Abstract
Objectives:
To assess combined hormonal contraceptives (CHC) use and adolescent women’s peak areal bone mineral density (BMD) accrual.
Methods:
We enrolled 527 randomly selected women across Canada (2004-6) divided by age into adolescents (16-19) and young adults (20-24) and by CHC use to ever (E-CHC)/never (N-CHC) users. At baseline and year 2 we measured height, weight, and BMD at lumbar spine (L1-4), femoral neck, and total hip sites. Interviewer-administered questionnaires addressed menarche age, cigarette and alcohol use, calcium/vitamin D intakes, physical activity and estrogen dose (≤30/>30 micrograms). Linear regression models examined associations of CHC use with 2-year BMD change adjusted for bone-related variables.
Results:
Of 307 women with complete data, 229 (75%) used CHC. N-CHC adolescents gained significantly more unadjusted total hip BMD +0.012 g/cm2/2-y (95% C.I.: 0.001, 0.023) with similar trends at all sites. N-CHC adolescents tended to have greater adjusted femoral neck BMD gain: mean difference +0.009 g/cm2 (95% CI:-0.002; 0.021). In young women N-CHC, however, adjusted femoral neck BMD decreased significantly more -0.021 g/cm2 (95%CI:-0.006; -0.036) with similar trends at other sites. BMD changes were unrelated to estrogen dose and age at starting CHC.
Conclusions:
Adolescent CHC users in a random population demonstrated less hip region peak BMD accrual than non-users. This requires randomized control trial confirmation.
Keywords: Combined Hormonal Contraception, Population-Based, Adolescent and Young Women, Areal Bone Mineral Density Change, Hip Peak Bone Mass
Introduction
Combined hormonal contraceptives (CHC, oral, vaginal ring, patch) are increasingly used by adolescent and young adult women who are maturing toward peak areal bone mineral density (BMD) and toward achieving adult regular, normal length menstrual cycles with normal ovulation[1,2]. At baseline (1995-7) in the population-based Canadian Multicentre Osteoporosis Study (CaMos), 87% of adult women aged 25-45 years reported having used CHC for at least 3 months at some point in time[3]. Areal bone mineral density (BMD) at the lumbar spine (L1-4) and trochanter was significantly lower in adult CHC ever-users than in non-users[3].
Normal values of peak BMD are considered essential to prevent later-life fracturing osteoporosis[4-8]. Prospective data from several cohorts show peak hip region bone mass is accrued during the adolescent years[9,10]. Prospective data in the population-based Canadian Multicentre Osteoporosis Study (CaMos) women aged 16-40 years from the Youth (2004-2006)[11] and Adult[12] Cohorts showed peak BMD at the femoral neck and total hip was accrued during ages 16-19 years and for lumbar spine between ages 33-40 years[13].
Bone modeling is required to achieve peak BMD. CHC use with ethinyl estradiol doses from 20 to 35 micrograms suppresses both bone formation and resorption in young women[14]. Thus CHC may suppress modeling and impair BMD accrual during the critical period of adolescent achievement of peak bone mass and optimal growth[4]. When CHC was introduced more than 50 years ago, it was presumed to be positive for bone health since menopausal ovarian hormone therapy increased BMD; it now is proven that this therapy prevents bone fractures[15]. CHC-related BMD effects, however, likely differ in adolescent women who have not yet accrued their peak BMD versus those having more mature skeletons[4].
Several prospective studies reported that adolescent CHC users gained less spinal BMD than non-users[16-18]; others reported no differences in BMD change[19,20]. Recently, our Centre for Menstrual Cycle and Ovulation Research conducted a meta-analysis of prospective studies of CHC use and BMD accrual in adolescents (ages 12 to 19 years) having sufficient data for quantitative synthesis. We documented that CHC-using adolescents from multiple cohorts and multiple countries attained significantly less spinal BMD than non-using controls (Goshtasebi, in review 7/2017, personal communication). Thus, prospective, population-based data are needed to more accurately describe any relationship between CHC use and BMD change in adolescent and young adult women.
The primary objective of this investigation was to document 2-year BMD changes related to CHC ever versus never use in women in the randomly sampled Youth Cohort of the Canadian Multicentre Osteoporosis Study (CaMos) and to separately describe BMD change in adolescent (16-19 years) and young adult (20-24 years) women. We also assessed whether BMD change related to CHC ethinyl estradiol dose or the age at which CHC users first started taking CHC. We hypothesized that BMD accrual would be impaired in adolescent CHC users, and that age at starting CHC, but not estrogen dose, would be negatively associated with BMD changes.
Materials and methods
Between the years 2004 and 2006, CaMos recruited women and men aged 16-24 years into a Youth Cohort; 527 women randomly selected from the population around each of the nine centres across Canada were enrolled as previously described[11]. Ethics approval was obtained from each centre’s research ethics board plus from the board at McGill University for the entire study. All participants signed informed consent (if at or above the age of majority, or by a parent/guardian if younger) and assent (for those younger). Using the same sampling approach as for the adult CaMos cohort[21], we mailed invitation letters to randomly selected households within 50 kilometers around our nine research centres: Vancouver, Calgary, Saskatoon, Toronto, Hamilton, Kingston, Quebec, Halifax, and St. John’s. We subsequently telephoned to determine whether a person of the appropriate age/sex lived there.
In this 2-year longitudinal study, each participant, at baseline and after two years, completed an interviewer-administered, validated[22], comprehensive CaMos questionnaire that assessed CHC use by brand name (from which we could assess the ethinyl estradiol dose), age at and the main reason for first CHC use, age at menarche, parity, dietary and supplemental (total) calcium and vitamin D intakes, recreational and occupational physical activity, alcohol and cigarette use. After one year, participants completed a postal questionnaire inquiring about medications including CHC use.
