Abstract
Small cell carcinoma (SCC) of the larynx is a rare type of neuroendocrine carcinoma (NEC), few cases of which have been described in the literature. The prognosis for this type of carcinoma is poor, with a survival time typically not exceeding two years. We describe the case of a 54-year-old male patient with a primary tumor in the right ventricular band and a biopsy compatible with SCC. The patient underwent radiotherapy (RT) and concomitant chemotherapy (QT) and, after a relapse at 17 months, underwent total laryngectomy with bilateral neck dissection. The survival time was 47 months. Further studies are required to elucidate the possible causes of the better prognosis in some cases.
Keywords: Small cell, Neuroendocrine carcinoma, Larynx, Survival
Highlights
-
•
The incidence of SCC of the larynx has increased in recent decades, although it still remains rare.
-
•
The survival rates are 16% for two years and 5% for five years.
-
•
We describe the case of a 54-year-old male who is still alive, a survival time of more than 44 months.
1. Introduction
NEC arise in neuroendocrine cells and may be either neural or epithelial in origin. The WHO has characterized neuroendocrine tumor of the larynx into four types: typical carcinoid tumor, atypical carcinoid tumor, small-cell neuroendocrine tumor, and paraganglioma. SCC is defined as tumor cells with small size (generally less than the diameter of 3 small resting lymphocytes), scant cytoplasm, high cell proliferation (Ki-67), and frequent necrosis [[1], [2], [3], [4]].
Certain bibliographies may refer to another group of epithelial carcinomas, known as combined SCC, associated with squamous carcinoma or adenocarcinoma, only 14 cases of which have been described in the literature [5].
The first case of NEC was published by Goldman et al., in 1969 [6] and was an AC. The first authors to describe a case of small cell carcinoma of the larynx (SCCL) were Olofsson and Van Nostram in 1972, the second being described by Ferlito in 1974 [7,8].
NEC are rare, the atypical type being more frequent and the carcinoid type being less frequent. Five hundred cases of primary NEC of the larynx have been reported in the literature [9,10], of which 160 cases were classified as SCC [11,12].
The commonest site for NEC is the gastrointestinal tract (70%), followed by the respiratory tract (25%). They occur rarely in the skin, paranasal sinuses, nasal cavity, genitourinary tract, salivary glands or larynx [2,7]. NEC account for less than 1% of neoplasms arising in the larynx [7]. The prognosis is related with the level of differentiation, being worse for the least differentiated types [1,2].
The treatment of choice for C or AC is surgery, whereas RT and QT seem to be the treatment of choice for SCCL. Surgery offers little hope and is reserved for cases of relapse after RT and QT. SSCL has the worst prognosis, the 5-year survival rate is 48% and 16% for two years and 5% for five years [7,9].
The incidence of SCC has increased in recent decades, although it still remains rare. In 1972 just two cases were reported [7,8]. In 1983, increased to 43 [13] and in 1985 the number of cases rose to 58 14. Prevalence increased thereafter, with 160 cases being described in 2004 [11] and 183 in 2015 [15]. Also in 2015, the University of Wisconsin published a case study of 298 patients with SCCL identified from the US National Cancer Database [16]. In the past two years, the incidence of small cell carcinoma of the larynx has doubled. The reasons for the increase in prevalence over the past 45 years, and particularly in the past two years, require further study.
2. Presentation of case
We present the case of a male patient, aged 54 years, smoker of one packet a day. He presented with dysphonia as his only symptom in November 2013. Fibroscopy revealed paralysis of the right vocal fold and an edematous lesion of the right ventricular band. No cervical lymphadenopathies were present in the physical examination.
