Abstract
To fertilize an egg, sperm must reside in the female reproductive tract to undergo several maturational changes that are collectively referred to as capacitation. From a molecular point of view, the HCO3−-dependent activation of the atypical soluble adenylyl cyclase (ADCY10) is one of the first events that occurs during capacitation and leads to the subsequent cAMP-dependent activation of protein kinase A (PKA). Capacitation is also accompanied by hyperpolarization of the sperm plasma membrane. We previously reported that PKA activation is necessary for CFTR (cystic fibrosis transmembrane conductance regulator channel) activity and for the modulation of membrane potential (Em). However, the main HCO3− transporters involved in the initial transport and the PKA-dependent Em changes are not well known nor characterized. Here, we analyzed how the activity of CFTR regulates Em during capacitation and examined its relationship with an electrogenic Na+/HCO3− cotransporter (NBC) and epithelial Na+ channels (ENaCs). We observed that inhibition of both CFTR and NBC decreased HCO3− influx, resulting in lower PKA activity, and that events downstream of the cAMP activation of PKA are essential for the regulation of Em. Addition of a permeable cAMP analog partially rescued the inhibitory effects caused by these inhibitors. HCO3− also produced a rapid membrane hyperpolarization mediated by ENaC channels, which contribute to the regulation of Em during capacitation. Altogether, we demonstrate for the first time, that NBC cotransporters and ENaC channels are essential in the CFTR-dependent activation of the cAMP/PKA signaling pathway and Em regulation during human sperm capacitation.
Keywords: sperm, bicarbonate, epithelial sodium channel (ENaC), fertilization, sodium transport, Capacitation, ENaC, human sperm, membrane potential, NBC
Introduction
Mammalian sperm are unable to fertilize an egg soon after they are ejaculated. Fertilization only becomes possible after the sperm have spent time in the female reproductive tract. During this time, sperm undergo several molecular and cellular changes in a process known as capacitation (1, 2). At the cellular level, capacitation prepares sperm to develop hyperactivated motility and to undergo acrosomal exocytosis (3, 4). At the molecular level, once sperm enter the seminal plasma and the female reproductive tract, they are exposed to a higher HCO3− concentration (∼15–25 mm) (5, 6), which results in activation of the atypical soluble adenylyl cyclase (ADCY10) that in turn leads to cAMP synthesis and activation of protein kinase A (PKA)3 (7). The activation of this signaling pathway is followed by downstream events such as hyperpolarization of the plasma membrane (8–10). In addition to stimulating ADCY10, the uptake of HCO3− itself triggers cytoplasmic alkalinization and membrane hyperpolarization in mouse sperm (11, 12). However, the mechanisms by which HCO3− is transported into the sperm are not well established. In mice, it is postulated that a Na+-dependent electrogenic HCO3− incorporation through members of the Na+/HCO3− cotransporter (NBC) is responsible for the initial anion entrance (11). It is also claimed that in mice the cystic fibrosis transmembrane conductance regulator channel (CFTR) works in association with other Cl−/HCO3− cotransporters to provide a sustained uptake of HCO3− (13, 14). On the other hand, the mechanism of HCO3− entrance in human sperm is completely unknown. Recent evidence from our group demonstrated that inhibition of CFTR affects HCO3− uptake resulting in low PKA activity and inhibition of cAMP/PKA-downstream events such as the increase in tyrosine phosphorylation (pTyr), hyperactivated motility, and acrosome reaction (9). It is well established in several systems that CFTR phosphorylation by PKA is required for its activation (15–17). For this reason, we postulated that an initial increase in PKA activity is necessary to activate CFTR channels and produce a sustained increase in HCO3− and cAMP. The identity of this initial HCO3− transport in human sperm that results in PKA-dependent activation of CFTR remains elusive.
Previously, we showed that CFTR and PKA activity are necessary for Em regulation during human sperm capacitation (9). The regulation of membrane potential (Em) is an important event for fertility (18, 19). Brown and co-workers (19) showed that depolarized sperm Em values correlate with a higher percentage of in vitro fertilization (IVF) failure. Previous evidence suggests the participation of both SLO1 and SLO3 channels in the hyperpolarization associated with capacitation in human sperm (20–23). Conversely, we observed that inhibition of CFTR results in Em depolarization that can be partially reversed by cAMP permeable analogs (9). It is reported in many cell types that CFTR regulates epithelial Na+ channels (ENaC) (24–27). In addition, it has been demonstrated that ENaC is involved in controlling Em in mouse sperm (28). Thus, we hypothesize that CFTR activity is necessary for ENaC inhibition, and therefore, for maintaining of lower Na+ permeability and regulation of Em during capacitation.
Our working hypothesis is that HCO3− is initially and rapidly incorporated in human sperm by NBC, leading to activation of PKA and CFTR during capacitation. Activation of CFTR is coupled to the inhibition of Na+ transport by ENaC, resulting in membrane hyperpolarization (27, 29, 30). Thus, our goal is to study the role of NBC and ENaC in the cAMP/PKA signaling pathway associated with capacitation and its participation in the regulation of Em in human sperm.
Results
NBC cotransporters are necessary for activating the cAMP/PKA pathway
We have previously demonstrated the role of CFTR in the uptake of HCO3− during capacitation (9). However, because CFTR requires phosphorylation by PKA to be active, we postulate that an initial HCO3− transport occurs in human sperm to stimulate ADCY10 and produce the cAMP-dependent activation of PKA. Previous studies in mice postulated that NBC cotransporters are responsible for the initial HCO3− entrance during capacitation (11). To test this hypothesis in human sperm, we used a specific and reversible NBC inhibitor, S0859 (31). To the best of our knowledge, this inhibitor has never been used in sperm. We first evaluated the effect of NBC inhibition in mouse sperm, where there is previous evidence of its function during capacitation. As shown in Fig. 1A, there was a concentration-dependent decrease in the levels of phosphorylation in PKA substrates (pPKA) and tyrosine residues (pTyr) with S0859. Similarly, human sperm incubated with an increasing concentration of S0859 also displayed lower levels of pPKA and pTyr (Fig. 1B). This effect was not due to an impairment in viability as shown in Fig. 1C.
Figure 1.
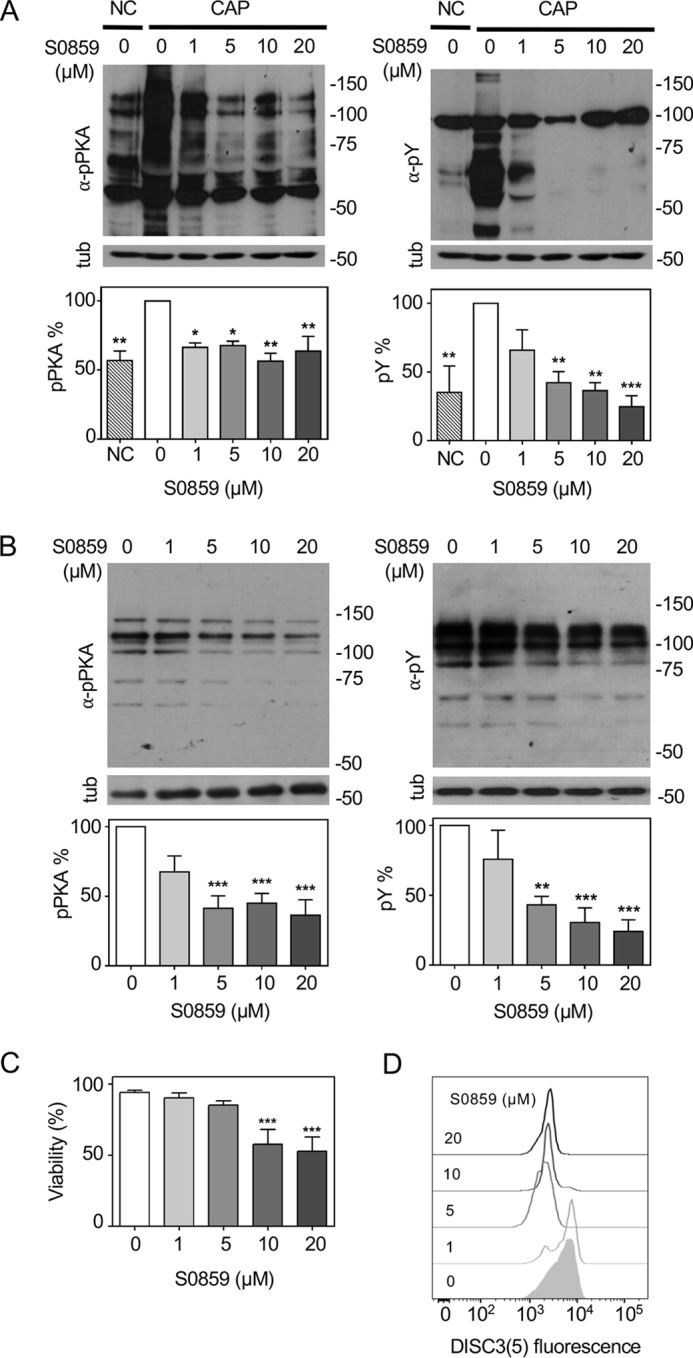
NBC is necessary for activation of the cAMP/PKA pathway and Em regulation during human sperm capacitation. A, mouse sperm were incubated in CAP for 90 min with different concentrations of the NBC inhibitor S0859. Sperm were also incubated under noncapacitating condition (NC); in the absence of HCO3− and BSA. Aliquots from each condition were processed for Western blotting with anti-pPKA (left panel) or anti-pTyr (right panel) antibodies and then membranes were reblotted with an anti-β-tubulin antibody for loading control (lower panel). Blots were quantified as described under “Experimental procedures” (bottom panel). Values represent the mean ± S.E. of at least 3 experiments. ***, p < 0.001; **, p < 0.01; *, p < 0.05. B, human sperm were incubated in capacitating medium with different concentrations of the NBC inhibitor S0859. Aliquots from each condition were processed for Western blotting with anti-pPKA (left panel) or anti-pTyr (right panel) antibodies and then membranes were reblotted with an anti-β-tubulin antibody for loading control (lower panel). Blots were quantified and values represent the mean ± S.E. of at least 3 experiments (bottom panel). ***, p < 0.001; **, p < 0.01; *, p < 0.05. C, human sperm were incubated with different concentrations of the NBC inhibitor S0859 and the percentage of live cells was assessed by Eosin-Y staining. ***, p < 0.001 (n = 4). D, histograms of percentage of the maximum (% max) versus DISC3(5) fluorescence of BCECF positive cells. Human sperm were incubated in medium that supports capacitation with different concentrations of the NBC inhibitor S0859. Subsequently, aliquots from each condition were processed by flow cytometry to evaluate Em with DISC3(5) and with BCECF-AM to estimate viability.
