Abstract
Mohs micrographic surgery (MMS), a specialized surgical excision technique used primarily in the treatment of skin cancers, is tissue sparing and provides optimal margin control through evaluation of 100% of both the peripheral and deep margin. The use of MMS for the treatment of malignant melanoma (MM) and melanoma in situ (MIS) has been slow in gaining the same widespread acceptance that it has for keratinocyte carcinomas despite its cost-effectiveness and the growing body of evidence demonstrating similar or improved cure rates to standard wide local excision. However, modern advances in immunohistochemical staining have continued to greatly enhance the ability of Mohs surgeons to interpret MMS frozen sections of melanoma specimens – the primary concern of most opponents of MMS for melanoma. These advances, coupled with an increased recognition by professional organizations of the utility of MMS in treating MM and MIS, have led to a rise in the use of MMS for melanoma in recent years. Given the expanding role of MMS in the treatment of cutaneous melanoma, this manuscript will describe how MMS is performed, discuss the rationale and current evidence regarding the use of MMS for MM and MIS, review the immunohistochemical stains currently available for use in MMS, and consider special situations and future directions in this area of growing interest.
Keywords: Mohs micrographic surgery, melanoma, melanoma in situ, immunohistochemical stains, evidence, review
Introduction
Mohs micrographic surgery (MMS), a specialized surgical excision technique used primarily in the treatment of skin cancers, is tissue sparing and provides optimal margin control through evaluation of 100% of both the peripheral and deep margin. Eponymously named after Frederic Mohs who developed the procedure in the 1930s, MMS was initially named “chemosurgery” for its utilization of the chemical fixative paste zinc chloride prior to excision of the lesion.1 Although the original 1941 publication of his technique reported primarily on basal and squamous cell carcinomas,1 Mohs published a case series of twenty patients with cutaneous melanoma successfully treated with this chemosurgery less than a decade later.2,3 Despite the early evidence of the effectiveness of chemosurgery in treating both keratinocyte carcinomas and melanoma,1,2,4,5 the technique was not widely practiced until 1974 when a modified version (renamed MMS) using fresh tissue and frozen sections was first described.6,7 These modifications significantly reduced the time involved with the procedure and eliminated the additional discomfort caused by the zinc chloride paste without sacrificing cure rates.6–8 Today, MMS has become a mainstay in the treatment of keratinocyte carcinomas.9
The use of MMS for the treatment of malignant melanoma (MM) and melanoma in situ (MIS) has been slow to gain the same widespread acceptance that it has for keratinocyte carcinomas3,10 despite its cost-effectiveness11 and the growing body of evidence demonstrating similar or improved cure rates to standard wide local excision (WLE).1–5,7,8,10,12–28 Ninety percent of all MIS lesions are treated with WLE21 – the recommended standard of care by expert panels29,30 – although the difficulty in clearing MIS with WLE is becoming increasingly recognized.3,8,21,24,31–33 Meanwhile, modern advances in immunohistochemical staining have continued to greatly enhance the ability of Mohs surgeons to interpret MMS frozen sections of melanoma specimens3,8,34–38 which has been the primary concern of most opponents of MMS for melanoma.3,8,36 These advances, coupled with an increased recognition by professional organizations,9,29,30 have led to a rise in the use of MMS for melanoma in recent years.39 Given the expanding role of MMS in treating MM and MIS, this manuscript will describe how MMS is performed for melanoma, discuss the rationale and current evidence regarding the use of MMS for MM and MIS, review the immunohistochemical stains currently available for use in MMS, and consider future directions in this area of growing interest.
Mohs micrographic surgery for MM and MIS
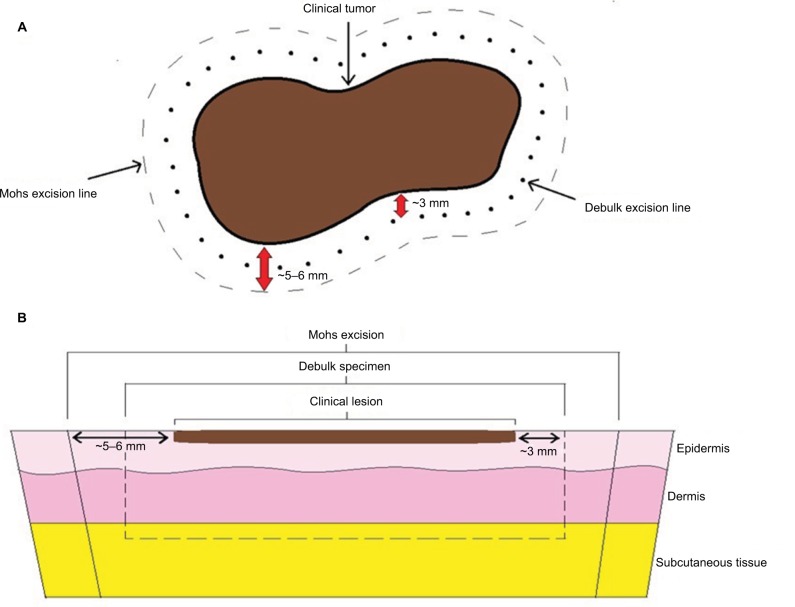
As previously noted, MMS is being increasingly used to treat both MM and MIS. From 2003 to 2008, the utilization of MMS to treat melanoma rose by 60% with 3.5% of all SEER-documented melanomas being treated with MMS during this time period.39 In a 2012 survey of 378 fellowship-trained Mohs surgeons in the US, 39% reported they currently perform MMS for MIS and 14% indicated they perform MMS on MM.35 While the general technique of MMS for melanomas (Figure 1) is similar to that for keratinocyte carcinomas, some important distinguishing features exist and will be described herein.
Figure 1.
Schematic of Mohs micrographic surgical excision for melanoma. (A) Clinical view (B) Cross-sectional view.
Fixed-tissue technique
The fixed-tissue technique has been largely replaced by fresh-tissue Mohs due to the additional discomfort and time involved with the procedure and is primarily of historical significance.40 Therefore, a detailed description of how the technique is performed will not be provided in this article but is summarized well by Hui et al.8
Fresh-tissue technique
The first step in MMS for melanoma is to identify and mark the lesion’s clinical borders (Figure 2).3,8 Many advocate for the use of a Wood’s lamp to assist in the identification of the tumor’s extent, particularly for lentigo maligna (LM) and lentigo maligna melanoma (LMM) as these lesions are notorious for their irregular and often indistinct clinical borders.3,8,29,41,42 After identifying and marking the precise clinical border of the lesion, the surgeon often then marks the initial margin which may be anywhere from 2 to 10 mm around the clinical border depending on the size of the lesion, anatomic location, and tumor stage. If tissue conservation is imperative due to anatomical or functional considerations (e.g., tip of nose and abutting tear duct), the surgeon may use the clinical borders of the lesion as the peripheral margin for the debulking specimen.43 However, it is generally recommended that an additional 2–3 mm of normal appearing tissue surrounding the clinical borders of the lesion be taken for the debulking specimen.19 Greater margins are suggested for high-risk tumors (American Joint Committee on Cancer [AJCC] 7th edition44 tumor stage T1b or greater).19
Figure 2.
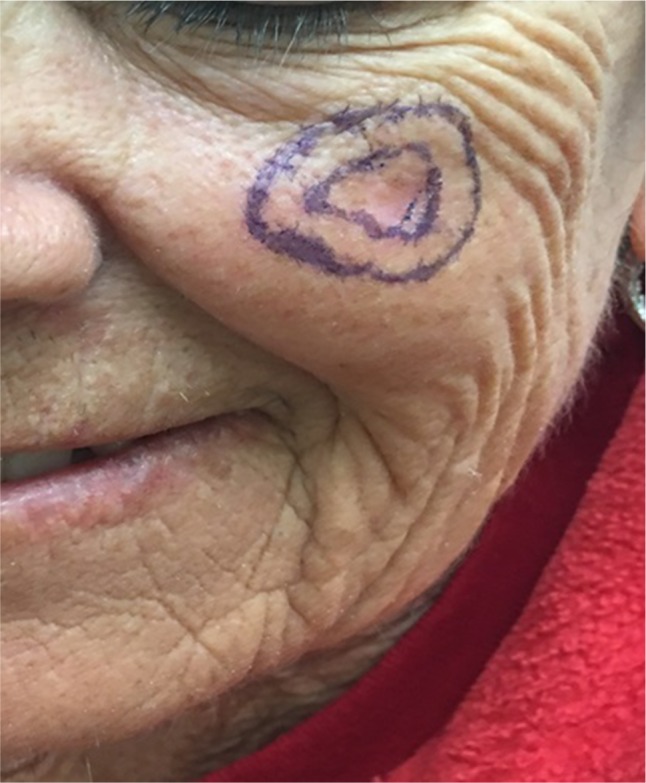
Pre-operative photograph of an MIS of the face outlining the clinical margins examined with a Wood’s lamp (inner marking) and Mohs excisional specimen (outer marking).
