Abstract
Photoenhanced toxicity is a distinct mechanism of petroleum toxicity that is mediated by the interaction of solar radiation with specific polycyclic aromatic compounds (PACs) in oil. Phototoxicity is observed as a 2 to greater than 1000 fold increase in chemical toxicity to aquatic organisms that have also been exposed to light sources containing sufficient quantity and quality of ultraviolet radiation (UV). When tested under natural sunlight or laboratory sources of UV, fresh and weathered middle distilates, crudes and heavy oils can exhibit phototoxicity. These same products do not exhibit phototoxicity in standard test protocols because of low UV irradiance in laboratory lighting. Fresh water, estuarine and marine waters have been shown to have sufficient solar radiation exposure to elicit photoehanced toxicity, and a diversity of aquatic invertebrate and fish species can exhibit phototoxicity when exposed to combinations of oil and UV. Risks of photoenhanced toxicity will be greatest to early life stages of aquatic organisms that are translucent to UV and that inhabit the photic zone of the water column and intertidal areas exposed to oil.
Introduction
Photoenhanced toxicity is the 2 to greater than 1000 fold increase in the toxicity of a chemical in the presence of ultraviolet light (UV), compared to toxicity elicited under conditions of minimal UV (Willis and Oris 2014). A variety of chemicals and chemical mixtures can cause phototoxicity, including drugs (e.g., tetracyclines), natural compounds (e.g., citrus oils, psoralens); pesticides (e.g., diquat), and polycyclic aromatic compounds (PACs) (e.g., anthracine, acridine) (Landrum et al. 1987; Barron 2007). Although there is considerable structural diversity of phototoxic chemicals, they share the ability to be activated by UV by either photomodification to more toxic intermediates or photoexcitation resulting in the formation of reactive oxygen species. The phototoxicity of PACs has been well established in laboratory, mesocosm, and field studies, and mechanistic predictive toxicity models based on principles of photochemistry and UV and PAC tissue dosimetry (Giesy et al. 2013; Willis and Oris 2014).
Petroleum is known to contain hundreds of PACs, some of which can be phototoxic to aquatic organisms. Although photoenhanced toxicity of PACs has been known for over 30 years (Landrum et al 1987), petroleum phototoxicity has only more recently been recognized by scientists and risk managers in the oil spill community (Barron and Ka’aihue, 2001; Kirby et al 2007). Multiple studies have indicated that laboratory tests that do not include UV may underestimate the environmental toxicity of oil to early life stages of aquatic organisms, and phototoxicity is generally not incorporated into testing protocols, spill response plans, or impact assessment models (Barron and Ka’aihue 2001; Barron and Ka’aihue 2003; Kirby et al. 2007; Alloy et al. 2016). This review summarizes key literature on the phototoxicity of petroleum to aquatic invertebrates and fish, including phototoxic components of oil, phototoxicity mechanisms, and environmental and biological factors controlling photoenhanced toxicity.
