Abstract
Background
Patients reporting penicillin allergy often receive unnecessary and costly broad-spectrum alternatives such as aztreonam with negative consequences. Penicillin allergy testing improves antimicrobial therapy but is not broadly used in hospitals due to insufficient testing resources and short-term expenses. We describe a clinical decision support (CDS) tool promoting pharmacist-administered penicillin allergy testing in patients receiving aztreonam and its benefits toward antimicrobial stewardship and costs.
Methods
A CDS tool was incorporated into the electronic medical record, directing providers to order penicillin allergy testing for patients receiving aztreonam. An allergy-trained pharmacist reviewed orders placed through this new guideline and performed skin testing and oral challenges to determine whether these patients could safely take penicillin. Data on tests performed, antibiotic utilization, and cost-savings were compared with patients tested outside the new guideline as part of our institution’s standard stewardship program.
Results
The guideline significantly increased penicillin allergy testing among patients receiving aztreonam from 24% to 85% (P < .001) while reducing the median delay between admission and testing completion from 3.31 to 1.05 days (P = 0.008). Patients tested under the guideline saw a 58% increase in penicillin exposure (P = .046). Institutional aztreonam administration declined from 2.54 to 1.47 administrations per 1000 patient-days (P = .016). Average antibiotic costs per patient tested before and after CDS decreased from $1265.81 to $592.08 USD, a 53% savings.
Conclusions
Targeting penicillin allergy testing to patients on aztreonam yields therapeutic and economic benefits during a single admission. This provides a cost-effective model for inpatient testing.
Keywords: aztreonam, clinical decision support, penicillin allergy, skin test, stewardship
Penicillin allergy skin testing (PAST) is gaining recognition as a key component of antimicrobial stewardship. The prevalence of reported penicillin allergy in hospitalized patients is approximately 10%–20%; however, fewer than 1 in 10 individuals carrying this diagnosis are truly allergic [1–5]. Many reaction histories are poorly documented, and in some cases, nonimmunologic side effects are recorded as allergies that persist in patients’ medical records [6]. In other instances, viral exanthems or urticaria are mistakenly blamed on concurrently administered penicillin [7]. Importantly, true immunoglobulin E (IgE)–mediated hypersensitivity to penicillin decreases with time, with more than half of skin test–positive patients losing sensitivity by 5 years and 80% of those with penicillin allergy histories losing sensitivity by 10 years [8, 9].
It is well established that many penicillin-“allergic” patients receive therapeutically inferior and potentially harmful alternative antibiotics, resulting in treatment failures and higher rates of Clostridium difficile, as well as drug-resistant infections such as vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus [1, 3, 10, 11]. Furthermore, these patients incur additional expense not only from antibiotic costs but also from increased length of hospitalizations [12]. PAST using penicilloyl-polylysine (PRE-PEN) and penicillin G combined with an amoxicillin challenge is the standard of care in most US centers and effectively rules out immediate-type hypersensitivity to penicillin with a negative predictive value of 99% [13]. Multiple studies demonstrate the efficacy of PAST in clearing misidentified penicillin allergies in hospitalized patients and promoting optimal antibiotic therapy [14–17].
Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America recommend PAST as part of antimicrobial stewardship protocols [18]. This has led to a number of novel initiatives aimed at increasing the availability of this tool in the hospital setting. Some institutions have reported the use of reflexive consultation and testing by allergy or infectious diseases services for penicillin-allergic inpatients [19–21]. Others enlist pharmacist collaboration with allergy and/or infectious diseases specialists to screen potential patients and integrate the testing process within the admission. Pharmacists performing PAST under allergist-directed protocols is a proven method of increasing the ranks of trained personnel needed to address the penicillin allergy epidemic [22–24]. Finally, clinical decision support (CDS) guidelines integrated in electronic medical records (EMRs) represent a sophisticated advancement in health information technology encompassing alerts and care reminders for staff, condition-specific order sets, and the incorporation of evidence-based guidelines that allow the user to efficiently make informed decisions. Such hospital-wide guidelines, when applied to penicillin allergy, enable primary care providers to determine when and how to use penicillin or other β-lactams before considering a full skin test. In recent studies, CDS increased β-lactam use while concurrently lowering that of alternative agents, with the potential benefit of preserving PAST resources for patients with stronger clinical indications [25, 26].
