ABSTRACT
We previously reported that mice intracerebrally inoculated with the mouse-adapted scrapie strain ME7 have markedly diminished T zones in the spleen due to the decreased expression of CCL19 and CCL21. In addition, follicular dendritic cell networks in germinal centers were larger in ME7-infected spleens compared to uninfected spleens. As an extension of that study, we set out to determine how ME7 infection affects spleen structure and follicular helper T (Tfh) cell responses in mice. For this study, mice were intraperitoneally inoculated with brain homogenate of the ME7 inoculum and spleens were analyzed 50, 130, and 200 days after inoculation and compared with those from uninfected mice. The result showed that ME7- infected mice had increased Tfh cell responses which were maintained until end-stage prion disease. Although CD4 T cells decreased in white pulps, they increased in germinal centers, and expressed higher levels of the Tfh-related genes, such as Bcl6, Il21, Cxcr5, Icos, and Pdcd1. In addition, ME7-infected spleens had increased numbers of CD4 memory T cells. These data indicate that although ME7 infection led to impaired splenic white pulp structure, CD4 memory T cells were increased and Tfh cell responses were required and prolonged to provide help for the replication and accumulation of pathogenic prion protein in germinal centers.
KEYWORDS: follicular helper T cells, germinal centers, ME7, prion, spleen
Introduction
The cellular prion protein (PrPC) is a glycosylphosphatidylinositol-anchored glycoprotein that is located at the cell surface. PrPC is mainly expressed in neurons within the central nervous system, as well as in lymphocytes [1,2], where it can be upregulated through T-cell activation [3]. PrPC can become misfolded into an abnormal conformation, called PrPSc, which is proteinase K-resistant. Importantly, PrPSc aggregation induces prion diseases, known as transmissible spongiform encephalopathies [4]. The role of PrPC in the propagation of PrPSc was identified using PrP-knockout mice, which showed no neuronal symptoms, even when exposed to mouse-adapted scrapie strains, including ME7, 22L, and 139A [5].
PrPSc replication is observed mainly in the central nervous system, but can also occur in lymphoreticular tissues [6]. Recently, it was shown that the lymphoreticular system is crucial for PrPSc replication, and PrPSc accumulation in lymphoid tissues can be detected at early clinical stage after prion infection in mice [1,2].
Follicular dendritic cells (FDC), which present native antigens to B cells and highly express PrPC, are key players in prion pathogenesis in the spleen, lymph nodes, and Peyer's patches [7]. ME7 infection of mice results in accumulation of PrPSc in FDCs, and previous studies have demonstrated that FDCs expressing PrPC are required for splenic PrPSc replication [8–10]. Spleen-derived prions access the brain through secondary lymphoid innervations that enable prions to enter the central nervous system [11]. Following infection of the brain, lymphoid PrPC replication and PrPSc accumulation occur quickly, accelerating end-stage disease and ultimate death [12,13].
In our previous study, and like many other studies on prion disease, we injected the mouse-adapted scrapie strain ME7 directly into the cerebrum because of the vulnerability of the brain to the effects of PrPSc [14–17]. However, in nature, it is probable that the more common mechanism of infection is through an oral route, whereby the proteins travel through blood vessels to the secondary lymphoid tissues where they replicate [18–20]. Therefore, in the current study, we injected the mouse-adapted scrapie strain ME7 into the peritoneum to target the spleen, and evaluated spleen structure and splenocyte responses. The main difference between intracerebral and intraperitoneal injection was the time to disease onset and length to end-stage disease. Mice infected by intracerebral injection died by approximately 150 days [21], while mice infected by intraperitoneal injection died in about 200 days. By the end stage of prion disease following intraperitoneal injection, we observed similar effects on spleen structure and responses, including advanced FDC networks, decreased T zones, and impaired white pulp structure. We found that CD4 T cell numbers were decreased, T zones were smaller or absent, but FDC networks and germinal centers were bigger in prion-infected mice. Therefore, we investigated activation of B cells and follicular helper T (Tfh) cells at early (day 50 after inoculation), middle (day 130), and end (day 200) stages of disease. In addition, since Tfh cells were highly activated at the end stage of ME7 infection, remaining CD4 T cells were analyzed and their change was traced.