Phototoxic Crude and Refined Oils
Over four decades of research has demonstrated that petroleum is phototoxic (e.g., Kochevar et al. 1982; Kosian et al 1998; Barron and Ka’aihue 2001; Lee 2003; Wernersson 2003; Kirby et al. 2007; Alloy et al. 2016). Studies with aquatic organisms have shown petroleum phototoxicity in water accommodated fractions (WAF) prepared by classic slow stir methods, as well as generator and oiled gravel columns, and field collected spill samples (e.g., Ho et al. 1999; Barron and Ka’aihue 2001; Wernersson 2003; Barron et al. 2003). For example, WAF of Arabian light crude oil, Prudhoe Bay crude oil, fuel oil #2, and Bunker C (fuel oil number 6) were phototoxic to shellfish embryos (Mulinia) and mysid (Mysidopsis) larvae (Pelletier et al., 1997). Ho et al. (1999) reported that water samples from a marine spill of fuel oil #2 were phototoxic to early life stages of bivalves and that phototoxicity persisted relative to no UV exposures. The toxicity of WAF of a heavily weathered middle distillate oil was both UV and oil dose-dependent in three species of aquatic organisms (Calfee et al. 1999; Little et al. 2000; Cleveland et al. 2000). WAF prepared from Alaska North Slope crude oil (ANSCO) and tested under limited natural sunlight was phototoxic to two field collected marine copepods (Duesterloh et al. 2002), and both physically and chemically dispersed ANSCO was phototoxic to Pacific herring (Clupea pallasii; Barron et al. 2003). Wernersson (2003) reported that WAF of 16 of 22 oil products tested were phototoxic to Daphnia magna including crude and fuel oils, whereas lighter petroleum products (gasolines, kerosenes, diesel oils) were not. Extracts of PACs from Canadian oil sands exhibited only minor phototoxicity to medaka (Oryzias latipes) embryos (Farwell et al. 2006). WAF of an intermediate fuel oil was phototoxic to two marine invertebrates, whereas no phototoxicity was observed for a heavy fuel oil from the Prestige spill (Saco-Alvarez et al. 2008). Incardona et al. (2012) reported phototoxicity of marine bunker oil WAF to Pacific herring embryos under natural sunlight, and evidence of environmental phototoxicity from a spill of the same heavy oil. WAF prepared from Hebei Spirit crude oil and chemically dispersed oil was only moderately phototoxic to clam embryos (Lee et al. 2013). More recently, WAF prepared from the Deepwater Horizon spill oil has been shown to be phototoxic to multiple species and life stages of aquatic organisms (e.g., Alloy et al. 2015; Alloy et al. 2016). Overall, the majority of studies over the last two decades demonstrate that multiple crudes oils, middle distillates, fuel oils, weathered oils, and chemically dispersed oils are phototoxic to a variety of aquatic organisms (Barron and Ka’aihue 2001; Lee 2003; Wernersson 2003; Kirby et al. 2007).
Phototoxic Components of Oil
Crude oil and petroleum products are complex mixtures of thousands of aliphatic and aromatic compounds. Multiple studies have now linked petroleum toxicity to PAC components of oil. Evidence for PAC mediated phototoxicity of petroleum includes: (1) presence of PAHs and heterocycles in phototoxic oils and WAF (e.g., Hatlen et al. 2010); (2) known phototoxic mechanisms of PACs requiring specific polycyclic aromatic ring conjugations (see below); (3) lack of phototoxicity of other major oil hydrocarbons including aliphatics, mono and di-aromatics, and asphaltenes (e.g., Kochevar et al. 1982; Spielmanna et al. 1998; Toyooka and Ibuki 2006), (4) apparent absence of other known phototoxic chemicals classes (e.g., Spielmanna et al. 1998); (5) significant correlations between phototoxic responses in aquatic organisms and PAC concentrations in either tissues or exposure water (Ankley et al. 1997, Duesterloh et al. 2002; Willis and Oris 2014); and (6) associations between PAC composition and oil phototoxicity. For example, middle distilates containing a greater abundance of tricylic PAHs are phototoxic, whereas oil products with primarily mono- and di-aromatics such as gasoline are not phototoxic (Wernersson 2003). Also, there is a general trend of greater phototoxic potency of heavier petroleum such as ANSCO and residual fuel oils that contain a larger fraction of 3 to 5 ring PACs (e.g., Wernersson 2003; Hatlen 2010).
Multiple investigations of individual compounds and chemical mixtures indicate that specific 3 to 5 ring polycyclic aromatic hydrocarbons and heterocycles are responsible for petroleum phototoxicity, because of their abundance in oil and WAF, ability to absorb specific wavelengths of UV, and demonstrated phototoxicity at part per billion concentrations. Single compound and structure activity modeling studies have shown that phototoxic PACs include parent and alkylated homologs of anthracenes, fluoranthenes, chrysenes, pyrenes, benzochrysenes, benzopyrenes, and oxygen, nitrogen and sulfur analogs of these compounds such as dibenzothiophenes and acridines (e.g., Pelletier et al. 1997; Lee 2003; Wiegman et al. 2001). Ring conjugation and conformation are critical to determining phototoxic potency of PACs. For example, the three ring PAH anthracene can exhibit a UV-mediated 1000 fold increase in toxicity to aquatic invertebrates and fish, whereas its three ring analog phenanthrene is not phototoxic. Mixture studies have shown that PAC phototoxicity is additive and that phototoxicity can be predicted based on specific physical-chemical features (e.g., Vieth et al. 1995; Ankley et al. 1997; Willis and Oris 2014). The alkyl homologs of PACs predominate in crude oils and petroleum products, and alkyl substitution is likely to either have no effect or increase the degree of phototoxicity (Vieth et al., 1995; Finch et al. in press).