Reference marks or suture mapping are often included for orientation purposes, and the debulking specimen is excised down to at least the subcutaneous fat and sent for permanent/paraffin-embedded processing for vertical sectioning at 2–3 mm intervals.19 Alternatively, some surgeons will remove a 1 mm strip of tissue from the central portion of the debulk specimen and process it via frozen section to confirm the Breslow depth and serve as a positive control, sending the remainder of the specimen for permanent sections.43 Regardless of the strategy used, it is critically important to evaluate this central debulking specimen to confirm the final depth of tumor invasion for final staging of the melanoma. Since MMS only examines the peripheral and deep margins of the tumor, an invasive or desmoplastic melanoma within the debulking specimen could be missed if it is not evaluated using vertical sectioning. For this reason, the debulking specimen is necessary for staging the tumor, which in turn is used to decide if adjuvant therapeutic or diagnostic/staging procedures, such as sentinel lymph node biopsy, are warranted.45
The first Mohs layer is subsequently removed around the debulk defect including a peripheral margin of at least 2–3 mm of normal appearing tissue. The surgeon should be careful to excise down to at least the deep subcutaneous fat for MIS lesions (to ensure being under hair follicles) and to the fascia for MM lesions.29 This margin may be extended for high-risk lesions (AJCC 7th edition tumor stage T1b or greater) as described for the debulking stage, but formal guidelines regarding margin width do not yet exist. In such cases, Etzkorn et al19 recommend a combined initial margin of 1 cm (debulk margin added to first Mohs layer margin), unless anatomical or functional restrictions exist. When excising Mohs layers for MM of MIS lesions, some have advocated against the usual 45° scalpel beveling in favor of a less pronounced 60–75° bevel, noting that it diminishes the melanocyte density in the sections and thereby improves histological interpretation.10 For the Mohs layer, reference marks are made on the surface of the skin that overlap the perilesional skin and the Mohs specimen with nicks at 0°, 90°, 180°, and 270°.19 The Mohs layer is subsequently divided into a number of smaller subsections depending on the size of the specimen, and the free edges of each subsection are inked. A surgical map of the gross sectioning is drawn to help orient the surgeon in the event that residual tumor is identified.
The inked subsections can then be processed in a variety of ways, including traditional frozen sections, frozen sections with immunostaining, frozen sections with the last layer sent for permanent sections, or staged excision with rush en face permanent sections for each layer.3 All of these methods allow for evaluation of 100% of the peripheral and deep margins, and the rationale and evidence for these will be discussed later. For frozen sections, each subsection is oriented on a cold glass slide and impregnated with embedding medium. The subsections are then rapidly frozen using a cryostat object disc and ultimately cut into 4 µm (thinner) horizontal/en face sections.43 Appropriate freezing of the tissue ensures that there is minimal freeze artifact vacuolization, which can improve slide interpretation.3 Once adequate sections have been obtained, the tissue is stained with H&E and any immunohistochemical stains desired. If the Mohs surgeon detects frank residual tumor, nesting of more than three atypical melanocytes,3 pagetoid spread, multiple nested melanocytes, widespread contiguous melanocytes (> 9 confluent melanocytes), and/or adnexal involvement when analyzing the slides,3,46 the area(s) of residual melanoma is marked on the map and the surgeon takes another layer with at least 2–3 mm margins only in the involved area(s).3 Additionally, if signs of regression are evident at the margins (e.g., fibrosis, dermal macrophages, and/or a dense lymphohistiocytic infiltrate), the removal of an additional Mohs layer may be indicated.47 This process is repeated until histologically clear margins are achieved.
Rationale for the use of MMS for MM and MIS
The incidence of primary cutaneous melanoma has continued to rise over the past several decades. Fortunately, when detected early, the disease is almost always curable. Standard surgical excision with histologically clear margins is the current standard of care for the treatment of primary cutaneous melanomas of any thickness. In general, cutaneous melanoma can be categorized as in situ (MIS) or invasive disease (MM) depending on if the melanoma is or is not confined to the epidermis, respectively. Within the group of MIS, LM is the most common subtype, accounting for ~80% of all MIS lesions. The prevalence of LM varies greatly between different demographic groups but is most prevalent among white men aged 65 years or older, typically presenting as an asymmetric pigmented lesion on a background of chronically sun-damaged skin. The invasive counterpart of LM –LMM – presents similarly and comprises 4%–15% of cutaneous malignant melanomas.48
Although WLE remains the recommended standard of care for the treatment of MM and MIS,29,30 there are many features of MMS which may make it a more ideal treatment modality in certain situations (Table 1). As previously noted, MMS is a tissue sparing technique and may therefore be particularly suited to treat melanomas of large diameter and lesions occurring in areas where tissue preservation is critical either for cosmetic or functional reasons.8 Examples of such areas include the eyelids, cheeks, nose, lips, ears, and neck. In the case of LM and LMM, the majority of these lesions occur on these cosmetically sensitive areas.3 Given the asymmetric peripheral growth pattern of many MM and MIS lesions, the predetermined margins used in standard WLE may be both inadequate and excessive within the same lesion. With MMS, this issue is avoided, and tissue is spared because the surgical margins are tailored to what is found histologically. Furthermore, MMS is uniquely capable of evaluating 100% of both the peripheral and deep margins through its use of en face/horizontal tissue sections. This processing is in contrast to standard WLE in which the excision specimen is evaluated by stepwise transverse vertical sections, allowing histologic examination of only 0.1% of the total margin.8 In order to detect 100% of positive margins with this bread-loaf sectioning, Kimyai-Asadi et al extrapolated that stepwise sections would need to be performed every 0.1 mm.49 This approach would be logistically implausible and leaves the possibility of false-negatives with WLE. Furthermore, MMS allows for same day surgery, histopathologic evaluation, and reconstruction, which are often not possible with WLE.
Table 1.
Comparison of the benefits and drawbacks of Mohs micrographic surgery versus wide local excision for melanoma
| Mohs micrographic surgery (MMS) | Wide local excision (WLE) | |
|---|---|---|
| Benefits | • Equal or improved recurrence and 5-year survival rates vs WLE • 100% of peripheral and deep margins evaluated • Tissue sparing technique • Tumor removal, microscopic evaluation, and repair are performed on the same day (for fresh tissue frozen sections) • Potentially more cost-effective vs WLE • Particularly suited to treat LM/LMM due to high prevalence of subclinical spread |
• Considered the gold standard for the treatment of MM and MIS • Long history of success ○ More data available • Does not require specialized (e.g., fellowship) training to perform • Excision procedure is faster than MMS • Utilizes permanent sections which are considered to be the gold standard for melanocytic lesions |
|
| ||
| Drawbacks | • Inability to determine the extent of the cleared margins • Interpretation of frozen sections can be tenuous in settings of chronic sun damage, inflammation, and bordering pigmented lesions ○ Success is dependent on ability of surgeon to evaluate frozen sections • Requires a contiguous growth pattern of the tumor to be reliable • Uncertain role when microsatellites are identified in Mohs layer |
• Only 1% of total margins are evaluated with vertical sections • Some studies suggest higher rates of local recurrence vs MMS • Not designed to be tissue sparing • Delay between surgical excision and pathology results ○ If positive margins found, patient must return for further excision and wait again • May be more costly than MMS |
Abbreviations: LM, lentigo maligna; LMM, lentigo maligna melanoma; MM, malignant melanoma; MIS, melanoma in situ.
