Abstract
Background
The 13-valent pneumococcal conjugate vaccine (PCV13) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23) were both recommended to adults aged ≥65 years. The study examines adults ≥65 years for risk of adverse events (AEs) requiring medical attention following vaccination with PCV13 as compared with vaccination with PPSV23, a long-standing vaccine with a satisfactory safety profile.
Methods
The cohort study included 6 Vaccine Safety Datalink sites. The exposed person-time included follow-up time of the first PCV13 received by subjects age ≥65 years from January 1 to August 15, 2015. The comparator person-time included follow-up time after the first PPSV23 received by subjects of the same age during Janaury 1 to August 15 of each year of 2011–2015. The prespecified AEs included cardiovascular events, Bell’s palsy, Guillain-Barré syndrome, syncope, erythema multiforme, thrombocytopenia, cellulitis and infection, allergic reaction, and anaphylaxis. Inverse probability of treatment weighting–adjusted Poisson regression models was used to estimate the relative risk (RR) of each AE.
Results
A total of 313 136 doses of PCV13 and 232 591 doses of PPSV23 were included. The adjusted RRs comparing the incidence of AEs following PCV13 vs PPSV23 were all <1, except for anaphylaxis, which was insignificant with an RR of 1.32 (95% confidence interval, 0.30–5.79). Only 1 patient who received PCV13 and 4 other vaccines concomitantly was confirmed by medical chart review as having experienced anaphylaxis after vaccination.
Conclusions
These data do not support an increased rate of adverse events following PCV13 administration in elders compared with PPSV23 and should provide reassurance regarding continued use of PCV13.
Keywords: pneumococcal conjugate vaccine, adult vaccination, adverse events
The incidence of invasive pneumococcal disease (IPD) is strongly age-related, with 38% of cases occurring in children under 2 years of age and another 54% in adults older than 50 years of age [1]. In 1997, the Advisory Committee on Immunization Practices (ACIP) recommended use of 23-valent pneumococcal polysaccharide vaccine (PPSV23) for prevention of IPD among adults aged ≥65 years and those adults aged 19–64 years with underlying medical conditions. However, the durability of protection by PPSV23 against IPD in older adults seemed to be limited [2, 3]. Additionally, the ability of the vaccine to protect against pneumococcal community-acquired pneumonia (CAP) was not consistently demonstrated [4–6].
In December 2011, the 13-valent pneumococcal conjugate vaccine Prevnar 13 (PCV13) was approved for use among adults age 50 years and older to prevent pneumonia and invasive disease caused by S. pneumoniae serotypes contained in the vaccine. PCV13 addressed the unmet medical need for an alternative pneumococcal vaccine for elderly adults [7].
In August 2014, the ACIP recommended that both PCV13 and PPSV23 be administered routinely in series to all adults aged ≥65 years. Adults aged ≥65 years who have not previously received pneumococcal vaccine or whose previous vaccination history is unknown should receive a dose of PCV13 first, followed by a dose of PPSV23. The dose of PPSV23 should be given 6–12 months after a dose of PCV13. Adults aged ≥65 years who have previously received ≥1 doses of PPSV23 also should receive a dose of PCV13 if they have not yet received it. On June 25, 2015, the ACIP changed the recommended interval for PCV13 followed by PPSV23 (PCV13–>PPSV23 sequence) from 6–12 months to ≥1 year for immunocompetent adults aged ≥65 years [8, 9].
Clinical trials have shown no association between PCV13 and serious adverse events (SAEs) [10]. However, prelicensure trials may fail to detect serious but rare adverse events, and postlicensure monitoring is needed to assess real-world safety in a larger population.
The objective of this study was to examine a large cohort of adults age 65 years and older for evidence of increased risk of adverse events (AEs) requiring medical attention following vaccination with PCV13 as compared with vaccination with PPSV23.
METHODS
Study Population
The study population consisted of members of 6 managed care organizations (MCOs) participating in the Vaccine Safety Datalink (VSD) Project, a collaborative project between the Centers for Disease Control and Prevention and MCOs in the United States. Established in 1990 to conduct postmarketing evaluations of vaccine safety, the project has created an infrastructure that allows for high-quality research and surveillance [11]. Vaccination records and medical encounters were identified from electronic health record data prepared by each organization. The Institutional Review Board of each organization approved this study.
