rat gastric mucosa after ethanol injury. Lab Invest. 1984;51:573–583. [PubMed] [Google Scholar]
- 89.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993;236:280–296. doi: 10.1002/ar.1092360203. This was a landmark study that qualitatively and quantitatively illustrated the dynamics of gastric stem cells giving rise to the pit cell lineage. [DOI] [PubMed] [Google Scholar]
- 90.Mimuro H, et al. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe. 2007;2:250–263. doi: 10.1016/j.chom.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 91.Reinhart WH, Muller O, Halter F. Influence of long-term 16,16-dimethyl prostaglandin E2 treatment on the rat gastrointestinal mucosa. Gastroenterology. 1983;85:1003–1010. [PubMed] [Google Scholar]
- 92.Rokutan K, Yamada M, Torigoe J, Saito T. Transforming growth factor-beta inhibits proliferation and maturation of cultured guinea pig gastric pit cells. Am J Physiol. 1998;275:G526–533. doi: 10.1152/ajpgi.1998.275.3.G526. [DOI] [PubMed] [Google Scholar]
- 93.Abe S, et al. Immunohistochemical studies on EGF family growth factors in normal and ulcerated human gastric mucosa. Dig Dis Sci. 1997;42:1199–1209. doi: 10.1023/a:1018897922644. [DOI] [PubMed] [Google Scholar]
- 94.Nam KT, et al. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology. 2009;136:1288–1296. doi: 10.1053/j.gastro.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murayama Y, et al. Localization of heparin-binding epidermal growth factor-like growth factor in human gastric mucosa. Gastroenterology. 1995;109:1051–1059. doi: 10.1016/0016-5085(95)90562-6. [DOI] [PubMed] [Google Scholar]
- 96.Coffey RJ, Romano M, Polk WH, Dempsey PJ. Roles for transforming growth factor-alpha in gastric physiology and pathophysiology. Yale J Biol Med. 1992;65:693–704. [PMC free article] [PubMed] [Google Scholar]
- 97.Jones MK, Tomikawa M, Mohajer B, Tarnawski AS. Gastrointestinal mucosal regeneration: role of growth factors. Front Biosci. 1999;4:D303–309. doi: 10.2741/a428. [DOI] [PubMed] [Google Scholar]
- 98.Polk WH, Jr, et al. Increased production of transforming growth factor alpha following acute gastric injury. Gastroenterology. 1992;102:1467–1474. doi: 10.1016/0016-5085(92)91703-7. [DOI] [PubMed] [Google Scholar]
- 99.Pai R, Tarnawski A. Signal transduction cascades triggered by EGF receptor activation: relevance to gastric injury repair and ulcer healing. Dig Dis Sci. 1998;43:14S–22S. [PubMed] [Google Scholar]
- 100.Poulsen SS. On the role of epidermal growth factor in the defence of the gastroduodenal mucosa. Scand J Gastroenterol Suppl. 1987;128:20–23. doi: 10.3109/00365528709090965. [DOI] [PubMed] [Google Scholar]
- 101.Goldenring JR, et al. Overexpression of transforming growth factor-alpha alters differentiation of gastric cell lineages. Dig Dis Sci. 1996;41:773–784. doi: 10.1007/BF02213134. [DOI] [PubMed] [Google Scholar]
- 102.Takagi H, Jhappan C, Sharp R, Merlino G. Hypertrophic gastropathy resembling Menetrier’s disease in transgenic mice overexpressing transforming growth factor alpha in the stomach. J Clin Invest. 1992;90:1161–1167. doi: 10.1172/JCI115936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coffey RJ, Washington MK, Corless CL, Heinrich MC. Menetrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach. J Clin Invest. 2007;117:70–80. doi: 10.1172/JCI30491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Genta RM. Review article: Gastric atrophy and atrophic gastritis--nebulous concepts in search of a definition. Aliment Pharmacol Ther. 1998;12(Suppl 1):17–23. doi: 10.1111/j.1365-2036.1998.00003.x. [DOI] [PubMed] [Google Scholar]
- 105.Goldenring JR, Nam KT. Oxyntic atrophy, metaplasia, and gastric cancer. Prog Mol Biol Transl Sci. 2010;96:117–131. doi: 10.1016/B978-0-12-381280-3.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weis VG, Goldenring JR. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer. 2009;12:189–197. doi: 10.1007/s10120-009-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petersen CP, Mills JC, Goldenring JR. Murine Models of Gastric Corpus Preneoplasia. Cell Mol Gastroenterol Hepatol. 2017;3:11–26. doi: 10.1016/j.jcmgh.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goldenring JR, Nam KT, Mills JC. The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res. 2011;317:2759–2764. doi: 10.1016/j.yexcr.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmidt PH, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. This paper first identified a metaplastic lineage, termed Spasmolytic Polypeptide-Expressing Metaplasia (SPEM), from gastric corpus biopsies of human patients chronically infected with Helicobacter pylori. [PubMed] [Google Scholar]