The comprehensive margin control afforded by MMS is particularly useful in situations where the clinical borders of a melanoma are indistinct, as is characteristic of LM, LMM, and especially amelanotic LMM.22 In fact, one of the strongest arguments for the use of MMS in LM/LMM in particular is the issue of subclinical spread.3,8,10,13,16,18,24,25,28,37,50 Subclinical spread occurs in 12%–71% of LM cases and refers to the microscopic extension of the tumor beyond the clinically apparent borders.24,26,50,51
For many years, the recommended margins for standard surgical excision of MIS were 5 mm.3,8 Over time, a wealth of data began to accumulate which showed through the use of MMS that this recommendation was inadequate. In 1997, Zitelli et al28 reported that 9 mm margins would be necessary to clear 95% of MIS cases using MMS and suggested that 1–1.5 cm standard surgical excision margins should be employed, depending on tumor diameter and location. More recent studies by Zalla et al52 and Felton et al53 reported similar findings, noting that 1.5 cm margins would be required to clear 96%–97% of MIS lesions on the head and neck, while standard 5 mm margins would only clear 65% of these lesions.53 These findings have also been replicated for MIS lesions on relatively sun-protected skin (i.e., the trunk and proximal extremities) with Stigall et al24 and Valentin-Nogueras et al26 requiring 9 mm margins to achieve a 97% clearance rate. Finally, a literature review on the topic has reported 3–5-year recurrence rates of 6%–20% for LM following standard surgical excision with 5-mm margins.54
As a result of this evidence, the recommended standard surgical excision margin for MIS was recently changed to 0.5–1 cm, with the caveat that larger margins may be required for large diameter lesions and those of the LM subtype.29,30 Citing the lack of prospective studies on appropriate standard surgical margins for MIS and the common issue of subclinical spread, the appropriateness of MMS in treating MIS and LM in particular is becoming increasingly recognized.9,29 In an effort to better predict the situations in which subclinical spread is likely to occur, Shin et al collected clinical and histopathological data on 674 MIS lesions treated with MMS and evaluated by melanoma antigen recognized by T cells 1 (MART-1) stained frozen sections.50 The authors subsequently performed multivariable logistic regression analysis to identify the clinical factors associated with subclinical spread and found that a history of prior treatment; location on the head, neck, acral sites, or the pretibial leg; lesions greater than 1 cm in diameter; and lesions occurring in persons aged 60 years or older were all associated with increased risk of subclinical spread.50 However, in the aforementioned Felton et al’s53 study of primary MIS lesions of the head and neck, neither patient’s age nor preoperative tumor size was predictive of surgical margins. In both studies, histological subtype (i.e., LM vs non-LM) was not routinely documented and therefore could not be incorporated into their analysis. Further research in this area would be of value, and until such time, predicting subclinical spread in MIS remains a tenuous endeavor. Finally, the use of MMS to treat melanoma is supported by a substantial amount of evidence indicating that it provides cure rates equal to or exceeding those of WLE for MIS and MM.1–5,7,8,10,12–28
Evidence regarding the effectiveness of MMS versus WLE for MM and MIS
The use of standard surgical excision (WLE) for LM often leads to high rates of local recurrence (6%–20%)54–56 and has prompted several investigations into the efficacy of MMS as a treatment modality for these lesions. A common limitation of these studies and similar ones on MM is that there is often a failure to differentiate between histologic subtypes (i.e., LM vs non-LM, or LMM vs non-LMM). Therefore, when describing these studies herein, we have utilized more broad terminology (MIS, MM) unless the specific subtype was documented.
In a recent literature review, Kwon and Miller summarized the local recurrence rates for MIS treated with MMS and showed that regardless of the method of margin evaluation, local recurrence rates were superior to that of WLE.3 The only study reporting a poor local recurrence rate with MMS (33% at 118 months of follow-up) had a small sample size of 18 LM lesions which were evaluated via H&E-stained frozen sections.57 In the other larger studies utilizing H&E-stained frozen sections for margin evaluation, the local recurrence rate ranged from 0% (67 LM, 9 non-LM lesions) to 0.5% (184 MIS lesions) with 33– 60 months of follow-up.14,15,28 In the two studies using frozen sections with permanent sections for the final Mohs layer, recurrence was 1.8% (2.6% among 51 non-LM vs 1.4% among 116 LM lesions) at 63 months follow-up12 and 2.6% (38 LM/LMM lesions) at 58 months follow-up.17 Among studies that utilized frozen sections with immunostains, local recurrence rates of 0.3% at 38 months (human melanoma black-45 [HMB-45] staining of 261 MIS lesions)13 and 0.5% at 58 months of follow-up (Mel-5 staining of 158 LM lesions)15 were reported. For studies of staged-excision with permanent sections for each Mohs layer, local recurrence rates ranged from 0% to 7.3% over 3–57 months of follow-up with the majority of studies reporting on LM.57–68
Many of the studies of LM cited earlier also included data on LMM treated with MMS and noted similar recurrence rates.13,14,17,57,58,60,64,65,68 In the aforementioned Zitelli et al’s study,28 the authors also investigated the efficacy of MMS with H&E-stained frozen section evaluation in treating 369 primary MM lesions. From their study, they reported a local recurrence rate of 0.5% as compared to 3% from historical WLE controls. Lower rates of metastasis independent of tumor thickness and equivalent or improved 5-year survival rates were also observed compared to historical WLE controls (the 0.76–1.49 mm and > 4 mm thick groups had statistically improved survival rates).28
Since the review by Kwon and Miller,3 multiple large-scale studies have emerged which report improved or non-inferior outcomes for both MM and MIS treated with MMS compared to WLE. Etzkorn et al performed a retrospective analysis of 161 MM and 436 MIS lesions in 563 patients who underwent MMS with MART-1 frozen-section staining.19 Over a mean follow-up time of 2.8 years, the local recurrence rate was 0% for MM and 0.34% for the MIS group. A similar retrospective study by Valentin-Nogueras et al of cutaneous melanoma treated with MMS utilizing MART-1 staining found a local recurrence rate of 0.49% among 1,419 primary melanomas (0.35% among 863 MIS lesions vs 0.72% among 556 MM lesions) over a mean follow-up of 3.73 years, and a 5-year survival rate of 98.5% which was statistically better than historical WLE controls.26 Of note, they found that the surgical margin required to clear the lesion was not statistically related to Breslow thickness, on which guidelines for WLE are currently based upon,29 but rather was related to tumor location and size.26 Lastly, the first known directly comparative study of MMS versus WLE for the treatment of MIS was recently published by Nosrati et al.21 In this retrospective review of a prospective database from an academic tertiary care referral center, 277 patients with MIS were treated with frozen-tissue MMS with H&E staining while 385 were treated with WLE. The MMS group was statistically significantly older than the WLE group by an average of 5.4 years and their MIS was more likely to be located on the face, scalp, or neck than the WLE group. The study found no significant differences in the recurrence rate, overall survival, or melanoma-specific survival between MMS and WLE.21 While many studies in the current literature are unable to provide long-term survival data, the improvements in local recurrence rates have important implications as recurrent melanomas are likely to be at a more advanced stage than the original lesion. This was shown in a study by Debloom et al in which 23% of patients with MIS left at the margin progressed to MM, and 33% of patients with minimally invasive MM remaining at the margin experienced a mean progression in Breslow depth from 1.5 mm to 2.8 mm at the time of recurrence.69
In light of the current evidence, the Ad Hoc Task Force’s 2012 appropriate use criteria statement deemed the use of MMS “appropriate” for the treatment of all MIS and LM lesions with the exception of primary lesions on the trunk or extremities (“uncertain” appropriateness). Notably, the Task Force chose to omit any commentary on MM from their statement, citing the “complexity of the issue”.9
Challenges regarding the use of MMS for MM and MIS
The primary concern of critics of MMS for melanoma is the perceived inferiority of frozen sections to permanent paraffin sections in evaluating melanocytic lesions.3,8 Part of the reason permanent sections are considered the gold standard in evaluating melanocytic lesions is that melanocytes retain their pericytoplasmic vacuolization with this method, allowing them to be more readily identified.3 In comparison, frozen sections can be plagued by freeze artifact, tissue folding,8 and keratinocyte vacuolization resembling melanocytes which can lead to false positives.70
There have been conflicting results in studies where the accuracy of frozen sections has been compared to permanent sections. In these studies, the sensitivity and specificity of frozen sections for detecting melanoma in LM and LMM lesions have ranged from 59% to 100% and from 81% to 90%, respectively.47,71 Furthermore, diagnostic discrepancy between frozen and permanent sections for melanocytic lesions was found to be as high as 40% in one such study.72 Perhaps the most common factor contributing to the difficulty of interpreting frozen sections is distinguishing non-malignant melanocyte hyperplasia occurring in chronically sun-damaged skin from MIS.3,8,73,74 This issue is heightened at the outermost edges of the lesion where there is often single atypical melanocytes which could represent chronic sun damage or the edge of the MIS.3 In the literature, investigators disagree on how to deal with these single atypical melanocytes, with some arguing to treat them as benign actinic damage12,15,28 while others suggest they be excised and processed via permanent sections to rule out MIS.71
Multiple studies have tried to better classify the normal distribution and density of melanocytes histopathologically in chronically sun-damaged skin to help physicians more accurately differentiate MIS from actinic damage.46,75,76 In a 2006 study by Hendi et al where frozen section slides from patients undergoing MMS for keratinocyte carcinoma were randomly selected to undergo MART-1 staining, a mean melanocyte count of 15 per high-power field, superficial follicular extension (<1 mm), confluence of up to nine melanocytes, and scattered nonspecific staining of dermal cells were found to occur in sun-damaged skin.46 The authors noted that nesting and pagetoid spread were not present,46 a finding which was replicated in a later study by Barlow et al which added that vertical stacking of melanocytes should be considered a factor that warrants harvesting of another Mohs layer.76 A more recent investigation by Hendi et al replicated many of their original findings but added that differences in melanocyte density can be expected relative to geographic location (South > North), age (inverse relationship), and sex (male > female).75 Of note, the more recent study refuted the original finding that focal pagetosis is unique to melanoma, further illustrating how challenging differentiating chronic actinic damage from MIS can be.75
There are a variety of strategies to address these concerns discussed in the literature. One strategy to help with slide interpretation is the use of control biopsies taken from the patient’s skin adjacent to the lesion, which serve as a measure of the patient’s background melanocytic density.10,16,42,52,62,74 Albertini et al favor shallow shave biopsies harvested from both perilesional and distant skin and also describe the use of a mapping technique which matches subtle pigmented lesions clinically and histologically.10 With this mapping technique, the authors report a decrease in superfluous removal of Mohs layers in their clinical practice.