Mechanisms of Petroleum Phototoxicity
Two mechanisms of the phototoxicity of petroleum and PACs have been reported in multiple studies and are consistent with principles of photochemistry: 1) photomodification to directly toxic intermediates, and 2) photoexcitation of bioaccumulated residues resulting in tissue damage known as photosensitization. Photooxidation of petroleum and PACs is well understood and was an important degradation process during the Deepwater Horizon oil spill (Aeppli et al. 2012). Photoxidation of PACs appears to occur through photocyclo-addition reactions with subsequent oxidations or rearrangements (McConkey et al. 2002). Photooxidation can generate a variety of oxidized petrogenic compounds including alcohols, ketones, aldehydes, carboxylic acids, esters, epoxides, quinones, and sulfoxides and sulfones (Lee 2003; Aeppli et al. 2012). Photooxidized PACs are more water soluble, can exhibit greater toxicity than the parent compound, and cause mortality in aquatic organisms and induce malformations and oxidative stress in fish embryos (Lampi et al. 2006; Knecht et al. 2013). However, photooxidation can also result in lower phototoxicity through degradation of phototoxic PACs (Choi and Oris 2003).
The principle mechanism of petroleum and PAC phototoxicity to aquatic invertebrates and fish appears to be phototosensitization (Little et al. 2000; Barron et al. 2003). In photosensitization, phototoxic oil residues are first bioaccumulated, then photoactivated by the UV reaching the unmodified chemical within the organism’s tissues. Multiple studies have shown that the degree of phototoenhanced toxicity is dependent on the spectrum of UV, with wavelengths in the UVA region (320–400 nm) most associated with phototoxicity in aquatic organisms. Thus phototosensitization can only occur with sufficient phototoxic chemical and wavelength-specific UV dose within organism tissue. PAC phototoxicity has been shown to be a function of the product of tissue residues of PAHs and UVA dose, and follows a reciprocity relationship over a range of PAC and UV levels (Ankley et al. 1997). The specific mechanism of phototsensitization includes: (1) initial wavelength-specific energy absorption from UV by the PAC, (2) molecular excitation of the outer orbital electron to a higher energy orbital, (3) passing of energy to oxygen molecules when the excited molecule returns to ground state, (4) formation of reactive oxygen species, free radicals, or other reactive products, (5) subsequent cycling of free radical reactions causing oxidative damage, (6) a cascade of lipid peroxidation and cell death, and (7) mortality with sufficient duration and intensity of exposure (Willis and Oris 2014).
Photosensitization of crude oils and refined products have been shown in several studies that first expose organisms to oil to allow PAC bioaccumulation, followed by later UV exposure either in clean water or WAF. For example, Little et al. (2000) and Barron et al. (2003) demonstrated photosensitization of a weathered middle distillate and ANSCO, respectively, in larval fish first exposed to oil followed by limited UV or natural sunlight exposure in clean water. No toxicity was observed in oil-only and UV-only treatments, nor in oil first exposed to UV then tested in fish. Duesterloh et al. (2002) observed opaque lipid sacs of a marine copepod in treatments with oil and limited sunlight, suggesting peroxidative lipid damage from photoactivation of bioaccumulated petrogenic PACs. Mechanistic studies with zebrafish embryos have shown separate etiologies in oil-only compared to oil followed by UV exposure (Hatlen et al. 2010). Oil-only exposures with a heavy fuel oil resulted in stereotypical cardiac toxicity linked to the fraction of tricylic aromatic compounds. In contrast, subsequent UV exposure resulted in rapid onset cell-lethal toxicity not observed with oil-only exposures (Hatlen et al. 2010). Overall, these studies indicate that petroleum phototoxicity primarily occurs through photosensitization of the PAC components of crude oils and refined products.