Results
ME7-infected spleens at end-stage prion disease have increased germinal center reactions
In our previous study, we reported that the white pulp regions of spleens of mice infected with ME7 scrapie strain have significantly decreased T zones due to decreased expression of the T zone chemokines, CCL19 and CCL21 [21]. Here, we set out to determine how scrapie infection of mice affects spleen structure. To do, we injected the ME7 scrapie strain into the peritoneum of 6-week-old C57BL/6 mice to allow the prion proteins to be absorbed into blood vessels and migrate to the secondary lymphoid tissues to replicate. Firstly, we measured spleen size and weight at the end stage of prion disease (Fig. 1A and B). Compared to control, ME7-infected mice showed smaller spleens: on average, ME7-infected spleens were 1.25 cm in size and 0.030 g in weight, whereas controls were 1.60 cm in size and 0.105 g in weight. Next, we investigated spleen structure. Normal spleens have well segregated T zone and B zone where the lymphocytes are predominantly T cells and B cells, respectively. After antigen stimulation, germinal centers around FDC networks in B zone were formed for intense B-cell proliferation, selection, and affinity maturation. At the early stage of prion disease (50 days after injection), ME7-infected spleens were not significantly different than controls (data not shown). At the middle stage (130 days after injection), ME7-infected spleens had more developed germinal centers and retained clearly segregated T and B zone structures (Fig. 1C). Compared to control spleens at the end stage (200 days after injection), ME7-infected spleens had smaller white pulp regions with significantly diminished T zones (Fig. 1C). However, at this stage, ME7-infected spleens had clearly formed germinal centers and FDC networks (Fig. 1C and Fig. 2). Importantly, even though T zones were significantly diminished, the clearly developed germinal centers contained many T cells, B cells, and FDCs.
Figure 1.
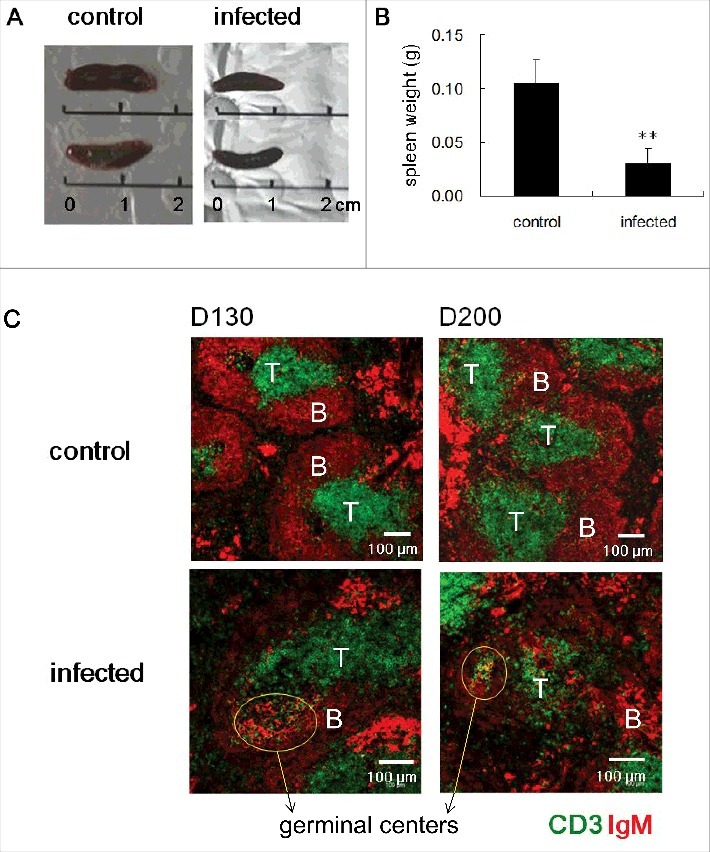
ME7-infected spleens at end-stage prion disease were smaller and have impaired T-zone structure and increased germinal center reactions. (A) Size and of (B) weight the spleens of control and ME7-infected mice at the end stage of prion disease. **, p < 0.01. (C) Confocal images of spleen sections from control and ME7-infected mice at the indicated times (130 and 200 days after injection). Immunofluorescent staining indicates CD3 (green, T cell marker) and IgM (red, B cell marker), and scale bar represents 100 μm. Yellow circles indicate germinal centers. N = 6 control and 6 ME7-infected mice.
