Abstract
Recent biomedical advances inspire hope that an end to the epidemic of HIV is in sight. Adopting new approaches and paradigms for treatment and prevention in terms of both messaging and programming is a priority to accelerate progress. Defining the key sequential steps that comprise engagement in HIV care has provided a useful framework for clinical programs and motivated quality improvement initiatives. Recently, the same approach has been applied to use of pre-exposure prophylaxis for HIV prevention. Building on the various prevention and care continua previously proposed, we present a novel schematic that incorporates both people living with HIV and people at risk, making it effectively “status-neutral” in that it proposes the same approach for engagement, regardless of one’s HIV status. This multidirectional continuum begins with an HIV test and offers 2 divergent paths depending on the results; these paths end at a common final state. To illustrate how this continuum can be utilized for program planning as well as for monitoring, we provide an example using data for New York City men who have sex with men, a population with high HIV incidence and prevalence.
Keywords: antiretrovirals, continuum, HIV, prevention, pre-exposure prophylaxis
The HIV epidemic has evolved over the past 3 decades; its end is now in sight. Yet, despite major progress and the existence of epidemic-ending technology, HIV continues to spread, with at least 37 000 new diagnoses in the United States in 2014 [1]. These new diagnoses add to the more than 1.1 million persons living with HIV (PLWH) in the United States [1]. Given these staggering numbers, adopting new approaches and paradigms for treatment and prevention messaging and programming is critical. This is especially true in the era of “treatment as prevention,” where it is now empirically clear that achievement of viral load suppression has implications for both individual and public health [2–4], and where pre-exposure prophylaxis (PrEP) represents a viable, highly effective biomedical intervention for HIV prevention [5–8].
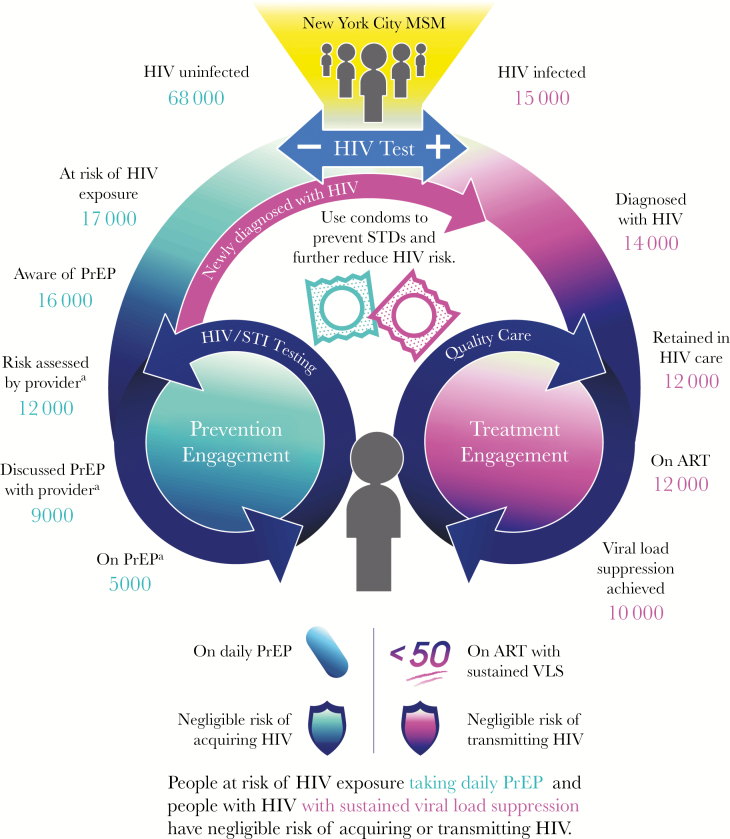
Building on earlier innovative HIV prevention and care continua [9–17] following the original care continuum proposed by Gardner [9] and colleagues, we present a novel schematic of the current care environment that incorporates both PLWH and people at risk of HIV exposure (Figure 1). This multidirectional continuum begins with an HIV test and proposes 2 dynamic, divergent paths depending on the test results (“HIV Primary Prevention Engagement” on the left for those testing negative; “HIV Treatment Engagement” on the right for those testing positive) that end at a common final state: engaged in clinical care, with either sustained viral load suppression (VLS) or taking daily PrEP, reflecting that the risk of either HIV transmission or acquisition is negligible in this state. Such a continuum is effectively “HIV status-neutral” in that it proposes the same approach for engagement, regardless of one’s HIV status.
Figure 1.