Study Design
This was a retrospective cohort study of patients aged 65 years and older who were vaccinated with either PPSV23 or PCV13 between January 1, 2011, and August 15, 2015. We tested the hypothesis that the incidence of prespecified AEs following PCV13 was no more common than that following PPSV23. The exposed person-time included follow-up time after the first dose of PCV13 was received during January 1, 2015, to August 15, 2015, by members age 65 years and older at each VSD site. This period was determined based on the timing of implementation of the ACIP recommendation, taking advantage of the large number of early catch-up doses and avoiding the potential discrepancies associated with the transition from International Classification of Diseases, Ninth Revision (ICD-9) codes to Tenth Revision (ICD-10) codes on October 1, 2015. The comparator (comparison) person-time included follow-up time after the first dose of PPSV23 received during January 1 to August 15 of each year between 2011 and 2015 by active members of the same age group. The restriction of months of vaccination among comparators allowed better control for seasonality and concomitant vaccination by providing alignment with the PCV13 vaccination period. For both cohorts, members were required to have at least 12 months of continuous enrollment (allowing a 31-day gap) before vaccination.
Prespecified Events of Interest
Nine major groups of adverse events, outlined in Table 1, were evaluated in this study. All conditions except thrombocytopenia were identified by ICD-9 diagnosis codes. Thrombocytopenia was identified using platelet laboratory data because of its accuracy over ICD-9 codes in the VSD setting.
Table 1.
Prespecified Adverse Events Following 13-Valent Pneumococcal Conjugate Vaccination in Elderly ≥65 Years Old
| Adverse Event Group/Adverse Event | ICD-9 Codesa | ICD-9 Code Description | Exclude if Diagnosis Occurs in the Specified Period Before Vaccination in any Care Setting | Type of Encounter | Risk Window |
|---|---|---|---|---|---|
| Group 1. Cardiovascular events | |||||
| Acute myocardial infarction | 410 | Acute myocardial infarction | 12 mo | Inpatient and ED | 1–42 d |
| 411 | Other acute and subacute forms of ischemic heart disease | ||||
| Acute pericarditis; myocarditis | 420 | Acute pericarditis | 12 mo | Inpatient and ED | 1–42 d |
| 422.0 | Myocarditis in diseases classified elsewhere | ||||
| 422.90 | Acute myocarditis, unspecified | ||||
| 422.91 | Idiopathic myocarditis | ||||
| 422.99 | Other and unspecified acute myocarditis | ||||
| 429.0 | Myocarditis, unspecified | ||||
| Atrial fibrillation | 427.31 | Atrial fibrillation | 12 mo | Inpatient and ED | 1–42 d |
| Cardiomyopathy; heart failure (defined as heart failure code plus cardiomyopathy code) | 425.4 | Other primary cardiomyopathies, NOS | 12 mo | Inpatient and ED | 1–42 d |
| 425.8 | Cardiomyopathy in other diseases classified elsewhere | ||||
| 425.9 | Secondary cardiomyopathy, unspecified | ||||
| 429.3 | Cardiomegaly—dilation, hypertrophy, ventricular dilation | ||||
| 428.0 | Congestive heart failure, unspecified | ||||
| 428.1 | Left heart failure | ||||
| 428.2 | Systolic heart failure | ||||
| 428.4 | Combined systolic and diastolic heart failure | ||||
| 428.9 | Heart failure, unspecified | ||||
| Group 2. Bell’s palsy | 351.0 | Bell’s palsy | 12 mo | Outpatient, inpatient, and ED | 1–42 d |
| Group 3. Guillain-Barré syndrome | 357.0 | Acute infective polyneuritis Guillain-Barré syndrome Postinfectious polyneuritis | 12 mo Also exclude if 357.81 (chronic inflammatory demyelinating polyneuritis) |
Outpatient, inpatient, and ED | 1–42 d |
| Group 4. Syncope | 780.2 | Syncope and collapse | 30 d | Inpatient and ED | 0 d |
| Group 5. Erythema multiforme | 695.1 | Erythema multiforme Stevens-Johnson syndrome Toxic epidermal necrolysis |
12 mo | Inpatient and ED | 1–42 d |
| Group 6. Thrombocytopenia | Lab value | Exclude if code below appears on same day as lab value | 12 mo | Outpatient, inpatient, and ED | 1–28 d |
| Thrombocytopenia I | 2 platelet counts of ≤50 000 within 7 d of each other (not necessarily consecutive) | Known illnesses causing thrombocytopenia: 140.0–208.0 (malignant neoplasms), 228 (hemangioma and lymphangioma, any site), 279 (disorders involving the immune mechanism), 283 (acquired hemolytic anemias), 284 (aplastic anemia and other bone marrow failure syndromes), 286.6 (defibrination syndrome), 570 (acute and subacute necrosis of liver), 571 (chronic liver disease and cirrhosis), 742.59 (other specified anomalies of spinal cord) | |||
| Thrombocytopenia II | 2 platelet counts of ≤100 000 within 7 d of each other (not necessarily consecutive) | Same as above | |||
| Group 7. Cellulitis and infection | 30 d | Outpatient, inpatient, and ED | 1–7 d | ||
| 682.3 | Cellulitis, upper arm and forearm | ||||
| 682.9 | Cellulitis, unspecified site | ||||
| 729.5 | Pain in limb | ||||
| 729.81 | Limb swelling | ||||
| 999.3 | Infection following infusion, infection, or vaccination | ||||
| 999.9 | Complication of medical care | ||||
| 289.3 | Lymphadenitis, unspecified, except mesenteric | ||||
| 683 | Acute lymphadenitis | ||||
| 785.6 | Enlargement of lymph nodes | ||||
| Group 8. Allergic reaction | 30 d | Outpatient, inpatient, and ED | 1–7 d | ||
| 995.2 | Adverse effects of drug | ||||
| 995.3 | Allergy, unspecified | ||||
| 708.0 | Allergic urticaria | ||||
| 708.1 | Idiopathic urticaria | ||||
| 708.9 | Urticaria, unspecified | ||||
| 995.1 | Angioneurotic edema | ||||
| 999.5 | Serum reaction | ||||
| Group 9. Anaphylaxis | 30 days | Outpatient, inpatient, and ED | 0–1 d | ||
| 999.4 | Anaphylactic shock due to serum | ||||
| 995.0 | Other anaphylactic shock | ||||
aFor 3-digit codes, include all fourth and fifth digits with that code; for 4-digit codes, include all fifth digits with that code.