The authors of this study previously published a model framework for proactive PAST at Parkland Hospital, an 870-bed public hospital serving Dallas County, Texas, academically affiliated with the University of Texas Southwestern Medical Center. This protocol utilizes an allergy-trained pharmacist assisted by a computer-driven protocol to identify, prioritize, and test admitted patients labeled as penicillin-allergic [24]. A census of these patients is generated daily in the EMR and processed through a series of filters. Eligibility for testing includes the absence of antihistamine use and discharge orders, active antibiotic therapy, and the presence of an immune-compromising condition predisposing to future infection such as HIV, diabetes mellitus, or malignancy. This service was also publicized through hospital-wide and department events to function as a traditional consultant service seeing patients by physician request. The pharmacist is certified in the administration of penicillin skin testing, oral challenges, and the treatment of allergic reactions while functioning as an extender of the allergist in the hospital with telephone support by the allergy service in case specific questions arise. This approach affords the benefits of PAST to a group of patients likely to acutely benefit from de-labeling of their allergy. To date, the service has screened more than 1500 charts and tested more than 400 patients, with a per-protocol clearance rate of 97%, a significant reduction in non-β-lactam use, and no serious adverse events. It is now a standard component in Parkland’s antibiotic stewardship program.
There remains a need to further minimize exposure to alternative agents and demonstrate a cost benefit to PAST in the acute care setting. The monobactam aztreonam has no cross-reactivity with penicillin and is therefore prescribed for penicillin-allergic patients at our institution. However, overall resistance against aztreonam is higher in comparison with piperacillin-tazobactam for common gram-negative organisms, making this a less than ideal agent for empiric treatment [27, 28]. In addition, aztreonam is one of the costliest antimicrobials, with an average wholesale price (AWP) of $39.54 and $80.34 USD for 1- and 2-gram doses, respectively, which is amplified by its standard 3-times-daily dosing. Piperacillin-tazobactam costs approximately $11.34 for 3.375 grams, and ampicillin-sulbactam costs $7.50 for 3 grams. Many common cephalosporins, which some clinicians still avoid in penicillin-allergic individuals, are also substantially cheaper (Supplementary Table 1). As most individuals reporting an allergy to penicillin in fact tolerate it, the majority of aztreonam use is expensive and avoidable. Prior pharmacist-led studies targeting aztreonam have reviewed allergy histories and directed providers’ therapeutic decisions without resolving the allergy label in the medical record [29, 30]. We sought to implement an institutional CDS guideline facilitating penicillin allergy testing in aztreonam recipients to assess its impact on antibiotic stewardship and cost.
METHODS
This was a quasi-experimental study evaluating admitted patients at Parkland carrying a penicillin allergy diagnosis. Our intervention linked all aztreonam orders with the pharmacist PAST consultation, guiding providers to obtain testing if penicillin allergy contributed to their antibiotic choice (AS-CDS). This CDS guideline was visible whether aztreonam was ordered independently or through an order set and was active by default, though providers could opt out at their discretion before signing. Being an academic, tertiary care institution, Parkland’s standard of care already restricts certain broad-spectrum antibiotics including aztreonam, meropenem, and daptomycin through a pharmacy or infectious diseases verification and integrates routine screening for penicillin allergy, as previously discussed. The comparator group included penicillin-allergic patients undergoing active screening (AS-only) by the service pharmacist per the published institutional protocol without accessory coupling of aztreonam to a pharmacist PAST consultation. Consultations were reviewed on weekdays during daytime hours by the pharmacist, who then preferentially approached these patients for testing, excluding those receiving antihistamines or actively being discharged. The required duration between receipt of last histamine antagonist and intended PAST is shown in Supplementary Table 2 and is based on approximately 4 elimination half-lives. The inclusion criteria were a documented penicillin allergy and an active order for aztreonam. Patients with histories of severe cutaneous adverse reactions, recent anaphylaxis within 4 weeks, and severe cardiac or pulmonary comorbidities were also excluded.
The AS-only group underwent evaluation from January 2015 to March 2016. The AS-CDS intervention ran from April to December 2016. For both groups, testing was performed by the service pharmacist at the patient’s bedside on medical/surgical floors and intensive care units. All patients had skin testing to the major determinant PRE-PEN (ALK, Round Rock, TX) used as per package insert, minor determinant penicillin G potassium at a concentration of 10 000 units/mL diluted in normal saline, histamine 6 mg/mL positive control, and a saline negative control (Hollister-Stier, Spokane, WA). Patients with negative skin tests were challenged to amoxicillin 500 mg orally with 60 minutes’ observation by the testing pharmacist. Intramuscular epinephrine and diphenhydramine were always carried in case of reaction. The on-call allergy fellow was available via telephone for each case if necessary. If no reaction occurred after the challenge, the negative test was documented, the allergy was deleted from the chart, and the primary physician was informed of the result. The penicillin allergy evaluation process took approximately 2 hours per patient from start to finish.