Figure 2.
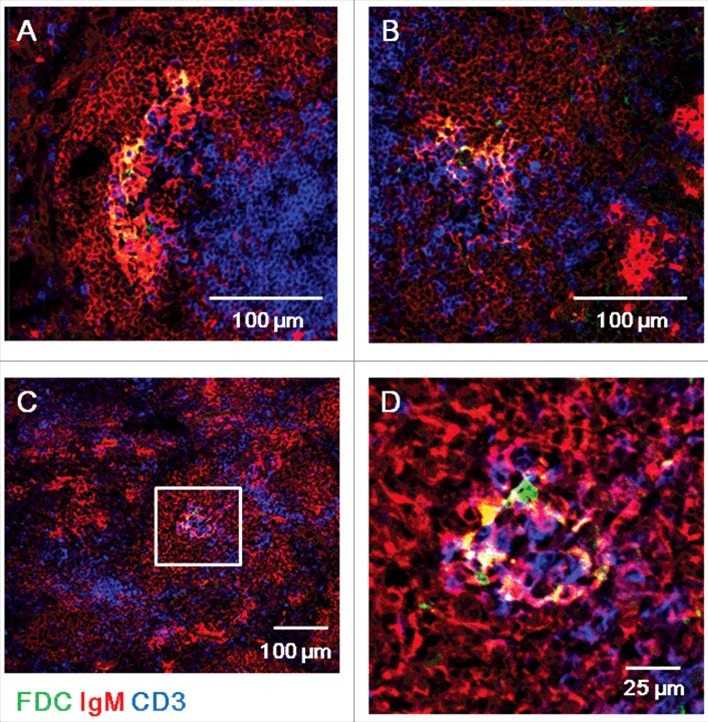
ME7-infected spleens at end-stage prion disease have larger FDC networks in germinal centers. Confocal images of ME7-infected mouse spleen sections 200 days after ME7 infection. Immunofluorescent staining indicates IgM (red), CD3 (blue), and FDC (green). (A, B, and C) Low-power confocal images of germinal centers. Scale bar represents 100 μm. (D) High-power confocal images of the area boxed in white in (C). Scale bar, 25 μm. N = 6 control and 6 ME7-infected mice.
Tfh cell responses are increased in the spleens of ME7-infected mice
Because Tfh cells are important for the generation of B cell–mediated germinal center differentiation, we examined the mRNA expression of genes related to Tfh cell responses (Fig. 3). We found that the expression of Bcl6, the master transcriptional regulator for Tfh differentiation [22,23], increased 130 and 200 days after ME7 infection: 43.4% in control and 49.2% in ME7-infected groups on day 130, and 38.7% in control and 45.1% % in ME7-infected groups on day 200 (Fig. 3A). In addition to Bcl6, the expression of Il21, which is expressed by Tfh cells and critical for germinal center formation [24], also increased 130 and 200 days after ME7 infection: 4.7% in control and 6.2% in ME7-infected mice on day 130, and 4.8% in control and 6.9% in ME7-infected mice on day 200 (Fig. 3B). However, the expression of Il6 was not significantly different between control and ME7-infected mice (Fig. 3C). The expression of Cxcr5, a receptor involved in B follicle homing and germinal center formation [25], was two-fold increased in ME7-infected groups on day 200 compared to control mice (2.8% in control and 5.5% in ME7-infected mice) (Fig. 3D). Finally, the expression of inducible T cell co-stimulator (Icos) and Pdcd1 was 1.63-fold (Fig. 3E) and 1.68-fold (Fig. 3F), respectively, increased in ME7-infected mice on day 200 compared to control mice.
Figure 3.
