Abstract
Background
Clinical trials have shown very modest short-term improvements in glycemic control among participants with diabetes after periodontitis treatment. Few longitudinal studies suggest that periodontitis may be related to prediabetes/diabetes risk.
Methods
We evaluated 1,206 diabetes free participants in the San Juan Overweight Adults Longitudinal Study (SOALS) and 941 with complete 3-year follow-up data were included. The National Health and Nutrition Examination Survey (NHANES) methods were used to assess periodontitis. Diabetes and prediabetes were classified using American Diabetes Association cutoffs for fasting and 2-hour post-load glucose and HbA1c. We used Poisson regression adjusting for baseline age, gender, smoking, education, family history of diabetes, physical activity, waist circumference, and alcohol intake.
Results
Over the 3-year follow-up, 69 (7.3%) of the 941 individuals developed type 2 diabetes, and 142 (34.9%) of the 407 with normal glycemia at baseline developed prediabetes. In multivariable models, greater mean pocket depth and mean attachment loss at baseline were associated with lower risk of developing prediabetes/diabetes over the follow-up (IRR=0.81; 95% CI: 0.67–0.99, and IRR=0.86; 95% CI: 0.74–0.99, respectively). Increase in periodontal attachment loss from baseline to follow-up was associated with higher prediabetes/diabetes risk (multivariate IRR=1.25; 95% CI: 1.09–1.42), and increase in pocket depth was associated with >20% fasting glucose increase (multivariate IRR=1.43; 95% CI: 1.14–1.79). The inverse associations persisted after additionally adjusting for baseline income, sugar-sweetened beverages, number of teeth, oral hygiene, glycemia, or previous periodontal therapy.
Conclusions
There is no association between periodontitis and risk of prediabetes/diabetes in this longitudinal study.
Keywords: Periodontal research, diabetes, prediabetes, insulin resistance, prospective cohort study, gingivitis
INTRODUCTION
Periodontitis is a chronic inflammation of the tissues surrounding the teeth that leads to formation of pockets between the teeth and gums, loss of surrounding tissue and bone (attachment loss and bone loss) which could lead to tooth mobility and subsequently the need for extraction of the teeth. A bi-directional association has been postulated between periodontitis and diabetes. However, the majority of studies showing associations are cross-sectional. In two longitudinal studies, periodontitis was associated with poor glycemic control among people with diabetes [1, 2]. Recent reviews of clinical trials assessing the effect of periodontitis treatment on glycosylated hemoglobin (HbA1c) levels among subjects with type 2 diabetes showed very modest short-term reductions over 3–4 months [3–8], with differing results across the studies. Most studies did not show sustained HbA1c reductions by six months [6–8]. One large well-controlled trial showed no improvements in glycemic control among participants with diabetes after periodontitis treatment [9]. The associations between periodontitis and diabetes are further supported by literature relating periodontitis with complications such as ischemic stroke [10]. Many risk factors for diabetes including obesity, inflammatory markers, low adiponectin, and dyslipidemia have also been related to periodontitis. Periodontitis is linked with increased systemic inflammation/endothelial dysfunction, dyslipidemia and low adiponectin, which in turn could lead to increased risk of prediabetes/diabetes. Figure 1 shows potential pathways linking periodontitis and glucose abnormalities, including these mediators proposed in the medical literature, and common risk factors that could be potential confounders.
Figure 1.