Prespecified incident events of interest were selected based on PCV13 clinical trial safety data and the reports of Vaccine Adverse Event Reporting System (VAERS) collected between January 1, 2014, and July 31, 2015, for PCV13 among adults age 65 years or older. The VAERS is a national reporting system to detect possible safety problems in licensed vaccines. VAERS accepts reports of adverse events after a person has received a vaccination. Anyone can report to VAERS. From clinical trials, the most frequently reported SAEs following PCV13 in subjects age 65 years and older were cardiac disorders [12]. Among the 12 deaths occurring among adult PCV13 recipients, 5 were due to cardiac failure or other cardiac disorders [13]. In VAERS, there were 5 reports of atrial fibrillation, 2 reports of cardiac arrest, 2 reports of myocardial infarction, 2 reports of cardiomegaly, 2 reports of tachycardia, 1 report of congestive heart failure, 1 report of cardiac murmur, and 1 report of pericardial effusion. There were 2 reports of seventh cranial nerve paralysis and 1 report of facial paresis in VAERS. From clinical trials, there was 1 report of Guillain-Barré syndrome (GBS) in a 78-year-old female that was considered possibly related to PCV13 [12]. There were 3 reports of GBS in VAERS. There were 7 reports of syncope and 3reports of loss of consciousness in VAERS. From clinical trials, 1 case of erythema multiforme occurred 34 days after receipt of a second dose of PCV13 [13]. From clinical trials, there was 1 case of idiopathic thrombocytopenic purpura (ITP) possibly related to PCV13 vaccination in an 81-year-old male [12]. In VAERS reports, there were 3 reports of decreased platelet count. Injection site reactions and systemic events were the most often reported events in PCV13 clinical trials. There were several reports of cellulitis, infection, and injection site reaction in VAERS. In VAERS, there was 1 case of anaphylactic reaction reported.
Risk Window
Postvaccination risk windows were defined for each event separately (Table 1). These were prespecified time periods following vaccination in which the risk of events could hypothetically or biologically be affected by vaccination. Shorter windows were defined for events with more immediate onset. The follow-up time in the risk window was censored at membership disenrollment or receipt of another vaccine. Preexisting cases, determined by documentation of the AE in a prespecified period before vaccination, were excluded.
Statistical Analysis
Descriptive statistics were used to summarize the distribution of demographic characteristics including age at vaccination, sex, VSD site, Charlson comorbidity score (assessed from the 12 months before vaccination), health care utilization (eg, number of outpatient visits, emergency department [ED] visits, and hospitalizations in the 12 months before vaccination), concomitant vaccine (yes or no), and calendar month of vaccination. Chi-square tests (for categorical variables) or t tests (for continuous variables) were used to assess the comparability between the exposed and comparator cohorts. All analyses were conducted by using SAS (version 9.3 for Windows, SAS Institute, Cary, NC).
To adjust estimated relative risks (RRs) for the differences between the exposed (PCV13) and comparator (PPSV23) cohorts that may be associated with the risk of adverse events, we conducted propensity score analyses with the inverse probability of treatment weighting (IPTW) approach [12]. First, we estimated the probability of receiving PCV13 (ie, the propensity scores), as predicted by age, sex, site, Charlson comorbidity score, health care utilization, concomitant vaccination, and calendar month of vaccination using a logistic regression model. The weight for each subject was set to the inverse of the estimated probability of receiving his/her own exposure (PCV13 or PPSV23). The weight was normalized by dividing by the average weight of each PCV13 or PPSC23 group. Standardized difference scores were used to assess whether balance of covariates was achieved between the PCV13 and PPSV23 groups. Standardized difference is a unified approach to quantifying the magnitude of difference between groups regardless of sample size, where an absolute value less than 0.10 is considered a negligible difference [14]. The standardized difference scores before and after weighting were examined graphically and in the covariates table. Second, a weighted Poisson regression was conducted to estimate the relative risk of each adverse event, comparing the recipients of PCV13 vs PPSV23. Chart reviews were conducted to validate presumptive cases if there was a statistically significantly increased risk of an adverse event or if determined important by investigators.