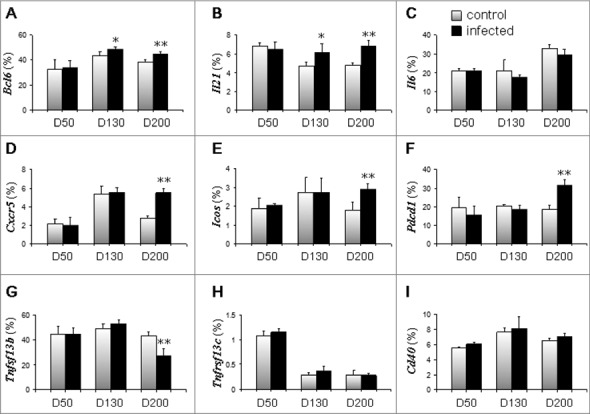
Tfh cell responses are increased in the spleens of ME7-infected mice. The mRNA expression levels of genes related to Tfh cell responses in the spleens of control and ME7-infected mice at the indicated times (50, 130, and 200 days after injection). (A) Bcl6, (B) Il21, (C) Il6, (D) Cxcr5, (E) Icos, (F) Pdcd1, (G) Tnfsf13b, (H) Tnfrsf13c, and (I) Cd40. Relative mRNA expression was measured by qRT-PCR and normalized to ActB signals. The data represent the average expression over six separate experiments, using six mice. Error bars represent mean values ± standard deviation. *, p < 0.05, and **, p < 0.01.
Since germinal center formation was retained, we analyzed the activation of B cells. Expression of Tnfsf13b (also known as Baff) was not affected in early and middle stages of ME7 infection, but was significantly decreased from 43.5% to 27.4% by end-stage disease (Fig. 3G). Additionally, expression of the Tnfrsf13c (also known as Baff receptor) by B cells was not affected until the end stage of infection, although its expression decreased in both control and infected mice (Fig. 3H). Finally, ME7 infection did not affect the expression of Cd40 in B cells until the end stage of infection (Fig. 3I).
ME7 infection increases CD4 memory T cells
Although CD4 T cells are decreased by end-stage ME7 infection, Tfh cells were highly activated. Therefore, we analyzed the remaining CD4 T cells over the time course of prion disease (Fig. 4). We found that the expression of CCR7, a T zone chemokine receptor, was similar in both groups: on day 50, 84.3% of CD4 T cells were positive for CCR7 in control mice, while 87.3% were positive in ME7-infected mice. On day 130, 77.8% of CD4 T cells were positive in control and 73.5 % were positive in ME7-infected mice. However, the expression of CCR7 decreased to 64.6% in infected mice on day 200 compared with 75.2% in control mice. The expression of CD44, a marker for memory T cells, was positive in 42.6% of CD4 T cells in control mice and in 35.4% in infected mice on day 50. On day 130 after infection, 44.9% of CD4 T cells were positive for CD44 in control mice and 55.2% were positive in infected mice. The CD44 expression was gradually increased until the end stage of prion disease, and was expressed 1.5-fold more in ME7-infected mice (62.0%) on day 200 compared to control mice (42.0%). In addition, CD44+ CD4 T cells that did not express CCR7 also were increased in ME7-infected mice (32.9%) on day 200 compared to control mice (21.7%).
Figure 4.
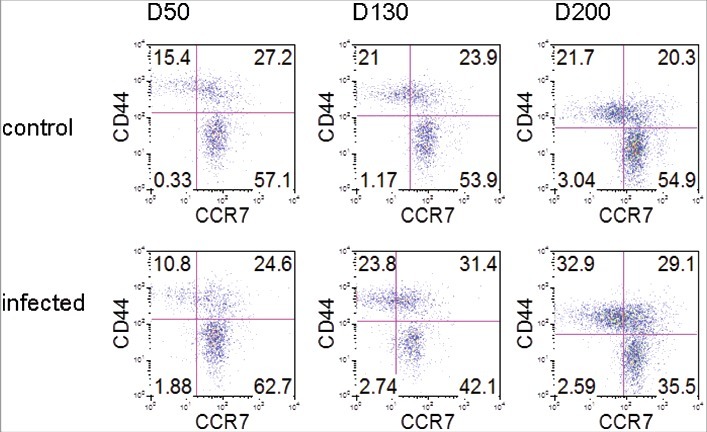
ME7 infections increases CD4 memory T cells. FACS analyses of CCR7 and CD44 expression by CD4+CD3+ gated cells in the spleens of control and ME7-infected mice after injection at the indicated times (50, 130, and 200 days after injection). The data represent the average expression over six separate experiments, using six mice.