To preserve the population-representativeness of the sample, available data from all participants were included unless they had been pregnant in the last year, were within one year of starting lactation[23] [n=6] or reported taking injectable medroxyprogesterone contraception (Depot-medroxyprogesterone) before, at baseline or during the study [n=10])[24]. We excluded no others since few of these young women had major illnesses (e.g. inflammatory bowel disease) or used BMD-altering medications (such as pharmacological glucocorticoids). We previously reported in this cohort that neither asthma nor prevalent fractures were related to baseline BMD[11].
Clinical examinations at baseline and after two years included height and weight measurements in light clothing without shoes with which we calculated body mass index (BMI, kg/m2). We also assessed areal BMD (g/cm2) by dual energy X-ray absorptiometry scans of the lumbar spine (L1-4), femoral neck, and total hip. All bone measuring centres conducted daily/weekly quality control. A phantom was circulated to each centre during each bone measurement-year; all BMD data were calibrated to this common phantom as reported[12].
We defined women as never CHC users (N-CHC) if they did not report past or current use of CHC at baseline, after one or two years. Women who reported CHC use on any questionnaire were considered ever CHC users (E-CHC). E-CHC users could change agents or use CHC intermittently or continuously.
Statistical analysis
We performed analyses for the whole 16-24 year old cohort of women and also stratified into adolescent (16-19 years) and young adult women (20-24 years). Means and 95% confidence intervals (CI) were computed for continuous variables and t-tests were used to compare baseline characteristics of E-CHC and N-CHC groups. We determined each E-CHC woman’s average ethinyl estradiol dose in micrograms based on self-reported CHC formulations; the 17% who reported no brand/dose were omitted from this calculation; only two women used the 15 micrograms ethinyl estradiol-releasing vaginal ring CHC and only for a portion of the duration of the study.
We fitted a multivariable linear regression model to examine associations between current study CHC use (never/ever) and 2-year BMD change at each skeletal site: lumbar spine, femoral neck and total hip. Regression diagnostics included visual inspection of graphs of residuals. A positive change indicated a 2-year BMD increase; a negative change, a decrease.
We included the following baseline variables as covariates in our models: age, height, body mass index (BMI), menarche age, total calcium intake (dietary and supplements in categories: <800, 800-1200, >1200 mg/day), 24 hour physical activity (kcal/d doing moderate, strenuous or vigorous exercise), alcohol consumption (beverages per week) and current smoking (yes/no). Models were further adjusted for baseline site-specific BMD and two year change in BMI. We fitted similar models with E-CHC users stratified according to estrogen dose: less than and greater than 30 micrograms (since there was little range in doses and they were not normally distributed). We also fitted multivariable models to examine the association between age at starting CHC and BMD change, adjusted for the same variables as above. Data are reported as plus or minus standard deviation (±SD) or as 95% confidence intervals (95%CI). Statistical analyses were performed using SAS 9.4 (Cary, NC, USA).
Results
Of 527 women aged 16-24 years recruited, 307 women provided complete baseline, 2-year BMD change and CHC use information (Figure 1). Youth Cohort women’s participation rate was 24.2%[11]. Many enrolled women (n=220) did not complete a 2-year questionnaire and second bone density measurement; this was primarily related to changing residence due to education, employment, relationships and other priorities. Those included (n=307) compared with not included women (n=220), started CHC use at a similar age, had similar baseline weight, height, BMI, menarche age, physical activities and baseline BMD values at all sites (Supplemental Table). However, those not included were older, had lower average baseline total calcium and vitamin D intakes, and were more likely to currently smoke and to drink >1 alcohol serving per month.
Figure 1.
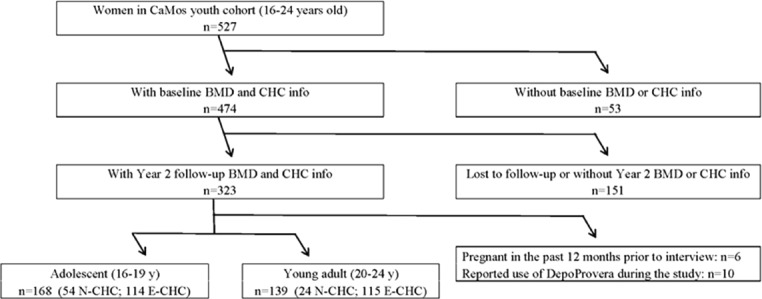
Flow of participants through the Canadian Multicentre Osteoporosis Study (CaMos) Youth Cohort by combined hormonal contraception (CHC) use and change in areal bone mineral density (BMD) in women 16-24 years of age.
Supplemental Table 1.
Comparison of descriptive and areal bone mineral density (BMD) data at the lumbar spine (L1-4), femoral neck (FN) and total hip (TH) between included women with two-year data (n=307) and those not included (n=220) in the Canadian Multicentre Osteoporosis Study (CaMos) Youth Cohort study of prospective data in women by combined hormonal contraception (CHC) use-total cohort (n=527, aged 16-24 years) (significant differences are indicated by bolding of the 95 percent confidence interval of the mean [95% CI]).