Environmental and Biological Modulation of Phototoxicity
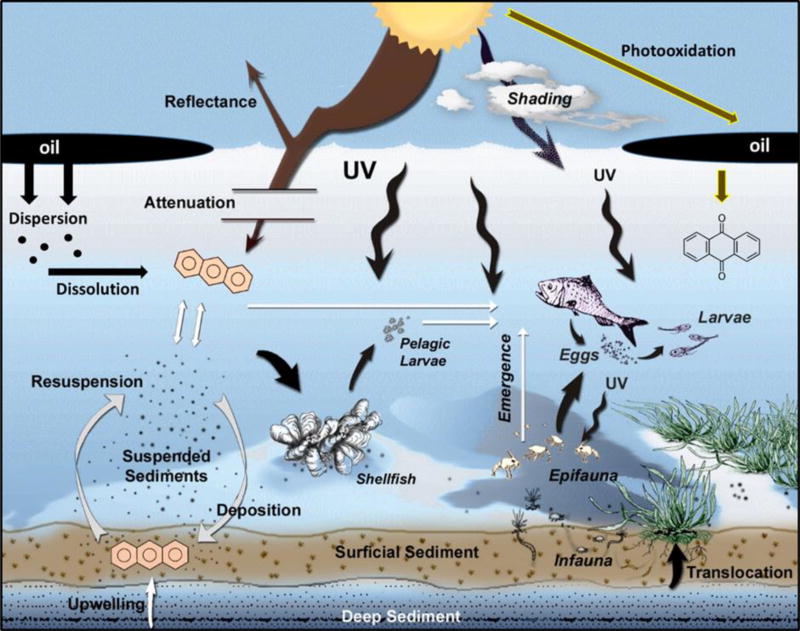
Over the last 30 years, multiple studies with oils and PACs have shown phototoxicity at environmentally realistic part per billion concentrations and at UVA intensities that occur in a diversity of fresh water, estuarine and marine environments (Landrum et al. 1987; Barron et al. 2000; Kirby et al. 2007; Barron et al. 2008). However, a complex interaction of factors control the environmental phototoxicity of petroleum to aquatic invertebrates and fish through modulation of phototoxic PAC exposure and the quantity and spectra of the absorbed UV dose (Fig. 1). Factors include phototoxic chemical dispersion and dissolution from spilled oil, translocation, partitioning, and degradation. The incident quantity and quality of UV at the water surface is determined by spatial and temporal variation in photoperiod, sun angle, and environmental conditions. Loss of incident UV can occur through surface reflectance, and depth and water quality specific attenuation (Landrum et al. 1987; Barron et al. 2000; Diamond 2003; Barron and Ka’ahue 2001). In general, light transmittance decreases with decreasing wavelength, with UVB (280–320 nm) showing greater attenuation than UVA and visible light (400–700 nm). The attenuation of UV within aquatic habitats is wavelength-specific and can range from a few centimeters to greater than 10 meters in clear colorous ocean water. Dissolved organic carbon, suspended solids, and chlorophyll increase UV attenuation and will lower phototoxicity risks in aquatic habitats.
Figure 1.
Conceptual model of phototoxicity showing ultraviolet radiation (UV) and phototoxic chemical exposure pathways to aquatic invertebrates and fish. Modified from Barron (2007).