- 110.Lauwers GY. In: Surgical pathology of the GI tract, liver, biliary tract, and pancreas. Odze RD, Goldblum JR, editors. Chapter 23. Saunders/Elsevier; Amsterdam: 2009. [Google Scholar]
- 111.Saenz JB, Burclaff J, Mills JC. Modeling Murine Gastric Metaplasia Through Tamoxifen-Induced Acute Parietal Cell Loss. Methods Mol Biol. 2016;1422:329–339. doi: 10.1007/978-1-4939-3603-8_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nomura S, et al. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G362–375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 113.Huh WJ, et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burclaff J, Osaki LH, Liu D, Goldenring JR, Mills JC. Targeted Apoptosis of Parietal Cells Is Insufficient to Induce Metaplasia in Stomach. Gastroenterology. 2017;152:762–766. doi: 10.1053/j.gastro.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Demitrack ES, et al. NOTCH1 and NOTCH2 regulate epithelial cell proliferation in mouse and human gastric corpus. Am J Physiol Gastrointest Liver Physiol. 2017;312:G133–G144. doi: 10.1152/ajpgi.00325.2016. This group had previously demonstrated the role for NOTCH1/2 in the gastric antrum and now show that NOTCH1/2 signaling promotes stem and progenitor cell proliferation in the gastric corpus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shiotani A, et al. Evidence that loss of sonic hedgehog is an indicator of Helicobater pylori-induced atrophic gastritis progressing to gastric cancer. Am J Gastroenterol. 2005;100:581–587. doi: 10.1111/j.1572-0241.2005.41001.x. [DOI] [PubMed] [Google Scholar]
- 117.Merchant JL, Ding L. Hedgehog Signaling Links Chronic Inflammation to Gastric Cancer Precursor Lesions. Cell Mol Gastroenterol Hepatol. 2017;3:201–210. doi: 10.1016/j.jcmgh.2017.01.004. This review nicely summarizes the role of Hedgehog signaling in promoting a metaplastic milieu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fox JG, et al. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–1890. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 119.Zavros Y, et al. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–2366. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- 120.Todisco A. Regulation of Gastric Metaplasia, Dysplasia, and Neoplasia by Bone Morphogenetic Protein Signaling. Cell Mol Gastroenterol Hepatol. 2017;3:339–347. doi: 10.1016/j.jcmgh.2017.01.014. Transgenic expression of the BMP inhibitor noggin results in gastric epithelial changes consistent with SPEM, implicating a role for BMP signaling in gastric epithelial homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shinohara M, et al. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology. 2010;139:2050–2060. doi: 10.1053/j.gastro.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Syu LJ, et al. Transgenic expression of interferon-gamma in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am J Pathol. 2012;181:2114–2125. doi: 10.1016/j.ajpath.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Serizawa T, et al. Gastric Metaplasia Induced by Helicobacter pylori Is Associated with Enhanced SOX9 Expression via Interleukin-1 Signaling. Infect Immun. 2015;84:562–572. doi: 10.1128/IAI.01437-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Waghray M, et al. Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. Gastroenterology. 2010;138:562–572. doi: 10.1053/j.gastro.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Buzzelli JN, et al. IL33 Is a Stomach Alarmin That Initiates a Skewed Th2 Response to Injury and Infection. Cell Mol Gastroenterol Hepatol. 2015;1:203–221. doi: 10.1016/j.jcmgh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Petersen CP, et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut. 2017 doi: 10.1136/gutjnl-2016-312779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rugge M, Genta RM, Group O. Staging gastritis: an international proposal. Gastroenterology. 2005;129:1807–1808. doi: 10.1053/j.gastro.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 128.Sepulveda AR, Patil M. Practical approach to the pathologic diagnosis of gastritis. Arch Pathol Lab Med. 2008;132:1586–1593. doi: 10.5858/2008-132-1586-PATTPD. [DOI] [PubMed] [Google Scholar]