New York City’s HIV status-neutral prevention and treatment cycle with estimates derived from HIV surveillance and local surveys. Data for the HIV-positive side of the continuum are derived from NYC surveillance data on men who have sex with men (MSM) aged 18–40 years, combining 2015 data from the surveillance registry with 2014 data from the NYC Medical Monitoring Project; data for the HIV-negative side of the continuum are derived from the Sexual Health Survey, conducted in Spring 2016 among NYC MSM aged 18–40 years who report anal sex with another man in the past six months and any of the following in previous 6 months, rendering them potentially at risk of HIV exposure and eligible for pre-exposure prophylaxis: condomless anal sex, stimulant or injection drug use, transactional sex, PEP use, HIV-positive sexual partner, or STI diagnosis in the past year. Numbers are rounded to the thousands. aPast 6 months. Abbreviations: ART, antiretroviral therapy; MSM, men who have sex with men; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection; STD, sexually transmitted disease; VLS, viral load suppression.
A key characteristic of this “cycle” is its nonlinearity. Continuous preventive and quality care services are highlighted as part of an ongoing effort by patient and provider to maintain engagement in clinical preventive care or treatment. The end point is not a final state but a dynamic one requiring continued attention by all parties. The figure emphasizes the consistent return among the uninfected to HIV testing, with a resultant trajectory into and through the continuum, as appropriate, depending on test results (and on the appropriateness of PrEP for those testing negative).
We illustrate how this continuum can be utilized by applying data for men who have sex with men (MSM) aged 18–40 years from NYC, a population known to have both a high incidence and prevalence of HIV infection attributed to sexual transmission. For the HIV Treatment Engagement cohort (Figure 1), we use NYC surveillance data on MSM, drawing on 2015 data from the NYC Department of Health and Mental Hygiene (DOHMH) surveillance registry and 2014 data from NYC’s Centers for Disease Control and Prevention (CDC) Medical Monitoring Project (MMP) limited to respondents from NYC [18, 19]. The “denominator” for all continuum steps is the number of cisgender MSM aged 18–40 years in NYC who are estimated to be HIV-infected (n = 15 000). Subsequent steps in the continuum are well described and defined when using population-based data: those who are diagnosed with HIV, followed by those retained in care, those prescribed antiretroviral therapy, and, finally, those achieving VLS [9, 20, 21]. The largest drop-off (33% relative decrease) is between those who are prescribed antiretrovirals and those who achieve VLS, highlighting the importance of medical and social interventions focused on maintenance of care and adherence.
For the HIV-negative Primary Prevention Engagement cohort, to derive the “denominator” of all cisgender MSM aged 18–40 years in NYC who are estimated to be HIV-uninfected (n = 68 000), we used data from the 2015 and 2016 Community Health Surveys [22], creating a weighted average estimate of all cisgender MSM aged 18–40 years (n = 83 000; 95% CI, 66 000–100 000), and subtracted the cisgender MSM aged 18–40 years estimated to be HIV-infected (n = 15 000). For most subsequent steps, we used data from the NYC DOHMH Sexual Health Survey (SHS), conducted semiannually online and annually in-person among NYC MSM aged 18–40 years who report anal sex with another man in the past 6 months [23–25]. The figure includes data from the Spring 2016 survey among respondents who reported any of the following in the previous 6 months, rendering them potentially at risk of HIV exposure and eligible for PrEP: condomless anal sex, stimulant or injection drug use, transactional sex, postexposure prophylaxis (PEP) use, HIV-positive sexual partner, or sexually transmitted infection (STI) diagnosis in past year.
For this HIV-negative cohort, the steps in the continuum are derived from NYC DOHMH work, as well as steps set forth by other colleagues [10–13]. We begin with those who report behavior consistent with PrEP “candidacy,” defined according to alignment with NYS PrEP prescribing guidance [26]; it has been previously estimated that approximately 25% of HIV-negative MSM have indications for PrEP [27] (n = 17 000, or 25% of the “denominator” for the HIV-negative cohort). From there, the steps include both client- and intervention-centric approaches; each is actionable from a public health perspective [15]; and estimates are derived using SHS.
This approach defines an arc from individual awareness to engagement in health care to taking daily PrEP. Further, we disaggregated critical aspects of PrEP-related clinical engagement, separating risk assessment conducted by a provider (operationalized as having had a provider visit at which a sexual history was taken) and discussing PrEP with a provider (regardless of who initiated the conversation). Awareness of PrEP is the only step that is not time bound; other steps refer to the past 6 months. Clearly, the steps for the HIV-negative cohort lack the inexorable, rigid progression that is characteristic of the HIV-positive side of the continuum. Specifically, individuals may become aware of PrEP from their provider. But awareness is often a recognized precursor to subsequent clinical engagement, so we present it as such herein.