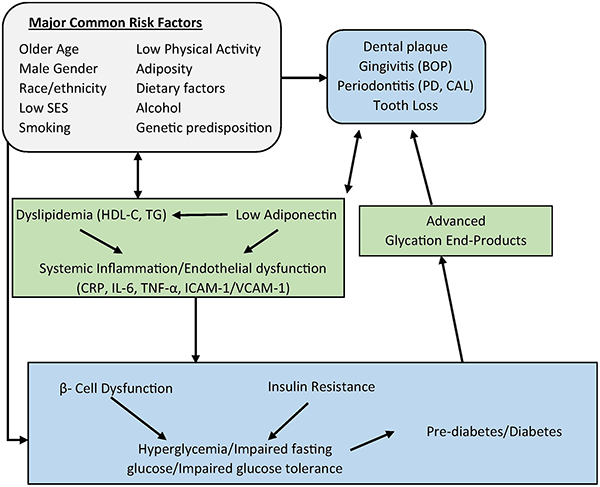
Pathways linking periodontitis and diabetes. Abbreviations: C-reactive protein (CRP), Interleukin 6 (IL-6), Tumor necrosis factor alpha (TNF-α), Intercellular adhesion molecule 1 (ICAM-1), Vascular cell adhesion protein 1 (VCAM-1), Socioeconomic status (SES), Pocket depth (PD), Clinical attachment loss (CAL), Bleeding on probing (BOP), High-density lipoprotein cholesterol (HDL-C), Triglycerides (TD)
Only seven recently published longitudinal studies to date have directly evaluated whether periodontitis is associated with increased risk of developing prediabetes and/or diabetes among people without diabetes [11–17]. Periodontal disease was associated with increased risk of prediabetes/diabetes in most studies, but most longitudinal studies were all limited in the exposure and/or outcome assessment [14–17]. Prediabetes/diabetes was self-reported in three studies and only based on fasting glucose in one study, and three of these studies did not assess standard pocket depth (PD) and clinical attachment loss (CAL) measures. Of the two longitudinal studies evaluating impaired fasting glucose, one did and one did not show significant associations with periodontitis, which was defined in an unconventional manner [12, 13]. Associations between periodontitis and other precursors of diabetes (insulin resistance or hyperglycemia) have only been evaluated in few cross-sectional studies [18–22], and in two longitudinal studies from Japan evaluating impaired fasting glucose [12, 13]. Hence, there is a need for longitudinal studies evaluating periodontitis in relation to precursors of diabetes using standard measures. Therefore, we assessed longitudinally whether periodontitis is associated with a higher progression of insulin resistance, glucose abnormalities, and development of prediabetes/diabetes within a large cohort over a three-year period.
MATERIALS AND METHODS
The San Juan Overweight Adults Longitudinal Study (SOALS) was initiated in 2011 to assess the bi-directional association between periodontitis and prediabetes, and to evaluate specific potential mediators. Adults aged 40–65 years, free of diagnosed diabetes, were recruited by advertising (e.g. flyers, word of mouth). SOALS was approved by the University of Puerto Rico Institutional Review Board and is reported following STROBE guidelines. SOALS baseline exclusion criteria, detailed in a prior publication [23], consisted mainly of factors precluding a valid periodontal exam (less than four teeth, orthodontic appliances), and factors that may put participants at risk (e.g. cardiovascular conditions and bleeding disorders). Participants were excluded from these analyses if they had fasting, 2-hour glucose, or HbA1c above the American Diabetes Association (ADA) thresholds for diabetes at the baseline exam [24]. Out of the 1,206 participants without diabetes, 255 did not complete the 3-year follow-up. A total of 951 completed the follow-up visit (79% retention rate), and 10 were further excluded for missing data.
Outcome: Progression of glucose abnormalities and development of prediabetes/diabetes
At both baseline and follow-up exams, glucose and insulin were assessed at fasting and after a 75-gram glucose load at 30, 60, and 120 minutes. All other tests were conducted on fasting samples. Glucose was assessed using a Vitros System 250 instrument with intra-assay coefficient of variation (CV) of 1.21% and inter-assay CV of 3.06% with diffraction spectrometric technology. An immuno-enzymometric assay was used to determine plasma insulin using a TOSOH analyzer (intra-assay CV=1.49%; inter-assay CV=4.42%). The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as fasting insulin (mU/L) × fasting glucose (mmol/L)/22.5. HbA1c was assessed by a latex immunoagglutination inhibition methodology with monoclonal antibody using a Siemens Kit for DCA 2000 and DCA Vantage Analyzer.