Another conservative strategy is to use staged excisions with rush permanent sections.57,58,60,63,64,68,71,72 In this approach, each Mohs layer is sent for permanent paraffin sectioning, and a pathologist reads the slides. Due to the tissue processing and evaluation time involved with this method, the surgery is substantially prolonged and may even require several days to weeks to achieve clear margins – hence the nickname of “slow Mohs”.3 Recently, Mallipeddi et al77 described a novel 2-hour method for preparing permanent sections of MIS for MMS through microwave tissue processing with comparable results – whether or not this has gained widespread use is yet to be seen. Despite its time-consuming nature, permanent sectioning has long been considered the gold standard for melanocytic lesion evaluation for the reasons previously described. However, fast and reliable immunohistochemical stains are now increasingly being used as an adjunct to H&E-stained frozen sections3,8,35,36,38 and can provide identical information to permanent sections.3,8,35,36,38,42,73,78,79
Immunohistochemical stains for melanoma
Over the past few decades, the expansion in the number of immunohistochemical stains available and the development of expedited staining protocols have led to increased utilization of immunostaining in Mohs surgery.36 In addition to its use for the challenging situations previously described, immunostaining for various tumors is useful in a variety of situations such as when the tumor is poorly differentiated, exhibits single cell spread, tracks along nerves or vessels, or when pagetoid distribution is present.36 Regardless of the immunostain or staining protocol used, 4-µm thin tissue sections are preferred as they provide the best staining results without masking cellular detail.36 Immunostaining can be applied to both permanent and frozen sections3,8 and is performed concurrently with H&E staining. The amount of literature regarding immunostains and staining protocols used in MMS for melanoma is so extensive that entire review articles have been dedicated to the topic.35,36,38
In 2012, 378 fellowship-trained US Mohs surgeons were surveyed on their use and attitudes toward immunostains for melanoma.35 Despite the largely positive attitudes surrounding the use of MMS for melanoma (90% felt that immunostains can be reliably used in Mohs, with more than half considering themselves advocates for the use of immunostains for MIS), only one-fifth of the 44% of surgeons performing MMS on melanocytic lesions reported using immunostains.35 The most common reasons for not using immunostains cited by respondents were: 1) too time consuming (45%), 2) lack of education (43%), and 3) startup and/or maintenance costs (42%).35 Interestingly, the percentage of Mohs surgeons who reported exposure to immunohistochemical staining during their fellowship training nearly doubled from trainees prior to 2000 (12%) compared to post-2000 (22%) but has since stagnated (26% for trainees from 2009 to 2012), with only 36% of all the Mohs surgeons surveyed reported feeling comfortable interpreting MART-1 stained frozen sections – the most commonly used immunostain for melanoma.35
For the purposes of this review, the specific details of staining protocols will not be discussed except to say that new staining protocols have reduced staining times from several hours to under 45 minutes – the time it takes to prepare traditional H&E-stained frozen sections.3,36 The major immunohistochemical stains have been summarized in Table 2, and additional discussion of MART-1 and SOX10 staining has been provided in the following section.
Table 2.
Summary of common immunohistochemical stains used in Mohs micrographic surgery for melanoma
| Immunostaining antibodies | Cellular targets | Stained cells | Strengths | Weaknesses |
|---|---|---|---|---|
| S-100 | • Intracellular calcium | • Melanocytes • Neural crest derivatives • Histiocytes • Chondrocytes • Lipocytes • Muscle |
• Best sensitivity for melanoma • Stain of choice for spindle cell and desmoplastic melanoma • Strong positive staining for deep melanoma components |
• Variable staining of epidermis ○ Problematic for MIS • Poor specificity ○ High background noise |
|
| ||||
| Human melanoma black-45 (HMB-45) | • gp100 glycoprotein on cytoplasmic premelanosomes | • Immature or proliferating melanocytes • Other cells containing melanosomes |
• Greater specificity than S-100 | • Less sensitive than S-100 (85%–97%) • Inconsistent staining of pseudonevoid nests ○ False negatives • Poor staining of spindle cell and desmoplastic melanomas |
|
| ||||
| Mel-5 | • g75 pigment-associated glycoprotein on cytoplasmic melanosomes | • Melanocytes • Other cells with melanosomes |
• Stains both proliferating and mature melanocytes • Greater specificity and staining intensity than S-100 |
• Less specific than HMB-45 • Stains nonmelanocytic pigmented cells (e.g., basal epithelial cells, pigmented AKs) • May miss amelanotic or desmoplastic melanomas |
|
| ||||
| Micropthalmia transcription factor (MiTF) | • Transcription factor (nuclear) | • Melanocytes • Schwann cells • Histiocytes • Fibroblasts • Lymphocytes • Smooth muscle |
• Great sensitivity and high specificity (88%–100%) • Nuclear staining ○ Clearly delineates individual cells ○ Ideal for melanocyte quantification and nucleus diameter measurement ○ Improves differentiation of chronic sun damage from MIS • Stains epithelioid and spindle cell melanoma • Useful for metastatic melanoma |
• Poor staining of desmoplastic melanoma |
|
| ||||
| Melanoma antigen recognized by T cells (MART-1) | • MART-1 glycoprotein on cytoplasmic melanosomes | • Melanocytes • Other cells with melanosomes ○ Adrenal cortex ○ Testis, ovary ○ Retina |
• Most useful immunostain • High sensitivity and specificity • Easiest to interpret, crisp staining of melanocytes • Fast immunostaining protocols • Useful for metastatic melanoma |
• Does not distinguish benign from malignant melanocytes • Not reliable for desmoplastic or spindle cell melanoma • Severely sun damaged or inflamed skin can lead to false positives |
|
| ||||
| Sry-related HMG-BOX gene 10 (SOX10) | • Transcription factor (nuclear) | • Melanocytes • Schwann cells • Eccrine glands |
• High sensitivity and better specificity than MiTF or S100 • Useful for desmoplastic and spindle cell melanoma • Nuclear staining ○ Clearly delineates individual cells ○ Ideal for melanocyte quantification and nucleus diameter measurement ○ Improves differentiation of chronic sun damage from MIS ○ Useful for metastatic melanoma |
• Does not distinguish benign from malignant melanocytes |
Melanoma antigen recognized by T cells (MART-1) immunostain
Each immunostain has its strengths and weaknesses (Table 2), but MART-1 (also called Melan-A) has been considered by many to be one of the most accurate and reliable stains for melanoma.8,10 MART-1 has excellent sensitivity for both primary (97%) and metastatic (81%–89%) melanoma,8,36 demonstrating homogenous staining in roughly 75% of tumor cells in the majority of melanoma lesions.80 The use of MART-1 as an adjunct to H&E-frozen sections is increasingly common3,8,36 due to the advent of rapid (20 minutes or less) automated staining protocols42,73,81,82 and multiple studies demonstrating its ability to detect junctional and focal dermal melanocytic proliferation on frozen sections.10,81 Furthermore, investigators have shown that the information gleaned from immunostained frozen sections and permanent sections is equivalent73,78,79 and that frozen sections stained with MART-1 are easier to interpret than H&E-stained permanent sections.42 While critics of MMS with frozen sections for melanoma have historically argued for staged excision by permanent sections, these data suggest that immunostained frozen sections are just as reliable and similarly easily interpretable as traditional gold standard permanent sections. Since MART-1 stains normal background melanocytes and malignant melanocytes, internal controls are inherent with the use of MART-1.36 A notable benefit of MART-1 is its ability to clearly differentiate atypical clear cells resulting from freeze artifact and pagetoid keratinocyte atypia from malignant melanocytes.36 In head-to-head studies of immunohistochemical stains (MART-1, HMB-45, Mel-5, S100), MART-1 was found to be the easiest to interpret and provides the most reliable epidermal staining and identification of melanocytes at frozen section margins (Figure 3).10,52
Figure 3.