NBC is necessary for the regulation of Em during capacitation
To evaluate if inhibition of NBC affects the human sperm Em, sperm were incubated in medium that supports capacitation in the presence of increasing concentrations of S0859. As shown in Fig. 1D, a concentration above 5 μm S0859 caused the maximum depolarization effect. The concentration of 5 μm for S0859 was chosen for all the following experiments because the levels of pTyr and pPKA were significantly decreased, and there is a maximum effect in Em depolarization without affecting viability with respect to the control condition. These results suggest that NBC are involved in HCO3− uptake to trigger activation of the cAMP/PKA pathway and regulation on Em.
S0859 has no direct effect in SLO1 and SLO3 channels
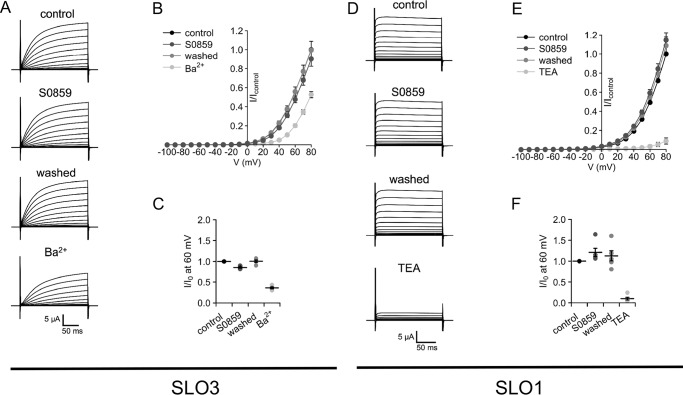
Previous reports indicate that human SLO3 and SLO1 K+ channels participate in the regulation of sperm Em (22, 32). Thus, we determined whether SLO3 and SLO1 channel activity could be nonspecifically altered by the NBC inhibitor S0859. We tested the effect of 5 μm S0859 on heterologously expressed human SLO3 and SLO1 channels. Our results showed that SLO3 and SLO1 currents expressed in Xenopus oocytes were not significantly inhibited by 5 μm S0859 (Fig. 2, A–F). As a control, trimethylammonium (TEA) and Ba2+ (two well-known inhibitors o SLO1 and SLO3 channels, respectively) decreased the recorded currents.
Figure 2.
S0859 did not affect SLO1 and SLO3 channels. A, SLO3 recordings. The currents were evoked by voltage pulses from −100 to +80 mV in 10-mV steps at a holding potential of −70 mV. Traces represent currents of the same oocyte subsequently recorded in: control conditions (ND96 solution in the bath); after the application of 5 μm S0859, after the wash with ND96 and treated with 1 mm Ba2+. B, mean current-voltage relationships ± S.E. in control conditions and in the presence of S0859 and Ba2+ with respect to the control condition at −80 mV (n = 4 oocytes). C, summarizes the current measurements at 60 mV normalized to control condition (I0). D, SLO1 recordings. The currents were evoked by voltage pulses from −100 to +80 mV in 10-mV steps at a holding potential of −70 mV in control conditions (ND96 solution in the bath), after the application of 5 μm S0859, after the wash with ND96 and treated with 5 mm TEA. E, mean current-voltage relationships ± S.E. in control conditions and during the application of S0859 and TEA with respect to control condition at −80 mV (n = 4 oocytes). F, summarizes the current measurements at 60 mV normalized by control condition (I0).
Inhibition of NBC by S0859 resulted in lower levels of [Na+]i
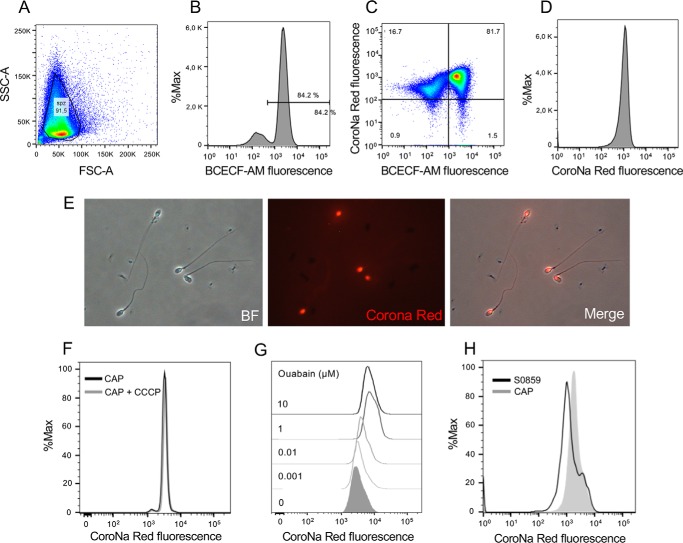
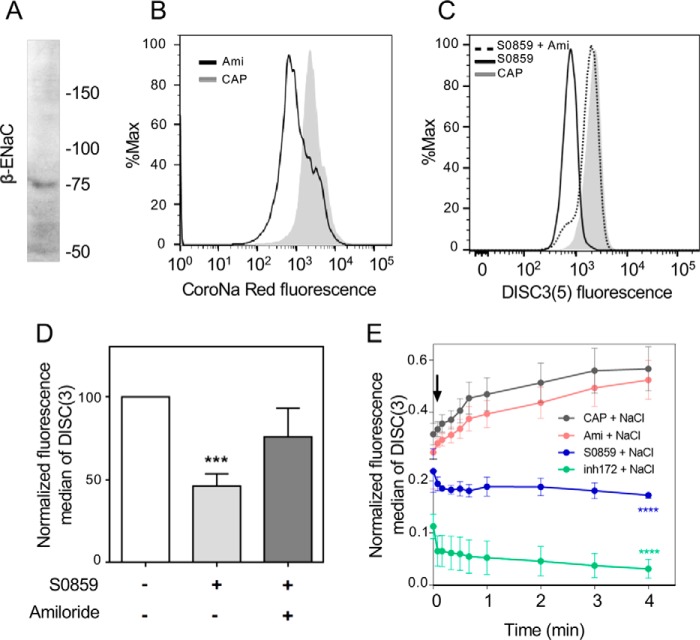
We next aimed to determine whether inhibition of NBC by S0859 resulted in lower levels of [Na+]i, as described under “Experimental procedures,” sperm were loaded with the Na+ probe CoroNa Red and BCECF-AM were then analyzed by flow cytometry as shown in Fig. 3, A–D. CoroNa Red was concentrated in the equatorial and postacrosomal regions of human sperm (Fig. 3E) as previously reported in mouse sperm (33). Because CoroNa Red detects mitochondrial [Na+] in other cells (34), mitochondrial uncoupler CCCP was incorporated to evaluate if mitochondria contributes to [Na+]i estimation. As shown in Fig. 3F, addition of 1 μm CCCP did not significantly affect CoroNa Red fluorescence. Because Na+/K+-ATPase pumps Na+ out of cells while pumping K+ into cells, both against their concentration gradients, we incubated sperm cells with different concentrations of ouabain to increase [Na+]i. As expected, CoroNa Red fluorescence increased after addition of ouabain (Fig. 3G). The maximum effect was observed at 1 μm ouabain, a concentration that affects both α1 and α4 isoforms (35). Finally, it was observed that 5 μm S0859 resulted in lower levels of [Na+]i as indicated by CoroNa Red fluorescence assessed by flow cytometry (Fig. 3H).
Figure 3.
NBC inhibition decreased the concentration of [Na+]i assessed by CoroNa Red fluorescence and flow cytometry. A, FSC and SSC fluorescence data were collected from 20,000 events per sample. Threshold levels for FSC-A and SSC-A were set to exclude signals from cellular debris or abnormal morphology for all samples. B, because BCECF-AM is only incorporated into living cells, BCECF-AM positive cells were used as a control of the viability for CoroNa Red experiments. C and D, cells were loaded with 0.5 μm BCECF-AM and 2 μm CoroNa Red and its fluorescence was evaluated using the FITC (530/30) and PE (575/26) lasers, respectively. Sperm positive for BCECF fluorescence were analyzed for CoroNa Red fluorescence to estimate [Na+]i. E, bright field (BF) and its corresponding CoroNa Red epifluorescence images. F, histogram of percentage of the maximum (% max) versus CoroNa Red fluorescence before and after 5 min addition of 1 μm CCCP. G, histogram of percentage of the maximum (% max) versus CoroNa Red fluorescence before and after the addition of increasing concentrations of ouabain. H, the inhibitor S0859 (5 μm) decreased the concentration of [Na+]i assessed by CoroNa Red fluorescence (n = 3).