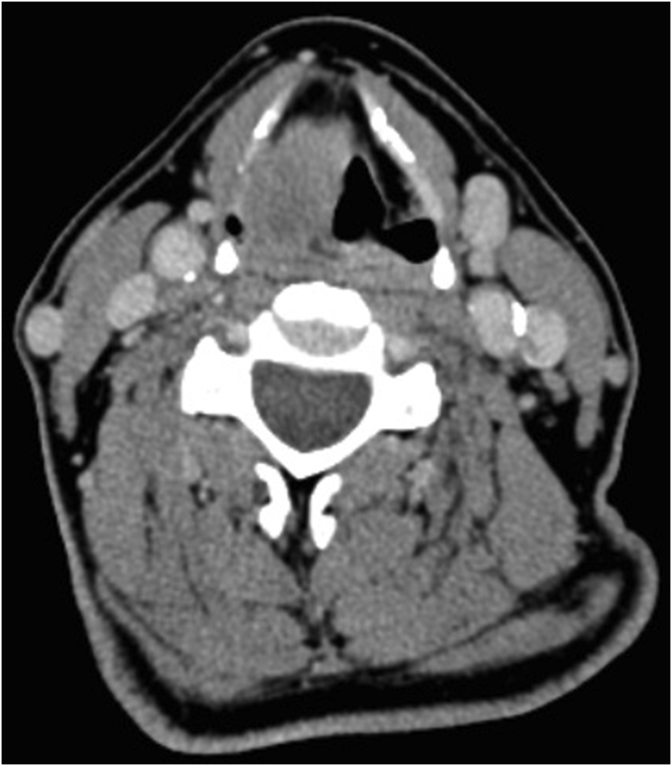
A cervical computed tomography (CT) scan revealed a mass of 32 × 18 × 29 mm in the right ventricular band, vocal fold and parapharyngeal space, as well as obliteration of the retropharyngeal fat plane. No cervical lymphadenopathies were observed. (Fig. 1).
Fig. 1.
Cervical CT. Tumor in the right hemilarynx, without cervical adenopathy.
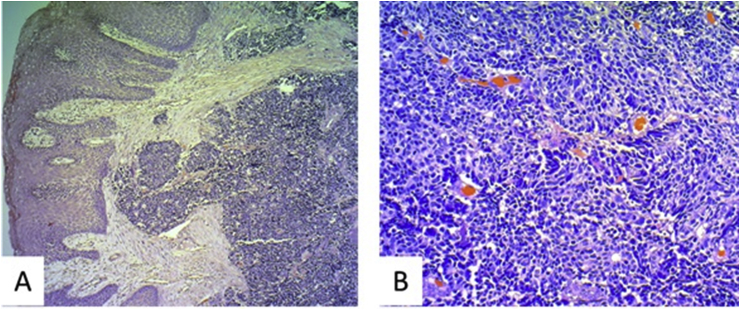
Biopsy revealed a squamous layer with a proliferation of small cells in the subepithelial tissue, with a predominantly solid pattern organized into trabeculae. The neoplastic cells had low cytoplasm levels and shaped nuclei with marginated, salt-and-pepper chromatin, with no evident nucleolus. Necrosis, apoptotic bodies and abundant mitosis were also observed (Fig. 2). SCC was diagnosed.
Fig. 2.
Right arytenoid cartilage, coated in squamous epithelium with pseudoepitheliomatous hyperplasia and stroma infiltrated by a neoplastic mass. (A). Haematoxylin and eosin (H&E) stain (5×) showing small cells, solid pattern (B). H&E stain (20×).
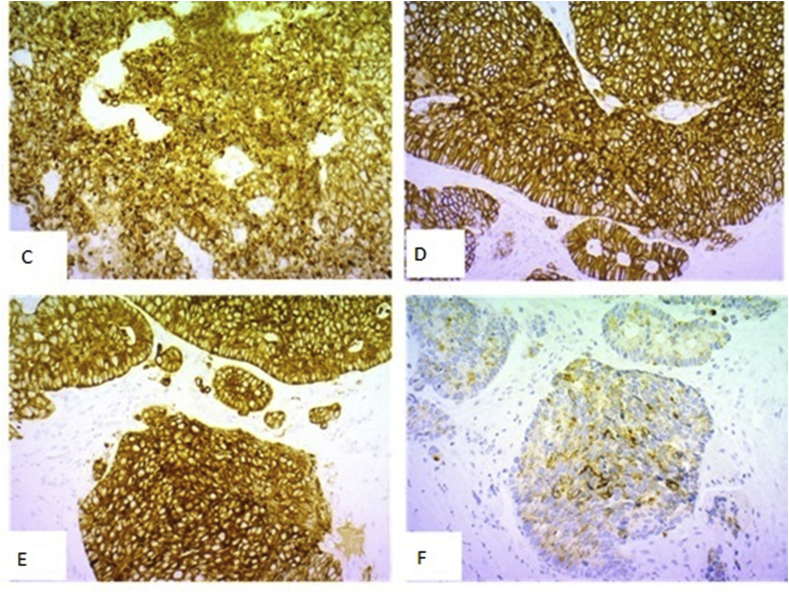
Immunohistochemistry of SSC requires neuroendocrine markers positive (CD56, synaptophysin and chromogranin A), epithelial cell markers positive; negative for p53 and p63 (squamous cell carcinoma is positive), and positive Ki-67 proliferation in 75% of tumorous cells (Fig. 3).