The 2 largest drop-offs in the HIV-negative Primary Prevention Continuum are between having a sexual history taken and having a PrEP discussion with a provider (25% relative decrease), and from having a PrEP discussion with a provider to having initiated PrEP (44% relative decrease). There are any number of explanations for each of these substantial drop-offs, including both provider and patient factors that require elucidation through additional investigation. Importantly, in terms of patient factors, we expect that patient choice will play a critical role; PrEP will not be right for every person who meets existing criteria. One limitation of these data is that they are not population-based (as the HIV surveillance data are) or a representative sample of all NYC MSM.
This extension of the continuum framework to visualize treatment as prevention has several key implications. First, the continuum makes clear that HIV testing is the ultimate gateway to prevention and care. Any HIV test result spurs action. Clinical protocols in settings that provide care to vulnerable, high-incidence populations can build this philosophy into workflows by following HIV testing with the offer of antiretrovirals as treatment or as either PrEP or PEP depending on the HIV test result and the recentness of any possible exposure to HIV.
This “status-neutral” continuum also serves as a reminder that the same approaches used for achieving VLS for treatment will be necessary for HIV prevention, supporting more integrated prevention and care programs. The cyclic aspect of this visualization emphasizes that PrEP and other prevention engagement must provide a seamless entrée into the care system in the event that individuals engaged in primary prevention are newly diagnosed with HIV. The new continuum also highlights that approaches to serving people taking prophylaxis and people taking treatment are virtually indistinguishable both clinically and programmatically. The “double cycle” equates the person living with HIV who is consistently virally suppressed to the individual taking PrEP daily, thereby supporting a vision in which the clinical and social HIV “divide” is nonexistent. Normalizing both treatment and prevention serves to destigmatize both.
In our own Health Department, the continuum has engendered status-neutral messaging, starting with a sex-positive HIV prevention social marketing campaign, PlaySure [28], which was simultaneously geared toward those living with HIV and those at risk [28]; a subsequent campaign, StaySure [29], explicitly promoted treatment as prevention. The Health Department’s #PlaySure Kit physically embodies that message, providing discrete transport for safer sex supplies, such as a “prevention” pill (HIV treatment or PrEP), condoms, and lubricant [30].
Extensive status-neutral programming followed, including the transformation of publicly funded sexually transmitted diseases clinics into more culturally competent sexual health clinics [31] that offer more comprehensive HIV services, including immediate antiretroviral therapy for those testing HIV-positive and pre-exposure prophylaxis for those testing HIV-negative, with navigation to clinical sites in the community for ongoing care or prevention [32]. And new programs were also developed citywide to offer navigation services through a robust referral network, sharing a name with the PlaySure campaign, for all persons regardless of HIV status at a combination of community-based organizations and clinical sites [33]. Additional programs, paid for with city funding, have been built on the Ryan White Care Coordination model to expand services to HIV-negative persons for engagement in PrEP, mental health, and substance use services. We recently rebid our portfolio of HIV testing contracts to align them with this approach and formally incorporate them into the PlaySure Network. A status-neutral, Health Department–convened community collaborative to improve testing and navigation services to black and Latino MSM in Brooklyn was also launched as part of a CDC-supported demonstration project (THRIVE) [34]. Further, New York Knows, the large-scale HIV testing initiative borne out of an earlier Bronx-specific campaign [35, 36], already focused on HIV testing and linkage to care, was expanded to provide technical assistance with PrEP implementation among 266 partner organizations [37]. A hands-on workshop to support diverse clinical sites to incorporate PrEP into existing workflows has been successful in supporting the development of PrEP-related protocols, including sites formerly focused on HIV care provision [38]. Future plans include developing protocols to initiate immediate, field-based antiretroviral therapy (as treatment or prevention) through partner services.
In the context of the ambitious goals for HIV prevention and care both locally [39] and nationally [40], tools are needed that stimulate and guide thought and action and measure progress on a range of outcomes. We believe the synergies and dynamism inherent in our new status-neutral continuum help bring us closer to our critical goals: “virtually eliminating new HIV infections, effectively supporting all people with HIV to lead long and healthy lives, and eliminating the disparities that persist among some populations.” [40]
Acknowledgments
The authors wish to thank Paul Salcuni and Anisha Gandhi for assistance with the estimate of cisgender MSM in NYC and Davida Farhat for assistance with preparation of the manuscript.