Several sensitivity analyses were performed. The first sensitivity analysis restricted the comparator cohort to 2014–2015 data to minimize the possibility of bias from using a historical comparison group, as the exposed and comparator cohorts were temporally close. We also performed a secular trend analysis for rates of diagnoses of each prespecified event to evaluate if there was significant variation over the study period. The second sensitivity analysis adjusted separately for the 17 individual indicators (specific conditions or disease groups) that contributed to the Charlson comorbidity score to evaluate if there was significant residual confounding by adjustment using Charlson score only. Finally, additional subgroup analyses by VSD site (3 levels: 2 largest sites separated and 4 remaining smaller sites combined) and age group (65–69, ≥70 years) were also conducted to evaluate if there was any interaction by these variables.
RESULTS
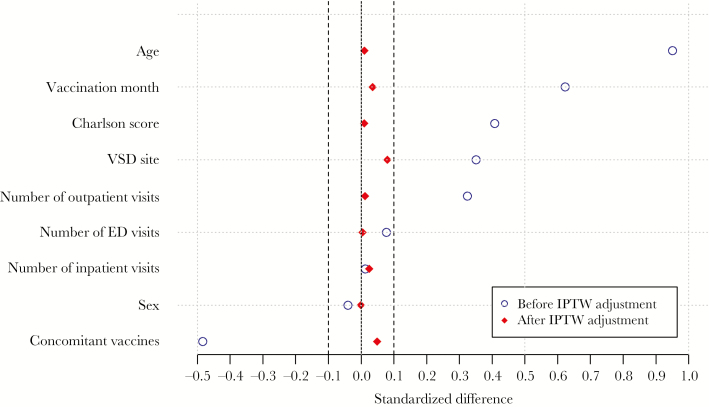
There were 313 136 doses of PCV13 and 232 591 doses of PPSV23 included in the study. Before the propensity score weighting process, the exposed and comparator cohorts differed on several variables. The PCV13 cohort was older, had more females, was more likely to have a higher Charlson score, had more inpatient, ED, and outpatient visits, and was less likely to receive concomitant vaccines. For age, site, Charlson score, number of outpatient visits in the past 12 months, concomitant vaccine indicator, and vaccination month, the absolute values of the standardized difference scores were greater than 0.20. After propensity score weighting, similar covariate distributions were achieved between the PCV13 and PPSV23 groups. The standardized difference score was less than 0.10 for all covariates, suggesting minimal differences in the distribution of these variables between the 2 groups after weighting (Table 2 and Figure 1).
Table 2.
Summary of Baseline Characteristics Among Cohort of 545 727 Doses by Vaccination Status
| Before IPTWa | After IPTW | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCV13b (n = 313 136), No. (%) | PPSV23c (n = 232 591), No. (%) | Total (n = 545 727), No. (%) | P Value | Standardized Difference | PCV13 (n = 313 136), % | PPSV23 (n = 232 591), % | Total (n = 545 727), % | P Value | Standardized Difference | |
| Age group, y | <.01 | 0.95 | <.01 | 0.01 | ||||||
| 65–69 | 93 934 (30.0) | 168 390 (72.4) | 262 324 (48.1) | 49.9 | 49.9 | 49.9 | ||||
| 70–74 | 80 319 (25.7) | 30 752 (13.2) | 111 071 (20.4) | 19.7 | 20.0 | 19.8 | ||||
| 75–79 | 61 341 (19.6) | 15 684 (6.7) | 77 025 (14.1) | 13.6 | 13.5 | 13.6 | ||||
| 80+ | 77 542 (24.8) | 17 765 (7.6) | 95 307 (17.5) | 16.9 | 16.6 | 16.7 | ||||
| Sex | <.01 | –0.04 | .73 | 0.00 | ||||||
| Female | 173 632 (55.5) | 124 276 (53.4) | 297 908 (54.6) | 54.4 | 54.3 | 54.3 | ||||
| Male | 139 504 (44.6) | 108 315 (46.6) | 247 819 (45.4) | 45.6 | 45.7 | 45.7 | ||||