Discussion
Our previous work demonstrated that ME7 infection via intracerebral inoculation causes decreased T zones in the spleens of mice, which is associated with decreased CCL19 and CCL21 expression [21]. Additionally, we previously showed that ME7-infected spleens display larger FDC networks, which accumulate PrPSc during scrapie infection [21]. Consistent with this previous work, we show here that C57BL/6 mice intraperitoneally inoculated with ME7 showed largely developed germinal centers and FDC networks. In addition, Tfh cell responses were increased and prolonged in germinal centers until the end stage of prion disease.
Formation of immunological memory depends on the cooperation of T and B cells. In particular, Tfh cells are important for germinal center formation and maintenance, as well as for their specialized role in the differentiation of B cells in germinal centers [26]. In this study, although CD4 T cells decreased in white pulps, they increased in germinal centers and, following 200 days of ME7 infection, expressed higher levels of Tfh-related genes, including Bcl6, Il21, Cxcr5, Icos, and Pdcd1 compared to controls. The employment of FDCs by infectious prions for their replication in germinal centers likely results in continuous activation of B cells, which requires T cell help. Therefore, based on our data, we suggest that Tfh cells in germinal centers are maintained for this purpose, while T zones in white pulps are reduced.
In addition, our previous study showed that B zone chemokine, CXCL13, and LTα, factors involved in B cell zone development, do not change in response to ME7 infection [21]. Here, we extend these findings by reporting the intact development of germinal centers in ME7-infected spleens and expression of additional genes related to B cell activation. The expression of the receptor for Baff, which is a B-cell activating factor, was notably decreased in both control and infected mice, and the expression of Baff itself in infected mice was significantly decreased at the end stage of disease compared to control mice. Developing B cells that leave the bone marrow mature in the secondary lymphoid tissues and receive survival signals through Baff receptors. Because ligation of the Baff receptor and Baff has a dominant role in peripheral B cell maturation and survival [27] rather than germinal center formation or maintenance in which matured and activated B cells are involved, their decreased expression does not appear to affect B zone structure after ME7 infection. In addition, expression of CD40, which is important for Tfh cell interaction [28], did not change, and B cells constitutively expressed CD40 until the end stage of disease.
Finally, we observed that although overall numbers of CD4 T cells were decreased, Tfh cells were highly activated at the end stage of ME7 infection. Therefore, we analyzed the remaining CD4 T cells over the timecourse of prion disease. We found that the white pulps of ME7-infected spleens had increased numbers of CD44-expressing memory T cells and decreased numbers of CD44-negative naïve cells than control spleens. These data indicate that although naïve CD4 T cells fail to home to splenic T zones, the remaining CD4 T cells are memory T cells.
In summary, by the end stage of prion disease, ME7-infected mice had impaired splenic white pulp structure and displayed failed naïve CD4 T cell homing to T zones, although memory T cells increased. Additionally, we suggest that prolonged activation of Tfh cell responses in response to prion infection may help germinal center B cells, where FDCs are utilized for the replication and accumulation of PrPSc.
Materials and methods
Mice
Six-week-old C57BL/6 mice were purchased from Young Bio (Seongnam, Republic of Korea). The original stock of the ME7 scrapie strain was kindly provided by Dr. Alan Dickinson of the Agriculture and Food Research Council and Medical Research Council Institute (Neuropathogenesis Unit, Edinburgh, UK). For scrapie infection, mice were intraperitoneally inoculated with 100 μl of 1% w/v brain homogenate of the ME7 inoculum in phosphate-buffered saline (pH 7.4). Uninfected control mice were injected with 100 μl of 1% w/v normal brain homogenate. Scrapie-inoculated and uninfected control mice were sacrificed 50, 130, and 200 days post-inoculation. All experiments were performed according to Korean laws and with the approval of the Hallym Medical Center Institutional Animal Care and Use Committee (HMC2011-0-0115-07).