Biological factors determining UV and oil exposure include species and life stage specific habitat use (e.g., pelagic versus benthic), ecological life history, and phenotypic traits such as behavioral avoidance or skeletal armoring (Barron 2007). Phototoxicity of oil and PACs has been demonstrated in over 30 species of aquatic organisms, including crustaceans, mollusks, oligochaetes, and fish (e.g., Boese et al 1997; Spehar et al. 1999; Barron and Ka’ahue 2001; Barron 2007). General biological characteristics associated with sensitivity include small size and translucence typical of the early life stages of invertebrates and fish. These organisms have limited pigment or armoring and an epidermis of only a few layers that allows UV penetration to tissues with bioaccumulated PACs (Hunter et al., 1980; Barron 2007). Species and life stages that are generally insensitive to phototoxicity have larger body size and/or extensive pigmentation or armoring of the exoskeleton (e.g, Barron et al 2005; Barron 2007). Behavioral avoidance or ecological life histories such as sediment burial may limit exposure to UV despite intrinsic sensitivity (Barron 2007).
Conclusions
A variety of fresh and weathered middle distilates, crudes and fuel oils are phototoxic to aquatic invertebrates and fish at environmentally relevant levels of UV and petroleum exposure. The PAC composition determines the phototoxic potency of an oil, along with environmental and biological modulation of UV and chemical exposure. Solar radiation in a diversity of aquatic habitats is sufficient to induce phototoxicity, with the degree of phototoxicity dependent on the absorbed dose of the phototoxic chemical and the absorbed dose of the active wavelengths of UV (Diamond 2003). Phototoxicity risks will be greatest to embryo and larval stages of aquatic organisms because they are relatively translucent to UV and inhabit the upper water column water column and nearshore areas that receive the greatest UV and petrogenic PAC exposure (Barron and Ka’ahue 2001).
Although the photoenhanced toxicity of PACs has been known for over 30 years (Landrum et al 1987), petroleum phototoxicity has only more recently been recognized by scientists and risk managers in the oil spill community (Barron and Ka’aihue, 2001; Kirby et al 2007). Risks of petroleum and petrogenic PAC exposure may be substantially underestimated without the explicit consideration of phototoxicity (Willis and Oris 2014; Alloy et al 2016). The choice of counter measures and spill response operations will influence the concentration and persistence of spilled oil in the water column, thus the potential for phototoxicity should be an additional factor that is weighed in spill response decisions (Carriger and Barron 2011). Incorporation of phototoxicity into oil testing protocols and spill response and impact assessment models is recommended (Barron and Ka’ahue 2003; Kirby et al. 2007).
Acknowledgments
Thanks to Mike Lewis, Jill Awkerman and Susan Yee for review of a draft of the manuscript, Lee Courtney for the base Figure graphic, to my many colleagues that have contributed to the knowlegebase of the photoenhanced toxicology of petroleum, and especially to Steve Diamond for introducing me to the field of phototoxicity and many fruitful discussions over the years. The conclusions may not necessarily reflect the views of EPA and no official endorsement should be inferred.
References