- 129.Miftahussurur M, Yamaoka Y, Graham DY. Helicobacter pylori as an oncogenic pathogen, revisited. Expert Rev Mol Med. 2017;19:e4. doi: 10.1017/erm.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rugge M, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631–636. doi: 10.1136/gut.2006.106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gjeorgjievski M, Cappell MS. Portal hypertensive gastropathy: A systematic review of the pathophysiology, clinical presentation, natural history and therapy. World J Hepatol. 2016;8:231–262. doi: 10.4254/wjh.v8.i4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Komorowski RA, Caya JG. Hyperplastic gastropathy. Clinicopathologic correlation. Am J Surg Pathol. 1991;15:577–585. doi: 10.1097/00000478-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 133.Gun F, Abbasoglu L, Celik A. Acute gastric perforation after acid ingestion. J Pediatr Gastroenterol Nutr. 2002;35:360–362. doi: 10.1097/00005176-200209000-00023. [DOI] [PubMed] [Google Scholar]
- 134.Ovenden C, et al. Occult upper gastrointestinal mucosal abnormalities in critically ill patients. Acta Anaesthesiol Scand. 2017;61:216–223. doi: 10.1111/aas.12844. [DOI] [PubMed] [Google Scholar]
- 135.Andersen LP. Colonization and infection by Helicobacter pylori in humans. Helicobacter. 2007;12(Suppl 2):12–15. doi: 10.1111/j.1523-5378.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 136.Sugano K, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–2210. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis--pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol. 2013;10:529–541. doi: 10.1038/nrgastro.2013.101. This review concisely and comprehensively summarizes the clinical, endoscopic, and histopathologic aspects of autoimmune gastritis. [DOI] [PubMed] [Google Scholar]
- 139.Claeys D, Faller G, Appelmelk BJ, Negrini R, Kirchner T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998;115:340–347. doi: 10.1016/s0016-5085(98)70200-8. [DOI] [PubMed] [Google Scholar]
- 140.Negrini R, et al. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996;111:655–665. doi: 10.1053/gast.1996.v111.pm8780570. [DOI] [PubMed] [Google Scholar]
- 141.Appelmelk BJ, Faller G, Claeys D, Kirchner T, Vandenbroucke-Grauls CM. Bugs on trial: the case of Helicobacter pylori and autoimmunity. Immunol Today. 1998;19:296–299. doi: 10.1016/s0167-5699(98)01281-x. [DOI] [PubMed] [Google Scholar]
- 142.Bergman MP, et al. The story so far: Helicobacter pylori and gastric autoimmunity. Int Rev Immunol. 2005;24:63–91. doi: 10.1080/08830180590884648. [DOI] [PubMed] [Google Scholar]
- 143.Hooi JKY, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 144.Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology. 2006;130:188–206. doi: 10.1053/j.gastro.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 145.Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615–640. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- 146.NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 147.Kuipers EJ, Thijs JC, Festen HP. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment Pharmacol Ther. 1995;9(Suppl 2):59–69. [PubMed] [Google Scholar]
- 148.Malfertheiner P. The intriguing relationship of Helicobacter pylori infection and acid secretion in peptic ulcer disease and gastric cancer. Dig Dis. 2011;29:459–464. doi: 10.1159/000332213. [DOI] [PubMed] [Google Scholar]
- 149.Bertaux-Skeirik N, et al. CD44 variant isoform 9 emerges in response to injury and contributes to the regeneration of the gastric epithelium. J Pathol. 2017 doi: 10.1002/path.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kuipers EJ. Review article: exploring the link between Helicobacter pylori and gastric cancer. Aliment Pharmacol Ther. 1999;13(Suppl 1):3–11. doi: 10.1046/j.1365-2036.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Y, et al. Clinical Significance of Spasmolytic Polypeptide-expressing Metaplasia and Intestinal Metaplasia in EBV-associated and EBV-negative Gastric Cancer. Hum Pathol. 2017 doi: 10.1016/j.humpath.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 152.Hansson LE, et al. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335:242–249. doi: 10.1056/NEJM199607253350404. [DOI] [PubMed] [Google Scholar]
- 153.Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191–5204. doi: 10.3748/wjg.v20.i18.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Graham DY, Asaka M. Eradication of gastric cancer and more efficient gastric cancer surveillance in Japan: two peas in a pod. J Gastroenterol. 2010;45:1–8. doi: 10.1007/s00535-009-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gifford GB, et al. Notch1 and Notch2 receptors regulate mouse and human gastric antral epithelial cell homoeostasis. Gut. 2017;66:1001–1011. doi: 10.1136/gutjnl-2015-310811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Merrell AJ, Stanger BZ. Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat Rev Mol Cell Biol. 2016;17:413–425. doi: 10.1038/nrm.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Burclaff J, Mills JC. Nature. 2017 doi: 10.1038/nature23534. In press. [DOI] [PubMed] [Google Scholar]