Fig. 3.
Immunoreactivity of microcystic neoplasia for CKAE1/AE3 (D1), CD 56 (D2), synaptophysin (D3) and chromogranin A (D4).
PET/CT ruled out metastasis (cT3N0M0). After assessment by the Head and Neck Tumors Committee of our Health Area, RT plus concomitant QT was agreed. In 2014, prophylactic percutaneous endoscopic gastrostomy was inserted (as per the current protocols of our Medical Oncology Unit) [16], completing RT up to a total dose of 54 Gy on bilateral cervicals II-III-VI and 66 Gy on the larynx. The patient underwent four cycles of concomitant cisplatin plus etoposide, between 20 January 2014 and 24 March 2014. Re-assessment with PET/CT and fibroscopy (no paralysis of the right vocal fold and no edematous lesion of the right ventricular band) showed a complete clinical response.
Our Head and Neck Cancer Unit, using multidisciplinary otorhinolaryngology and oncology testing, maintaining complete remission according to fibroscopy and CT, evaluated the patient every month for 11 months.
In December 2014, a chest CT scan was ordered, showing a nodule in the left lower lobe (LLL). Core needle biopsy was compatible with SCC, although fibrosopy remained clear of relapse of the larynx and a PET/CT scan showed no metastasis. RT (55 Gy) was completed on LLL by mid-January 2015.
Fibroscopy examination in April 2015 showed paralysis of the right hemilarynx with edema of the right aryepiglottic fold and arytenoid. Cervical CT and biopsies were compatible with SCCL, and a PET/CT scan suggested a small lesion (solitary metastasis) in the right upper lobe (RUL). After assessment by the Head and Neck Tumors Committee, in June 2015 a total laryngectomy was performed with bilateral neck dissection, pectoralis major flap reconstruction and insertion of a voice prosthesis. RT was applied to the new pulmonary lesion, followed by four cycles of carboplatin plus etoposide between August and November 2015.
After a neoplasia-free interval of four months, a PET/CT scan showed lesions compatible with malignancy at the base of the tongue, right cervical lymph node, right paratracheal stripe and both lung roots. Fine needle aspiration confirmed SCC metastasis.
This relapse was not considered to be treatable by radical means, so in May 2016 weekly palliative treatment was begun with gemcitabine/irinotecan, temozolomide, carboplatin/etoposide and praclitaxel, the final cycle being administered on June 1 2017.
The patient died in November 2017. Loco-regional disease was the cause of the death.
3. Discussion
We have seen that SCCL affects predominantly men [17,18] aged 50–70 years, with a high incidence of a positive history of cigarette smoking [8,17]. Accordingly, we need to study the causal relationship between sex, age and tobacco history with regard to the appearance of SCCL.
Most authors agree that, in spite of the range of treatments applied (surgery, RT, QT), the prognosis is bleak, even in the initial stages. A 1978 review suggested surgery plus RT as the treatment of choice [19]. In later reviews, the treatment offering the best survival rates was found to be RT concomitant with cisplatin and etoposide [7,9,14,15,[17], [18], [19]]. Some authors have used surgery of the larynx as a rescue therapy in cases of local relapse [7,14].
A 1978 bibliographical review conducted by Dr Ferlito described six patients with survival times of six months [19]. In his 1985 review, Dr Coakley described a two-year survival rate of 8.5% [20]. Ferlito et al. published a new review in 1998, showing a two-year survival rate of 16% and a five-year survival rate of 5% [9]. In 2003, Ferlito and Rinaldo published a new paper, maintaining the same survival rates as in 1998 [8]. Mikic et al. published a new paper in 2009, maintaining a two-year survival rate of 5% [7].
In 2015, Van der Laan et al. conducted an exhaustive review of 436 cases of NEC of the larynx, suggesting a five-year survival rate of 19,3% for SCCL [15].
In 2017 Pointer et al. performed a review of SCCL, Pointer et al. suggested a higher survival rate than those published previously, specifying the survival rate by disease stage. Thus, stage I/II patients had a mean survival time of 29.1 months, with a two-year survival rate of 65.3%. III/IVA/IVB patients had a mean survival time of 19.1 months, with a two years survival rate of 42.3%, while IVC patients had a mean survival time of 10.8 months, with a two-year survival rate of 14.2% [17].