- Aeppli C, Carmichael CA, Nelson RK, Lemkau KL, Graham WM, Redmond MC, Valentine DL, Reddy CM. Oil weathering after the Deepwater Horizon disaster led to the formation of oxygenated residues. Environ Sci Technol. 2012;46:8799–8807. doi: 10.1021/es3015138. [DOI] [PubMed] [Google Scholar]
- Alloy MM, Boube I, Griffitt RJ, Oris JT, Roberts AP. Photo-induced toxicity of Deepwater Horizon slick oil to blue crab (Callinectes sapidus) larvae. Environ Tox Chem. 2015;34:2061–2066. doi: 10.1002/etc.3026. [DOI] [PubMed] [Google Scholar]
- Alloy M, Baxter D, Stieglitz J, Mager E, Hoenig R, Benetti D, Grosell M, Oris J, Roberts A. Ultraviolet radiation enhances the toxicity of deepwater horizon oil to mahi-mahi (Coryphaena hippurus) embryos. Environ Sci Technol. 2016;50:2011–2017. doi: 10.1021/acs.est.5b05356. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Erickson RJ, Sheedy BR, Kosian PA, Mattson VR, Cox JS. Evaluation of models for predicting the phototoxic potency of polycyclic aromatic hydrocarbons. Aquat Tox. 1997;37:37–50. [Google Scholar]
- Barron MG, Little EE, Calfee RD, Diamond S. Quantifying solar spectral irradiance in aquatic habitats for the assessment of photoenhanced toxicity. Environ Toxicol Chem. 2000;19:920–925. [Google Scholar]
- Barron MG, Ka’aihue L. Potential for photenhanced toxicity of spilled oil in Prince William Sound and Gulf of Alaska waters. Mar Poll Bull. 2001;43:86–92. doi: 10.1016/s0025-326x(01)00037-6. [DOI] [PubMed] [Google Scholar]
- Barron MG, Kaaihue L. Critical evaluation of CROSERF test methods for oil dispersant toxicity testing under subarctic conditions. Marine Poll. Bull. 2003;46:1191–1199. doi: 10.1016/S0025-326X(03)00125-5. [DOI] [PubMed] [Google Scholar]
- Barron MG, Carls MG, Short JW, Rice SD. Photoenhanced toxicity of aqueous phase and chemically dispersed weathered Alaska North Slope crude oil to Pacific herring eggs and larvae. Environ Tox Chem. 2003;22:650–660. [PubMed] [Google Scholar]
- Barron MG, Carls MG, Short JW, Heintz R, Rice SD. Assessment of the phototoxicity of weathered Alaska North Slope crude oil to juvenile pink salmon. Chemosphere. 2005;60:105–110. doi: 10.1016/j.chemosphere.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Barron MG. Sediment-associated phototoxicity to aquatic organisms. Human Ecol Risk Assess. 2007;13:317–321. [Google Scholar]
- Barron MG, Vivian D, Yee, Diamond S. Temporal and spatial variation in solar radiation and photoenhanced toxicity risks of spilled oil in Prince William Sound, Alaska. Environ Toxicol Chem. 2008;27:227–236. doi: 10.1897/07-317.1. [DOI] [PubMed] [Google Scholar]
- Boese BL, Lamberson JO, Swartz RC, Ozretich RJ. Photoinduced toxicity of fluoranthene to seven marine benthic crustaceans. Arch Environmental Contam Tox. 1997;32:389–393. doi: 10.1007/s002449900201. [DOI] [PubMed] [Google Scholar]
- Calfee RD, Little EE, Cleveland L, Barron MG. Photoenhanced toxicity of a weathered oil to Ceriodaphnia dubia reproduction. Environ Sci Poll Res. 1999;6:207–212. doi: 10.1007/BF02987329. [DOI] [PubMed] [Google Scholar]
- Carriger J, Barron MG. Minimizing risks from spilled oil to ecosystem services using influence diagrams: The Deepwater Horizon spill response. Environ Sci Technol. 2011;45:7631–7639. doi: 10.1021/es201037u. [DOI] [PubMed] [Google Scholar]
- Choi J, Oris JT. Assessment of the toxicity of anthracene photo-modification products using the topminnow (Poecilopsis lucida) hepatoma cell line (PLHC-1) Aquat Tox. 2003;65:243–251. doi: 10.1016/s0166-445x(03)00139-5. [DOI] [PubMed] [Google Scholar]
- Cleveland L, Little EE, Calfee RD, Barron MG. Photoenhanced toxicity of a weathered oil to Mysidopsis bahia. Aquat Tox. 2000;49:63–76. doi: 10.1016/s0166-445x(99)00071-5. [DOI] [PubMed] [Google Scholar]