Before beginning the study, approval was obtained from the Institutional Review Board at Parkland and UT Southwestern to screen hospitalized patients carrying a penicillin allergy diagnosis. The Pharmacy and Therapeutics Committee at Parkland also approved pharmacist-administered testing. Our institution uses Hyperspace (Epic Systems Corporation, Verona, WI) as its EMR. All patient data were collected from the medical record by the service pharmacists (S.A.T. and K.S.A.) and hosted on secure workstations at Parkland. Collected patient data included age, sex, race, admission date, discharge date, primary discharge diagnosis, primary inpatient service, allergy history, and medication administration records. The dates of consultation, testing, and the patient’s physical location at the time of each were also noted. Cost-savings were calculated from average wholesale price and medication administration record reviews of inpatient days on antibiotic therapy. Primary outcomes were the number of patients tested, the proportion of inpatients on aztreonam receiving a PAST consult, time from admission to testing completion, and whether the consultation occurred in the emergency department or an inpatient unit. Secondary outcomes were the differences in antibiotic administration during the admission, the antibiotic costs per patient, and hospital-wide rates of aztreonam use following the addition of CDS. Beta-lactam antibiotics were categorized into penicillins, cephalosporins, and carbapenems, with penicillins including those combined with beta-lactamase inhibitors. Statistical differences in times to test and aztreonam administration were analyzed using IBM SPSS 19 and STATA 14.1; t tests and Wilcoxon rank-sum tests were used, respectively, for normally and non–normally distributed groups.
RESULTS
There were 250 unique hospitalized patients with active aztreonam orders during the AS-only period and 91 during the AS-CDS period. After initiating CDS guidance, a significantly higher percentage of penicillin-allergic aztreonam recipients received PAST consultations compared with the AS-only group (77/91 [85%] vs 59/250 [24%]; P < .001) (Table 1). Linking the test to the aztreonam order led to earlier awareness of eligible candidates by the testing team, with 58 of 77 (75%) consultations placed concurrently with initial antibiotic orders in the emergency department. In the AS-only group, consultations were not initiated until the patient was already admitted to an inpatient service, typically accumulating at least 1 dose of aztreonam in that time.
Table 1.
Characteristics of Penicillin-Allergic Aztreonam Recipients
| AS-Only | AS-CDS | |
|---|---|---|
| Patients on aztreonam | 250 | 91 |
| Patients on aztreonam receiving PAST Consultation (%)* | 59 (24) | 77 (85) |
| PAST consult placed in emergency department, n (%)* | 0 (0) | 58 (75) |
| Patients completing PAST (%)* | 22 (9) | 21 (27) |
| Patients with negative PAST (%) | 22 (100) | 21(100) |
Abbreviations: AS-CDS, active screening with clinical decision support guideline; AS-only, active screening by pharmacist per hospital protocol; PAST, penicillin allergy skin testing.
* P < .05.