Na+ transport is necessary for the activation of capacitation-associated cAMP/PKA pathway and Em regulation
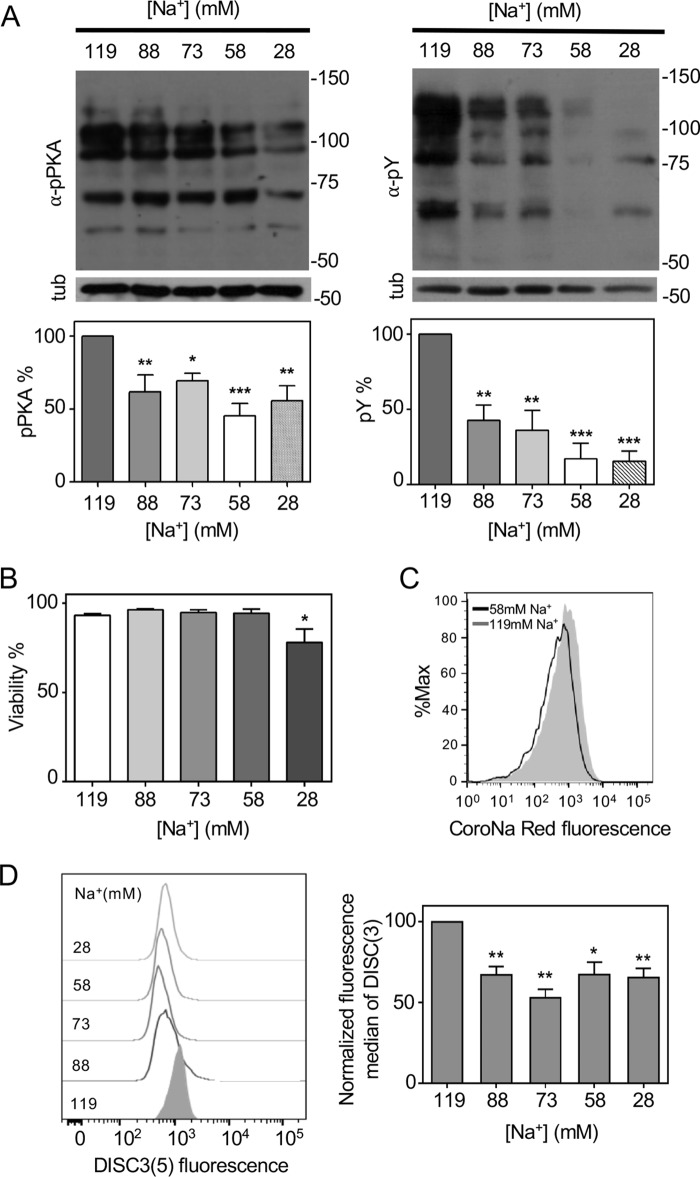
To further support the role of Na+/HCO3− cotransporters in human sperm, we replaced NaCl in capacitation medium with choline chloride. As shown in Fig. 4A, reducing the Na+ concentration resulted in decreased levels of pPKA and pTyr. Decreasing the [Na+] to 58 mm did not significantly affect the viability of the cells (Fig. 4B).
Figure 4.
[Na+]e is required for the capacitation-associated increased activation of the cAMP/PKA pathway and Em regulation during capacitation. A, sperm were incubated in capacitating medium with different Na+ concentrations. Aliquots from each condition were processed for Western blotting with anti-pPKA (left panel) or anti-pTyr (right panel) antibodies and then the membranes were reblotted with an anti-β-tubulin antibody for loading control (lower panel). Blots were quantified as described under “Experiment procedures” (bottom panel). Values represent the mean ± S.E. of at least 3 experiments. ***, p < 0.001; **, p < 0.01; *, p < 0.05. B, human sperm were incubated with different Na+ concentrations and the percentage of live cells was assessed using Eosin-Y. *, p < 0.05 (n = 3). C, histograms of percentage of the maximum (% max) versus CoroNa Red fluorescence of BCECF-stained sperm are shown. Human sperm were incubated for 5 h in medium that supports capacitation containing 58 mm Na+. Subsequently, aliquots from each condition were processed by flow cytometry to evaluate [Na+]i with CoroNa Red and BCECF to estimate viability. These experiments were repeated at least 3 times with similar results. D, histograms of percentage of the maximum (% max) versus DISC3(5) fluorescence of BCECF stained sperm are shown (left). Human sperm were incubated in medium that supports capacitation with different Na+ concentrations. Subsequently, aliquots from each condition were processed by flow cytometry to evaluate Em with DISC3(5) and with BCECF-AM to estimate viability. Fluorescence median of DISC3(5) was normalized with respect to 119 mm Na+ (right). Values represent the mean ± S.E. of 4 experiments. **, p < 0.01; *, p < 0.05 versus the sample with 119 mm Na+.
To demonstrate that reducing [Na+]e resulted in lower [Na+]i, we used flow cytometry to assess the levels of [Na+]i in sperm incubated in 58 mm Na+ in the capacitating medium. As shown in Fig. 4C, sperm exposed to lower levels of extracellular Na+ ([Na+]e) displayed lower CoroNa Red fluorescence.
We previously demonstrated that inhibition of the cAMP/PKA pathway causes a depolarization in human sperm (9). To evaluate if decreasing the [Na+]e impacts the human sperm Em, incubations in medium that supports capacitation with decreasing [Na+]e were performed. Estimation of the sperm Em was assessed by flow cytometry using DISC3(5) as previously described (9). As shown in Fig. 4D decreasing the [Na+]e concentration produced Em depolarization in a concentration-dependent manner. Altogether, these results may suggest the participation of an electrogenic NBC in the activation of PKA, and hence CFTR.
cAMP analogs partially rescued the inhibitory effects caused by S0859 on phosphorylation pathways and Em
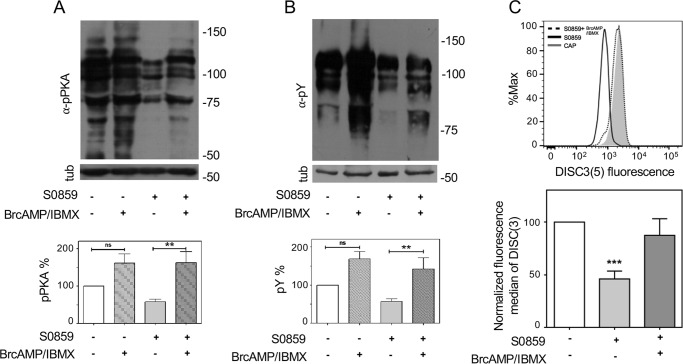
The presence of NBC inhibitor S0859 during capacitation reduced both pPKA and pTyr levels (Fig. 1B). Addition of permeable cAMP analogs (Br-cAMP) in combination with phosphodiesterase inhibitors (IBMX) restored phosphorylation of PKA substrates (Fig. 5A) and pTyr (Fig. 5B) affected by the presence of S0859. These results are in accordance with the role of NBC upstream PKA activation. Similarly, Br-cAMP/IBMX rescued the depolarization caused by NBC inhibition using S0859 (Fig. 5C). Altogether, these results suggest that NBC participate in the initial uptake of HCO3− upstream for the activation of PKA.
Figure 5.
cAMP agonists rescued the inhibitory effects on cAMP/PKA pathway and Em caused by NBC inhibitor S0859. Human sperm were incubated in medium that support capacitation in the presence or absence of 5 μm S0859 and cAMP analogs (1 mm BrcAMP + 0.2 mm IBMX). Aliquots from each condition were processed for Western blotting with anti-pPKA (A) or anti-pTyr (B) antibodies (upper panel) and then membranes were reblotted with an anti-β-tubulin antibody for loading control (lower panel). Blots displayed in Fig. 1, A and B, were quantified and values represent the mean ± S.E. of at least 6 experiments (bottom panel). **, p < 0.01. C, human sperm were incubated in medium that support CAP in the absence or presence of 5 μm S0859. Subsequently, aliquots from each condition were processed to evaluate Em with DISC3(5) and with BCECF-AM to estimate viability by flow cytometry. cAMP analogs (1 mm Br-cAMP and 0.2 mm IBMX, dotted line) rescued the decrease in Em produced by S0859 (5 μm, solid line) (upper panel). Fluorescence median of DISC3(5) was normalized with respect to CAP condition (bottom panel). Values represent the mean ± S.E. of 5 experiments. ***, p < 0.001 versus CAP condition.
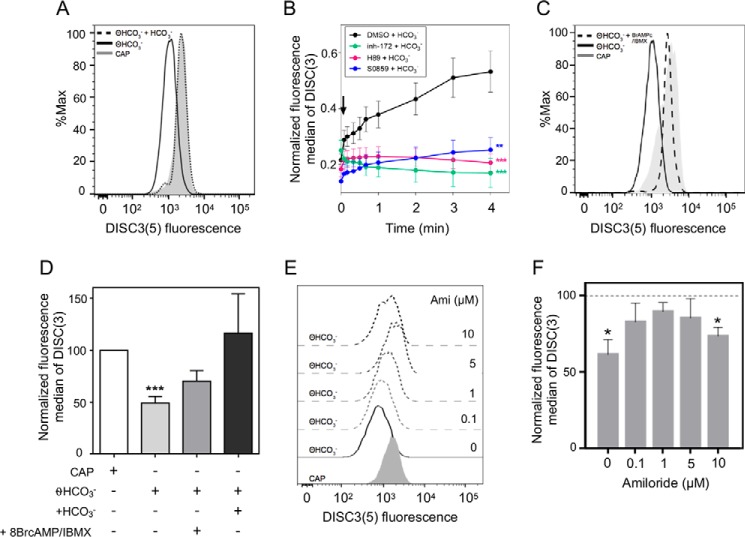
Addition of HCO3− produced a membrane hyperpolarization sensitive to inh-172, H89, and S0859 and amiloride rescued the depolarization caused by absence of HCO3−
When human sperm were incubated in medium lacking HCO3−, the population showed a more depolarized Em compared with the capacitating control condition. Addition of HCO3− to these HCO3−-deprived cells hyperpolarized the plasma membrane to a similar extent to that observed in a capacitated population where HCO3− was present in the medium during incubation (Fig. 6A). To determine the kinetics of this hyperpolarization, we monitored the changes in DISC3(5) upon addition of HCO3− by flow cytometry. In these experiments, as soon as the initial fluorescence value was registered (time = 0 s, prior to the addition of HCO3−), the cells were continuously monitored within the following 4 min after addition of 25 mm HCO3−. In each experiment, the fluorescence median was relativized to maximum fluorescence reached after addition of 1 μm valinomycin, because this Em is assumed to correspond to a constant K+ equilibrium potential value under our conditions. As shown in Fig. 6B, the membrane hyperpolarization caused by addition of HCO3− was noticeable within the first 4 min.