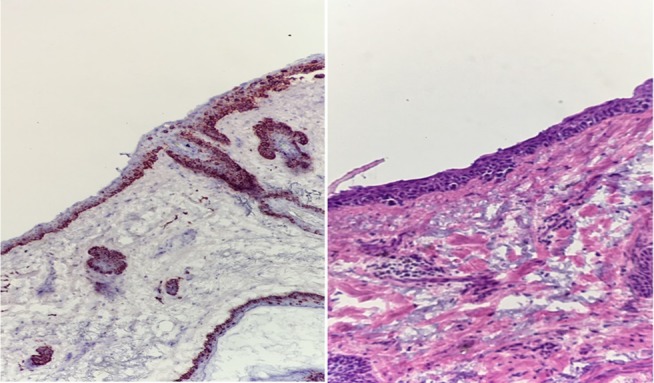
Photomicrographs of positive margins on MART-1 immunostain (left) versus H&E-stained (right) frozen sections displaying how MART-1 improves interpretability of MMS frozen sections for melanoma.
MART-1 staining has some shortcomings, including increased false-positive rates in the setting of pigmented actinic keratoses,83 severely sun-damaged skin,84 or inflamed skin,85 and the inability to distinguish benign from malignant melanocytes.36 In the setting of sun damage or inflammation, melanocytes may degenerate and the resultant debris surrounding keratinocytes and engulfed by macrophages can lead to artifact that can be mistaken for melanocytic nests.84,85 Additionally, MART-1 poorly stains desmoplastic and spindle cell melanomas.36 In situations where diffuse staining is problematic due to extensive solar lentigines, melanocyte hyperplasia, or darker skin types, there is some evidence to suggest that micropthalmia transcription factor (MiTF) may be helpful as an adjunct or alternative to MART-1.36,86 Since MiTF only stains the nucleus, the stain burden is decreased and slide interpretation may be improved.36,86 Although MART-1 remains the mainstay immunostain used in MMS for melanoma, SOX10 is gaining popularity.
SRY-related HMG-box 10 (SOX10) immunostain
SOX10 is a nuclear transcription factor expressed by cells of neural crest lineage.87–89 On formalin-fixed sections, SOX10 immunostain has demonstrated sensitivities approaching 100% for primary, in situ, desmoplastic, and spindle cell melanomas.87,89 In contrast to S-100 which has historically been considered the gold standard for desmoplastic and spindle cell melanomas,10,52 SOX10 has markedly better specificity.87 Similar to MiTF, SOX10 displays a crisp nuclear staining pattern which improves differentiation of LM from chronically sun-damaged skin89 – one of the major weaknesses of MART-1 staining. Furthermore, SOX10 is more specific than MiTF and is particularly useful for excision scars as it has been found to be less likely to stain background histiocytes and fibroblasts than either S-100 or MiTF.88 Recently, successful rapid SOX10 immunostaining of frozen sections for MMS has been described in the literature.89 Due to its versatility and relatively few weaknesses, SOX10 has the potential to become the cornerstone immunostain in MMS for melanoma in the near future. Further comparative studies of SOX10 to other common immunostains are warranted.
Special situations
Upstaging occurs when the debulking excision specimen meets criteria for an increased T category in the AJCC 7th edition melanoma staging classification system compared to the original biopsy specimen.19 In a review by Kwon and Miller, the prevalence of upstaging during MMS for LM was found to range from 5% to 67%.3 In an effort to understand the clinical and patient characteristics associated with upstaging, Iorizzo et al looked for differences in the characteristics of upstaged MIS lesions (8.1% of 173 cases analyzed) compared to non-upstaged lesions treated with MMS and found no statistically significant predictors of upstaging.45 These findings illustrate the importance of sending the debulking specimen for permanent sections without exception.
In cases where upstaging occurs, and the Breslow depth is found to be ≥0.76 mm, the patient becomes a candidate for sentinel lymph node biopsy (SLNBx) and should be engaged in a conversation regarding its role in their management. Whenever possible, reconstruction should be deferred until after SLNBx to preserve normal lymphatic drainage as best as possible, but MMS should be continued until completion.19
Limitations of Mohs for melanoma
The use of MMS for melanoma is not without limitations. First, MMS is unable to assess how much margin of clearance is achieved which may or may not have implications for recurrence. Second, the success of MMS is highly dependent on the surgeon’s ability to accurately interpret frozen sections. There have been several studies in recent years which have demonstrated over 99.4% concordance between Mohs surgeon and dermatopathologist interpretation of non-melanoma skin cancer frozen sections.90–93 While these studies suggest that Mohs surgeons are as capable as dermatopathologists in frozen section interpretation, similar larger-scale studies assessing melanoma frozen section interpretation would be helpful. Third, MMS is dependent on the tumor having a contiguous growth pattern without any skip areas, and uncertainty exists regarding if and how one should proceed with MMS when microsatellites are discovered within MMS sections.8 If microsatellites are seen, the growth pattern of the tumor can no longer be considered contiguous and it becomes impossible to call MMS margins “clear” with any certainty. Additionally, the presence of microsatellites is associated with increased risk of regional node involvement and the role of SLNBx should be considered.8
Future directions
Recent research has indicated that in vivo reflectance confocal microscopy, through its ability to identify atypical melanocytes and other features of melanoma at a cellular level, may have a future role in assisting in determining the ideal area within a suspected LM or LMM to take a biopsy and could also be used adjunctively during MMS.94,95 To our knowledge, patient satisfaction with MMS versus WLE and its role in the surgical management of melanoma have yet to be investigated and would be of value. Although the preliminary evidence to suggest MMS is more cost-effective than WLE for melanoma,11 larger and more detailed analyses incorporating recurrence and survival data into the cost-savings calculations are warranted. Future research on this topic would benefit from clearly delineating histological subtypes of melanoma when possible (e.g., LM vs superficial spreading), as this is currently a common limitation in the literature. Doing so will provide more specific outcomes data upon which the most accurate treatment recommendations can be made. Finally, there are currently no randomized clinical trials comparing long-term recurrence and survival for melanoma treated with MMS versus WLE – such a study could be influential in shaping attitudes and guidelines surrounding the surgical management of melanoma.