| Variable | Inclusion status | N | Mean | Standard Deviation | 95% CI of the Mean |
|---|---|---|---|---|---|
| Age (years) | Not Included | 220 | 20.1 | 2.5 | 19.7, 20.4 |
| Included | 307 | 19.5 | 2.7 | 19.2, 19.8 | |
| Height (cm) | Not Included | 216 | 164.7 | 6.5 | 163.8, 165.5 |
| Included | 306 | 164.6 | 6.7 | 163.9, 165.4 | |
| Weight (kg) | Not Included | 216 | 63.6 | 13.1 | 61.8, 65.3 |
| Included | 306 | 61.9 | 11.8 | 60.6, 63.3 | |
| Body Mass Index (kg/m2) | Not Included | 216 | 23.4 | 4.4 | 22.8, 24.0 |
| Included | 306 | 22.8 | 4.2 | 22.4, 23.3 | |
| Menarche age | Not Included | 212 | 12.5 | 1.2 | 12.3, 12.6 |
| Included | 305 | 12.5 | 1.3 | 12.4, 12.6 | |
| Age started CHC | Excluded | 166 | 17.1 | 2.0 | 16.8, 17.4 |
| Included | 229 | 17.5 | 2.3 | 17.2, 17.8 | |
| Total Ca (mg/day) | Not Included | 218 | 974 | 595 | 894, 1053 |
| Included | 305 | 1132 | 637 | 1061, 1204 | |
| Total Vitamin D (µg/day) | Not Included | 219 | 4.5 | 4.7 | 3.9, 5.2 |
| Included | 307 | 5.5 | 5.1 | 5.0, 6.1 | |
| Physical activity (kcal/d) | Excluded | 216 | 4956 | 3829 | 4443, 5470 |
| Included | 306 | 4569 | 3925 | 4127, 5010 | |
| Alcohol (# servings/week) | Not Included | 220 | 1.8 | 3.1 | 1.4, 2.2 |
| Included | 307 | 1.2 | 2.0 | 1.0, 1.4 | |
| L1-L4 BMD (g/cm2) | Not Included | 207 | 1.030 | 0.11 | 1.015, 1.045 |
| Included | 306 | 1.028 | 0.12 | 1.014, 1.042 | |
| FN BMD (g/cm2) | Not Included | 209 | 0.872 | 0.11 | 0.857, 0.887 |
| Included | 307 | 0.869 | 0.11 | 0.856, 0.881 | |
| TH BMD (g/cm2) | Not Included | 205 | 0.978 | 0.11 | 0.962, 0.993 |
| Included | 303 | 0.978 | 0.12 | 0.964, 0.992 |
Note-more of those not included drank >1 alcohol serving per month; among those, however, the amount consumed was the same. Similarly, more non-included women at baseline currently smoked; among smokers, however, the average number of cigarettes per day was similar.
Of the 307 women in the prospective cohort (168 adolescent, 139 young adult), 61.9 % (n=190) were using CHC at baseline and a further 39 women began using CHC during the study. Thus, over two years, 74.6% (n=229) ever used CHC and 25.4% (n=78) never used CHC. Of the E-CHC users, 91 were intermittent and 138 were continuous users. Estrogen dose, available in 190 CHC users, averaged 26.5 micrograms per day (range= 15 to 35).
[Table 1] provides baseline demographic, reproductive, nutritional, lifestyle and lumbar spine, femoral neck and total hip BMD data for the whole cohort plus BMI change data. Mean baseline age at starting CHC was 17.2 years (SD=2.3); when including women who subsequently started CHC, however, average age at first E-CHC was 17.5 y (SD=2.3). Average age at starting CHC was earlier in adolescents (16.6, SD=1.6 years) compared with young adults (18.4, SD=2.6 years) (95% CI of the difference: -1.2, -2.4). Only 36 young women smoked and these averaged 8.0 cigarettes/day (SD=5.5). At baseline 50.5% of women drank ≥ one alcoholic beverage per month. Most of these Youth Cohort women, roughly reflecting the characteristics of the Canadian population, were Caucasian (88.6%) with East Asians being the second most prevalent race/ethnicity.
Table 1.
Baseline descriptive and areal bone mineral density (BMD) data at the lumbar spine (L1-4), femoral neck (FN) and total hip (TH) for Canadian Multicentre Osteoporosis Study (CaMos) Youth Cohort women taking and not taking combined hormonal contraceptives (CHC) with complete data at baseline and year two (n=307, aged 16-24 years) including the 95 percent confidence intervals (95% CI).
| Variable | N | Mean | Standard Deviation | 95% CI |
|---|---|---|---|---|
| Age (years) | 307 | 19.5 | 2.7 | 19.2, 19.8 |
| Height (cm) | 306 | 164.6 | 6.7 | 163.9, 165.4 |
| Weight (kg) | 306 | 61.9 | 11.8 | 60.6, 63.3 |
| Body Mass Index (kg/m2) | 306 | 22.8 | 4.2 | 22.4, 23.3 |
| Menarche age (years) | 305 | 12.5 | 1.3 | 12.4, 12.6 |
| Age started CHC* | 229 | 17.5 | 2.3 | 17.2, 17.8 |
| Total Calcium (mg/day) | 305 | 1132 | 637 | 1061, 1204 |
| Total Vitamin D (mcg/day) | 307 | 5.5 | 5.1 | 5.0, 6.1 |
| Physical activity (kcal/day) | 306 | 4569 | 3925 | 4127, 5010 |
| Alcohol (# servings/week) | 307 | 1.2 | 2.0 | 1.0, 1.4 |
| L1-L4 BMD (gm/cm2) | 306 | 1.028 | 0.12 | 1.014, 1.041 |
| FN BMD (gm/cm2) | 307 | 0.869 | 0.11 | 0.856, 0.881 |
| TH BMD (gm/cm2) | 303 | 0.978 | 0.12 | 0.964, 0.992 |
| BMI change (kg/m2/2-year) | 306 | 0.28 | 1.84 | 0.08, 0.49 |
| Race/Ethnicity | Caucasian | Asian | African Canadian | Other (including Indigenous) |
| N=307 | 272 (88.60%) | 14 (4.56%) | 4 (1.30%) | 17 (5.54%) |
values from those 229 who were taking CHC at baseline.
[Table 2] provides baseline demographic and BMD average data comparing those who were N-CHC with E-CHC users. Those who ever used CHC were significantly older and taller but did not differ from N-CHC in weight or BMI. Menarche age and total calcium intakes were similar. Total vitamin D intake was greater in E-CHC; however, in each group at baseline, few used vitamin D supplements (15.0%, n=46; nine N-CHC [7.27 micrograms per day], and 37 E-CHC [8.57], mean 8.31 micrograms per day). Likewise, at baseline only a small proportion of women were taking calcium supplements (3.3%; n=10; one N-CHC [175 mg/d] and nine E-CHC [394 milligrams per day]; mean 372 milligrams per day). Alcohol intake was significantly higher in the E-CHC group as was the prevalence of smoking, with only one N-CHC smoking three cigarettes/d but 35 E-CHC smoking a mean of eight (8.1) cigarettes per day (SD=5.5).