Figure 6.
HCO3−-induced a rapid Em hyperpolarization in human sperm sensitive to S0859, inh-172, and H89. A, human sperm were incubated in the presence (CAP) or absence of HCO3− for 3 h. After that, cells were loaded with DISC3(5) and BCECF-AM to evaluate Em and viability, respectively. Cells that were not exposed to HCO3− displayed more depolarized Em compared with the condition incubated with HCO3−. Addition of HCO3− (25 mm) induced a rapid Em hyperpolarization in human sperm. B, human sperm were incubated in the absence of HCO3− for 3 h. After that, the cells were loaded with DISC3(5) to evaluate Em. DISC3(5) fluorescence median was recorded before (time = 0 min) and after (black arrow) addition of 25 mm HCO3−. In each experiment, the fluorescence median was normalized to fluorescence maximum reached after 4 min of addition of 1 μm valinomycin to sperm incubated in capacitating medium. Values represent the mean ± S.E. of at least 3 experiments. Two-way ANOVA with Dunnett's post test. ***, p < 0.001; **, p < 0.01 versus NC at 4 min, ns at 0 min (before HCO3− addition). C, human sperm were incubated in the presence (CAP) or absence of HCO3− for 3 h. Then, the cells were loaded with DISC3(5) and BCECF-AM to evaluate Em and viability, respectively. Cells that were not exposed to HCO3− displayed more depolarized Em than those incubated with HCO3−. Addition of cAMP analogs (1 mm Br-cAMP and 0.2 mm IBMX, dotted line) could rescue Em depolarization. D, fluorescence median of DISC3(5) shown in A and C was normalized with respect to CAP condition. Values represent the mean ± S.E. of at least 3 experiments. ***, p < 0.001 versus CAP condition. E, human sperm were incubated in the presence (CAP) or absence of HCO3− for 3 h. Then, the cells were loaded with DISC3(5) and BCECF-AM to evaluate Em and viability, respectively. Cells that were not exposed to HCO3− displayed more depolarized Em that could be rescued with increasing concentrations of amiloride (Ami). F, fluorescence median of DISC3(5) shown in E was normalized with respect to CAP condition. Values represent the mean ± S.E. *, p < 0.05 (n = 4) versus CAP condition.
Some isoforms of NBC are electrogenic such as NBC1 (SLC4A4, 2HCO3−:1Na+) and NBC2 (SLC4A5, 2HCO3−:1Na+), whereas some are electroneutral such as NBCn1 (SLC4A7) and NBCn2 (SLC4A10), and an electroneutral Na+-driven Cl−/HCO3− exchanger NDCBE (SLC4A8 2 HCO3−:1 Na+). To investigate if an electrogenic NBC contributes to the observed membrane hyperpolarization, cells were incubated with the NBC inhibitor S0859 and challenged with HCO3−. In this condition, the rapid membrane hyperpolarization was inhibited (Fig. 6B, blue line). Furthermore, when sperm were preincubated with CFTR and PKA inhibitors (inh-172 and H89, respectively), hyperpolarization of Em in response to HCO3− was also inhibited (Fig. 6B, green and red lines). This observation indicates that events downstream from NBC activation are required for the HCO3−-mediated rapid hyperpolarization. To further support these observations, human sperm incubated in the absence of HCO3− were challenged with cAMP analogs. According to our hypothesis, the analogs would act downstream of NBC and upstream of PKA and CFTR. As expected, addition of Br-cAMP/IBMX partially rescued the depolarization caused by the absence of HCO3− (Fig. 6C). Quantification of these results are summarized in Fig. 6D.
Although CFTR is a Cl− channel, because the Cl− equilibrium potential is close to the sperm resting Em, the opening of CFTR cannot explain a hyperpolarization of the sperm plasma membrane by itself. As we mentioned before (24, 27, 28), it has been shown in other systems and also proposed for mouse sperm that CFTR can interact with ENaC channels causing their inhibition. This inhibition of ENaC channels can in turn trigger membrane hyperpolarization. To test this hypothesis, human sperm not previously exposed to HCO3− were treated with increasing concentrations of the ENaC inhibitor amiloride. Supporting our hypothesis, we observed that the ENaC blocker amiloride partially reverted the depolarization caused by the absence of HCO3− (Fig. 6E). Its maximum effect was observed at 1 μm amiloride (Fig. 6G).
Because amiloride may also inhibit NHE (Na+/H+ exchanger) at higher concentrations (36), we also estimate intracellular pH (pHi) at different concentrations of amiloride by using flow cytometry with the pH-sensitive probe BCECF-AM (Fig. S1). Between the range of 0.1 and 1 μm amiloride pHi was not affected. The concentration of 5 μm resulted in intracellular alkalinization, suggesting that lower intracellular Na+ concentrations caused by ENaC closure could affect H+/Na+ exchange. On the contrary, 10 μm amiloride resulted in acidification probably due to the direct inhibition of NHE. Altogether, these results suggest that a rapid membrane hyperpolarization induced by HCO3− is mediated by both an electrogenic NBC and downstream events that result in closing ENaC.
ENaC contribution to the regulation of Em in human sperm
To further support the hypothesis that an ENaC-type channel influences Em, we explored the expression of the β-subunit of ENaC channel in human sperm. By immunoblotting, we observed a single band with a molecular size of ∼75 kDa, which is close to the 73 kDa predicted molecular mass of this subunit (Fig. 7A). In addition, because ENaC channels are selectively permeable to Na+, we tested if inhibition of ENaC by 1 μm amiloride decreases the levels of [Na+]i. As expected, amiloride reduced the levels of [Na+]i as estimated with CoroNa Red fluorescence by flow cytometry (Fig. 7B).
Figure 7.
Human sperm display functional ENaCs sensitive to amiloride. A, human sperm proteins were analyzed by 8% SDS-PAGE and immunoblotted using antibodies against the β subunit of ENaC. The lane contained 5 × 106 sperm. B, amiloride (1 μm) decreased the concentration of [Na+]i assessed by CoroNa Red fluorescence and flow cytometry. C, the ENaC inhibitor amiloride (1 μm) rescued the inhibitory effect of S0859 (5 μm). D, fluorescence median of DISC3(5) shown in C was normalized with respect to CAP condition. Values represent the mean ± S.E.; ***, p < 0.001 (n = 5). E, human sperm were incubated in capacitating conditions (CAP) in the presence or absence of 1 μm amiloride, 5 μm S0859, or 5 μm inh-172. Then, the cells were loaded with DISC3(5) to evaluate Em. DISC3(5) fluorescence median was registered before (time = 0 min) and after (black arrow) addition of 40 mm NaCl. In each experiment, the fluorescence median was relativized to maximum fluorescence reached between 4 min addition of 1 μm valinomycin. Values represent the mean ± S.E. of at least 3 experiments. Two-way ANOVA with Dunnett's post test. ****, p < 0.0001 versus CAP at 4 min.
As previously shown, sperm incubated in the presence of the NBC inhibitor S0859 displayed a more depolarized membrane potential compared with the capacitating control condition. Supporting our hypothesis, inhibition of ENaC by amiloride also partially rescued the depolarization caused by S0859 (Fig. 7, C and D). To further strengthen these observations, we monitored the changes in DISC3(5) fluorescence by flow cytometry upon addition of 40 mm NaCl. In these experiments, before the addition of NaCl, the initial fluorescence value was recorded. The cells were continuously monitored for 4 min after addition of 40 mm NaCl and the fluorescence median was normalized to the maximum fluorescence reached after addition of 1 μm valinomycin. In agreement with our hypothesis, when ENaC is closed due to amiloride (Ami) inhibition, or during capacitation where the activation of CFTR leads to ENaC closure, in both conditions the addition of NaCl would favor the uptake of Na+ and HCO3− through an electrogenic NBC isoform. This uptake resulted in membrane hyperpolarization (Fig. 7E). In contrast, incubation of sperm in conditions where ENaC is open due to either CFTR or NBC inhibition (with inh-172 or S0859, respectively), the addition of NaCl caused Na+ entrance through ENaC resulting in a rapid depolarization (Fig. 7E).
Discussion
When sperm leave the epididymis and enter the seminal plasma, the Na+ and HCO3− concentrations change abruptly. Although in the epididymis [HCO3−] is ∼2–4 mm (5) and [Na+] is ∼30 mm (37), in seminal plasma they increase 6- and 4-fold, respectively ([HCO3−] ∼25 mm (38) and [Na+] ∼102–143 mm (39)). We hypothesize that these changes in concentration of both ions can facilitate the initial influx of HCO3− by coupling its transport with the Na+ gradient. In this work, we demonstrate for the first time in human sperm that the participation of NBC cotransporter in the initial HCO3− transport is necessary for activation of the cAMP/PKA pathway and essential for capacitation. In addition, we have provided supporting evidence that ENaC channels contributes to the regulation of Em in human sperm.