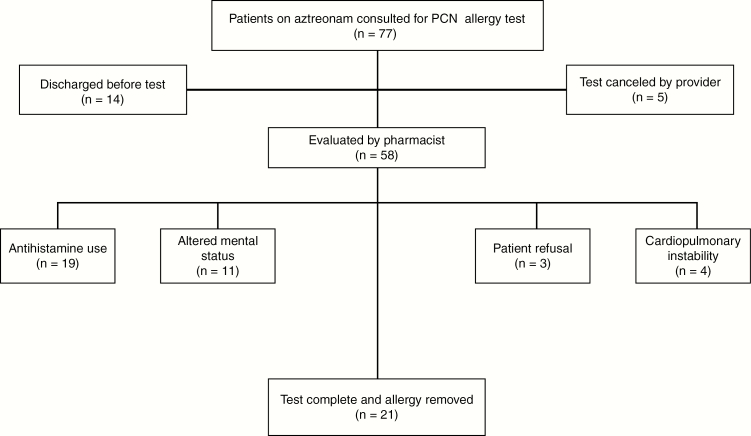
Not all potential patients were tested, for reasons including antihistamine use, discharge before evaluation, altered mental status, cardiopulmonary instability, and patient refusal. Under the AS-only protocol, 32 patients successfully completed the PAST procedure and an oral challenge to amoxicillin. There were no positive skin tests or challenges. Twenty-one patients on aztreonam were tested via the guideline order set in the AS-CDS period. Again, all could tolerate a penicillin challenge and had their allergy removed (Figure 1). Among tested patients, the demographics were similar in average age and sex, with a higher proportion of Hispanic patients in the AS-only group and a higher proportion of black patients in the AS-CDS group. The most commonly self-reported reaction to penicillin in both groups was urticaria and/or angioedema. Approximately 60% of tested patients had an immune-compromising condition. The AS-CDS period saw more expedient identification and evaluation of eligible patients, reducing the median time from admission to test completion (IQR) to 1.05 (0.90–3.43) days, compared with 3.31 (1.68–6.32) days for those tested in the AS-only group (P = .008) (Table 2).
Figure 1.
Outcomes of patients screened by the aztreonam clinical guideline. Abbreviation: PCN: penicillin.
Table 2.
Characteristics of Aztreonam Recipients Completing Penicillin Allergy Skin Testing
| AS-Only (n = 22) | AS-CDS (n = 21) | |
|---|---|---|
| Patient age, y | 54.4 | 59.3 |
| Female sex, n (%) | 14 (63) | 14 (67) |
| Race, n (%) | ||
| White | 5 (23) | 7 (33) |
| Black | 11 (50) | 14 (67) |
| Hispanic | 6 (27) | 0 (0) |
| Allergy history, n (%)a | ||
| Nonurticarial rash | 2 (9) | 6 (28) |
| Urticaria/angioedema | 7 (32) | 6 (28) |
| Respiratory | 7 (32) | 2 (9) |
| Gastrointestinal | 2 (9) | 0 (0) |
| Cardiovascular | 1 (5) | 3 (14) |
| Unknown | 3 (14) | 5 (24) |
| Primary infection by system, n (%) | ||
| Pulmonary | 7 (32) | 13 (62) |
| Urinary | 8 (36) | 2 (9) |
| Musculoskeletal/skin | 4 (18) | 2 (9) |
| Gastrointestinal | 2 (9) | 0 (0) |
| Other | 1 (5) | 4 (19) |
| Immune compromised, n (%)b | 13 (59) | 13 (62) |
| Median days from admission to test (IQR)* | 3.31 (1.68–6.32) | 1.05 (0.9–3.43) |
Abbreviations: AS-only, active screening by pharmacist per hospital protocol; AS-CDS, active screening with clinical decision support guideline; IQR, interquartile range.
aMore than 1 reaction type may be reported. Reactions involving the respiratory, gastrointestinal, or cardiovascular systems are defined as anaphylactic.
bConcurrent diagnosis of HIV, diabetes mellitus, or malignancy.
* P < .05.
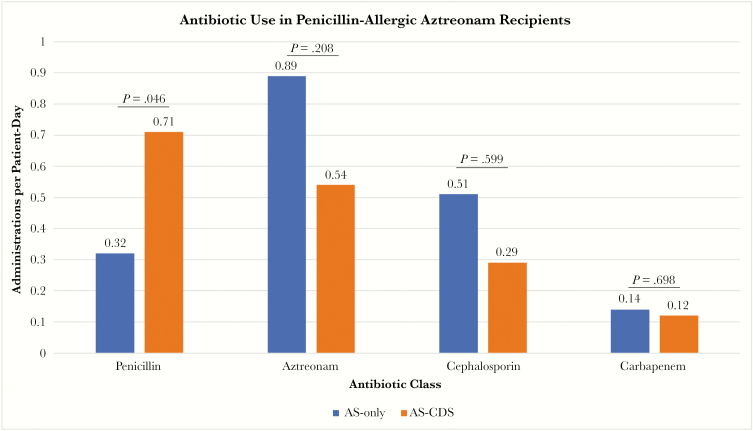
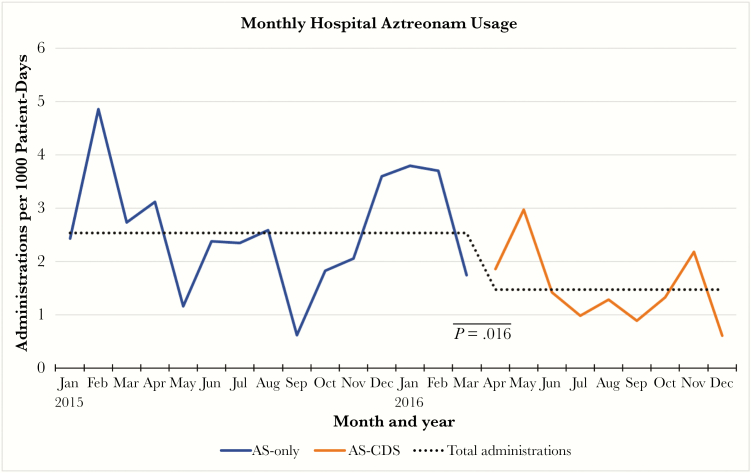
CDS-driven identification of eligible patients was associated with improvements in antibiotic stewardship over the hospital course. The use of penicillins among these “allergic” patients increased from 0.32 to 0.71 (P = .046) administrations per patient-day, whereas aztreonam use decreased from 0.89 to 0.54 (P = .2085) administrations per patient-day. CDS was also associated with declines in cephalosporin (0.51 to 0.29 administrations per patient-day; P = .599) and carbapenem use (0.14 to 0.12 administrations per patient-day; P = .698), though these did not reach significance (Figure 2). The patients tested via the CDS guideline went on to accumulate 121 doses of penicillins and 21 doses of cephalosporins in lieu of aztreonam. Average antibiotic costs among aztreonam recipients decreased from $1265.81 to $592.08 USD per patient following CDS implementation. The cost of per-protocol testing was $220 per patient, with $113 from supplies and $107 for 2 hours of pharmacist labor. When evaluating the overall inpatient population, a statistically significant decline in the monthly aztreonam usage rate was seen following CDS (2.54 to 1.47 administrations per 1000 patient-days; P = .016) (Figure 3).