- Diamond SA. Photoactivated toxicity in aquatic environments. In: Helbling EW, Zagarese H, editors. UV Effects in Aquatic Organisms and Ecosystems. The Royal Society of Chemistry; Cambridge, UK: 2003. pp. 219–250. [Google Scholar]
- Duesterloh S, Short J, Barron MG. Photoenhanced toxicity of weathered Alaska North Slope crude oil to two species of marine calanoid zooplankton. Environ Sci Technol. 2002;36:3953–3959. doi: 10.1021/es020685y. [DOI] [PubMed] [Google Scholar]
- Farwell AJ, Nero V, Croft M, Rhodes S, Dixon DG. Phototoxicity of oil sands–derived polycyclic aromatic compounds to japanese medaka (Oryzias latipes) embryos. Environ Tox Chem. 2006;25:3266–3274. doi: 10.1897/05-637r1.1. [DOI] [PubMed] [Google Scholar]
- Finch Evaluation of the Phototoxicity of Unsubstituted and alkylated PAH compounds to mysid shrimp (Americamysis bahia): validation of predictive models. Env Tox Chem (in press) doi: 10.1002/etc.3733. in press. [DOI] [PubMed] [Google Scholar]
- Giesy JP, Newsted JL, Oris JT. Photoenhanced toxicity: serendipity of a prepared mind and flexible program management. Environ Tox Chem. 2013;32:969–971. doi: 10.1002/etc.2211. [DOI] [PubMed] [Google Scholar]
- Hatlen K, Sloan CA, Burrows DG, Collier TK, Scholz NL, Incardona JP. Natural sunlight and residual fuel oils are an acutely lethal combination for fish embryos. Aquat Tox. 2010;99:56–64. doi: 10.1016/j.aquatox.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Ho KT, Patton L, Latimer JS, Pruell RJ, Pelletier M, McKinney R, Jayaraman S. The chemistry and toxicity of sediment impacted by the North Cape Oil Spill in Rhode Island Sound. Mar Poll Bull. 1999;38:314–323. [Google Scholar]
- Hunter JR, Kaupp SE, Taylor JH. Assessment of effects of UV radiation on marine fish larvae. In: Calkins J, editor. The Role of Solar Radiation in Marine Ecosystems. Plenum Press; NY: 1980. pp. 459–493. [Google Scholar]
- Incardona JP, Vines CA, Linbo TL, Myers MS, Sloan CA, Anulacion BF, Boyd D, Collier TK, Morgan S, Cherr GN, Scholz NL. Phototoxicity of Marine Bunker Oil to Translucent Herring Embryos after Prolonged Weathering. PLOSone. 2012;7:e30116. doi: 10.1371/journal.pone.0030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby MF, Lyons BP, Barry J, Law RJ. The toxicological impacts of oil and chemically dispersed oil: UV mediated phototoxicity and implications for environmental effects, statutory testing and response strategies. Mar Poll Bull. 2007;54:472–475. doi: 10.1016/j.marpolbul.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Knecht AL, Goodale BC, Truong L, Simonich MT, Swanson AJ, Matzke MM, Anderson KA, Waters KM, Tanguay RL. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Tox Appl Pharm. 2013;271:266–275. doi: 10.1016/j.taap.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosian P, Makynen EA, Monson PD, Mount DR, Spacie A, Mekenyan OG, Ankley GT. Application of toxicity-based fractionation techniques and structure-activity relationship models for the identification of phototoxic polycyclic aromatic hydrocarbons in sediment pore water. Environ Tox and Chem. 1998;17:1021–1033. [Google Scholar]
- Kochevar IE, Armstrong RB, Einbinder J, Walther RR, Harber LC. Coal tar phototoxicity: active compounds and action spectra. Photochem Photobiol. 1982;36:65–69. doi: 10.1111/j.1751-1097.1982.tb04341.x. [DOI] [PubMed] [Google Scholar]