The survival rate for SCCL has obviously improved since Van der Laan's review. Further studies would be necessary to correlate the improved survival rates with improvements in treatment, particularly RT, as the standard QT option is still cisplatin plus etoposide.
We did find references to longer survival times, as in a case reported by Dr Solé et al., in 1992, with a survival time of 53 months [14].
Gidding et al. published an article in which 4 cases of SCCL had longer survival time (7 years), those patients were treated with chemotherapy and radiation therapy, but the sample reported (49 patients) was small [21].
Another study by Zhu et al., in 2015 reported two patients surviving longer than 47 months, namely one who had a survival time of 129 months and another of 72 months [22].
A fourth study conducted in 2015 by Iqbal et al. reported a patient with a survival time of 99 months [23].
These eight patients with survival times of longer than 47 months had been treated with RT concomitant to cisplatin and etoposide, as had the patients described in other studies whose survival time had not been that long. Although chemotherapy plus radiotherapy seems to be the best treatment, it still remains controversial.
4. Conclusion
SCCL is a very rare tumor, the small number of cases of which makes its study difficult. Probably for this reason, the treatment for this disease has not changed over the past forty years. We encourage the medical community to report new cases of SCCL, conduct clinical trials and try therapeutic alternatives to improve survival time.
Consent of patient
Yes.
Ethical approval
Research ethics approval was provided by the National Research Ethics Service (12/LO/1985).
Sources of funding
Nil.
Author contribution
Study Design: Alberto Raposo.
Data Collection: Alfonso Marco.
Statistical Analysis Jerónimo Lajara.
Data Interpretation Maria J. Martínez-Ortíz, Francisco Garcia-Purriños.
Writing first draft of manuscript: Alberto Raposo, Maria E. García-Solano.
Critical review and approval of manuscript: All.
Conflicts of interest
No conflicts to declare.
Research registration number
UIN: researchregistry3194.
Guarantor
Alberto Raposo Jiménez.
Contributor Information
A. Raposo, Email: albertoraposo@hotmail.com.
A. Marco, Email: alfonsojmarco@gmail.com.
M.E. García-Solano, Email: garciasolano2002@yahoo.com.
M.J. Martínez-Ortiz, Email: mjmartinezortiz@hotmail.com.
F. García-Purriños, Email: fgpurrinhos@yahoo.es.
J. Lajara, Email: jlajara@ucam.edu.
References
- 1.D. Carten, J.K. Creenson, V.E. Reuter, M.H. Stoler, Diagnostic Surgical Pathology, Virginia, T.
- 2.Thompson L., Nelson B.L., Gannon F.H., Cassarino D., Wenig B.M., Muller S., Torske K.R., Gale N. Marban; Madrid: 2013. Diagnóstico y Patología. Cabeza y cuello; pp. 74–75 p.. [Google Scholar]
- 3.Thomassin J.M., Deveze A., Chrestian M.A. Elsevier; Paris: 2002. Système neuroendocrinien disséminé et pathologie cervicofaciale. Encyclopédie Médico-Chirurgicale. E-20-945-A-10 pp. [Google Scholar]
- 4.Xu B., Chetty R., Perez-Ordoñez B. Neuroendocrine neoplasms of the head and neck: some suggestions for the new WHO classification of head and neck tumors. Head Neck Pathol. 2014;8:24–32. doi: 10.1007/s12105-014-0531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ereño C., La nueva clasificación de la O.M.S. Lesiones precursoras y los tumores de la laringe, hipofaringe y tráquea. Rev. Esp. Patol. 2005;2007(40):3–10. [Google Scholar]
- 6.Goldman N.C., Hood C.I., Singleton G.T. Carcinoid of the larynx. Arch. Otolaryngol. 1969;90(1):64–67. doi: 10.1001/archotol.1969.00770030066013. [DOI] [PubMed] [Google Scholar]
- 7.Mikic A. Small cell neuroendocrine tumor of the larynx. Coll. Antropol. 2012;36(2):201–204. [PubMed] [Google Scholar]