Figure 2.
Antibiotic usage rates among aztreonam recipients before and after clinical decision support. Abbreviations: AS, active screening; CDS, clinical decision support.
Figure 3.
Aztreonam administration rates by month and throughout the active screening and AS-CDS periods. Abbreviations: AS, active screening; CDS, clinical decision support.
DISCUSSION
Inpatient PAST has proven useful in reducing superfluous broad-spectrum antibiotic use, but widespread implementation is hindered by a relative lack of testing capacity and the upfront cost of testing. Given the prevalence of penicillin allergy in the US population, there are simply inadequate numbers of allergy specialists to perform these evaluations [31]. Tackling the penicillin allergy epidemic requires cooperation with ancillary health care providers and greater utilization of technology in the hospital environment. Our initiative synergizes physician–pharmacist collaboration and CDS guidelines for prescribers, and we expect both innovations to play a major role in future efforts.
Implementing a CDS guideline for aztreonam clearly led to more penicillin use in eligible patients, preserving a valuable agent for infections in which it is truly indicated. Although per-patient decreases in aztreonam, cephalosporin, and carbapenem exposure did not reach statistical significance, a modest but significant reduction in aztreonam use occurred hospital-wide after its introduction despite the study group being a small minority of the total inpatient census. Even though aggressive stewardship measures including regular penicillin allergy testing were already in place, a convenient EMR-based testing tool expedited elimination of false penicillin allergy even beyond the standard of care. It is reasonable to expect a greater relative reduction in aztreonam use if this intervention were introduced at a hospital without existing programs for reducing broad-spectrum antibiotic use.
This study differs from previous pharmacist-led efforts to lower aztreonam use by disproving the penicillin allergy and removing it from the medical record [28, 29]. As aztreonam is expensive and often reserved for penicillin-allergic individuals, resolving the allergy in this specific inpatient population has great potential for immediate cost reductions, along with long-term savings obtained through future avoidance of this medication. The initial monetary investment is comparable to published values in the ambulatory setting, and considering the price difference between aztreonam and most comparable β-lactams, avoidance of even 1 inpatient day on the former justifies the cost of testing [32]. Prior studies suggest a near-term financial benefit from targeting other high-cost penicillin alternatives such as linezolid and daptomycin, so similar guidelines might also aid patients receiving these agents [21]. Recently published long-term follow-up of patients completing PAST at other institutions indicates continued reduction in broad-spectrum antibiotic exposure across outpatient and inpatient health care encounters [33]. Therefore, this program should benefit patients and the public at large well into the future.