The NBC family consists of at least four isoforms, which cotransports Na+ and HCO3− with different stoichiometries. Some of which are electrogenic, whereas others are electroneutral (40–42). The electroneutral isoforms ARNm NDCBE, NBCn2 were detected in human testis (43) as well as the electrogenic NBC2 (44). Jensen and co-workers (45, 46) showed the expression of the NBC1 protein in rat sperm. From a functional point of view, the role of NBC in the initial HCO3− transport was first reported in mouse sperm by Demarco and co-workers (11), who also found evidence indicating that the uptake of HCO3− is electrogenic and Na+-dependent. Na+-coupled HCO3− cotransporters have also been involved in sperm pHi regulation. In mouse sperm, the pHi depends on extracellular Na+, HCO3−, and Cl− and DIDS, a nonspecific inhibitor of NBC affects pHi recovery following imposition of an acid load (12).
We have previously reported the role of CFTR in controlling the sustained HCO3− entrance during human sperm capacitation (9). However, because phosphorylation by PKA is essential for CFTR activity, we postulated the existence of an initial HCO3− uptake. Based on previous results in mice, we hypothesized that NBC was responsible for that HCO3− uptake. The results presented in this article support that initial HCO3− uptake: (a) is Na+-dependent; (b) is inhibited by a NBC-specific inhibitor S0859; and (c) if inhibited, it can be rescued by drugs that act downstream, such as cAMP analogs. Based on these results, we postulate that both human and mouse sperm (and probably other mammalian species) perform the initial HCO3− uptake through these cotransporters. This transport may occur when sperm are exposed to higher HCO3− concentrations in the seminal plasma at the time of ejaculation or while migrating to the site of fertilization in the female reproductive tract. In this regard, it was reported that uterine secretions of oestrous female mice contained higher HCO3− concentrations compared with those in dioestrous (47). This increase correlates with CFTR expression indicating that this channel is up-regulated hormonally (47).
Interestingly, whereas the initial HCO3− transport seems to be conserved at least in both mouse and human sperm, the molecular steps that occur downstream of NBC to produce a sustained HCO3− incorporation might be different in both species. We have recently shown that CFTR is essential for the sustained HCO3− uptake by using two specific inhibitors of this channel, which abrogated the phosphorylation of PKA substrates and in tyrosine residues. On the contrary, these inhibitors did not produce any effect on phosphorylation of PKA substrates or tyrosine residues in mouse sperm (47) because the sustained HCO3− transport occurs through NKCC transporters (48). Supporting this concept, inhibitors of NKCC such as bumetanide did not abolish pTyr in human sperm (Fig. S2).
In addition to the HCO3− transport through the aforementioned mechanisms, it is also proposed that carbonic anhydrases (CA), which catalyze the reversible hydration of CO2 to HCO3− are involved in HCO3− homeostasis. In mouse, targeted deletion of CA isoforms II and IV (49, 50) display a HCO3− disequilibrium and significant alterations in sperm motility. In addition, CAII/CAIV double knockout mice display subfertility and reduced sperm motility (49). Sperm from this double KO display normal tyrosine phosphorylation because these cells show a reduced and delayed response to HCO3−, which may be sufficient to support these capacitation-related events. In humans, the role of these proteins is scarce but by using a pharmacological approach, it was recently claimed that capacitated human sperm strongly depend on CA activity to support normal motility (51). Overall, evidence presented in these papers as well as previous work by others may suggest that several concurrent mechanisms contribute to the HCO3− homeostasis necessary for sperm function.
One of the most interesting events that occurs during mammalian capacitation is the Em hyperpolarization. This phenomenon has been observed in at least 3 different mammalian species including mouse, bovine, and human (8, 20, 52, 53). The membrane hyperpolarization in mouse sperm seems to be essential for the occurrence of the acrosomal exocytosis and for the Ca2+ entry through CatSper channels (52, 54). Previous results from several laboratories have postulated the involvement of ENaC, CFTR, K+ channels, and other Cl− transporters in mouse sperm hyperpolarization. The activation of the SLO3 K+ channel may be the principal mechanism whereby the murine sperm plasma membrane hyperpolarizes during capacitation (32). However, it is still not clear which channel is responsible of the main regulation of the Em during human sperm capacitation. A previous report have postulated that SLO1 is the principal K+ channel of human sperm (22), whereas others have claimed that the K+ current of human sperm is mediated by SLO3 (23). Our group has previously studied the role of K+ channels SLO1 and SLO3 in capacitation (55). By using a combination of K+ channels inhibitors, membrane hyperpolarization is abrogated, suggesting that both members of the SLO family may potentially be involved in the regulation of Em. Despite all this evidence about the participation of K+ channels in the regulation of Em, the role of other channels such as ENaCs remains unclear. What is observed in both species is that the activation of PKA that occurs during capacitation is critical for membrane hyperpolarization (9, 10). Reports in mouse sperm indicate that Src kinase may be one of the connecting players between PKA activation and hyperpolarization through SLO3 K+ channels (56). The mechanism of regulation requires further experimentation. In human sperm, we have previously observed that in addition to PKA activity, CFTR is essential for regulating the Em.
We hypothesize that activation of CFTR results in closing the ENaC channels. ENaC is a heteromultimeric channel that can be formed by the combination of four subunits: α, β, γ, and/or δ, where α or δ formed the channel pore (57, 58). In humans, previous reports demonstrated the presence of the ENaC-β subunit in testis (59), Hernández González and co-workers (28) mentioned the detection of ENaC-α in human spermatozoa and Kong and co-workers (60) showed the presence of ENaC-α by Western blotting and detected its presence in the midpiece of human sperm by immunocytochemistry, the same region as reported for CFTR (61–64). The latter authors also showed that the treatment of human sperm with EIPA (5-(N-ethyl-N-isopropyl)-amiloride), an inhibitor of ENaC, improves sperm motility in both healthy donors and asthenospermic patients. No ENaC current has been shown in electrophysiological examination of ejaculated cells so far. However, it is worth mentioning that in a recent study where 81 subfertile patients undergoing IVF/ICSI were investigated, one patient that presented depolarized Em associated with low IVF showed a large inward leak conductance (probably Na+) (19). Unfortunately, this patient did not give permission for genetic analysis.
It has been shown that CFTR negatively regulates Na+ channels containing ENaC-β or ENaC-γ by modulating their gating, specifically by extending the time in the closed state (27). Here we demonstrated for the first time the expression of the ENaC-β subunit in human sperm. We also found that amiloride, which prevents Na+ permeation through ENaC, produces hyperpolarization of the human sperm plasma membrane. In particular, the effect of amiloride was evident when cells were incubated in the absence of HCO3−. In this condition, ENaC is predicted to be open, because according to our model, CFTR remains inactive (Fig. 8). This concept is also supported by experiments using NaCl. In a condition where ENaC is open, addition of Na+ produced a rapid depolarization. Similarly, the connection between NBC–PKA–CFTR–ENaC in human spermatozoa is shown by incubating the spermatozoa in the capacitating medium with lower [Na+]e. In contrast to what occurs in mouse sperm where the decrease of [Na+]e induces Em hyperpolarization, in human spermatozoa, decreasing [Na+]e induces a depolarization of Em. This can be explained in two ways. First, because addition of HCO3− results in a rapid hyperpolarization, we postulated that NBC is electrogenic. As a consequence, a net negative charge is incorporated into the cell. Second, the lower HCO3− transport induces a decrease in PKA activity that impairs CFTR causing the opening of ENaC. Considering [Na+]i in human sperm is between ∼3 (65) and ∼17.8 mm (66) at 28, 58, 73, 88, and 119 mm [Na+]e, the Na+ equilibrium potential calculated by the Nernst equation for both intracellular concentrations (+12.1, +31.5 + 35.7 + 42.7 + 50.7 and +59,6 + 79.1 + 85.3 + 90.3 + 98.3 mV) predicts Na+ influx and therefore explains the depolarization observed under these conditions (resting ∼−40 mV (67); ∼−58 mV (66)).
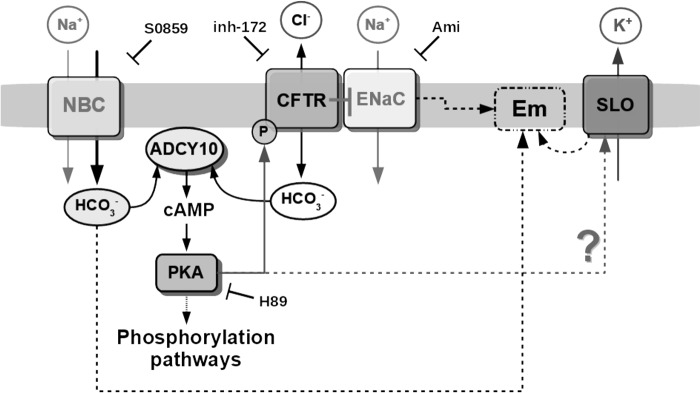
Figure 8.
Proposed model for the HCO3− transport during human sperm capacitation. An initial HCO3− influx through electrogenic NBC causes the stimulation of ADCY10, resulting in cAMP production and activation of PKA. Then PKA activity is necessary to phosphorylate and activate CFTR. Activation of CFTR results in sustained HCO3− influx and closing of ENaC. ENaC consequently contributes to the regulation of Em during capacitation together with SLO channels (SLO1 and/or SLO3). In the case of SLO3, its activity may be modulated by PKA as previously proposed in mouse sperm, although this possibility remains to be explored.
Results presented in this work together with previous reports from others are summarized in our current working model depicted in Fig. 8. We show for the first time that NBC and ENaC are essential in the CFTR-dependent activation of the cAMP/PKA signaling pathway. We postulate that together with SLO channels, ENaC is important for regulation of Em during human sperm capacitation. Although additional investigation is needed, it is possible that cAMP-dependent activation of PKA plays a central role by simultaneously stimulating SLO3 and CFTR. Both cAMP-dependent effects may contribute to regulate Em during human sperm capacitation.