- 8.Ferlito A., Rinaldo A. Small cell neuroendocrine carcinoma of the larynx: a preventable and frustrating disease with a highly aggressive lethal behavior. ORL J. Otorhinolaryngol. Relat. Spec. 2003;65(3):131–133. doi: 10.1159/000072249. [DOI] [PubMed] [Google Scholar]
- 9.Ferlito A., Barnes L., Rinaldo A., Gnepp D.R., Milroy C.M. A review of neuroendocrine neoplasms of the larynx: update on diagnosis and treatment. J. Laryngol. Otol. 1998;112(9):827–834. doi: 10.1017/s0022215100141830. [DOI] [PubMed] [Google Scholar]
- 10.LaBryer L., Sawh R., McLaurin C., Scofield Hal. Calcitonin-secreting neuroendocrine carcinoma of larynx with metastasis to thyroid. Case Rep. Endocrinol. 2015:1–6. doi: 10.1155/2015/606389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaiswal V.R., Hoang M.P. Primary combined squamous and small cell carcinoma of the larynx: a case report and review of the literature. Arch. Pathol. Lab Med. 2004;128(11):1279–1282. doi: 10.5858/2004-128-1279-PCSASC. [DOI] [PubMed] [Google Scholar]
- 12.Pytala M., Olejniczak I., Kupnickic P., Trenda-Lewyd I., Michaleckie L. The small cell carcinoma neuroendocrine type of the larynx. Case report. Polish Ann. Med. 2017;24(1):60–63. [Google Scholar]
- 13.Gnepp D.R., Ferlito A., Hyams V. Primary anaplastic small cell (oat cell) carcinoma of the larynx. Review of the literature and report of 18 cases. Cancer. 1983;51(9):1731–1745. doi: 10.1002/1097-0142(19830501)51:9<1731::aid-cncr2820510929>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Solé J., Jürgens A., Musulén E., Lacasta A., Guedea F., Quer M., Leon X., Lopez Pousa A., Lerma E. Small cell carcinoma of the larynx: results of therapy. Bull. Canc. Radiother. 1994;81(1):45–48. [PubMed] [Google Scholar]
- 15.Van der Laan T.P., Plaat B., Van der Laan B., Halmos G. Clinical recommendations on the treatment of neuroendocrine carcinoma of the larynx: a meta-analysis of 436 reported cases. Head Neck. 2015;37(5):707–715. doi: 10.1002/hed.23666. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Liu M., Liu Ch, Ye Y., Huang G. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for patients with head and neck cancer: a systematic review. J. Radiat. Res. 2014;55:559–567. doi: 10.1093/jrr/rrt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pointer K.B., Ko H.C., Brower J.V., Witek M.E., Kimple R.J., Lloyd R.V., Harari Paul M., Baschnagel A.M. Small cell carcinoma of the head and neck: an analysis of the National Cancer Database. Oral Oncol. 2017;69:92–98. doi: 10.1016/j.oraloncology.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma J.D., Baishya N., Rahman T., Krishnatreya M., Kataki ACh, Singh A. Primary small cell carcinoma of the larynx. Clin. Canc. Invest. J. 2014;3(6):542–544. [Google Scholar]
- 19.Ferlito A. Primary oat-cell carcinoma of the larynx following supraglottic laryngectomy for squamous-cell carcinoma. J. Am. Geriatr. Soc. 1978;26(6):278–283. doi: 10.1111/j.1532-5415.1978.tb02403.x. [DOI] [PubMed] [Google Scholar]
- 20.Coakley J.F. Primary oat cell carcinoma of the larynx. J. Laryngol. Otol. 1985;99(3):301–304. doi: 10.1017/s0022215100096730. [DOI] [PubMed] [Google Scholar]
- 21.Gidding N.A., Kennedy T.L., Vrabec D.P. Primary small cell carcinoma of the larynx: analysis of treatment. J. Otolaryngol. 1987;16(3):157–166. [PubMed] [Google Scholar]
- 22.Zhu Y., Gao L., Meng Y., Diao W., Zhu X., Li G., Gao Z., Chen X. Laryngeal neuroendocrine carcinomas: a retrospective study of 14 cases. BioMed Res. Int. [Internet] 2015 doi: 10.1155/2015/832194. https://www.hindawi.com/journals/bmri/2015/832194/ [Accepted 2 July 2015]; 4 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iqbal M.S., Paleri V., Moor J., Dobrowsky W., Kelly C., Kovarik J. Small cell neuroendocrine carcinoma of larynx: case series and literature review. J. Laryngol. Otol. 2015;129(9):910–915. doi: 10.1017/S0022215115001668. [DOI] [PubMed] [Google Scholar]