This study had several limitations. The most significant limitation was the inability to coordinate testing for all potential patients receiving aztreonam during their stay. The most common reason for ineligibility among consulted patients was concurrent antihistamine use, and this is likely expected to occur across institutions. Although instructions to withhold antihistamines could be integrated into the guideline, inpatient providers may be unfamiliar with all histamine-antagonizing agents such as psychotropics and antiemetics. This reinforces the advantage of involving pharmacists, as they are familiar with reviewing medications in detail to identify the optimal testing candidates. The second most frequent reason for losing patients was discharge before evaluation by the testing service. As our service employed a single tester during weekdays, we subsequently trained additional personnel in the event that the primary tester is unavailable. Another issue was refusal of testing either by the patient or the primary service. It was not possible to discern individual reasons for declining the test, but this reiterates the need for continuing education among allergy, infectious diseases, and other inpatient specialists to present a unified message to patients on the importance of penicillin allergy testing. During the AS-CDS period, we skin-tested 27% of eligible patients, an improvement over our previous study, in which patients were manually screened by the study team [24].
In summary, this study combines EMR-based clinical guidelines and active removal of penicillin allergy to provide therapeutic and financial benefits to both hospitals and patients. By directing resources to patients at greater risk of accumulating disproportionate antibiotic costs, facilities with limited allergy testing capacity can benefit economically from penicillin allergy de-labeling. Alternatively, existing stewardship measures including active inpatient PAST can be supplemented by targeting aztreonam or similarly high-cost β-lactam alternatives. Whether used as an adjunct or standalone effort, we present a cost-effective model by which varying institutions may combat inappropriate antibiotic use.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank L. Steven Brown of the Department of Health Systems Research at Parkland Hospital for assistance with statistical analysis. The efforts of the many staff in the Department of Pharmacy Services and Infectious Diseases Division in making this intervention possible are acknowledged.
Financial support. S. A. Tarver is supported by the Texas 1115 Medicaid Transformation Waiver.
Potential conflicts of interest. Conflicts of interest: J. R. Chen has received travel support from the American Academy of Allergy, Asthma, and Immunology and is a member of the speakers’ bureau for ALK-Abello. S. A. Tarver and K. S. Alvarez have received a Texas 1115 Medicaid Transformation Waiver from the Center for Medicaid and CHIP Services. W. Wei declares no relevant conflicts of interest. D. A. Khan is on the Aimmune Data Safety Monitoring Committee and has received speakers’ fees from Genentech.
References
- 1. Lee CE, Zembower TR, Fotis MA, et al. . The incidence of antimicrobial allergies in hospitalized patients: implications regarding prescribing patterns and emerging bacterial resistance. Arch Intern Med 2000; 160:2819–22. [DOI] [PubMed] [Google Scholar]
- 2. Picard M, Bégin P, Bouchard H, et al. . Treatment of patients with a history of penicillin allergy in a large tertiary-care academic hospital. J Allergy Clin Immunol Pract 2013; 1:252–7. [DOI] [PubMed] [Google Scholar]
- 3. MacFadden DR, LaDelfa A, Leen J, et al. . Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis 2016; 63:904–10. [DOI] [PubMed] [Google Scholar]
- 4. Solensky R, Khan DA. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105:259–73. [DOI] [PubMed] [Google Scholar]
- 5. del Real GA, Rose ME, Ramirez-Atamoros MT, et al. . Penicillin skin testing in patients with a history of beta-lactam allergy. Ann Allergy Asthma Immunol 2007; 98:355–9. [DOI] [PubMed] [Google Scholar]
- 6. Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc 2014; 35:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caubet JC, Kaiser L, Lemaître B, et al. . The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol 2011; 127:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blanca M, Torres MJ, García JJ, et al. . Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol 1999; 103:918–24. [DOI] [PubMed] [Google Scholar]