| HMO site | <.01 | 0.35 | <.01 | 0.08 | ||||||
| A | 77 382 (24.7) | 85 794 (36.9) | 163 176 (29.9) | 32.7 | 32.1 | 32.5 | ||||
| B | 23 875 (7.6) | 15 278 (6.6) | 39 153 (7.2) | 7.5 | 8.3 | 7.8 | ||||
| C | 12 645 (4.0) | 4604 (2.0) | 17 249 (3.2) | 3.1 | 3.5 | 3.3 | ||||
| D | 25 063 (8.0) | 12 256 (5.3) | 37 319 (6.8) | 7.0 | 8.7 | 7.7 | ||||
| E | 160 958 (51.4) | 96 227 (41.4) | 257 185 (47.1) | 43.4 | 41.2 | 42.5 | ||||
| F | 13 213 (4.2) | 18 432 (7.9) | 31 645 (5.8) | 6.3 | 6.2 | 6.3 | ||||
| Charlson Index | <.01 | 0.41 | <.01 | 0.01 | ||||||
| 0 | 108 563 (34.7) | 124 477 (53.5) | 233 040 (42.7) | 43.3 | 42.8 | 43.1 | ||||
| 1 | 62 830 (20.1) | 41 993 (18.1) | 104 823 (19.2) | 19.1 | 19.1 | 19.1 | ||||
| ≥2 | 141 743 (45.3) | 66 121 (28.4) | 207 864 (38.1) | 37.7 | 38.1 | 37.9 | ||||
| No. of inpatient visits in past 12 mo | <.01 | 0.01 | <.01 | 0.03 | ||||||
| 0 | 256 798 (82.0) | 191 346 (82.3) | 448 144 (82.1) | 81.3 | 80.4 | 80.9 | ||||
| 1 | 35 681 (11.4) | 26 620 (11.4) | 62 301 (11.4) | 11.9 | 12.3 | 12.1 | ||||
| ≥2 | 20 657 (6.6) | 14 625 (6.3) | 35 282 (6.5) | 6.8 | 7.3 | 7.0 | ||||
| No. of ER visits in past 12 mo | <.01 | 0.08 | .28 | 0.00 | ||||||
| 0 | 232 854 (74.4) | 180 026 (77.4) | 412 880 (75.7) | 75.5 | 75.3 | 75.4 | ||||
| 1 | 48 280 (15.4) | 33 132 (14.2) | 81 412 (14.9) | 14.9 | 15.0 | 15.0 | ||||
| ≥2 | 32 002 (10.2) | 19 433 (8.4) | 51 435 (9.4) | 9.6 | 9.7 | 9.6 | ||||
| No. of outpatient visits in past 12 mo | <.01 | 0.32 | <.01 | 0.01 | ||||||
| 0 | 11 019 (3.5) | 18 358 (7.9) | 29 377 (5.4) | 5.9 | 5.6 | 5.8 | ||||
| 1 to 4 | 83 798 (26.8) | 86 423 (37.2) | 170 221 (31.2) | 31.6 | 31.5 | 31.6 | ||||
| >4 | 218 319 (69.7) | 127 810 (55.0) | 346 129 (63.4) | 62.5 | 62.9 | 62.7 | ||||
| Concomitant vaccine | <.01 | –0.48 | <.01 | 0.05 | ||||||
| No | 284 671 (90.9) | 169 305 (72.8) | 453 976 (83.2) | 79.7 | 81.7 | 80.6 | ||||
| Yes | 28 465 (9.1) | 63 286 (27.2) | 91 751 (16.8) | 20.3 | 18.3 | 19.4 | ||||
| Vaccination month | <.01 | 0.62 | <.01 | 0.04 | ||||||
| Jan | 23 108 (7.4) | 34 433 (14.8) | 57 541 (10.5) | 10.8 | 10.4 | 10.6 | ||||
| Feb | 22 820 (7.3) | 30 432 (13.1) | 53 252 (9.8) | 10.1 | 9.6 | 9.9 | ||||
| Mar | 26 407 (8.4) | 32 377 (13.9) | 58 784 (10.8) | 11.1 | 10.8 | 11.0 | ||||
| Apr | 23 369 (7.5) | 31 049 (13.4) | 54 418 (10.0) | 10.4 | 10.2 | 10.3 | ||||
| May | 26 734 (8.5) | 31 271 (13.4) | 58 005 (10.6) | 10.8 | 10.9 | 10.8 | ||||
| Jun | 76 108 (24.3) | 28 654 (12.3) | 104 762 (19.2) | 18.5 | 18.5 | 18.5 | ||||
| Jul | 77 192 (24.7) | 29 239 (12.6) | 106 431 (19.5) | 18.9 | 19.7 | 19.2 | ||||
| Aug | 37 398 (11.9) | 15 136 (6.5) | 52 534 (9.6) | 9.4 | 10.0 | 9.7 | ||||
Abbreviations: IPTW, inverse probability of treatment weighting; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Figure 1.
Abbreviations: ED, emergency department; IPTW, inverse probability of treatment weighting; VSD, Vaccine Safety Datalink.