- 158.Mills JC, Sansom OJ. Reserve stem cells: Differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal. 2015;8:re8. doi: 10.1126/scisignal.aaa7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Demitrack ES, Samuelson LC. Notch as a Driver of Gastric Epithelial Cell Proliferation. Cell Mol Gastroenterol Hepatol. 2017;3:323–330. doi: 10.1016/j.jcmgh.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hayakawa Y, Fox JG, Wang TC. The Origins of Gastric Cancer From Gastric Stem Cells: Lessons From Mouse Models. Cell Mol Gastroenterol Hepatol. 2017;3:331–338. doi: 10.1016/j.jcmgh.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–279. doi: 10.1002/ar.1092360202. The morphologic characteristics of the isthmal stem cell were described in this pivotal ultra-structural analysis. [DOI] [PubMed] [Google Scholar]
- 162.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993;236:297–313. doi: 10.1002/ar.1092360204. This was another landmark study that described the morphologic characteristics of the epithelial lineages from the neck and base regions of the gastric corpus unit as well as the dynamics of epithelial differentiation in the corpus gland. [DOI] [PubMed] [Google Scholar]
- 163.Ramsey VG, et al. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 164.Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol. 2002;283:G767–777. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- 165.Barker N, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. This was one of the first lineage tracing experiments in the stomach and identified Lgr5 as an antral stem cell marker. [DOI] [PubMed] [Google Scholar]
- 166.Hayakawa Y, et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64:544–553. doi: 10.1136/gutjnl-2014-307190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sigal M, et al. Helicobacter pylori Activates and Expands Lgr5(+) Stem Cells Through Direct Colonization of the Gastric Glands. Gastroenterology. 2015;148:1392–1404. doi: 10.1053/j.gastro.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 168.Lee ER, Leblond CP. Dynamic histology of the antral epithelium in the mouse stomach: II. Ultrastructure and renewal of isthmal cells. Am J Anat. 1985;172:205–224. doi: 10.1002/aja.1001720304. This study represents the first systematic analysis of stem cell dynamics and differentiation in the gastric antrum. [DOI] [PubMed] [Google Scholar]
- 169.Leushacke M, et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol. 2017 doi: 10.1038/ncb3541. Lgr5-positive chief cells are recruited to fuel epithelial renewal following glandular injury, suggesting that chief cells can acquire stemness. [DOI] [PubMed] [Google Scholar]
- 170.Lee ER, Leblond CP. Dynamic histology of the antral epithelium in the mouse stomach: IV. Ultrastructure and renewal of gland cells. Am J Anat. 1985;172:241–259. doi: 10.1002/aja.1001720306. [DOI] [PubMed] [Google Scholar]
- 171.Hayakawa Y, et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell. 2015;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kretzschmar K, Watt FM. Lineage tracing. Cell. 2012;148:33–45. doi: 10.1016/j.cell.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 173.Li N, Nakauka-Ddamba A, Tobias J, Jensen ST, Lengner CJ. Mouse Label-Retaining Cells Are Molecularly and Functionally Distinct From Reserve Intestinal Stem Cells. Gastroenterology. 2016;151:298–310. doi: 10.1053/j.gastro.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Qiao XT, et al. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Arnold K, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tsukamoto T, et al. Down-regulation of a gastric transcription factor, Sox2, and ectopic expression of intestinal homeobox genes, Cdx1 and Cdx2: inverse correlation during progression from gastric/intestinal-mixed to complete intestinal metaplasia. J Cancer Res Clin Oncol. 2004;130:135–145. doi: 10.1007/s00432-003-0519-6. [DOI] [PubMed] [Google Scholar]
- 177.Sigal M. Stromal R-spondin orchestrates gastric epithelial homeostasis in the healthy and Helicobacter pylori-infected stomach. Nature. 2017 doi: 10.1038/nature23642. This study identifies Lgr4 as a marker of isthmal stem cells in the antrum and highlights the relative potential of various cell populations along the antral unit to serve as stem cells. [DOI] [PubMed] [Google Scholar]