Table 2.
Baseline descriptive and areal bone mineral density (BMD) data at the lumbar spine (L1-4), femoral neck (FN) and total hip (TH) in Canadian Multicentre Osteoporosis Study (CaMos) Youth Cohort women with complete data at baseline and year 2 (n=307, aged 16-24 years) by never use of combined hormonal contraceptives (N-CHC) versus ever-use (E-CHC) during this two-year study. (Bold= significant N-CHC vs E-CHC difference).
| CHC Never Use (n=78) | CHC Ever Use (n=229) | Mean difference | 95% CI of the difference | |||
|---|---|---|---|---|---|---|
| Continuous variable | Mean | 95% CI | Mean | 95% CI | ||
| Age (years) | 18.5 | 18.0, 19.1 | 19.8 | 19.5, 20.2 | -1.3 | -2.0; -0.6 |
| Height (cm) | 163 | 161.2, 164.8 | 165.2 | 164.4, 166.0 | -2.2 | -4.1; -0.2 |
| Weight (kg) | 60.7 | 57.7, 63.7 | 62.3 | 60.9, 63.8 | -1.7 | -5.0; 1.7 |
| Body Mass Index (kg/m2) | 22.8 | 21.7, 23.8 | 22.9 | 22.3, 23.4 | -0.1 | -1.2; 1.0 |
| Menarche age (years) | 12.5 | 12.2, 12.8 | 12.5 | 12.3, 12.7 | 0.0 | -0.4; 0.3 |
| Total Calcium (mg/day) | 1054 | 908, 1200 | 1159 | 1076, 1241 | -105 | -270; 60 |
| Total Vitamin D (mcg/day) | 4.5 | 3.4, 5.6 | 5.9 | 5.2, 6.6 | -1.4 | -2.7; -0.1 |
| Physical activity (kcal/d) | 3933 | 3183, 4682 | 4786 | 4252, 5320 | -853 | -1769; 63 |
| Alcohol (# servings/week) | 0.3 | 0.0, 0.5 | 1.5 | 1.3, 1.8 | -1.3 | -1.6; -0.9 |
| L1-L4 BMD (g/cm2) | 1.022 | 0.990, 1.054 | 1.030 | 1.015, 1.045 | -0.008 | -0.043; 0.027 |
| FN BMD (g/cm2) | 0.854 | 0.826, 0.882 | 0.874 | 0.860, 0.887 | -0.02 | -0.048; 0.009 |
| TH BMD (g/cm2) | 0.964 | 0.932, 0.997 | 0.982 | 0.967, 0.998 | -0.018 | -0.053; 0.018 |
| Body Mass Index change (kg/m2/2-year. | 0.62 | 0.26, 0.98 | 0.17 | -0.08, 0.40 | 0.45 | -0.03; 0.92 0.92 |
| Categorical variable (Race/Ethnicity) | N | % | N | % | % difference | 95% CI of % difference |
| Caucasian | 56 | 71.8 | 216 | 94.3 | -22.5 | -32.9; -12.1 |
Baseline BMD values were not different at any site between N-CHC and E-CHC users (Table 2). Over two-years body mass index increased significantly in N-CHC women by 0.62 kg/m2 (SD 1.6 [95% CI 0.26; 0.98]) whereas BMI did not change significantly in E-CHC users (0.17, SD 1.9, 95% CI -0.08; 0.42). N-CHC users were more likely to be non-Caucasian (28.2%) than E-CHC users (5.7%). We did not include race in multivariate models due to the small numbers of non-Caucasian CHC users (n=13). In addition, we found non-significant change in BMD results in univariate models of Caucasians/non-Caucasians at any site (data not shown).
Figure 2 illustrates individual data for absolute, unadjusted BMD change (in grams per cm2) across the 2-year study in adolescents and young adults by their CHC use group. Adolescent N-CHC users demonstrated a significantly more positive total hip average BMD change versus E-CHC users (difference= +0.012 g/cm2/two years; 95% CI +0.001, +0.023 g/cm2/2-y). There were trends for N-CHC users to also have more positive BMD gains at the femoral neck and the lumbar spine sites. In young adults, unadjusted BMD changes at all sites were similar between N-CHC and E-CHC.
Figure 2.
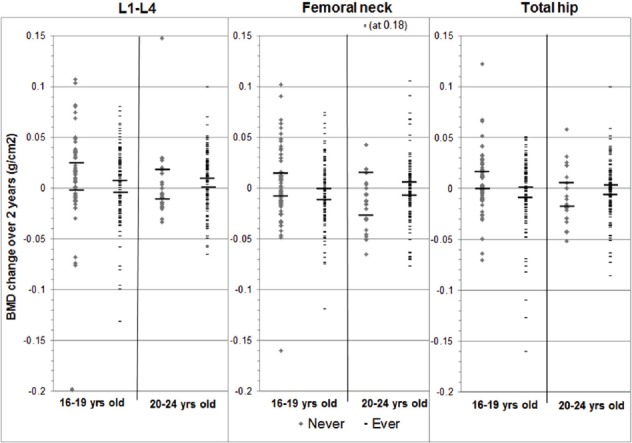
Scatterplot of two-year absolute (unadjusted) changes in areal bone mineral density (BMD) at the lumbar spine (L1-4), femoral neck (FN) and total hip (TH) by use of combined oral contraceptives (CHC, Never and Ever users) in adolescent (ages 16-19 years) and young adult (ages 20-24 years) women in the population-based Canadian Multicentre Osteoporosis Study (CaMos) Youth Cohort. Short horizontal bars are 95% Confidence Intervals.