Quantitative PCR
The isolation of mRNA, cDNA preparation, and quantitative real-time PCR (qRT-PCR) (Takara, Shiga, Japan) were performed as previously described [29,30]. The relative expression of the ActB control signal was calculated as 2–ΔCt × 102. Expression of each target was normalized to the ActB signal (ActB = 100%). The primer sequences used are as follows (synthesized by Bioneer [Daejeon, Korea]):
ActB
(forward CGTGAAAAGATGACCCAGATCA) (reverse TGGTACGACCAGAGGCATACAG),
Il6
(forward TAGTCCTTCCTACCCCAATTTCC) (reverse TTGGTCCTTAGCCACTCCTTC),
Il21
(forward ACTCAGTTCTGGTGGCATGG) (reverse TGAATCATCTTTTGAAGGAGCCA),
Cxcr5
(forward ATGAACTACCCACTAACCCTGG) (reverse TGTAGGGGAATCTCCGTGCT), Tnfsf13b
(forward CCTCGCATCTCCCACAAACA) (reverse GGTGTTGCTGAACCTCGGTA), Tnfrsf13c
(forward ATCAGACCGAGTGCTTCGAC) (reverse ACATTTTCCAGGGACTCTTGCT), Bcl6
(forward CCGGCACGCTAGTGATGTT) (reverse TGTCTTATGGGCTCTAAACTGCT),
Icos
(forward TCTGCGAACTCACCAAGACC) (reverse ATCAAGTCAGGTGCCCTGTG), Pdcd1
(forward TGGGGCCTAAGCCTATGTCT) (reverse CTCCCAAGGGTGGCTTTAGG),
Cd40
(forward TTGTTGACAGCGGTCCATCT) (reverse TCTCAAGAGCTGTGCAGTGG)
Confocal images
Immunofluorescence and confocal imaging was perfomed as described previously [21]. The following primary antibodies were used in our analyses: FITC-conjugated anti-mouse CD3 (145-2C11, eBioscience, San Diego, CA), purified anti-mouse FDC (FDC-M1, BD Biosciences, San Jose, CA), rhodamine-conjugated anti-mouse IgM (Jackson ImmunoResearch, West Grove, PA), and AMCA-conjugated anti-mouse IgM (Jackson ImmunoResearch). Purified FDC antibodies were detected with donkey-anti-rat IgG FITC (Jackson ImmunoResearch). FITC-conjugated antibodies were detected with AF488-conjugated rabbit anti-FITC (Molecular Probes, Carlsbad, CA), and FITC-conjugated donkey anti-rabbit IgG (Molecular Probes). Sections were mounted using ProLong Gold Antifade Reagent (Molecular Probes), and confocal images were obtained using an LSM 700 Meta microscope (Carl Zeiss, GmbH, Germany), equipped with 405, 488, 555, and 639 nm lasers. Images were analyzed using Zeiss LSM software (Carl Zeiss).
Flow cytometry
Monoclonal Abs (mAbs) for CD3 (145-2c11), CD44 (IM7), and CCR7 (4B12) were purchased from eBioscience (San Diego, CA), and anti-CD4 (RM4-5) was purchased from BD Biosciences (San Jose, CA). Data were collected with FACSCaliber (BD Biosciences), and analyzed using Flowjo software (TreeStar, San Carlos, CA).
Statistical analysis
For all experiments, significance was calculated using the Student's t-test and one–way ANOVA, with p < 0.05 considered significant; *: p < 0.05, and **: p < 0.01, as compared to control.
Funding Statement
Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, NRF- 2016R1D1A1B01011371
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF- 2016R1D1A1B01011371).