Unadjusted analyses to evaluate the risk of prespecified events of interest between the PCV13 and PPSV23 groups showed a significantly elevated risk for atrial fibrillation and significantly reduced risks for syncope, thrombocytopenia, and allergic reaction in the PCV13 group (Table 3). No significantly increased risk was found in the PCV13 group in adjusted analyses for any of the prespecified events of interest, including atrial fibrillation. Anaphylaxis was the only event in adjusted analyses with an RR greater than 1 (1.32; 95% confidence interval, 0.30–5.79). All anaphylaxis diagnoses at 0–1 days following vaccination were chart-reviewed, including 5 following PCV13 and 4 following PPSV23. Among these 9 presumptive cases, 8 were codes for historical events, and only 1 was confirmed as having experienced anaphylaxis after vaccination. This was a patient from the PCV13 cohort who received 4 other vaccines concomitantly, including hepatitis A; hepatitis B; tetanus, diphtheria, and pertussis; and typhoid vaccines. Within 15 minutes of immunization, the patient felt the tongue swollen/thick and experienced mild difficulty in breathing and swallowing. The patient had no rash hives or facial swelling, no nausea or abdominal pain, and no confusion. The patient had a history of anaphylaxis to other agents such as eggs and intravenous contrast and experienced the same symptoms.
Table 3.
Adverse Event Rates and Relative Incidence Comparing PCV13 and PPSV23 in the Elderly, ≥65 Years Old
| PCV13 (n = 313 136) |
PPSV23 (n = 232 591) |
Relative Incidence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adverse Event Groups | No. of Adverse Events | Total Follow-up Time in Risk Window, Person-Days | No. of Adverse Events | Total Follow-up Time in Risk Window, Person-Days | Unadjusted | 95% Confidence Interval | IPTW Weighted | 95% Confidence Interval | ||
| Group 1. Cardiovascular events | ||||||||||
| Acute myocardial infarction | 534 | 13 024 960 | 375 | 9 572 008 | 1.05 | 0.92 | 1.19 | 0.73 | 0.61 | 0.88 |
| Acute pericarditis | 6 | 13 024 960 | 8 | 9 572 008 | 0.55 | 0.19 | 1.59 | 0.90 | 0.27 | 2.94 |
| Atrial fibrillation | 702 | 13 024 960 | 430 | 9 572 008 | 1.20 | 1.06 | 1.35 | 0.67 | 0.57 | 0.80 |
| Cardiomyopathy; heart failure (defined as heart failure code plus cardiomyopathy code) | 838 | 13 024 960 | 566 | 9 572 008 | 1.09 | 0.98 | 1.21 | 0.62 | 0.54 | 0.72 |
| Group 2. Bell’s palsy | 69 | 13 024 960 | 57 | 9 572 008 | 0.89 | 0.63 | 1.26 | 0.69 | 0.41 | 1.15 |
| Group 3. Guillain-Barré syndrome | 4 | 13 024 960 | 8 | 9 572 008 | 0.37 | 0.11 | 1.22 | 0.21 | 0.05 | 0.78 |
| Group 4. Syncope | 22 | 313 136 | 75 | 232 591 | 0.22 | 0.14 | 0.35 | 0.13 | 0.07 | 0.25 |
| Group 5. Erythema multiforme | 2 | 13 024 960 | 2 | 9 572 008 | 0.73 | 0.10 | 5.22 | 0.94 | 0.13 | 6.71 |
| Group 6. Thrombocytopenia | ||||||||||
| Thrombocytopenia I | 17 | 8 718 620 | 21 | 6 428 155 | 0.60 | 0.31 | 1.13 | 0.66 | 0.25 | 1.76 |
| Thrombocytopenia II | 96 | 8 718 620 | 100 | 6 428 155 | 0.71 | 0.53 | 0.94 | 0.44 | 0.31 | 0.61 |
| Group 7. Cellulitis and infection | 1915 | 2 188 604 | 1393 | 1 621 515 | 1.02 | 0.95 | 1.09 | 0.89 | 0.81 | 0.98 |
| Group 8. Allergic reaction | 49 | 2 188 604 | 70 | 1 621 515 | 0.52 | 0.36 | 0.75 | 0.47 | 0.30 | 0.73 |
| Group 9. Anaphylaxis | 5a | 626 152 | 4 | 464 888 | 0.93 | 0.25 | 3.46 | 1.32 | 0.30 | 5.79 |
Abbreviations: IPTW, inverse probability of treatment weighting; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
aAll anaphylaxis diagnoses at 0–1 days following vaccination were chart-reviewed, and 1 patient receiving 5 vaccines concomitantly, including PCV13, was confirmed as anaphylaxis.