[Table 3] documents BMD 2-year adjusted change estimates with their 95% CI for N-CHC versus E-CHC for the whole cohort and separately within adolescent and young adult women groups. Adolescent N-CHC users, after adjusting for bone-related covariates, tended to gain more femoral neck BMD compared with E-CHC (+0.009 g/cm2; 95%CI:-0.002; +0.021). After adjusting for covariates, adolescent change in total hip and lumbar spine BMD was not significantly different between groups. However, in the young adult adjusted models, N-CHC users experienced significantly less positive femoral neck BMD change than E-CHC (-0.021 g/cm2; 95% CI: -0.037, -0.006); similar trends occurred at the total hip and lumbar spine sites.
Table 3.
Estimates and 95% confidence intervals (CI) for multivariable regression analysis of two-year areal bone mineral density (BMD) change at the lumbar spine (L2-4), femoral neck (FN) and total hip (TH) for: A. never (N-CHC) versus ever (E-CHC) use of combined hormonal contraceptives (CHC); and B. age at first CHC use, in all women in the Canadian Multicentre Osteoporosis Study (CaMos) Youth Cohort (ages 16-24 years) and in Adolescent (ages 16-19 years) and Young Adult (aged 20-24 years) sub-cohorts with 95 percent confidence intervals (95% CI). Results in bold are statistically significant.
| A. +Adjusted 2-y BMD change estimates (95% CI) for N-CHC users versus E-CHC users | ||||||
|---|---|---|---|---|---|---|
| CaMos Youth Cohort 16-24 years | Adolescent 16-19 years | Young Adult 20-24 years | ||||
| estimate | 95% CI | estimate | 95% CI | estimate | 95% CI | |
| L1-L4 | 0.002 | (-0.104; 0.091) | 0.004 | (-0.008; 0.015) | -0.007 | (-0.020; 0.006) |
| FN | -0.001 | (-0.010; 0.008) | 0.009 | (-0.002; 0.021) | -0.021 | (-0.036; -0.006) |
| TH | -0.001 | (-0.009; 0.006) | 0.002 | (-0.008; 0.012) | -0.013 | (-0.026; 0.000) |
| B. +Adjusted 2-year BMD change estimates (95% CI) for age CHC started among CHC users | ||||||
| CaMos Youth Cohort 16-24 years | Adolescent 16-19 years | Young Adult 16-24 years | ||||
| estimate | 95% CI | estimate | 95% CI | estimate | 95% CI | |
| L1-L4 | 0.001 | (-0.001; 0.003) | -0.001 | (-0.005; 0.004) | -0.007 | (-0.020; 0.006) |
| FN | 0.000 | (-0.002; 0.002) | -0.001 | (-0.005; 0.003) | 0.000 | (-0.002; 0.003) |
| TH | 0.000 | (-0.002; 0.002) | 0.000 | (-0.004; 0.004) | 0.000 | (-0.002; 0.002) |
+ Adjusted for baseline age, height, BMI, menarche age, total calcium intake, 24 hour physical activity (kcal/d), alcohol consumption, current smoking, BMD and Body Mass Index change over 2-years.
[Table 3] also presents adjusted 2-year BMD change estimates from the multivariable regression model for age at first use of CHC for the whole cohort including both adolescent and young adult women. Age at first CHC use was not associated with BMD change at any of the sites.
In the 190 women with available CHC estrogen doses, adjusted 2-year BMD change was not significantly associated with average estrogen levels of less than 30 micrograms (n=119) compared with average doses of equal to or more than 30 micrograms (n=71) at any site [spine: -0.002 g/cm2 (95% CI -0.011; +0.008); femoral neck: -0.003 g/cm2 (-0.012; +0.007); total hip: -0.001 g/cm2 (-0.009; +0.007)].
Discussion
In this first population-based prospective study describing associations between CHC use and changes in BMD in adolescent and young women ages 16-24 years, 75% of women used CHC for some or all of the time during the 2-year study. Average age at starting CHC in adolescents (16.6 years) was significantly younger than in young adults (18.4 years). Adolescents who used CHC had significantly less positive unadjusted BMD change at the total hip after two years with a trend toward less positive adjusted BMD change at the femoral neck. By contrast, adjusted femoral neck 2-year BMD changes were significantly more positive in E-CHC versus N-CHC users with trends toward similar differences at the lumbar spine and total hip BMD sites. In the entire CaMos Youth cohort (ages 16-24 y), adjusted BMD changes were not related to CHC estrogen dose (less than 30 versus equal to or more than 30 micrograms) nor to age at first CHC use.
This study confirms the literature[16-18] that in adolescents CHC use may interfere with the gain to peak BMD. In previous prospective studies, adolescent CHC users demonstrated less gain in lumbar spine BMD compared with non-CHC using controls[20,25,26]. However, in the present study, CaMos adolescents had no significant unadjusted or adjusted CHC-related 2-y spine BMD changes; reasons for this are unclear. There are several prospective studies in adolescents that measured BMD changes at the levels of femoral neck or total hip sites. Similar to our findings, Pikkarainen et al., showed less femoral neck BMD (or bone mineral content) gain in CHC users versus non-users[17]. Cromer et al. showed significantly less femoral neck BMD accrual in CHC adolescent users (12-20 years old) compared to controls after one year[27] but this difference was no longer significant after two years[26]. Similarly, a Chinese study reported no statistically significant difference in femoral neck BMD changes after two years of CHC use or non-use in adolescents[20].
The only available population-based study of adolescent E-CHC and N-CHC users was cross-sectional[28]. Like results of the present study, it showed no baseline BMD differences between groups[28].