Footnotes
Consent for publication
Written informed consent was obtained to publish the patient image included in this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mohs FE. Chemosurgery: a microscopically controlled method of cancer excision. Arch Surg. 1941;42(2):279–295. [Google Scholar]
- 2.Mohs FE. Chemosurgical treatment of melanoma: a microscopically controlled method of excision. Arch Derm Syphilol. 1950;62(2):269–279. doi: 10.1001/archderm.1950.01530150091011. [DOI] [PubMed] [Google Scholar]
- 3.Kwon SY, Miller SJ. Mohs surgery for melanoma in situ. Dermatol Clin. 2011;29(2):175–183. vii–viii. doi: 10.1016/j.det.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Mohs FE. Chemosurgery for the microscopically controlled excision of cutaneous cancer. Head Neck Surg. 1978;1(2):150–166. doi: 10.1002/hed.2890010209. [DOI] [PubMed] [Google Scholar]
- 5.Mohs FE. Chemosurgery for melanoma. Arch Dermatol. 1977;113(3):285–291. [PubMed] [Google Scholar]
- 6.Tromovitch TA, Stegman SJ. Microscopically controlled excision ofskin tumors: chemosurgery (Mohs): fresh tissue technique. Arch Dermatol. 1974;110:231–232. [PubMed] [Google Scholar]
- 7.Nagi C, O’Grady TC, Izadpanah A. Mohs micrographically controlled surgery and the treatment of malignant melanoma. Semin Oncol. 2002;29(4):336–340. doi: 10.1053/sonc.2002.34111. [DOI] [PubMed] [Google Scholar]
- 8.Hui AM, Jacobson M, Markowitz O, Brooks NA, Siegel DM. Mohs micrographic surgery for the treatment of melanoma. Dermatol Clin. 2012;30(3):503–515. doi: 10.1016/j.det.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Hoc Task Ad F. Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531–550. doi: 10.1016/j.jaad.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Albertini JG, Elston DM, Libow LF, Smith SB, Farley MF. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28(8):656–665. doi: 10.1046/j.1524-4725.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RP, Butala N, Alam M, Lawrence NA. Retrospective case-matched cost comparison of surgical treatment of melanoma and non-melanoma skin cancer in the outpatient versus operating room setting. Dermatol Surg. 2017;43(7):897–901. doi: 10.1097/DSS.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 12.Bene I, Healy C, Coldiron BM. Mohs micrographic surgery is accurate 95.1% of the time for melanoma in situ: a prospective study of 167 cases. Dermatol Surg. 2008;34(5):660–664. doi: 10.1111/j.1524-4725.2007.34124.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhardwaj SS, Tope WD, Lee PK. Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma using Mel-5 immunostaining: University of Minnesota experience. Dermatol Surg. 2006;32(5):690–696. doi: 10.1111/j.1524-4725.2006.32142.x. discussion 696–697. [DOI] [PubMed] [Google Scholar]
- 14.Bienert TN, Trotter MJ, Arlette JP. Treatment of cutaneous melanoma of the face by Mohs micrographic surgery. J Cutan Med Surg. 2003;7(1):25–30. doi: 10.1007/s10227-002-1161-7. [DOI] [PubMed] [Google Scholar]
- 15.Bricca GM, Brodland DG, Ren D, Zitelli JA. Cutaneous head and neck melanoma treated with Mohs micrographic surgery. J Am Acad Dermatol. 2005;52(1):92–100. doi: 10.1016/j.jaad.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 16.Cohen LM, McCall MW, Hodge SJ, Freedman JD, Callen JP, Zax RH. Successful treatment of lentigo maligna and lentigo maligna melanoma with Mohs’ micrographic surgery aided by rush permanent sections. Cancer. 1994;73(12):2964–2970. doi: 10.1002/1097-0142(19940615)73:12<2964::aid-cncr2820731213>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.Cohen LM, McCall MW, Zax RH. Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. A follow-up study. Dermatol Surg. 1998;24(6):673–677. doi: 10.1111/j.1524-4725.1998.tb04226.x. [DOI] [PubMed] [Google Scholar]
- 18.Dawn ME, Dawn AG, Miller SJ. Mohs surgery for the treatment of melanoma in situ: a review. Dermatol Surg. 2007;33(4):395–402. doi: 10.1111/j.1524-4725.2007.33085.x. [DOI] [PubMed] [Google Scholar]
- 19.Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72(5):840–850. doi: 10.1016/j.jaad.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Newman J, Beal M, Schram SE, Lee PK. Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma using Mel-5 immunostaining: an update from the University of Minnesota. Dermatol Surg. 2013;39(12):1794–1799. doi: 10.1111/dsu.12356. [DOI] [PubMed] [Google Scholar]
- 21.Nosrati A, Berliner JG, Goel S, et al. Outcomes of melanoma in situ treated with Mohs micrographic surgery compared with wide local excision. JAMA Dermatol. 2017;153(5):436–441. doi: 10.1001/jamadermatol.2016.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponzo MG, Crawford RI, Kossintseva I. Amelanotic lentigo maligna melanoma: Mohs surgery as the definitive treatment of an invisible tumour. J Cutan Med Surg. 2018;22(1):51–57. doi: 10.1177/1203475417719046. [DOI] [PubMed] [Google Scholar]
- 23.Snow SN, Mohs FE, Oriba HA, Dudley CM, Leverson G, Hetzer M. Cutaneous malignant melanoma treated by Mohs surgery. Review of the treatment results of 179 cases from the Mohs Melanoma Registry. Dermatol Surg. 1997;23(11):1055–1060. doi: 10.1111/j.1524-4725.1997.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 24.Stigall LE, Brodland DG, Zitelli JA. The use of Mohs micrographic surgery (MMS) for melanoma in situ (MIS) of the trunk and proximal extremities. J Am Acad Dermatol. 2016;75(5):1015–1021. doi: 10.1016/j.jaad.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Temple CL, Arlette JP. Mohs micrographic surgery in the treatment of lentigo maligna and melanoma. J Surg Oncol. 2006;94(4):287–292. doi: 10.1002/jso.20305. [DOI] [PubMed] [Google Scholar]
- 26.Valentin-Nogueras SM, Brodland DG, Zitelli JA, Gonzalez-Sepulveda L, Nazario CM. Mohs micrographic surgery using MART-1 immunostain in the treatment of invasive melanoma and melanoma in situ. Dermatol Surg. 2016;42(6):733–744. doi: 10.1097/DSS.0000000000000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whalen J, Leone D. Mohs micrographic surgery for the treatment of malignant melanoma. Clin Dermatol. 2009;27(6):597–602. doi: 10.1016/j.clindermatol.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Zitelli JA, Brown C, Hanusa BH. Mohs micrographic surgery for the treatment of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37(2 Pt 1):236–245. doi: 10.1016/s0190-9622(97)80131-4. [DOI] [PubMed] [Google Scholar]
- 29.Bichakjian CK, Halpern AC, Johnson TM, et al. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65(5):1032–1047. doi: 10.1016/j.jaad.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Coit DG, Thompson JA, Algazi A, et al. NCCN Guidelines Insights: Melanoma, Version 3.2016. J Natl Compr Canc Netw. 2016;14(8):945–958. doi: 10.6004/jnccn.2016.0101. [DOI] [PubMed] [Google Scholar]
- 31.McKenna JK, Florell SR, Goldman GD, Bowen GM. Lentigo maligna/lentigo maligna melanoma: current state of diagnosis and treatment. Dermatol Surg. 2006;32(4):493–504. doi: 10.1111/j.1524-4725.2006.32102.x. [DOI] [PubMed] [Google Scholar]