We classified participants as having diabetes at the baseline or follow-up examination tests based on fasting glucose ≥ 126 mg/dl, 2-hour post-load glucose ≥ 200 mg/dl, or HbA1c ≥ 6.5%. Participants were classified as having prediabetes if fasting glucose levels were 100–125 mg/dl, 2-hour post load glucose were 140–199 mg/dl, or HbA1c was 5.7%−6.4%, or as having normal glycemia if all these values were below the mentioned thresholds for prediabetes [24]. Changes from normal glycemia to prediabetes or diabetes, or from prediabetes to diabetes based on study assessments, or reported physician diagnosed diabetes during the follow-up period were considered as development of prediabetes/diabetes. HOMA-IR was evaluated using 20% or greater change, as there is no conventional cut-off.
Exposures: Periodontitis and bleeding on probing
Full mouth periodontal exams were conducted at baseline and 3-year follow-up using NHANES procedures by one of three examiners [25, 26]. Periodontitis was assessed by clinical measurements of probing PD and recession at six sites (disto-buccal, mid-buccal, mesio-buccal, disto-lingual, mid-lingual, and mesio-lingual) for all 28 teeth excluding the third molars. All measurements were taken with a periodontal probe (product number PCP2; Hu-Friedy, Chicago, IL, USA) and rounded upwards to the nearest millimeter. The CAL was calculated as the sum of PD and gingival recession. Missing teeth were noted. The NHANES reference examiner (Dr. Bruce Dye) trained and calibrated the SOALS examiners. Gingivitis, was evaluated as bleeding on probing (BOP) [27]. About 20 seconds after probing, BOP was marked as present if bleeding was detected at the lingual and/or buccal surfaces respectively. BOP was classified as high for each individual if 30% or more of buccal and/or lingual surfaces showed BOP [23, 28–30].
Periodontitis was classified according to the CDC/AAP definition [31, 32]. Severe periodontitis was defined as having two or more interproximal sites with CAL ≥ 6 mm (not on the same tooth), and at least one interproximal site with PD ≥ 5 mm. Moderate periodontitis was defined as having two or more interproximal sites with CAL ≥ 4 mm (not on the same tooth), or two or more interproximal sites with PD ≥ 5 mm (not on the same tooth). Mild periodontitis was defined as having at least two interproximal sites with CAL ≥ 3 mm and at least two interproximal sites with PD ≥ 4 mm (not on the same tooth) or one site with PD ≥ 5 mm. Several additional definitions of periodontitis were considered as follows: mean PD, mean CAL, ≥ 1 site with PD ≥ 7 mm, and ≥ 1 site with CAL ≥ 7 mm. Since severe periodontitis could lead to tooth loss, we also evaluated baseline number of teeth comparing the extremes (4–10 compared to 2528 teeth), and severe periodontitis combined with tooth loss (> 1 site with CAL > 7 mm and/or < 10 teeth), as well as incident tooth loss during the follow-up (any versus none). We also investigated change measures (follow-up minus baseline) for mean PD and mean CAL, further excluding 49 people ineligible for the follow-up periodontal exam, and 21 who could not complete the follow-up oral exam for logistical reasons.
For quality assurance [26], 40 participants were evaluated by two of the three examiners (examiner 1, examiner 2, and examiner 3) to assess reliability. The intra-class correlation coefficient was 0.89 (95% CI: 0.81, 0.94) for mean CAL, and 0.93 (95% CI: 0.88, 0.96) for mean PD using inter-proximal sites, suggesting good inter-examiner reliability. For the CDC/AAP measures (none or mild versus moderate or severe), examiners 1 and 2 were in perfect agreement for the 9 participants they both evaluated, whereas examiners 2 and 3 showed a kappa statistic of 0.53 (95% CI: 0.24, 0.82) based on the 31 participants they both evaluated. All examiners were in perfect agreement for the classification of number of teeth. For intra-examiner reliability, we evaluated the percent of participants similarly classified on both visits when repeat dental exams were conducted, i.e. periodontal disease with CDC/AAP definition none or mild versus moderate or severe. The percent within examiner agreement was 79% and the kappa 0.58 (95%CI 0.330.84).