CHC use has consistently been shown to suppress bone biomarkers[14,19,29-32]. Unlike previous studies[19,27,33], we found no evidence suggesting that women who used CHC preparations with lower estrogen doses had less positive BMD changes. Adjusted data also showed that in CHC users the average age at starting CHC was not related to BMD change; cross-sectional results in the CaMos Adult Cohort were similar[3].
Young adult E-CHC users aged 20-24 years in this study showed no unadjusted differences in BMD changes compared with N-CHC users. However, unexpectedly E-CHC young adult women demonstrated significantly greater adjusted 2-year BMD gains at the femoral neck compared with same-aged N-CHC users. This has not previously been reported. Reasons for this are unclear and further exploration of this is needed.
Increased risk for fragility fracture in later life is a potential and important clinical outcome of lower peak BMD accrual in women who were CHC users[4-6]. A current Cochrane meta-analysis of fracture related to CHC use in younger women found insufficient data to allow any conclusion[34]. However, three large retrospective observational studies of menopausal women who reported previous CHC use all showed that incident fractures were approximately 20% higher in past CHC users compared with never-CHC using controls[35-37].
We highlight several strengths of this study including the Canada-wide random population sampling, 2-year prospective design and collection of an array of important bone covariates. We used an interviewer-administered questionnaire, obtained direct measures of height and weight and standardized BMD measures to a common phantom. We collected data on CHC formulations from which we determined E-CHC user’s average estrogen doses. We also avoided the loss of power that would have resulted from stratification to continuous and intermittent CHC use.
This study was limited not only by a low participation rate (common in studies of young people) but also by loss to two-year follow-up. Retention of adolescent and young adult participants is challenging given their geographic mobility and major life transitions. However, baseline included and non-included women did not differ in BMD or BMI. Bone biomarkers or three-dimensional bone microstructure and strength were also not feasible in this study. Finally, it was difficult to assess the exact durations of 2-year CHC use hence categorization as ever versus never CHC use in our analysis.
In conclusion, in this population-based Canadian cohort, adolescent users of CHC demonstrated less 2-year peak BMD accrual than adolescent non-users of CHC. Adolescent CHC-users also tended toward less gain in adjusted BMD at the femoral neck compared with N-CHC adolescents. Furthermore, at the total hip, the adolescent E-CHC group also demonstrated significantly more negative unadjusted BMD changes and a trend toward less BMD gain at lumbar spine and femoral neck in comparison with the adolescent N-CHC group. However, young adult E-CHC users had more positive adjusted BMD change at the femoral neck compared with N-CHC using women.
Mean age at first CHC use was 19.8 years in Canadian women aged 25-45 years in 1998[3], but was significantly younger at 17.5 years (-2.5 y; 95% CI -2.0; -2.8) in those aged 16-24 in this cohort. This decrease in age at first CHC use is of public health concern given the high prevalence of CHC use (75%) in this cohort of adolescent and young adult women. Thus, more young women who have not yet reached peak BMD are likely to be exposed to the potentially detrimental effects of CHC on peak BMD accrual. Randomized controlled trials are urgently needed to investigate the possibility that CHC use in the early years post-menarche has a negative influence on optimal bone accrual, particularly at the femoral neck, and thus on future hip fracture risk.
Acknowledgements
This project’s CaMos Data Analysis and Publication proposal was originally written by the then internal medicine resident, Juliya Iosfina Hemmett MD. We thank Ramanjit Sidhu, MD for her review of all combined hormonal contraceptives used by CaMos Youth Cohort women with tabulation of the ethinyl estradiol doses of each. We are also very grateful for the critical reviews and support from Azita Goshtasebi MD, MPH, PhD.
We appreciate that the funding for data acquisition for the Canadian Multicentre Osteoporosis Study (CaMos) Youth Cohorts and for statistical analysis for this manuscript was by the Canadian Institutes of Health Research (CIHR). CaMos has, in the past, been additionally supported by Merck Frosst Canada Ltd.; Eli Lilly Canada Inc.; Novartis Pharmaceuticals Inc.; The Alliance: sanofi-aventis & Procter and Gamble Pharmaceuticals Canada Inc.; Servier Canada Inc.; Amgen Canada Inc.; The Dairy Farmers of Canada; and The Arthritis Society. There was no additional funding for this analysis/manuscript.
Footnotes
The authors have no conflict of interest.
Edited by: F. Rauch
Contributorship
This study was designed by JCP and CB and approved by the Canadian Multicentre Osteoporosis Study (CaMos) Data Analysis and Publication Committee. JCP, DH, JDA and CK acquired the data. Statistical analysis and database management was by CB. It was written by TSB and JCP with CB’s assistance. All authors have critically reviewed the manuscript and approve it for publication.
Transparency
JCP, as senior author, affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained. CB is accountable for all statistical analyses.
Data sharing
The Canadian Multicentre Osteoporosis Study (CaMos) database (including both Adult and Youth Cohorts) is possible to interrogate in collaboration with a CaMos investigator.
CaMos Research Group
David Goltzman (co-principal investigator, McGill University, Montreal, Quebec, Canada), Nancy Kreiger (co-principal investigator, University of Toronto, Toronto, Ontario, Canada)
McGill University, Montreal, Quebec: Elham Rahme (biostatistician), J. Brent Richards (investigator), Suzanne N. Morin (investigator).
CaMos Coordinating Centre: Claudie Berger (study statistician), Suzanne Godmaire (research assistant), Silvia Dumont (research assistant).
Memorial University, St. John’s Newfoundland: Carol Joyce (director), Christopher S. Kovacs (co-director), Minnie Parsons (coordinator).
Dalhousie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie M. Kaiser (co-directors), Barbara Stanfield (coordinator).
Laval University, Quebec City, Quebec: Jacques P. Brown (director), Louis Bessette (co-director), GRMO, Jeanette Dumont (coordinator), Martin Després (imaging IT technician).