The results of the sensitivity analysis restricting PPSV23 data to the 2014–2015 period were very similar to the main analysis (2011–2015). The results were unstable for rare events with large variation, but the point estimates were consistent for more common events. The IPTW method could not balance all covariates in the sensitivity analysis, and therefore unbalanced variables (site, number of inpatient visits) were included in the multivariable model (Appendix Table 1). The secular trends of the prespecified events of interest over the study period (2011–2015) are presented in Appendix Figure 1. For acute myocardial infarction and GBS, there was an increasing trend in more recent years (eg, 2015) that was largely due to the trend in 1 of the VSD sites. For 3 outcomes (pericarditis/myocarditis, syncope, allergic reaction), there was a decreasing trend in recent years (eg, 2013–2015). The trends were within 2 standard deviations of the mean. We also disaggregated the Charlson comorbidity score into its individual disease categories and repeated the main analyses, and the findings remained unchanged. Finally, we conducted additional stratified analyses by VSD site (3 categories) and age group (65–69, ≥70 years). The results were consistent with the findings from the main analysis.
DISCUSSION
In this study, we evaluated the incidence rates of prespecified AEs following PCV13 and found that they were no more common than those following PPSV23 in the elderly population. PPSV23 was a logical comparison group because it has been used in this age group for decades without major safety concerns. Our results indicate that there is no significantly elevated risk of cardiovascular events, Bell’s palsy, Guillain-Barré syndrome, syncope, erythema multiforme, thrombocytopenia, cellulitis and infection, or allergic reaction compared with PPSV23. Among all the anaphylaxis diagnoses that were reviewed, 1 patient receiving 5 vaccines concomitantly, including PCV13, was confirmed as having experienced anaphylaxis.
Prelicensure safety of PCV13 was evaluated in approximately 6000 adults age 50 years and older across 6 phase III clinical trials in European countries and the United States. Common adverse reactions reported with PCV13 were pain, redness, and swelling at the injection site, limitation of movement of the arm in which the injection was given, fatigue, headache, chills, decreased appetite, generalized muscle pain, and joint pain. Similar reactions were observed in adults who received PPSV23 [12]. Overall incidence of SAEs reported within 1 month of an initial study dose of PCV13 or PPSV23 did not differ between the 2 vaccines (0.2%–1.4% of 5055 subjects vaccinated with PCV13 and 0.4%–1.7% of 1124 subjects vaccinated with PPSV23). From 1 month to 6 months after the initial study dose, the overall incidence of SAEs ranged from 1.2% to 5.8% among subjects vaccinated with PCV13 and 2.4% to 5.5% among persons vaccinated with PPSV23 [12].
More recently, a randomized trial of 42237 adults age 65 years or older who received PCV13 with no prior pneumococcal vaccination history (the Community-Acquired Pneumonia Immunization Trial in Adults [CAPITA]) included an evaluation of the safety profile of PCV13 [10]. The frequencies of prespecified local reactions and systemic events reported by participants were higher in the PCV13 group than in the placebo group. Most local reactions and systemic events were mild or moderate in severity. There was no significant difference between the 2 groups in the frequencies of newly diagnosed chronic medical conditions, SAEs, or deaths. The significant difference in the percentage of participants who reported an adverse event reflected differences between the groups with respect to the prevalence of injection site reactions and of muscular pain. No vaccine-related SAEs were reported [10]. The study did not identify any safety concerns associated with the use of PCV13. These findings were consistent with observations from previous studies of PCV13 vaccination in adults [15–20].
The lower risk of adverse events in the PCV13 cohort deserves comment. This appeared to be independent of age, comorbidities, and other covariates included in the weighted models. As the trend of these selected events had been relatively stable over the study period, and analyses restricting the comparison cohort to doses used in years close to that of the PCV13 cohort yielded similar findings, it seems that the PCV13 cohort in this study might represent a group of early adopters of the ACIP recommendation who might be different in general due to self-selection or physician’s preference to vaccinate. We acknowledge that the inherent differences between the groups and the limited ability to adjust for confounding could contribute to the unexpectedly low relative risks. Future studies with designs using a different comparison group or a self-comparison approach might help elucidate the questions.
We checked prior pneumococcal vaccination history using KPSC data and found out that both cohorts (PCV13 and PPSV23) were almost PCV13 naïve before being included in the study. Also, the record showed that most PCV13 doses were given to subjects who were vaccinated with PPSV23 before. With the data obtained during the early implementation phase, it is difficult to evaluate the safety by sequential vaccination status (PCV13–>PPSV23 or PPSV23–>PCV13) as almost all subjects in the PCV13 cohort were vaccinated with PPSV23 before. Very few subjects were in the PCV13–>PPSV23 sequence.
In conclusion, we found no evidence of increased risk of adverse events requiring medical attention following vaccination with PCV13 as compared with vaccination with PPSV23 in adults age 65 years and older. PPSV23 has a well-established safety profile. As unconjugated polysaccharide vaccines likely do not confer long-lasting protection, and the incidence of IPD among adults increases dramatically with age, PCV13 has the advantage of offering higher levels of protection against the vaccine serotypes after vaccination and the ability to prolong the duration of protection in elderly adults, with no apparent increase in safety concerns.