Experimental procedures
Full details are provided as supporting information.
Ethical approval
The study protocol was approved by the Bioethics Committee of the Instituto de Biología y Medicina Experimental (IByME), Buenos Aires. The studies are in compliance with the Declaration of Helsinki principles. Human donors were provided with written information about the study prior to giving informed consent. Experiments involving animals were conducted according to guidelines of the institutional animal care and were reviewed and approved by the Ethical Committee of the Instituto de Biología y Medicina Experimental, Buenos Aires (CICUAL) and conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH).
Human sperm capacitation
Semen samples were obtained by masturbation from 15 healthy donors after 3–5 days of abstinence and analyzed following WHO recommendations (World Health Organization. 2010). All samples fulfilled semen parameters (total fluid volume, sperm concentration, motility, viability, and morphology) according to WHO normality criteria. Samples were allowed to liquefy for 1 h at room temperature. Then, ejaculated sperm were allowed to swim-up in noncapacitating medium at 37 °C for 1 h. The highly motile sperm recovered after swim-up, were washed for 5 min, centrifuged at 400 × g, and preincubated in 250 μl of noncapacitating medium containing inhibitor (or their corresponding vehicle) for 10 min. After preincubation, an equal volume (250 μl) of 2-fold concentrated capacitating medium (50 mm NaHCO3 and 1% (w/v) BSA) was added to a final cell concentration of 2–8 × 106 cells/ml and incubated for different time periods at 37 °C in an atmosphere of 5% (v/v) CO2. According to the experiment, sperm were capacitated for 1 h to evaluate PKA substrate phosphorylation (pPKA), or 3 h to evaluate pTyr hyperactivation, viability, pHi, or Em. Before extraction of sperm proteins and immunoblotting, sperm viability was evaluated by Eosin-Y staining (World Health Organization, 2010), Xenopus oocytes injection and voltage-clamp recordings and analysis. Xenopus laevis oocytes were purchased from EcoCyte Bioscience (TX). Oocytes were injected with 50 nl of SLO1 or SLO3 cRNA (0.4 μg/μl) using a Drummond Scientific nanoinjector. Injected oocytes were incubated at 18 °C in ND96 medium: 96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, and 5 mm HEPES, pH 7.5, with NaOH. Two-electrode voltage-clamp experiments were undertaken in ND96 3 days after injection. Voltage steps (−100 to 80 mV) were applied in 10-mV increments from a holding potential of −70 mV. Recording pipettes (1 mΩ) were filled with 3 m KCl. S0859 (5 μm) and the inhibitors TEA (5 mm) for SLO1 or BaCl2 (1 mm) for SLO3 were applied to the recording chamber by continuous perfusion. Whole cell recordings were acquired and analyzed with pClamp 9.0 (Molecular Devices). Data were analyzed as described before (68).
Determination of intracellular pH and Em by flow cytometry
Human sperm Em and pHi changes were assessed using DISC3(5) and BCECF-AM, respectively, according to López-González (20). After 3 h incubation on capacitating medium, samples were centrifuged at 400 × g for 5 min, re-suspended in 500 μl of noncapacitating medium, and the concentration adjusted to 1 × 106 cells/ml. Cells were then loaded with 0.5 μm BCECF-AM for 10 min or 50 nm DISC3(5) during 3 min, washed again, and re-suspended in 500 μl of noncapacitating medium with or without 25 mm HCO3−. Data were recorded as individual cellular events using a FACSCanto II TM cytometer (BD Bioscience). Forward scatter (FSC) and side scatter (SSC) fluorescence data were collected from 20,000 events per sample. Positive cells for BCECF were collected using the filter for fluorescein isothiocyanate (FITC; 525/50). Positive cells for BCECF were used to monitor viability for DISC3(5) as previously described (9). Positive cells for DISC3(5) were detected using the filter for allophycocyanine (585/40). Data were analyzed using FACS Diva and FlowJo software (Tree Star 7.6.2).
Determination of intracellular Na+ by flow cytometry
Sperm [Na+]i was assessed using CoroNa Red dye as previously described (33). After 5 h incubation on capacitating medium, samples were centrifuged at 400 × g for 5 min, re-suspended in 500 μl of noncapacitating medium, and the concentration was adjusted to 1 × 106 cells/ml. Sperm were loaded for 20 min at 37 °C in noncapacitating medium with 2 μm CoroNa Red. In the last 10 min, to select viable cells, BCECF-AM was incorporated as described before. To eliminate any unincorporated dye, sperm suspensions were then washed again. The resulting pellets were then resuspended in 500 μl of noncapacitating medium (for conditions without HCO3−) or medium with HCO3− without BSA (for conditions incubated in capacitating medium). As a control, sperm were incubated with the mitochondrial uncoupler CCCP to evaluate if mitochondria contributes to the [Na+]i estimation, and with increasing concentrations of the Na+/K+-ATPase ouabain to increase [Na+]i (33). To discriminate nonsperm particles passing through the flow cytometer detector, two-dimensional sideways (SSC)-forward (FSC) scatter dot plots were used. Once nonsperm events were excluded, BCECF was used as a control of the viability for CoroNa Red experiments because BCECF-AM is only incorporated into living cells. Then, two-dimensional fluorescence dot plots of CoroNa Red versus BCECF were created. Sperm positive for BCECF fluorescence were analyzed for CoroNa Red fluorescence to estimate [Na+]i (Fig. 3, A–D).
Data were recorded as individual cellular events using a MACS Quant Analyzer 10 cytometer. FSC and SSC fluorescence data were collected from 20,000 events per sample. Positive cells for BCECF were collected using the filter for FITC (530/30). Positive cells for CoroNa Red were detected using the filter for phycoerythrin (PE) (575/26). The two dyes were compensated accordingly. Data were analyzed using FACS Diva and FlowJo software (Tree Star 7.6.2).
Calculations and statistical analysis
Data are expressed as mean ± S.E. Western blotting values were normalized to the control conditions and the percentages were analyzed using a one sample t test against an hypothetical value (100; control condition). Viability was analyzed by one-way analysis of variance (ANOVA) with Dunnett's post test. Kinetics of DISC3(5) relative median of flow cytometry histograms were analyzed by two-way ANOVA with Dunnett's post test. Normalized fluorescence median of DISC3(5) relative to capacitating medium (CAP) were analyzed by one sample t test against a hypothetical value of 100 (CAP). Calculations were performed with Libre Office 4.3.2.2 spreadsheet and statistical analysis with GraphPad Prism version 4.00 for Windows, GraphPad Software (San Diego, CA). Independent experiments were carried out using different donors. A p value < 0.05 was considered statistically significant.
Author contributions
L. C. P. M., D. K., C. M. S., C. L. T., A. D., and M. G. B. conceptualization; L. C. P. M. and M. G. B. resources; L. C. P. M., N. A. P., and M. G. B. formal analysis; L. C. P. M., A. D., and M. G. B. supervision; L. C. P. M. and M. G. B. funding acquisition; L. C. P. M., N. A. P., N. I. T., A. L. G.-C., G. M. L., P. A. B., A. R., D. K., C. M. S., and C. L. T. investigation; L. C. P. M., N. A. P., and M. G. B. writing-original draft; L. C. P. M., G. M. L., A. R., and M. G. B. project administration; N. A. P., N. I. T., A. L. G.-C., G. M. L., A. R., D. K., C. M. S., L. C. P. M., and C. L. T. methodology; N. I. T., L. C. P. M., and A. L. G.-C. software; D. K., C. M. S., A. D., and M. G. B. validation.
Supplementary Material
Acknowledgments
We thank Gabriel Rabinovich, Juan Pablo Cerliani, Pablo Pomata, Mónica Montes, Maximilian D. Lyon, and Juan Jeremías Incicco for advice and technical assistance. We also thank the Fulbright Scholar Program and Rene Baron, Fortabat, and Williams Foundations.
This work was supported in part by Agencia Nacional de Promoción Científica y Tecnológica Grants PICT 2015–2294 (to M. G. B.) and PICT 2015–3164 (to D. K.), CONACyT-México Fronteras de la Ciencia 71 (to A. D. and C. T.), DGAPA/UNAM Grants IN203116 (to C. T.) and IN205516 (to A. D.), and National Institutes of Health Grants R01 HD069631 (to C. M. S.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- PKA
- protein kinase A
- NBC
- Na+/HCO3− cotransporter
- CFTR
- cystic fibrosis transmembrane conductance regulator channel
- Em
- membrane potential
- ENaC
- epithelial Na+ channels
- TEA
- trimethylammonium
- CCCP
- carbonyl cyanide p-chlorophenylhydrazone
- IBMX
- isobutylmethylxanthine
- [Na+]e
- extracellular Na+
- DIDS
- 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid
- CA
- carbonic anhydrases
- IVF
- in vitro fertilization
- FSC
- forward scatter
- SSC
- side scatter
- PE
- phycoerythrin
- ANOVA
- analysis of variance
- CAP
- capacitating medium
- BCECF-AM
- 2′,7′-bis(carboxyethyl)-S-(and 6)carboxyfluorescein acetoxy methylester.