- Lampi MA, Gurska J, McDonald KI, Xie F, Huang XD, Dixon DG, Greenberg BM. Photoinduced toxicity of polycyclic aromatic hydrocarbons to Daphnia magna: ultraviolet-mediated effects and the toxicity of polycyclic aromatic hydrocarbon photoproducts. Environ Toxicol Chem. 2006;25:1079–1087. doi: 10.1897/05-276r.1. [DOI] [PubMed] [Google Scholar]
- Landrum PF, Giesy JP, Oris JT, Allred PM. Photoinduced toxicity of polycyclic aromatic hydrocarbons to aquatic organisms. In: Vandermeulen JH, Hrudey SE, editors. Oil in Freshwater. Pergamon Press; NY: 1987. pp. 304–318. [Google Scholar]
- Lee RF. Photo-oxidation and photo-toxicity of crude and refined oils. Spill Sci Tech Bull. 2003;8:157–162. [Google Scholar]
- Lee C-H, Sung C-G, Kang S-K, Moon S-D, Le J-H, Lee J-H. Effects of ultraviolet radiation on the toxicity of water-accommodated fraction and chemically enhanced water-accommodated fraction of Hebei Spirit crude oil to the embryonic development of the Manila clam, Ruditapes philippinarum. Korean J Malcol. 2013;29:23–32. [Google Scholar]
- Little EE, Cleveland L, Calfee R, Barron MG. Assessment of the photoenhanced toxicity of a weathered oil to the tidewater silverside. Environ Tox Chem. 2000;19:926–932. [Google Scholar]
- McConkey BJ, Hewitt LM, Dixon DG, Greenberg BM. Natural Sunlight Induced Photooxidation of Naphthalene in Aqueous Solution. Water Air Soil Poll. 2002;136:347–359. [Google Scholar]
- Pelletier MC, Burgess RM, Ho KT, Kuhn A, McKinney RA, Ryba SA. Phototoxicity of individual polycyclic aromatic hydrocarbons and petroleum to marine invertebrate larvae and juveniles. Environ Tox Chem. 1997;16:2190–2199. [Google Scholar]
- Spehar RL, Poucher S, Brooke LT, Hansen DJ, Champlin D, Cox DA. Comparative toxicity of fluoranthene to freshwater and saltwater species under fluorescent and ultraviolet light. Arch Environmental Contam Tox. 1999;37:496–502. doi: 10.1007/s002449900544. [DOI] [PubMed] [Google Scholar]
- Saco-Álvarez L, Bellas J, Nieto O, Bayona JM, Albaigés J, Beiras R. Toxicity and phototoxicity of water-accommodated fraction obtained from Prestige fuel oil and Marine fuel oil evaluated by marine bioassays. Sci Total Env. 2008;394:275–282. doi: 10.1016/j.scitotenv.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Spielmanna H, Ballsb M, Dupuisc J, Paped WJ, Pechovitche G, de Silvaf O, Holzhütterg H-G, Clothierh R, Desollef P, Gerbericki F, Liebscha M, Lovella WW, Maurerg T, Pfannenbeckerd U, Potthastl JM, Csatol M, Sladowskim D, Steilingn W, Brantomo P. The International EU/COLIPA in vitro phototoxicity validation study: results of phase II (blind trial). Part 1: The 3T3 NRU phototoxicity test. Tox in Vitro. 1998;12:305–327. doi: 10.1016/s0887-2333(98)00006-x. [DOI] [PubMed] [Google Scholar]
- Toyooka T, Ibuki Y. New method for testing phototoxicity of polycyclic aromatic hydrocarbons. Environ Sci Technol. 2006;40:3603–3608. doi: 10.1021/es060182i. [DOI] [PubMed] [Google Scholar]
- Willis AM, Oris JT. Acute photo-induced toxicity and toxicokinetics of single compounds and mixtures of polycyclic aromatic hydrocarbons in zebrafish. Environ Toxicol Chem. 2014;33:2028–2037. doi: 10.1002/etc.2648. [DOI] [PubMed] [Google Scholar]
- Veith GD, Mekenyan OG, Ankley GT, Call DJ. A QSAR analysis of substituent effects on the photoinduced acute toxicity of PAHs. Chemosphere. 1995;30:2129–2142. [Google Scholar]
- Wernersson AS. Predicting petroleum phototoxicity. Ecotoxicol Environ Saf. 2003;54:355–365. doi: 10.1016/s0147-6513(02)00083-0. [DOI] [PubMed] [Google Scholar]
- Wiegman S, van Vlaardingen PLA, Bleeker EAJ, de Voogt P, Kraak MHS. Phototoxicity of azaarene isomers to the marine flagellate Dunaliella tertiolecta. Environ Tox Chem. 2001;20:1544–1550. [PubMed] [Google Scholar]