Queen’s University, Kingston, Ontario: Tassos P. Anastassiades (director), Tanveer Towheed (co-director), Wilma M. Hopman (investigator), Karen J. Rees-Milton (coordinator).
University of Toronto, Toronto, Ontario: Robert G. Josse (director), Angela M. Cheung (co-director), Barbara Gardner-Bray (coordinator).
McMaster University, Hamilton, Ontario: Jonathan D. Adachi (director), Alexandra Papaioannou (co-director).
University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P. Olszynski (director), K. Shawn Davison (co-director), Jola Thingvold (coordinator).
University of Calgary, Calgary, Alberta: David A. Hanley (director), Steven K. Boyd (co-director), Jane Allan (coordinator and Coordinator’s Representative to Executive Council).
University of British Columbia, Vancouver, British Columbia: Jerilynn C. Prior (director), Shirin Kalyan (co-director), Brian Lentle (investigator/radiologist), Bernice Liang (coordinator).
University of Alberta, Edmonton, Alberta: Stuart D. Jackson (medical physicist).
University of Manitoba, Winnipeg, Manitoba: William D. Leslie (investigator/nuclear medicine physician).
References
- 1.Prior JC. Adolescents'Use of Combined Hormonal Contraceptives for Menstrual Cycle-Related Problem Treatment and Contraception:Evidence of Potential Lifelong Negative Reproductive and Bone Effects. Women's Reproductive Health. 2016;3:73–92. [Google Scholar]
- 2.Jones RK. Beyond birth control:The overlooked benefits of oral contraceptive pills. Guttmacher Institute. 2011 [Google Scholar]
- 3.Prior JC, Kirkland S, Joseph L, Kreiger N, Murray TM, Hanley DA, Adachi JD, Vigna YM, Berger MS, Blondeau L, Jackson SA, Tenenhouse A. Oral contraceptive agent use and bone mineral density in premenopausal women:cross-sectional, population-based data from the Canadian Multicentre Osteoporosis Study. Can Med Assoc J. 2001;165:1023–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Tremollieres F. Impact of oral contraceptive on bone metabolism. Best Pract Res Clin Endocrinol Metab. 2013;27:47–53. doi: 10.1016/j.beem.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Bonjour JP, Chevalley T, Ferrari S, Rizzoli R. The importance and relevance of peak bone mass in the prevalence of osteoporosis. Salud Publica Mex. 2009;51(Suppl 1):S5–S17. doi: 10.1590/s0036-36342009000700004. [DOI] [PubMed] [Google Scholar]
- 6.Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O'Karma M, Wallace TC, Zemel BS. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors:a systematic review and implementation recommendations. Osteoporos Int. 2016;27:1281–386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–5. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 8.Boot AM, de Ridder MA, van der Sluis IM, van SI, Krenning EP, Keizer-Schrama SM. Peak bone mineral density, lean body mass and fractures. Bone. 2010;46:336–41. doi: 10.1016/j.bone.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Theintz G, Buchs B, Rizzoli R, Slosman D, Clavien H, Sizonenko PC, Bonjour JP. Longitudinal monitoring of bone mass accumulation in healthy adolescents:evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75:1060–5. doi: 10.1210/jcem.75.4.1400871. [DOI] [PubMed] [Google Scholar]
- 10.Kroger H, Kotaniemi A, Kroger L, Alhava E. Development of bone mass and bone density of the spine and femoral neck - a prospective study of 65 children and adolescents. Bone Miner. 1993;23:171–82. doi: 10.1016/s0169-6009(08)80094-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhou W, Langsetmo L, Berger C, Adachi JD, Papaioannou A, Ioannidis G, Webber C, Atkinson SA, Olszynski WP, Brown JP, Hanley DA, Josse R, Kreiger N, Prior J, Kaiser S, Kirkland S, Goltzman D, Davison KS. Normative bone mineral density z-scores for Canadians aged 16 to 24 years:the Canadian Multicenter Osteoporosis Study. J Clin Densitom. 2010;13:267–76. doi: 10.1016/j.jocd.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenenhouse A, Joseph L, Kreiger N, Poliquin S, Murray T.M, Prior JC, Blondeau L, Berger C CAMOS Research Group. Estimation of prevalence of low bone density in Canadian women and men using a population-specific DEXA reference standard:the Canadian Multicentre Osteoporosis Study (CaMos) Osteoporos Int. 2000;11:897–904. doi: 10.1007/s001980070050. [DOI] [PubMed] [Google Scholar]
- 13.Berger C, Goltzman D, Langsetmo L, Joseph L, Jackson S, Kreiger N, Tenenhouse A, Davison KS, Josse RG, Prior JC, Hanley DA. Peak bone mass from longitudinal data:implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res. 2010;25:1948–57. doi: 10.1002/jbmr.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ott SM, Scholes D, LaCroix AZ, Ichikawa LE, Yoshuda CK, Barlow WE. Effect of contraceptive use on bone biochemical markers in young women. J Clin Endocrinol Metab. 2001;86:179–85. doi: 10.1210/jcem.86.1.7118. [DOI] [PubMed] [Google Scholar]