References
- 1. Austin C. R. (1951) Observations on the penetration of the sperm in the mammalian egg. Aust. J. Sci. Res. 4, 581–596 [DOI] [PubMed] [Google Scholar]
- 2. Chang M. C. (1951) Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168, 697–698 10.1038/168697a0,10.1038/168697b0 [DOI] [PubMed] [Google Scholar]
- 3. Buffone M. G., Hirohashi N., and Gerton G. (2014) Unresolved questions concerning mammalian sperm acrosomal exocytosis. Biol. Reprod. 90, 112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stival C., Puga Molina L. del C., Paudel B., Buffone M. G., Visconti P. E., and Krapf D. (2016) Sperm capacitation and acrosome reaction in mammalian sperm. Adv. Anat. Embryol. Cell Biol. 220, 93–106 10.1007/978-3-319-30567-7_5 [DOI] [PubMed] [Google Scholar]
- 5. Okamura N., Tajima Y., Soejima A., Masuda H., and Sugita Y. (1985) Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J. Biol. Chem. 260, 9699–9705 [PubMed] [Google Scholar]
- 6. Luconi M., Porazzi I., Ferruzzi P., Marchiani S., Forti G., and Baldi E. (2005) Tyrosine phosphorylation of the A kinase anchoring protein 3 (AKAP3) and soluble adenylate cyclase are involved in the increase of human sperm motility by bicarbonate. Biol. Reprod. 72, 22–32 10.1095/biolreprod.104.032490 [DOI] [PubMed] [Google Scholar]
- 7. Buffone M. G., Wertheimer E. V., Visconti P. E., and Krapf D. (2014) Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim. Biophys. Acta 1842, 2610–2620 10.1016/j.bbadis.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeng Y., Clark E. N., and Florman H. M. (1995) Sperm membrane potential: hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev. Biol. 171, 554–563 10.1006/dbio.1995.1304 [DOI] [PubMed] [Google Scholar]
- 9. Puga Molina L. C., Pinto N. A., Torres Rodríguez P., Romarowski A., Vicens Sanchez A., Visconti P. E., Darszon A., Treviño C. L., and Buffone M. G. (2017) Essential role of CFTR in PKA-dependent phosphorylation, alkalinization, and hyperpolarization during human sperm capacitation. J. Cell Physiol. 232, 1404–1414 10.1002/jcp.25634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Escoffier J., Navarrete F., Haddad D., Santi C. M., Darszon A., and Visconti P. E. (2015) Flow cytometry analysis reveals that only a subpopulation of mouse sperm undergoes hyperpolarization during capacitation. Biol. Reprod. 92, 1–11 10.1095/biolreprod.114.127266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Demarco I. A., Espinosa F., Edwards J., Sosnik J., De La Vega-Beltrán J. L., Hockensmith J. W., Kopf G. S., Darszon A., and Visconti P. E. (2003) Involvement of a Na+/HCO cotransporter in mouse sperm capacitation. J. Biol. Chem. 278, 7001–7009 10.1074/jbc.M206284200 [DOI] [PubMed] [Google Scholar]
- 12. Zeng Y., Oberdorf J. A., and Florman H. M. (1996) pH regulation in mouse sperm: identification of Na+-, Cl−, and HCO3−-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev. Biol. 173, 510–520 10.1006/dbio.1996.0044 [DOI] [PubMed] [Google Scholar]
- 13. Chávez J. C., Hernández-González E. O., Wertheimer E., Visconti P. E., Darszon A., and Treviño C. L. (2012) Participation of the Cl−/HCO3−-exchangers SLC26A3 and SLC26A6, the Cl-channel CFTR, and the regulatory factor SLC9A3R1 in mouse sperm capacitation. Biol. Reprod. 86, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rode B., Dirami T., Bakouh N., Rizk-Rabin M., Norez C., Lhuillier P., Lorès P., Jollivet M., Melin P., Zvetkova I., Bienvenu T., Becq F., Planelles G., Edelman A., Gacon G., and Touré A. (2012) The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: a potential role during sperm capacitation. Hum. Mol. Genet. 21, 1287–1298 10.1093/hmg/ddr558 [DOI] [PubMed] [Google Scholar]
- 15. Gadsby D. C., Vergani P., and Csanády L. (2006) The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature 440, 477–483 10.1038/nature04712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorum B., Czégé D., and Csanády L. (2015) Timing of CFTR pore opening and structure of its transition state. Cell 163, 724–733 10.1016/j.cell.2015.09.052 [DOI] [PubMed] [Google Scholar]
- 17. Tabcharani J. A., Chang X.-B., Riordan J. R., and Hanrahan J. W. (1991) Phosphorylation-regulated CI-channel in CHO cells stably expressing the cystic fibrosis gene. Nature 352, 628–631 10.1038/352628a0 [DOI] [PubMed] [Google Scholar]
- 18. Calzada L., and Tellez J. (1997) Defective function of membrane potential (psi) on sperm of infertile men. Arch. Androl. 38, 151–155 10.3109/01485019708987892 [DOI] [PubMed] [Google Scholar]
- 19. Brown S. G., Publicover S. J., Mansell S. A., Lishko P. V., Williams H. L., Ramalingam M., Wilson S. M., Barratt C. L., Sutton K. A., and Da Silva S. M. (2016) Depolarization of sperm membrane potential is a common feature of men with subfertility and is associated with low fertilization rate at IVF. Hum. Reprod. 31, 1147–1157 10.1093/humrep/dew056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. López-González I., Torres-Rodríguez P., Sánchez-Carranza O., Solís-López A., Santi C. M., Darszon A., Treviño C. L., Lopez-Gonzalez I., Torres-Rodriguez P., Sanchez-Carranza O., Solis-Lopez A., Santi C. M., Darszon A., and Treviño C. L. (2014) Membrane hyperpolarization during human sperm capacitation. Mol. Hum. Reprod. 20, 619–629 10.1093/molehr/gau029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geng Y., Ferreira J. J., Dzikunu V., Butler A., Lybaert P., Yuan P., Magleby K. L., Salkoff L., and Santi C. M. (2017) A genetic variant of the sperm-specific SLO3 K+ channel has altered pH and Ca2+ sensitivities. J. Biol. Chem. 292, 8978–8987 10.1074/jbc.M117.776013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mannowetz N., Naidoo N. M., Choo S.-A., Smith J. F., Lishko P. V., Sara Choo S.-A., Smith J. F., and Lishko P. V. (2013) Slo1 is the principal potassium channel of human spermatozoa. Elife 2, e01009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brenker C., Zhou Y., Müller A., Echeverry F. A., Trötschel C., Poetsch A., Xia X.-M., Bönigk W., Lingle C. J., Kaupp U. B., and Strünker T. (2014) The Ca2+-activated K+ current of human sperm is mediated by Slo3. Elife 3, e01438–e01438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Briel M., Greger R., and Kunzelmann K. (1998) Cl− transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na+ channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J. Physiol. 508, 825–836 10.1111/j.1469-7793.1998.825bp.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hallows K. R., Raghuram V., Kemp B. E., Witters L. A., and Foskett J. K. (2000) Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J. Clin. Invest. 105, 1711–1721 10.1172/JCI9622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naren A. P., Cormet-Boyaka E., Fu J., Villain M., Blalock J. E., Quick M. W., and Kirk K. L. (1999) CFTR chloride channel regulation by an interdomain interaction. Science 286, 544–548 10.1126/science.286.5439.544 [DOI] [PubMed] [Google Scholar]
- 27. Berdiev B. K., Qadri Y. J., and Benos D. J. (2009) Assessment of the CFTR and ENaC association. Mol. Biosyst. 5, 123–127 10.1039/B810471A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hernández-González E. O., Sosnik J., Edwards J., Acevedo J. J., Mendoza-Lujambio I., López-González I., Demarco I. A., Wertheimer E., Darszon A., and Visconti P. E. (2006) Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J. Biol. Chem. 281, 5623–5633 10.1074/jbc.M508172200 [DOI] [PubMed] [Google Scholar]
- 29. Guggino W. B., and Stanton B. A. (2006) New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat. Rev. Mol. Cell Biol. 7, 426–436 10.1038/nrm1949 [DOI] [PubMed] [Google Scholar]
- 30. Kunzelmann K. (2003) ENaC is inhibited by an increase in the intracellular Cl− concentration mediated through activation of Cl-channels. Pflügers Arch. 445, 504–512 12548397 [Google Scholar]
- 31. Ch'en F. F., Villafuerte F. C., Swietach P., Cobden P. M., and Vaughan-Jones R. D. (2008) S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart. Br. J. Pharmacol. 153, 972–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santi C. M., Martínez-López P., de la Vega-Beltrán J. L., Butler A., Alisio A., Darszon A., Salkoff L., de la Vega-Beltrán J. L., De, Butler A., Darszon A., and Salkoff L. (2010) The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 584, 1041–1046 10.1016/j.febslet.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Escoffier J., Krapf D., Navarrete F., Darszon A., and Visconti P. E. (2012) Flow cytometry analysis reveals a decrease in intracellular sodium during sperm capacitation. J. Cell Sci. 125, 473–485 10.1242/jcs.093344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baron S., Caplanusi A., van de Ven M., Radu M., Despa S., Lambrichts I., Ameloot M., Steels P., and Smets I. (2005) Role of mitochondrial Na+ concentration, measured by CoroNa Red, in the protection of metabolically inhibited MDCK cells. J. Am. Soc. Nephrol. 16, 3490–3497 10.1681/ASN.2005010075 [DOI] [PubMed] [Google Scholar]