- 178.Dedhia PH, Bertaux-Skeirik N, Zavros Y, Spence JR. Organoid Models of Human Gastrointestinal Development and Disease. Gastroenterology. 2016;150:1098–1112. doi: 10.1053/j.gastro.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schlaermann P, et al. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut. 2016;65:202–213. doi: 10.1136/gutjnl-2014-307949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bartfeld S, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148:126–136. doi: 10.1053/j.gastro.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 182.Shimizu T, et al. Characterization of progressive metaplasia in the gastric corpus mucosa of Mongolian gerbils infected with Helicobacter pylori. J Pathol. 2016;239:399–410. doi: 10.1002/path.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jeong S, et al. Distinct metaplastic and inflammatory phenotypes in autoimmune and adenocarcinoma-associated chronic atrophic gastritis. United European Gastroenterol J. 2017;5:37–44. doi: 10.1177/2050640616644142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nguyen TL, et al. Autoimmune gastritis mediated by CD4+ T cells promotes the development of gastric cancer. Cancer Res. 2013;73:2117–2126. doi: 10.1158/0008-5472.CAN-12-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Khurana SS, et al. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem. 2013;288:16085–16097. doi: 10.1074/jbc.M112.445551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hayakawa Y, Fox JG, Wang TC. Isthmus Stem Cells Are the Origins of Metaplasia in the Gastric Corpus. Cell Mol Gastroenterol Hepatol. 2017;4:89–94. doi: 10.1016/j.jcmgh.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nam KT, et al. Spasmolytic polypeptide-expressing metaplasia (SPEM) in the gastric oxyntic mucosa does not arise from Lgr5-expressing cells. Gut. 2012;61:1678–1685. doi: 10.1136/gutjnl-2011-301193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nam KT, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. The authors demonstrate that metaplastic cells lineage trace from chief cells following acute or chronic glandular injury, suggesting that mature chief cells are the source of metaplasia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Choi E, Hendley AM, Bailey JM, Leach SD, Goldenring JR. Expression of Activated Ras in Gastric Chief Cells of Mice Leads to the Full Spectrum of Metaplastic Lineage Transitions. Gastroenterology. 2016;150:918–930. doi: 10.1053/j.gastro.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Matsuo J, et al. Identification of Stem Cells in the Epithelium of the Stomach Corpus and Antrum of Mice. Gastroenterology. 2017;152:218–231. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 191.Stange DE, et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. This study identifies Troy as a stem cell marker expressed by mature chief cells at the base of corpus units and highlights the plasticity of chief cells under certain forms of glandular injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Capoccia BJ, et al. The ubiquitin ligase Mindbomb 1 coordinates gastrointestinal secretory cell maturation. J Clin Invest. 2013;123:1475–1491. doi: 10.1172/JCI65703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Radyk MM, JC EMBO J. 2017 doi: 10.15252/embj.201797519. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lennerz JK, et al. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mills JC, Goldenring JR. Metaplasia in the Stomach Arises From Gastric Chief Cells. Cell Mol Gastroenterol Hepatol. 2017;4:85–88. doi: 10.1016/j.jcmgh.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Raven A, et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547:350–354. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Iovanna JL, Lechene de la Porte P, Dagorn JC. Expression of genes associated with dedifferentiation and cell proliferation during pancreatic regeneration following acute pancreatitis. Pancreas. 1992;7:712–718. doi: 10.1097/00006676-199211000-00013. [DOI] [PubMed] [Google Scholar]
- 198.Jensen JN, et al. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 199.Pinho AV, et al. Adult pancreatic acinar cells dedifferentiate to an embryonic progenitor phenotype with concomitant activation of a senescence programme that is present in chronic pancreatitis. Gut. 2011;60:958–966. doi: 10.1136/gut.2010.225920. [DOI] [PubMed] [Google Scholar]