- 15.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, Leboff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB. Effects of estrogen plus progestin on risk of fracture and bone mineral density:the Women's Health Initiative randomized trial. JAMA. 2003;290:1729–38. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 16.Scholes D, Hubbard RA, Ichikawa LE, LaCroix AZ, Spangler L, Beasley JM, Reed S, Ott SM. Oral contraceptive use and bone density change in adolescent and young adult women:a prospective study of age, hormone dose, and discontinuation. J Clin Endocrinol Metab. 2011;96:E1380–E1387. doi: 10.1210/jc.2010-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pikkarainen E, Lehtonen-Veromaa M, Mottonen T, Kautiainen H, Viikari J. Estrogen-progestin contraceptive use during adolescence prevents bone mass acquisition:a 4-year follow-up study. Contraception. 2008;78:226–31. doi: 10.1016/j.contraception.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Beksinska ME, Kleinschmidt I, Smit JA, Farley TM. Bone mineral density in a cohort of adolescents during use of norethisterone enanthate, depot-medroxyprogesterone acetate or combined oral contraceptives and after discontinuation of norethisterone enanthate. Contraception. 2009;79:345–9. doi: 10.1016/j.contraception.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lattakova M, Borovsky M, Payer J, Killinger Z. Oral contraception usage in relation to bone mineral density and bone turnover in adolescent girls. Eur J Contracept Reprod Health Care. 2009;14:207–14. doi: 10.1080/13625180902838828. [DOI] [PubMed] [Google Scholar]
- 20.Gai L, Jia Y, Zhang M, Gai P, Wang S, Shi H, Yu X, Liu Y. Effect of two kinds of different combined oral contraceptives use on bone mineral density in adolescent women. Contraception. 2012;86:332–6. doi: 10.1016/j.contraception.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Kreiger N, Tenenhouse A, Joseph L, Mackenzie MD, Poliquin S, Brown JP, Prior JC, Rittmaster RS. The Canadian Multicentre Osteoporosis Study (CaMos):Background, rationale, methods. Can J Aging. 1999;18:376–87. [Google Scholar]
- 22.Nadalin V, Bentvelsen K, Kreiger N. Reliability of self-reports:data from the Canadian Multi-Centre Osteoporosis Study (CaMos) Chronic Dis Can. 2004;25:28–31. [PubMed] [Google Scholar]
- 23.Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiological Reviews. 2016;96:449–547. doi: 10.1152/physrev.00027.2015. [DOI] [PubMed] [Google Scholar]
- 24.Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139–44. doi: 10.1001/archpedi.159.2.139. [DOI] [PubMed] [Google Scholar]
- 25.Biason TP, Goldberg TB, Kurokawa CS, Moretto MR, Teixeira AS, Nunes HR. Low-dose combined oral contraceptive use is associated with lower bone mineral content variation in adolescents over a 1-year period. BMC Endocr Disord. 2015;15:15. doi: 10.1186/s12902-015-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cromer BA, Bonny AE, Stager M, Lazebnik R, Rome E, Ziegler J, Camlin-Shingler K, Secic M. Bone mineral density in adolescent females using injectable or oral contraceptives:a 24-month prospective study. Fertil Steril. 2008;90:2060–7. doi: 10.1016/j.fertnstert.2007.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cromer BA, Stager M, Bonny A, Lazebnik R, Rome E, Ziegler J, Debanne SM. Depot medroxyprogesterone acetate, oral contraceptives and bone mineral density in a cohort of adolescent girls. J Adolesc Health. 2004;35:434–41. doi: 10.1016/j.jadohealth.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Winther A, Dennison E, Ahmed LA, Furberg AS, Grimnes G, Jorde R, Gjesdal CG, Emaus N. The Tromso Study:Fit Futures:a study of Norwegian adolescents'lifestyle and bone health. Arch Osteoporos. 2014;9:185. doi: 10.1007/s11657-014-0185-0. [DOI] [PubMed] [Google Scholar]
- 29.Gargano V, Massaro M, Morra I, Formisano C, Di CC, Nappi C. Effects of two low-dose combined oral contraceptives containing drospirenone on bone turnover and bone mineral density in young fertile women:a prospective controlled randomized study. Contraception. 2008;78:10–5. doi: 10.1016/j.contraception.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Rome E, Ziegler J, Secic M, Bonny A, Stager M, Lazebnik R, Cromer BA. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive. J Pediatr Adolesc Gynecol. 2004;17:373–7. doi: 10.1016/j.jpag.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Paoletti AM, Orru M, Floris S, Mannias M, Vacca AM, Ajossa S, Guerriero S, Melis GB. Evidence that treatment with monophasic oral contraceptive formulations containing ethinylestradiol plus gestodene reduces bone resorption in young women. Contraception. 2000;61:259–63. doi: 10.1016/s0010-7824(00)00104-9. [DOI] [PubMed] [Google Scholar]
- 32.Mais V, Fruzzetti F, Ajossa S, Paoletti AM, Guerriero S, Melis GB. Bone metabolism in young women taking a monophasic pil containing 20 mcg ethinylestradiol:A prospective study. Contraception. 1993;48:445–52. doi: 10.1016/0010-7824(93)90134-s. [DOI] [PubMed] [Google Scholar]
- 33.Cibula D, Skrenkova J, Hill M, Stepan JJ. Low-dose estrogen combined oral contraceptives may negatively influence physiological bone mineral density acquisition during adolescence. Eur J Endocrinol. 2012;166:1003–11. doi: 10.1530/EJE-11-1047. [DOI] [PubMed] [Google Scholar]
- 34.Lopez LM, Grimes DA, Schulz KF, Curtis KM. Steroidal contraceptives:effect on bone fractures in women. Cochrane Database Syst Rev. 2009;2:1–35. [Google Scholar]
- 35.Cooper C, Hannaford P, Croft P, Kay CR. Oral contraceptive pill use and fractures in women:a prospective study. Bone. 1993;14:41–5. doi: 10.1016/8756-3282(93)90254-8. [DOI] [PubMed] [Google Scholar]
- 36.Vessey M, Mant J, Painter R. Oral contraception and other factors in relation to hospital referral for fracture - findings in a large cohort study. Contraception. 1998;57:231–5. doi: 10.1016/s0010-7824(98)00026-2. [DOI] [PubMed] [Google Scholar]
- 37.Barad D, Kooperberg C, Wactawski-Wende J, Liu J, Hendrix SL, Watts NB. Prior oral contraception and postmenopausal fracture:a Women's Health Initiative observational cohort study. Fertil Steril. 2005;84:374–83. doi: 10.1016/j.fertnstert.2005.01.132. [DOI] [PubMed] [Google Scholar]