- 32.McKinnon JG, Starritt EC, Scolyer RA, McCarthy WH, Thompson JF. Histopathologic excision margin affects local recurrence rate: analysis of 2681 patients with melanomas < or =2 mm thick. Ann Surg. 2005;241(2):326–333. doi: 10.1097/01.sla.0000152014.89434.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng AK, Jones WO, Shaw JH. Analysis of local recurrence and optimizing excision margins for cutaneous melanoma. Br J Surg. 2001;88(1):137–142. doi: 10.1046/j.1365-2168.2001.01611.x. [DOI] [PubMed] [Google Scholar]
- 34.Christensen KN, Hochwalt PC, Hocker TL, et al. Comparison of MITF and Melan-A immunohistochemistry during Mohs surgery for lentigo maligna-type melanoma in situ and lentigo maligna melanoma. Dermatol Surg. 2016;42(2):167–175. doi: 10.1097/DSS.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 35.Trimble JS, Cherpelis BS. Rapid immunostaining in Mohs: current applications and attitudes. Dermatol Surg. 2013;39(1 Pt 1):56–63. doi: 10.1111/dsu.12015. [DOI] [PubMed] [Google Scholar]
- 36.Miller CJ, Sobanko JF, Zhu X, Nunnciato T, Urban CR. Special stains in Mohs surgery. Dermatol Clin. 2011;29(2):273–286.ix. doi: 10.1016/j.det.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Etzkorn JR, Cherpelis BS, Glass LF. Mohs surgery for melanoma: rationale, advances and possibilities. Expert Rev Anticancer Ther. 2011;11(7):1041–1052. doi: 10.1586/era.11.64. [DOI] [PubMed] [Google Scholar]
- 38.Stranahan D, Cherpelis BS, Glass LF, Ladd S, Fenske NA. Immunohistochemical stains in Mohs surgery: a review. Dermatol Surg. 2009;35(7):1023–1034. doi: 10.1111/j.1524-4725.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- 39.Viola KV, Rezzadeh KS, Gonsalves L, et al. National utilization patterns of Mohs micrographic surgery for invasive melanoma and melanoma in situ. J Am Acad Dermatol. 2015;72(6):1060–1065. doi: 10.1016/j.jaad.2015.02.1122. [DOI] [PubMed] [Google Scholar]
- 40.Lang PG, Braun M. Mohs surgery: fixed tissue technique. In: Nouri K, editor. Mohs Micrographic Surgery. London: Springer; 2012. pp. 78–81. [Google Scholar]
- 41.Reyes BA, Robins P. Wood’s lamp and surgical margins in malignant melanoma in situ. J Dermatol Surg Oncol. 1988;14(1):22. doi: 10.1111/j.1524-4725.1988.tb03336.x. [DOI] [PubMed] [Google Scholar]
- 42.Chang KH, Finn DT, Lee D, Bhawan J, Dallal GE, Rogers GS. Novel 16-minute technique for evaluating melanoma resection margins during Mohs surgery. J Am Acad Dermatol. 2011;64(1):107–112. doi: 10.1016/j.jaad.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 43.Cherpelis BS, Glass LF, Ladd S, Fenske NA. Mohs surgery for melanoma in situ: how we do it. J Drugs Dermatol. 2010;9(7):786–788. [PubMed] [Google Scholar]
- 44.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iorizzo LJ, 3rd, Chocron I, Lumbang W, Stasko T. Importance of vertical pathology of debulking specimens during Mohs micrographic surgery for lentigo maligna and melanoma in situ. Dermatol Surg. 2013;39(3 Pt 1):365–371. doi: 10.1111/dsu.12078. [DOI] [PubMed] [Google Scholar]
- 46.Hendi A, Brodland DG, Zitelli JA. Melanocytes in long-standing sun-exposed skin: quantitative analysis using the MART-1 immunostain. Arch Dermatol. 2006;142(7):871–876. doi: 10.1001/archderm.142.7.871. [DOI] [PubMed] [Google Scholar]
- 47.Zitelli JA, Moy RL, Abell E. The reliability of frozen sections in the evaluation of surgical margins for melanoma. J Am Acad Dermatol. 1991;24(1):102–106. doi: 10.1016/0190-9622(91)70020-3. [DOI] [PubMed] [Google Scholar]
- 48.Swetter SM, Boldrick JC, Jung SY, Egbert BM, Harvell JD. Increasing incidence of lentigo maligna melanoma subtypes: northern California and national trends 1990–2000. J Invest Dermatol. 2005;125(4):685–691. doi: 10.1111/j.0022-202X.2005.23852.x. [DOI] [PubMed] [Google Scholar]
- 49.Kimyai-Asadi A, Katz T, Goldberg LH, et al. Margin involvement after the excision of melanoma in situ: the need for complete en face examination of the surgical margins. Dermatol Surg. 2007;33(12):1434–1439. doi: 10.1111/j.1524-4725.2007.33313.x. discussion 1439–1441. [DOI] [PubMed] [Google Scholar]
- 50.Shin TM, Etzkorn JR, Sobanko JF, et al. Clinical factors associated with subclinical spread of in situ melanoma. J Am Acad Dermatol. 2017;76(4):707–713. doi: 10.1016/j.jaad.2016.10.049. [DOI] [PubMed] [Google Scholar]
- 51.Kunishige JH, Brodland DG, Zitelli JA. Surgical margins for melanoma in situ. J Am Acad Dermatol. 2012;66(3):438–444. doi: 10.1016/j.jaad.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 52.Zalla MJ, Lim KK, Dicaudo DJ, Gagnot MM. Mohs micrographic excision of melanoma using immunostains. Dermatol Surg. 2000;26(8):771–784. doi: 10.1046/j.1524-4725.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 53.Felton S, Taylor RS, Srivastava D. Excision margins for melanoma in situ on the head and neck. Dermatol Surg. 2016;42(3):327–334. doi: 10.1097/DSS.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 54.Erickson C, Miller SJ. Treatment options in melanoma in situ: topical and radiation therapy, excision and Mohs surgery. Int J Dermatol. 2010;49(5):482–491. doi: 10.1111/j.1365-4632.2010.04423.x. [DOI] [PubMed] [Google Scholar]
- 55.Coleman WP, 3rd, Davis RS, Reed RJ, Krementz ET. Treatment of lentigo maligna and lentigo maligna melanoma. J Dermatol Surg Oncol. 1980;6(6):476–479. doi: 10.1111/j.1524-4725.1980.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 56.Osborne JE, Hutchinson PE. A follow-up study to investigate the efficacy of initial treatment of lentigo maligna with surgical excision. Br J Plast Surg. 2002;55(8):611–615. doi: 10.1054/bjps.2002.3967. [DOI] [PubMed] [Google Scholar]
- 57.Walling HW, Scupham RK, Bean AK, Ceilley RI. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 2007;57(4):659–664. doi: 10.1016/j.jaad.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Johnson TM, Headington JT, Baker SR, Lowe L. Usefulness of the staged excision for lentigo maligna and lentigo maligna melanoma: the “square” procedure. J Am Acad Dermatol. 1997;37(5 Pt 1):758–764. doi: 10.1016/s0190-9622(97)70114-2. [DOI] [PubMed] [Google Scholar]
- 59.Hill DC, Gramp AA. Surgical treatment of lentigo maligna and lentigo maligna melanoma. Australas J Dermatol. 1999;40(1):25–30. doi: 10.1046/j.1440-0960.1999.00311.x. [DOI] [PubMed] [Google Scholar]
- 60.Clayton BD, Leshin B, Hitchcock MG, Marks M, White WL. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26(7):671–678. doi: 10.1046/j.1524-4725.2000.99235.x. [DOI] [PubMed] [Google Scholar]
- 61.Anderson KW, Baker SR, Lowe L, Su L, Johnson TM. Treatment of head and neck melanoma, lentigo maligna subtype: a practical surgical technique. Arch Facial Plast Surg. 2001;3(3):202–206. doi: 10.1001/archfaci.3.3.202. [DOI] [PubMed] [Google Scholar]
- 62.Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47(5):743–748. doi: 10.1067/mjd.2002.124085. [DOI] [PubMed] [Google Scholar]
- 63.Moller MG, Pappas-Politis E, Zager JS, et al. Surgical management of melanoma-in-situ using a staged marginal and central excision technique. Ann Surg Oncol. 2009;16(6):1526–1536. doi: 10.1245/s10434-008-0239-x. [DOI] [PubMed] [Google Scholar]
- 64.Bub JL, Berg D, Slee A, Odland PB. Management of lentigo maligna and lentigo maligna melanoma with staged excision: a 5-year follow-up. Arch Dermatol. 2004;140(5):552–558. doi: 10.1001/archderm.140.5.552. [DOI] [PubMed] [Google Scholar]
- 65.Huilgol SC, Selva D, Chen C, et al. Surgical margins for lentigo maligna and lentigo maligna melanoma: the technique of mapped serial excision. Arch Dermatol. 2004;140(9):1087–1092. doi: 10.1001/archderm.140.9.1087. [DOI] [PubMed] [Google Scholar]