References
- [1].Stahl N, Borchelt DR, Hsiao K, et al.. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987;51:229–40. [DOI] [PubMed] [Google Scholar]
- [2].Li R, Liu D, Zanusso G, et al.. The expression and potential function of cellular prion protein in human lymphocytes. Cell Immunol. 2001;207:49–58. [DOI] [PubMed] [Google Scholar]
- [3].Mabbott NA, Brown KL, Manson J, et al.. T-lymphocyte activation and the cellular form of the prion protein. Immunology. 1997;92:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chesebro B. Introduction to the transmissible spongiform encephalopathies or prion diseases. British Medical Bulletin. 2003;66:1–20. [DOI] [PubMed] [Google Scholar]
- [5].Aguzzi A, Lakkaraju AKK, Frontzek K. Toward Therapy of Human Prion Diseases. Annu Rev Pharmacol Toxicol. 2017;58:331–51. [DOI] [PubMed] [Google Scholar]
- [6].Srivastava S, Makarava N, Katorcha E, et al.. Post-conversion sialylation of prions in lymphoid tissues. Proc Natl Acad Sci U S A. 2015;112:E6654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Heinen E. [Follicular dendritic cells: phenotype, origin and functions]. Pathologie-Biologie. 1995;43:848–57. [PubMed] [Google Scholar]
- [8].Mould DL, Dawson AM, Rennie JC. Very early replication of scrapie in lymphocytic tissue. Nature. 1970;228:779–80. [DOI] [PubMed] [Google Scholar]
- [9].Kitamoto T, Muramoto T, Mohri S, et al.. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt-Jakob disease. J Virol. 1991;65:6292–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brown KL, Stewart K, Ritchie DL, et al.. Scrapie replication in lymphoid tissues depends on prion protein-expressing follicular dendritic cells. Nature medicine. 1999;5:1308–12. [DOI] [PubMed] [Google Scholar]
- [11].Cole S, Kimberlin RH. Pathogenesis of mouse scrapie: dynamics of vacuolation in brain and spinal cord after intraperitoneal infection. Neuropathol Appl Neurobiol. 1985;11:213–27. [DOI] [PubMed] [Google Scholar]
- [12].Rubenstein R, Merz PA, Kascsak RJ, et al.. Scrapie-infected spleens: analysis of infectivity, scrapie-associated fibrils, and protease-resistant proteins. J Infect Dis. 1991;164:29–35. [DOI] [PubMed] [Google Scholar]
- [13].Prinz M, Montrasio F, Furukawa H, et al.. Intrinsic resistance of oligodendrocytes to prion infection. J Neurosci : the official journal of the Society for Neuroscience. 2004;24:5974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Betmouni S, Perry VH, Gordon JL. Evidence for an early inflammatory response in the central nervous system of mice with scrapie. Neuroscience. 1996;74:1–5. [DOI] [PubMed] [Google Scholar]
- [16].Mabbott NA, Williams A, Farquhar CF, et al.. Tumor necrosis factor alpha-deficient, but not interleukin-6-deficient, mice resist peripheral infection with scrapie. J Virol. 2000;74:3338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang F, Wang X, Yuan CG, et al.. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Andreoletti O, Berthon P, Marc D, et al.. Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol. 2000;81:3115–26. [DOI] [PubMed] [Google Scholar]
- [19].Aguzzi A, Heikenwalder M. Pathogenesis of prion diseases: current status and future outlook. Nat Rev Microbiol. 2006;4:765–75. [DOI] [PubMed] [Google Scholar]
- [20].Mabbott NA, MacPherson GG. Prions and their lethal journey to the brain. Nat Rev Microbiol. 2006;4:201–11. [DOI] [PubMed] [Google Scholar]
- [21].Kim S, Han S, Lee HS, et al.. Impaired spleen structure and chemokine expression in ME7 scrapie-infected mice. Immunobiology. 2016;221:871–8. [DOI] [PubMed] [Google Scholar]
- [22].Nurieva RI, Chung Y, Martinez GJ, et al.. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zotos D, Coquet JM, Zhang Y, et al.. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schaerli P, Willimann K, Lang AB, et al.. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Crotty S. Follicular helper CD4 T cells (TFH). Ann Rev immunol. 2011;29:621–63. [DOI] [PubMed] [Google Scholar]
- [27].Craxton A, Draves KE, Gruppi A, et al.. BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J Exp Med. 2005;202:1363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Deenick EK, Ma CS, Brink R, et al.. Regulation of T follicular helper cell formation and function by antigen presenting cells. Curr Opin Immunol. 2011;23:111–8. [DOI] [PubMed] [Google Scholar]
- [29].Kim MY, Gaspal FM, Wiggett HE, et al.. CD4(+)CD3(−) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–54. [DOI] [PubMed] [Google Scholar]
- [30].Kim S, Han S, Kim MY. Effects of interleukin-15 on human CD3(−)CD117(+)CD56(−)OX40L(+) cell differentiation. Hum Immunol. 2010;71:745–50. [DOI] [PubMed] [Google Scholar]