- 9. Sullivan TJ, Wedner HJ, Shatz GS, et al. . Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981; 68:171–80. [DOI] [PubMed] [Google Scholar]
- 10. Jeffres MN, Narayanan PP, Shuster JE, Schramm GE. Consequences of avoiding β-lactams in patients with β-lactam allergies. J Allergy Clin Immunol 2016; 137:1148–53. [DOI] [PubMed] [Google Scholar]
- 11. Blumenthal KG, Shenoy ES, Huang M, et al. . The impact of reporting a prior penicillin allergy on the treatment of methicillin-sensitive Staphylococcus aureus bacteremia. PLoS One 2016; 11:e0159406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol 2014; 133:790–6. [DOI] [PubMed] [Google Scholar]
- 13. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract 2013; 1:258–63. [DOI] [PubMed] [Google Scholar]
- 14. Nadarajah K, Green GR, Naglak M. Clinical outcomes of penicillin skin testing. Ann Allergy Asthma Immunol 2005; 95:541–5. [DOI] [PubMed] [Google Scholar]
- 15. Arroliga ME, Wagner W, Bobek MB, et al. . A pilot study of penicillin skin testing in patients with a history of penicillin allergy admitted to a medical ICU. Chest 2000; 118:1106–8. [DOI] [PubMed] [Google Scholar]
- 16. Arroliga ME, Radojicic C, Gordon SM, et al. . A prospective observational study of the effect of penicillin skin testing on antibiotic use in the intensive care unit. Infect Control Hosp Epidemiol 2003; 24:347–50. [DOI] [PubMed] [Google Scholar]
- 17. Chen JR, Khan DA. Evaluation of penicillin allergy in the hospitalized patient: opportunities for antimicrobial stewardship. Curr Allergy Asthma Rep 2017; 17:40. [DOI] [PubMed] [Google Scholar]
- 18. Barlam TF, Cosgrove SE, Abbo LM, et al. . Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Banks TA, Ressner RA, Gada SM. Antibiotic reclamation: penicillin allergy, antibiotic stewardship, and the allergist. Ann Allergy Asthma Immunol 2015; 115:451–2. [DOI] [PubMed] [Google Scholar]
- 20. Heil EL, Bork JT, Schmalzle SA, et al. . Implementation of an infectious disease fellow-managed penicillin allergy skin testing service. Open Forum Infect Dis 2016; 3:ofw155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rimawi RH, Cook PP, Gooch M, et al. . The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med 2013; 8:341–5. [DOI] [PubMed] [Google Scholar]
- 22. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with β-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol 2016; 117:67–71. [DOI] [PubMed] [Google Scholar]
- 23. Wall GC, Peters L, Leaders CB, Wille JA. Pharmacist-managed service providing penicillin allergy skin tests. Am J Health Syst Pharm 2004; 61:1271–5. [DOI] [PubMed] [Google Scholar]
- 24. Chen JR, Tarver SA, Alvarez KS, et al. . A proactive approach to penicillin allergy testing in hospitalized patients. J Allergy Clin Immunol Pract 2017; 5:686–93. [DOI] [PubMed] [Google Scholar]
- 25. Blumenthal KG, Shenoy ES, Varughese CA, et al. . Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol 2015; 115:294–300.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blumenthal KG, Wickner PG, Hurwitz S, et al. . Tackling inpatient penicillin allergies: assessing tools for antimicrobial stewardship. J Allergy Clin Immunol 2017; 140:154–161.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Obritsch MD, Fish DN, MacLaren R, Jung R. National surveillance of antimicrobial resistance in Pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrob Agents Chemother 2004; 48:4606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009–2011). Diagn Microbiol Infect Dis 2014; 78:443–8. [DOI] [PubMed] [Google Scholar]
- 29. Swearingen SM, White C, Weidert S, et al. . A multidimensional antimicrobial stewardship intervention targeting aztreonam use in patients with a reported penicillin allergy. Int J Clin Pharm 2016; 38:213–7. [DOI] [PubMed] [Google Scholar]
- 30. Staicu ML, Brundige ML, Ramsey A, et al. . Implementation of a penicillin allergy screening tool to optimize aztreonam use. Am J Health Syst Pharm 2016; 73:298–306. [DOI] [PubMed] [Google Scholar]
- 31. Gerace KS, Karlin E, McKinnon E, Phillips E. Varying penicillin allergy testing practices in the United States: a time for consensus. J Allergy Clin Immunol Pract 2015; 3:791–3. [DOI] [PubMed] [Google Scholar]
- 32. Blumenthal KG, Li Y, Banerji A, et al. . The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract 2018; 6:1019–1027.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Macy E, Shu YH. The effect of penicillin allergy testing on future health care utilization: a matched cohort study. J Allergy Clin Immunol Pract 2017; 5:705–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.