- 200.Weis VG, et al. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am J Physiol Gastrointest Liver Physiol. 2017;312:G67–G76. doi: 10.1152/ajpgi.00326.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rubio CA, Jaramillo E, Suzuki G, Lagergren P, Nesi G. Antralization of the gastric mucosa of the incisura angularis and its gastrin expression. Int J Clin Exp Pathol. 2009;2:65–70. [PMC free article] [PubMed] [Google Scholar]
- 202.Xia HH, et al. Antral-type mucosa in the gastric incisura, body, and fundus (antralization): a link between Helicobacter pylori infection and intestinal metaplasia? Am J Gastroenterol. 2000;95:114–121. doi: 10.1111/j.1572-0241.2000.01609.x. [DOI] [PubMed] [Google Scholar]
- 203.Xia HH, et al. Topographic association of gastric epithelial expression of Ki-67, Bax, and Bcl-2 with antralization in the gastric incisura, body, and fundus. Am J Gastroenterol. 2002;97:3023–3031. doi: 10.1111/j.1572-0241.2002.07120.x. [DOI] [PubMed] [Google Scholar]
- 204.Xia HH, et al. Antralization of gastric incisura is topographically associated with increased gastric epithelial apoptosis and proliferation, but not with CagA seropositivity. J Gastroenterol Hepatol. 2004;19:1257–1263. doi: 10.1111/j.1440-1746.2004.03489.x. [DOI] [PubMed] [Google Scholar]
- 205.Genta RM, Rugge M. Review article: pre-neoplastic states of the gastric mucosa--a practical approach for the perplexed clinician. Aliment Pharmacol Ther. 2001;15(Suppl 1):43–50. doi: 10.1046/j.1365-2036.2001.00110.x. [DOI] [PubMed] [Google Scholar]
- 206.Genta RM. Atrophy and atrophic gastritis: one step beyond the Sydney system. Ital J Gastroenterol Hepatol. 1998;30(Suppl 3):S273–275. [PubMed] [Google Scholar]
- 207.Rogers AB, Houghton J. Helicobacter-based mouse models of digestive system carcinogenesis. Methods Mol Biol. 2009;511:267–295. doi: 10.1007/978-1-59745-447-6_11. [DOI] [PubMed] [Google Scholar]
- 208.Wright NA. Migration of the ductular elements of gut-associated glands gives clues to the histogenesis of structures associated with responses to acid hypersecretory state: the origins of “gastric metaplasia” in the duodenum of the specialized mucosa of barrett’s esophagus and of pseudopyloric metaplasia. Yale J Biol Med. 1996;69:147–153. [PMC free article] [PubMed] [Google Scholar]
- 209.Nozaki K, et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–522. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Van Zanten SJ, Dixon MF, Lee A. The gastric transitional zones: neglected links between gastroduodenal pathology and helicobacter ecology. Gastroenterology. 1999;116:1217–1229. doi: 10.1016/s0016-5085(99)70025-9. [DOI] [PubMed] [Google Scholar]
- 211.Stave R, Brandtzaeg P, Nygaard K, Fausa O. The transitional body-antrum zone in resected human stomachs. Anatomical outline and parietal-cell and gastrin-cell characteristics in peptic ulcer disease. Scand J Gastroenterol. 1978;13:685–691. doi: 10.3109/00365527809181782. [DOI] [PubMed] [Google Scholar]
- 212.Peterson WL. Review article: Helicobacter pylori and gastric adenocarcinoma. Aliment Pharmacol Ther. 2002;16(Suppl 1):40–46. doi: 10.1046/j.1365-2036.2002.0160s1040.x. [DOI] [PubMed] [Google Scholar]
- 213.Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15:306–316. doi: 10.1016/j.chom.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 214.Tsugawa H, et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–777. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 215.Greenfield LK, Jones NL. Modulation of autophagy by Helicobacter pylori and its role in gastric carcinogenesis. Trends Microbiol. 2013;21:602–612. doi: 10.1016/j.tim.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 216.Panella C, et al. Proliferative activity of gastric epithelium in progressive stages of Helicobacter pylori infection. Dig Dis Sci. 1996;41:1132–1138. doi: 10.1007/BF02088228. [DOI] [PubMed] [Google Scholar]