- 35. Sanchez G., Nguyen A.-N., Timmerberg B., Tash J. S., and Blanco G. (2006) The Na,K-ATPase α4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Mol. Hum. Reprod. 12, 565–576 10.1093/molehr/gal062 [DOI] [PubMed] [Google Scholar]
- 36. Wang D., King S. M., Quill T. A., Doolittle L. K., and Garbers D. L. (2003) A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat. Cell Biol. 5, 1117–1122 10.1038/ncb1072 [DOI] [PubMed] [Google Scholar]
- 37. Hinton B. T., Brooks D. E., Dott H. M., and Setchell B. P. (1981) Effects of carnitine and some related compounds on the motility of rat spermatozoa from the caput epididymidis. J. Reprod. Fertil. 61, 59–64 10.1530/jrf.0.0610059 [DOI] [PubMed] [Google Scholar]
- 38. Vishwakarma P. (1962) The pH and bicarbonate-ion content of the oviduct and uterine fluids. Fertil. Steril. 13, 481–485 10.1016/S0015-0282(16)34633-7 [DOI] [PubMed] [Google Scholar]
- 39. Owen D. H., and Katz D. F (2005) A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 26, 459–469 10.2164/jandrol.04104 [DOI] [PubMed] [Google Scholar]
- 40. Sassani P., Pushkin A., Gross E., Gomer A., Abuladze N., Dukkipati R., Carpenito G., and Kurtz I. (2002) Functional characterization of NBC4: a new electrogenic sodium-bicarbonate cotransporter. Am. J. Physiol. Cell Physiol. 282, C408–C416 10.1152/ajpcell.00409.2001 [DOI] [PubMed] [Google Scholar]
- 41. Soleimani M., and Burnham C. E. (2001) Na+:HCO3− cotransporters (NBC): cloning and characterization. J. Membr. Biol. 183, 71–84 10.1007/s00232-001-0055-8 [DOI] [PubMed] [Google Scholar]
- 42. Romero M. F., Hediger M. A., Boulpaep E. L., and Boron W. F. (1997) Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature 387, 409–413 10.1038/387409a0 [DOI] [PubMed] [Google Scholar]
- 43. Damkier H. H., Nielsen S., and Praetorius J. (2007) Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R2136–R2146 10.1152/ajpregu.00356.2007 [DOI] [PubMed] [Google Scholar]
- 44. Ishibashi K., Sasaki S., and Marumo F. (1998) Molecular cloning of a new sodium bicarbonate cotransporter cDNA from human retina. Biochem. Biophys. Res. Commun. 246, 535–538 10.1006/bbrc.1998.8658 [DOI] [PubMed] [Google Scholar]
- 45. Jensen L. J., Schmitt B. M., Berger U. V., Nsumu N. N., Boron W. F., Hediger M. A., Brown D., and Breton S. (1999) Localization of sodium bicarbonate cotransporter (NBC) protein and messenger ribonucleic acid in rat epididymis. Biol. Reprod. 60, 573–579 10.1095/biolreprod60.3.573 [DOI] [PubMed] [Google Scholar]
- 46. Gunaratne H. J., Nomura M., Moy G. W., and Vacquier V. D. (2006) A sodium bicarbonate transporter from sea urchin spermatozoa. Gene 375, 37–43 10.1016/j.gene.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 47. Mannowetz N., Wandernoth P., Hornung J., Ruffing U., Raubuch M., and Wennemuth G. (2011) Early activation of sperm by HCO3− is regulated hormonally in the murine uterus. Int. J. Androl. 34, 153–164 10.1111/j.1365-2605.2010.01067.x [DOI] [PubMed] [Google Scholar]
- 48. Wertheimer E. V., Salicioni A. M., Liu W., Trevino C. L., Chavez J. C., Hernández-González E. O., Darszon A., and Visconti P. E. (2008) Chloride is essential for capacitation and for the capacitation-associated increase in tyrosine phosphorylation. J. Biol. Chem. 283, 35539–35550 10.1074/jbc.M804586200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wandernoth P. M., Raubuch M., Mannowetz N., Becker H. M., Deitmer J. W., Sly W. S., and Wennemuth G. (2010) Role of carbonic anhydrase IV in the bicarbonate-mediated activation of murine and human sperm. PLoS ONE 5, e15061–e15061 10.1371/journal.pone.0015061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wandernoth P. M., Mannowetz N., Szczyrba J., Grannemann L., Wolf A., Becker H. M., Sly W. S., and Wennemuth G. (2015) Normal fertility requires the expression of carbonic anhydrases II and IV in sperm. J. Biol. Chem. 290, 29202–29216 10.1074/jbc.M115.698597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. José O., Torres-Rodríguez P., Forero-Quintero L. S., Chávez J. C., De la Vega-Beltrán J. L., Carta F., Supuran C. T., Deitmer J. W., and Treviño C. L. (2015) Carbonic anhydrases and their functional differences in human and mouse sperm physiology. Biochem. Biophys. Res. Commun. 468, 713–718 10.1016/j.bbrc.2015.11.021 [DOI] [PubMed] [Google Scholar]
- 52. De La Vega-Beltran J. L., Sánchez-Cárdenas C., Krapf D., Hernandez-González E. O., Wertheimer E., Treviño C. L., Visconti P. E., and Darszon A. (2012) Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J. Biol. Chem. 287, 44384–44393 10.1074/jbc.M112.393488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McPartlin L. A., Suarez S. S., Czaya C. A., Hinrichs K., and Bedford-Guaus S. J. (2009) Hyperactivation of stallion sperm is required for successful in vitro fertilization of equine oocytes. Biol. Reprod. 81, 199–206 10.1093/biolreprod/81.s1.199,10.1095/biolreprod.108.074880 [DOI] [PubMed] [Google Scholar]
- 54. Chavez J. C., Ferreira J. J., Butler A., De La Vega Beltrán J. L., Treviño C. L., Darszon A., Salkoff L., and Santi C. M. (2014) SLO3 K+ channels control calcium entry through CATSPER channels in sperm. J. Biol. Chem. 289, 32266–32275 10.1074/jbc.M114.607556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mansell S. A., Publicover S. J., Barratt C. L., and Wilson S. M. (2014) Patch clamp studies of human sperm under physiological ionic conditions reveal three functionally and pharmacologically distinct cation channels. Mol. Hum. Reprod. 20, 392–408 10.1093/molehr/gau003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stival C., La Spina F. A., Baró Graf C., Arcelay E., Arranz S. E., Ferreira J. J., Le Grand S., Dzikunu V. A., Santi C. M., Visconti P. E., Buffone M. G., and Krapf D. (2015) Src kinase is the connecting player between protein kinase A (PKA) activation and hyperpolarization through SLO3 potassium channel regulation in mouse sperm. J. Biol. Chem. 290, 18855–18864 10.1074/jbc.M115.640326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de la Rosa D. A., Canessa C. M., Fyfe G. K., and Zhang P. (2000) Structure and regulation of amiloride-sensitive sodium channels. Annu. Rev. Physiol. 62, 573–594 10.1146/annurev.physiol.62.1.573 [DOI] [PubMed] [Google Scholar]
- 58. Kellenberger S., and Schild L. (2002) Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 82, 735–767 10.1152/physrev.00007.2002 [DOI] [PubMed] [Google Scholar]
- 59. Waldmann R., Champigny G., Bassilana F., Voilley N., and Lazdunski M. (1995) Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J. Biol. Chem. 270, 27411–27414 10.1074/jbc.270.46.27411 [DOI] [PubMed] [Google Scholar]
- 60. Kong X. B., Bin, Ma H. G., Li H. G., and Xiong C. L. (2009) Blockade of epithelial sodium channels improves sperm motility in asthenospermia patients. Int. J. Androl. 32, 330–336 10.1111/j.1365-2605.2008.00864.x [DOI] [PubMed] [Google Scholar]
- 61. Diao R., Fok K. L., Zhao L., Chen H., Tang H., Chen J., Zheng A., Zhang X., Gui Y., Chan H. C., and Cai Z. (2013) Decreased expression of cystic fibrosis transmembrane conductance regulator impairs sperm quality in aged men. Reproduction 146, 637–645 10.1530/REP-13-0146 [DOI] [PubMed] [Google Scholar]
- 62. Xu W. M., Shi Q. X., Chen W. Y., Zhou C. X., Ni Y., Rowlands D. K., Yi Liu G., Zhu H., Ma Z. G., Wang X. F., Chen Z. H., Zhou S. C., Dong H. S., Zhang X. H., Chung Y. W., et al. (2007) Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc. Natl. Acad. Sci. U.S.A. 104, 9816–9821 10.1073/pnas.0609253104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hernández-González E. O., Treviño C. L., Castellano L. E., de la Vega-Beltrán J. L., Ocampo A. Y., Wertheimer E., Visconti P. E., and Darszon A. (2007) Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J. Biol. Chem. 282, 24397–24406 10.1074/jbc.M701603200 [DOI] [PubMed] [Google Scholar]
- 64. Li C.-Y., Jiang L.-Y., Chen W.-Y., Li K., Sheng H.-Q., Ni Y., Lu J.-X., Xu W.-X., Zhang S.-Y., and Shi Q.-X. (2010) CFTR is essential for sperm fertilizing capacity and is correlated with sperm quality in humans. Hum. Reprod. 25, 317–327 10.1093/humrep/dep406 [DOI] [PubMed] [Google Scholar]
- 65. Torres-Flores V., García-Sánchez N. L., and González-Martínez M. T. (2008) Intracellular sodium increase induced by external calcium removal in human sperm. J. Androl. 29, 63–69 10.2164/jandrol.107.003368 [DOI] [PubMed] [Google Scholar]
- 66. Patrat C., Serres C., and Jouannet P. (2002) Progesterone induces hyperpolarization after a transient depolarization phase in human spermatozoa. Biol. Reprod. 66, 1775–1780 10.1095/biolreprod66.6.1775 [DOI] [PubMed] [Google Scholar]
- 67. Linares-Hernández L., Guzmán-Grenfell A. M., Hicks-Gomez J. J., and González-Martínez M. T. (1998) Voltage-dependent calcium influx in human sperm assessed by simultaneous optical detection of intracellular calcium and membrane potential. Biochim. Biophys. Acta 1372, 1–12 10.1016/S0005-2736(98)00035-2 [DOI] [PubMed] [Google Scholar]
- 68. Li P., Stewart R., Butler A., Gonzalez-Cota A. L., Harmon S., and Salkoff L. (2017) GABA-B controls persistent Na+ current and coupled Na+-activated K+ current. eNeuro 4, 114–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.