Financial support. This work was supported by the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP) at the Centers for Disease Control and Prevention (grant number 5U62PS003639-05) and the Health Resources and Services Administration, HIV/AIDS Bureau, HIV Emergency Relief Grant (grant number H89HA00015-27).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Johnson AS, Ruiguang S, Hall I. State-level estimates of HIV incidence, prevalence, and undiagnosed infections. Paper presented at: Retroviruses and Opportunistic Infections; February 13–16, 2017; Seattle, WA. [Google Scholar]
- 2. Cohen MS, Chen YQ, McCauley M et al. HPTN 052 Study Team Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodger AJ, Cambiano V, Bruun T et al. PARTNER Study Group Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016; 316:171–81. [DOI] [PubMed] [Google Scholar]
- 4. Bavinton B, Grinsztejn B, Phanuphak F et al. HIV treatment prevents HIV transmission in male serodiscordant couples in Australia, Thailand and Brazil. Paper presented at: 9th IAS Conference on HIV Science; July 23–26, 2017; Paris, France. [Google Scholar]
- 5. Baeten JM, Donnell D, Ndase P et al. Partners PrEP Study Team Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thigpen MC, Kebaabetswe PM, Paxton LA et al. TDF2 Study Group Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 7. Choopanya K, Martin M, Suntharasamai P et al. Bangkok Tenofovir Study Group Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 8. McCormack S, Dunn DT, Desai M et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gardner EM, McLees MP, Steiner JF et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelley CF, Kahle E, Siegler A et al. Applying a PrEP continuum of care for men who have sex with men in Atlanta, Georgia. Clin Infect Dis 2015; 61:1590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu A, Colfax G, Cohen S et al. The spectrum of engagement in HIV prevention: proposal for a PrEP cascade. Paper presented at: 7th International Conference on HIV Treatment and Prevention Adherence; June 3–5, 2012; Miami, FL. [Google Scholar]
- 12. McNairy ML, El-Sadr WM. A paradigm shift: focus on the HIV prevention continuum. Clin Infect Dis 2014; 59(Suppl 1):S12–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nunn AS, Brinkley-Rubinstein L, Oldenburg CE et al. Defining the HIV pre-exposure prophylaxis care continuum. AIDS 2017; 31:731–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parsons JT, Rendina HJ, Lassiter JM et al. Uptake of HIV pre-exposure prophylaxis (PrEP) in a National Cohort of Gay and bisexual men in the United States. J Acquir Immune Defic Syndr 2017; 74:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garnett GP, Hallett TB, Takaruza A et al. Providing a conceptual framework for HIV prevention cascades and assessing feasibility of empirical measurement with data from east Zimbabwe: a case study. Lancet HIV 2016; 3:e297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson J. Forgotten negatives: the limits of treatment as prevention. TAGline 2014; 21:2–5. Available at: http://www.treatmentactiongroup.org/tagline/2014/spring/forgotten-negatives-limits-treatment-prevention. Accessed 1 December 2016. [Google Scholar]
- 17. Horn T, Sherwood J, Remien RH et al. Treatment Action Group and Foundation for Aids Research HIV Prevention Continuum Working Group Towards an integrated primary and secondary HIV prevention continuum for the United States: a cyclical process model. J Int AIDS Soc 2016; 19:21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McNaghten AD, Wolfe MI, Onorato I et al. Improving the representativeness of behavioral and clinical surveillance for persons with HIV in the United States: the rationale for developing a population-based approach. PLoS One 2007; 2:e550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. HIV Epidemiology and Field Services Program. Care and Clinical Status of People Newly Diagnosed With HIV and People Living With HIV/AIDS in NYC, 2015. New York: New York City Department of Health and Mental Hygiene; 2016. Available at: https://www1.nyc.gov/assets/doh/downloads/pdf/dires/hiv-related-medical-care.pdf. Accessed 8 March 2018. [Google Scholar]
- 20. Medland NA, McMahon JH, Chow EP et al. The HIV care cascade: a systematic review of data sources, methodology and comparability. J Int AIDS Soc 2015; 18:20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xia Q, Kersanske LS, Wiewel EW et al. Proportions of patients with HIV retained in care and virally suppressed in New York City and the United States: higher than we thought. J Acquir Immune Defic Syndr 2015; 68:351–8. [DOI] [PubMed] [Google Scholar]