- 217.Oh JD, Kling-Backhed H, Giannakis M, Engstrand LG, Gordon JI. Interactions between gastric epithelial stem cells and Helicobacter pylori in the setting of chronic atrophic gastritis. Curr Opin Microbiol. 2006;9:21–27. doi: 10.1016/j.mib.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 218.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Camargo MC, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60:1644–1649. doi: 10.1136/gut.2010.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.You WC, et al. Comparison of the anatomic distribution of stomach cancer and precancerous gastric lesions. Jpn J Cancer Res. 1992;83:1150–1153. doi: 10.1111/j.1349-7006.1992.tb02738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wanebo HJ, et al. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg. 1993;218:583–592. doi: 10.1097/00000658-199321850-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Guerra C, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 223.Collins MA, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–653. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Huang H, et al. Oncogenic K-Ras requires activation for enhanced activity. Oncogene. 2014;33:532–535. doi: 10.1038/onc.2012.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cassaro M, et al. Topographic patterns of intestinal metaplasia and gastric cancer. Am J Gastroenterol. 2000;95:1431–1438. doi: 10.1111/j.1572-0241.2000.02074.x. This study examined the extent and distribution of intestinal metaplasia in Colombian patients with and without gastric cancer and found that certain patterns of gastric intestinal metplasia correlated with an increased risk of developing gastric cancer. [DOI] [PubMed] [Google Scholar]
- 226.Odze RD. Unraveling the mystery of the gastroesophageal junction: a pathologist’s perspective. Am J Gastroenterol. 2005;100:1853–1867. doi: 10.1111/j.1572-0241.2005.50096.x. [DOI] [PubMed] [Google Scholar]
- 227.Chandrasoma P. RE: Odze RD. unraveling the mystery of the gastroesophageal junction: a pathologist’s perspective. Am J Gastroenterol. 2006;101:199. doi: 10.1111/j.1572-0241.2006.00393_1.x. [DOI] [PubMed] [Google Scholar]
- 228.Derdoy JJ, et al. The gastric cardia: to be or not to be? Am J Surg Pathol. 2003;27:499–504. doi: 10.1097/00000478-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 229.Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol. 2013;23:3–9. doi: 10.1016/j.semradonc.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cancer Genome Atlas Research N et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–175. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Odze RD. Pathology of the gastroesophageal junction. Semin Diagn Pathol. 2005;22:256–265. doi: 10.1053/j.semdp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 232.Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G211–218. doi: 10.1152/ajpgi.90250.2008. [DOI] [PubMed] [Google Scholar]
- 233.Chandrasoma P. Controversies of the cardiac mucosa and Barrett’s oesophagus. Histopathology. 2005;46:361–373. doi: 10.1111/j.1365-2559.2005.02088.x. [DOI] [PubMed] [Google Scholar]
- 234.Lavery DL, et al. The stem cell organisation, and the proliferative and gene expression profile of Barrett’s epithelium, replicates pyloric-type gastric glands. Gut. 2014;63:1854–1863. doi: 10.1136/gutjnl-2013-306508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.McDonald SA, Lavery D, Wright NA, Jansen M. Barrett oesophagus: lessons on its origins from the lesion itself. Nat Rev Gastroenterol Hepatol. 2015;12:50–60. doi: 10.1038/nrgastro.2014.181. [DOI] [PubMed] [Google Scholar]
- 236.Wang X, et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Quante M, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012;21:36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lee Y, et al. Gastrin stimulates a cholecystokinin-2-receptor-expressing cardia progenitor cell and promotes progression of Barrett’s-like esophagus. Oncotarget. 2017;8:203–214. doi: 10.18632/oncotarget.10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Spechler SJ, et al. A Summary of the 2016 James W. Freston Conference of the American Gastroenterological Association Intestinal Metaplasia in the Esophagus and Stomach: Origins, Differences, Similarities and Significance. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yoshizawa N, et al. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest. 2007;87:1265–1276. doi: 10.1038/labinvest.3700682. [DOI] [PubMed] [Google Scholar]
- 241.Jensen P, Dymecki SM. Essentials of recombinase-based genetic fate mapping in mice. Methods Mol Biol. 2014;1092:437–454. doi: 10.1007/978-1-60327-292-6_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 243.Grun D, et al. De Novo Prediction of Stem Cell Identity using Single-Cell Transcriptome Data. Cell Stem Cell. 2016;19:266–277. doi: 10.1016/j.stem.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Goldenring JR, Mills JC. Isthmus Time Is Here: Runx1 Identifies Mucosal Stem Cells in the Gastric Corpus. Gastroenterology. 2017;152:16–19. doi: 10.1053/j.gastro.2016.11.028. [DOI] [PubMed] [Google Scholar]