Supplementary Material
Acknowledgments
The authors thank the data managers, project managers, and abstractors at the VSD sites for their assistance with this study. Dr. Hung Fu Tseng had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This study was funded through the Vaccine Safety Datalink (VSD) under contract 200-2012-53580 from the Centers for Disease Control and Prevention.
Potential conflicts of interest. Hung Fu Tseng and Lina Sy report receiving research support from Novartis Vaccines, GlaxoSmithKline (GSK), and Novavax for studies unrelated to this publication. Sara Y. Tartof received funding from Merck, Novartis Vaccines, and GSK for studies unrelated to this publication. Lei Qian received research support from GSK for studies unrelated to this publication. Nicola P. Klein reports research support from Pfizer, GSK, Merck, Sanofi Pasteur, MedImmune, Protein Science, and Dynavax for studies unrelated to this publication. Elizabeth Liles reports research support from Pfizer for a study unrelated to this publication. Jennifer Nelson reports research support unrelated to this publication from inventive Health, a consortium of pharmaceutical companies carrying out Food and Drug Administration–mandated drug safety studies. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect 2014; 20(Suppl 5):45–51. [DOI] [PubMed] [Google Scholar]
- 2. Musher DM, Manof SB, Liss C et al. . Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis 2010; 201:516–24. [DOI] [PubMed] [Google Scholar]
- 3. Sankilampi U, Honkanen PO, Bloigu A, Leinonen M. Persistence of antibodies to pneumococcal capsular polysaccharide vaccine in the elderly. J Infect Dis 1997; 176:1100–4. [DOI] [PubMed] [Google Scholar]
- 4. Metersky ML, Dransfield MT, Jackson LA. Determining the optimal pneumococcal vaccination strategy for adults: is there a role for the pneumococcal conjugate vaccine?Chest 2010; 138:486–90. [DOI] [PubMed] [Google Scholar]
- 5. Jackson L, Neuzil K. Pneumococcal polysaccharide vaccines. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed Philadelphia, PA: Saunders, Elsevier;2008:570–604. [Google Scholar]
- 6. Centers for Disease Control and Prevention (CDC). Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010; 59:1102–6. [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–9. [PubMed] [Google Scholar]
- 8. Kobayashi M, Bennett NM, Gierke R et al. . Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015; 64:944–7. [DOI] [PubMed] [Google Scholar]
- 9. Tomczyk S, Bennett NM, Stoecker C et al. ; Centers for Disease Control and Prevention (CDC) Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–5. [PMC free article] [PubMed] [Google Scholar]
- 10. Bonten MJ, Huijts SM, Bolkenbaas M et al. . Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–25. [DOI] [PubMed] [Google Scholar]
- 11. Baggs J, Gee J, Lewis E et al. . The vaccine safety datalink: a model for monitoring immunization safety. Pediatrics 2011; 127(Suppl 1):S45–53. [DOI] [PubMed] [Google Scholar]
- 12. Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee (VRBPAC) Adult Indication Briefing Document: Prevnar 13. Silver Spring, MD: US Department of Health and Human Services; Food and Drug Administration; 2011. [Google Scholar]
- 13. Prevnar 13 (Pneumococcal 13-valent conjugate vaccine [diphtheria CRM-197 protein]) [package insert]. Philadelphia, PA: Wyeth Pharmaceutical Division of Wyeth Holdings Corporation, a subsidiary of Pfizer Inc; 2015. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=501 (accessed 28 February 2017). [Google Scholar]
- 14. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson LA, Gurtman A, Rice K et al. . Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2013; 31:3585–93. [DOI] [PubMed] [Google Scholar]
- 16. Jackson LA, Gurtman A, van Cleeff M et al. . Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine 2013; 31:3577–84. [DOI] [PubMed] [Google Scholar]
- 17. Greenberg RN, Gurtman A, Frenck RW et al. . Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine 2014; 32:2364–74. [DOI] [PubMed] [Google Scholar]
- 18. Schwarz TF, Flamaing J, Rümke HC et al. . A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged ≥65 years. Vaccine 2011; 29:5195–202. [DOI] [PubMed] [Google Scholar]
- 19. Schwarz T, Pauksens K, Juergens C et al. . Safety of a 13-valent pneumococcal conjugate vaccine in elderly adults previously immunized with a 23-valent pneumococcal polysaccharide vaccine: an open-label trial. World J Vaccines 2013; 3:123–9. [Google Scholar]
- 20. Haber P, Arana J, Pilishvili T et al. . Post-licensure surveillance of 13-valent pneumococcal conjugate vaccine (PCV13) in adults aged 19 years old in the United States, Vaccine Adverse Event Reporting System (VAERS), June 1, 2012–December 31, 2015. Vaccine 2016; 34:6330–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.