- 22. NYC DOHMH. Community Health Survey data. Available at: http://www1.nyc.gov/site/doh/data/data-sets/community-health-survey.page. Accessed 29 January 2018. [Google Scholar]
- 23. Rucinski KB, Mensah NP, Sepkowitz KA et al. Knowledge and use of pre-exposure prophylaxis among an online sample of young men who have sex with men in New York City. AIDS Behav 2013; 17:2180–4. [DOI] [PubMed] [Google Scholar]
- 24. Edelstein Z, Scanlin K, Findlater C et al. HIV Prevention Continuum among MSM, New York City, Spring 2016. Paper presented at: 12th International Conference on HIV Treatment and Prevention Adherence; June 4–6, 2017; Miami, FL. [Google Scholar]
- 25. Scanlin K, Edelstein Z, Findlater C et al. Trends in PrEP awareness and use and associations with use among men who have sex with men, New York City, 2012–2016. Paper presented at: 12th International Conference on HIV Treatment and Prevention Adherence; June 4–6, 2017; Miami, FL. [Google Scholar]
- 26. New York State Department of Health AIDS Institute. PrEP to prevent HIV acquisition guideline. HIV Clinical Resource. 2016. Available at: https://www.hivguidelines.org/prep-for-prevention/. Accessed 7 March 2018. [Google Scholar]
- 27. Smith DK, Van Handel M, Wolitski RJ et al. Vital signs: estimated percentages and numbers of adults with indications for preexposure prophylaxis to prevent HIV acquisition–United States, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:1291–5. [DOI] [PubMed] [Google Scholar]
- 28. Bellafante G. New York revamps safe sex. New York Times. December 20, 2015. [Google Scholar]
- 29. Health Department recognizes World AIDS Day and launches new HIV awareness campaign [press release]. New York, NY: December 1, 2016. [Google Scholar]
- 30. Health Department celebrates LGBT Pride Month with #PlaySure HIV/AIDS Awareness Media Campaign [press release]. New York, NY: June 2, 2016. [Google Scholar]
- 31. Treatment Action Group. Treatment action group lauds launch of NYC sexual health clinics as hubs of comprehensive HIV prevention and care. 2017. Available at: http://www.treatmentactiongroup.org/content/treatment-action-group-lauds-launch-nyc-sexual-health-clinics-hubs-comprehensive-hiv. Accessed 7 March 2018. [Google Scholar]
- 32. Daskalakis D. If you can make it there: ending the HIV epidemic in New York. Paper presented at: Conference on Retroviruses and Opportunistic Infections; February 13–16, 2017; Seattle, WA. [Google Scholar]
- 33. Saleh L, Estacio K, Dorsainvil M et al. Let’s PlaySure: PrEP’ing for Prevention Navigation SYNChronicity. Paper presented at: SYNChronicity 2018, April 22–24, 2018; Arlington, VA. [Google Scholar]
- 34. Jones R, Ortiz E.. NYC Project THRIVE. Paper presented at: The 2018 National African American MSM Leadership Conference on HIV/AIDS (NAESM); January 18, 2018; Atlanta, GA. [Google Scholar]
- 35. Myers JE, Braunstein SL, Shepard CW et al. Assessing the impact of a community-wide HIV testing scale-up initiative in a major urban epidemic. J Acquir Immune Defic Syndr 2012; 61:23–31. [DOI] [PubMed] [Google Scholar]
- 36. NYC DOHMH. The Bronx Knows HIV Testing Initiative: Final Report. New York City Department of Health and Mental Hygiene: New York, NY; 2011. Available at: https://www1.nyc.gov/assets/doh/downloads/pdf/ah/bronx-knows-summary-report.pdf. Accessed 8 March 2018. [Google Scholar]
- 37. NYC DOHMH. HIV testing initiatives: New York knows. Infectious Diseases: HIV Testing. 2017. Available at: https://www1.nyc.gov/site/doh/providers/health-topics/aids-hiv-new-york-knows.page. Accessed 15 December 2017. [Google Scholar]
- 38. Khawja A, Myers J, Wells A, Golub S. Long-term follow-up of participants attending a PrEP implementation Workshop in NYC. Paper presented at: 2016 United States Conference on AIDS (USCA); September 15–18, 2016; Hollywood, FL. [Google Scholar]
- 39. New York State Department of Health AIDS Institute. Ending the AIDS epidemic in New York State. AIDS Institute. 2017. Available at: https://www.health.ny.gov/diseases/aids/ending_the_epidemic. Accessed 15 December 2017. [Google Scholar]
- 40. The Office of National AIDS Policy. National HIV/AIDS Strategy for the United States: Updated to 2020. Washington, DC: Office of National AIDS Policy; 2015. [Google Scholar]