- 66.Mahoney MH, Joseph M, Temple CL. The perimeter technique for lentigo maligna: an alternative to Mohs micrographic surgery. J Surg Oncol. 2005;91(2):120–125. doi: 10.1002/jso.20284. [DOI] [PubMed] [Google Scholar]
- 67.Then SY, Malhotra R, Barlow R, et al. Early cure rates with narrow-margin slow-Mohs surgery for periocular malignant melanoma. Dermatol Surg. 2009;35(1):17–23. doi: 10.1111/j.1524-4725.2008.34377.x. [DOI] [PubMed] [Google Scholar]
- 68.Bosbous MW, Dzwierzynski WW, Neuburg M. Staged excision of lentigo maligna and lentigo maligna melanoma: a 10-year experience. Plast Reconstr Surg. 2009;124(6):1947–1955. doi: 10.1097/PRS.0b013e3181bcf002. [DOI] [PubMed] [Google Scholar]
- 69.DeBloom JR, 2nd, Zitelli JA, Brodland DG. The invasive growth potential of residual melanoma and melanoma in situ. Dermatol Surg. 2010;36(8):1251–1257. doi: 10.1111/j.1524-4725.2010.01618.x. [DOI] [PubMed] [Google Scholar]
- 70.Cook J. Surgical margins for resection of primary cutaneous melanoma. Clin Dermatol. 2004;22(3):228–233. doi: 10.1016/j.clindermatol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Barlow RJ, White CR, Swanson NA. Mohs’ micrographic surgery using frozen sections alone may be unsuitable for detecting single atypical melanocytes at the margins of melanoma in situ. Br J Dermatol. 2002;146(2):290–294. doi: 10.1046/j.1365-2133.2002.04661.x. [DOI] [PubMed] [Google Scholar]
- 72.Prieto VG, Argenyi ZB, Barnhill RL, et al. Are en face frozen sections accurate for diagnosing margin status in melanocytic lesions? Am J Clin Pathol. 2003;120(2):203–208. doi: 10.1309/J1Q0-V35E-UTMV-R193. [DOI] [PubMed] [Google Scholar]
- 73.Cherpelis BS, Moore R, Ladd S, Chen R, Glass LF. Comparison of MART-1 frozen sections to permanent sections using a rapid 19-minute protocol. Dermatol Surg. 2009;35(2):207–213. doi: 10.1111/j.1524-4725.2008.34411.x. [DOI] [PubMed] [Google Scholar]
- 74.Kelley LC, Starkus L. Immunohistochemical staining of lentigo maligna during Mohs micrographic surgery using MART-1. J Am Acad Dermatol. 2002;46(1):78–84. doi: 10.1067/mjd.2002.119197. [DOI] [PubMed] [Google Scholar]
- 75.Hendi A, Wada DA, Jacobs MA, et al. Melanocytes in nonlesional sun-exposed skin: a multicenter comparative study. J Am Acad Dermatol. 2011;65(6):1186–1193. doi: 10.1016/j.jaad.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 76.Barlow JO, Maize J, Sr., Lang PG. The density and distribution of melanocytes adjacent to melanoma and nonmelanoma skin cancers. Dermatol Surg. 2007;33(2):199–207. doi: 10.1111/j.1524-4725.2006.33039.x. [DOI] [PubMed] [Google Scholar]
- 77.Mallipeddi R, Stark J, Xie XJ, Matthews M, Taylor RS. A novel 2-hour method for rapid preparation of permanent paraffin sections when treating melanoma in situ with mohs micrographic surgery. Dermatol Surg. 2008;34(11):1520–1526. doi: 10.1111/j.1524-4725.2008.34316.x. [DOI] [PubMed] [Google Scholar]
- 78.Black WH, Thareja SK, Blake BP, Chen R, Cherpelis BS, Glass LF. Distinction of melanoma in situ from solar lentigo on sun-damaged skin using morphometrics and MITF immunohistochemistry. Am J Dermatopathol. 2011;33(6):573–578. doi: 10.1097/DAD.0b013e3182093b13. [DOI] [PubMed] [Google Scholar]
- 79.Bricca GM, Brodland DG, Zitelli JA. Immunostaining melanoma frozen sections: the 1-hour protocol. Dermatol Surg. 2004;30(3):403–408. doi: 10.1111/j.1524-4725.2004.30110.x. [DOI] [PubMed] [Google Scholar]
- 80.Jungbluth AA, Busam KJ, Gerald WL, et al. A103: An anti-melan-a monoclonal antibody for the detection of malignant melanoma in paraffin-embedded tissues. Am J Surg Pathol. 1998;22(5):595–602. doi: 10.1097/00000478-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 81.Kimyai-Asadi A, Ayala GB, Goldberg LH, Vujevich J, Jih MH. The 20-minute rapid MART-1 immunostain for malignant melanoma frozen sections. Dermatol Surg. 2008;34(4):498–500. doi: 10.1111/j.1524-4725.2007.34095.x. [DOI] [PubMed] [Google Scholar]
- 82.Novodiax I. Novodiax 10-Minute ihcDirect® CK5 and Mart-1 (Melan A) Assays Available for IVD Use. Hayward, CA: Novodiax; 2017. [Google Scholar]
- 83.El Shabrawi-Caelen L, Kerl H, Cerroni L. Melan-A: not a helpful marker in distinction between melanoma in situ on sun-damaged skin and pigmented actinic keratosis. Am J Dermatopathol. 2004;26(5):364–366. doi: 10.1097/00000372-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 84.Beltraminelli H, Shabrawi-Caelen LE, Kerl H, Cerroni L. Melan-a-positive “pseudomelanocytic nests”: a pitfall in the histopathologic and immunohistochemical diagnosis of pigmented lesions on sun-damaged skin. Am J Dermatopathol. 2009;31(3):305–308. doi: 10.1097/DAD.0b013e31819d3769. [DOI] [PubMed] [Google Scholar]
- 85.Maize JC, Jr, Resneck JS, Jr, Shapiro PE, McCalmont TH, LeBoit PE. Ducking stray “magic bullets”: a Melan-A alert. Am J Dermatopathol. 2003;25(2):162–165. doi: 10.1097/00000372-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 86.Kim J, Taube JM, McCalmont TH, Glusac EJ. Quantitative comparison of MiTF, Melan-A, HMB-45 and Mel-5 in solar lentigines and melanoma in situ. J Cutan Pathol. 2011;38(10):775–779. doi: 10.1111/j.1600-0560.2011.01763.x. [DOI] [PubMed] [Google Scholar]
- 87.Ferringer T. Immunohistochemistry in dermatopathology. Arch Pathol Lab Med. 2015;139(1):83–105. doi: 10.5858/arpa.2014-0075-RA. [DOI] [PubMed] [Google Scholar]
- 88.Ramos-Herberth FI, Karamchandani J, Kim J, Dadras SS. SOX10 immunostaining distinguishes desmoplastic melanoma from excision scar. J Cutan Pathol. 2010;37(9):944–952. doi: 10.1111/j.1600-0560.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- 89.Donaldson MR, Deeths MJ, Weber LA. Rapid SOX10 immunostain on fresh frozen tissue. Dermatol Surg. 2016;42(2):269–271. doi: 10.1097/DSS.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 90.Semkova K, Mallipeddi R, Robson A, Palamaras I. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39(11):1648–1652. doi: 10.1111/dsu.12320. [DOI] [PubMed] [Google Scholar]
- 91.Highsmith JT, Highsmith MJ, Monheit GD. Histologic accuracy of Mohs micrographic surgery. Dermatol Surg. 2018;44(3):350–353. doi: 10.1097/DSS.0000000000001352. [DOI] [PubMed] [Google Scholar]
- 92.Kesty K, Sangueza OP, Leshin B, Albertini JG. Mohs micrographic surgery and dermatopathology concordance: an analysis of 1421 Mohs cases over 17 years. J Am Acad Dermatol. doi: 10.1016/j.jaad.2017.11.055. Epub 2017 Dec 13. [DOI] [PubMed] [Google Scholar]
- 93.Mariwalla K, Aasi SZ, Glusac EJ, Leffell DJ. Mohs micrographic surgery histopathology concordance. J Am Acad Dermatol. 2009;60(1):94–98. doi: 10.1016/j.jaad.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahlgrimm-Siess V, Massone C, Scope A, et al. Reflectance confocal microscopy of facial lentigo maligna and lentigo maligna melanoma: a preliminary study. Br J Dermatol. 2009;161(6):1307–1316. doi: 10.1111/j.1365-2133.2009.09289.x. [DOI] [PubMed] [Google Scholar]
- 95.Wurm E, Pellacani G, Longo C, et al. The value of reflectance confocal microscopy in diagnosis of flat pigmented facial lesions: a prospective study. J Eur Acad Dermatol Venereol. 2017;31(8):1349–1354. doi: 10.1111/jdv.14171. [DOI] [PubMed] [Google Scholar]