Assessment of Covariates
Interviewer-administered questionnaires collected information on important covariates including age, gender, smoking, family history of diabetes, education, physical activity during a typical week (METs), diet, alcohol intake, physician-diagnosed hypertension, antihypertensive medication use, sleep breathing disorders, and oral hygiene practice including brushing, flossing, and mouthwash use. If people smoked at least 100 cigarettes and did not quit they were classified as current smokers; people who had smoked at least 100 cigarettes but had stopped smoking prior to the baseline visit were classified as former smokers; the rest were classified as nonsmokers. Presence of bacterial plaque was determined by visual assessment or after passing a periodontal probe around the tooth surface of six pre-selected teeth; each surface was scored as per the Silness and Loe criteria [33]. The plaque score for each individual was computed by averaging the scores from 4 surfaces on 6 teeth. Anthropometric measurements including height, weight, and body circumferences were taken 2–3 times according to the NHANES procedures and averaged [34]. Blood pressure was measured following the gold standard Korotkoff auscultatory method after five minutes of rest,[35] with intervals of one minute in between three measures, and averaged. Participants were classified as hypertensive if they reported physician diagnosis of hypertension, and/or high blood pressure medication, and/or high blood pressure at the baseline exam with systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg. They were classified as pre-hypertensive if no reported physician diagnosis of hypertension, and if systolic blood pressure was between 120 and 139 mm Hg and/or diastolic blood pressure between 80 and 89 mm Hg.
Serum lipids (high density lipoprotein cholesterol (HDL-C) and triglycerides) were processed spectrometrically from the fasting samples using standard commercial procedures, and low density lipoprotein cholesterol (LDL-C) was determined using the Friedewald equation. High-sensitivity C-reactive protein (hs-CRP) concentrations were measured by high sensitive latex turbidimetric method by Beckman Coulter AU5421 K-assay (Beckman Coulter, Inc., Brea, CA, USA). The accuracy, reportable range, and linearity of hs-CRP were examined using an instrument AU 5822 in a measure ranged from 0.0 to 167.51 mg/L. Adiponectin levels were analyzed with radioimmunoassay with a Millipore human adiponectin RIA kit (Billerica, MA). The inter- and intra-assay CVs from adiponectin methodology were 9.07% and 6.04%, respectively, and the minimum sensitivity was 0.50 ng/mL. The levels of interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion protein 1 (VCAM-1) were measured using the Mesoscale Discovery (MSD) multiplex method, an enzyme-linked immunosorbent assay (ELISA) that uses electrochemiluminescence as the signal to detect binding events. The inter- and intra-assay CV and the minimum sensitivity were as follows: IL-6: 9.65% and 6.67%, and 0.23 pg/mL respectively; TNF-α: 5.73% and 5.77%, and 1.30 pg/mL respectively; ICAM-1: 13.91% and 4.20%, respectively, and 1.72 ng/mL respectively; and VCAM-1: 10.80% and 3.43%, and 8.01 ng/mL respectively.
Statistical Analyses
Development of prediabetes/diabetes during the follow-up was modeled as the outcome with periodontal measures at baseline as the exposure using Poisson regression. We report the results as Incidence Rate Ratios (IRR) with 95% confidence intervals (95% CI) based on robust standard errors [36–38], accounting for variations in follow-up time [39, 40]. Analyses were performed using Stata for Windows version 13.
Baseline potential confounders were initially selected for inclusion based on the major risk factors for diabetes from the literature. The base model included age, gender, smoking status, family history of diabetes, education, physical activity [41], waist circumference, and alcohol consumption. We evaluated additional potential confounders using change in estimate procedures. The covariates in the base model were included for evaluation of all other associations.
Potential mediators including adiponectin, biomarkers of inflammation, endothelial dysfunction, and dyslipidemia were subsequently evaluated by adding each biomarker individually to the final models for the significant associations detected above. If the addition of the biomarker resulted in reducing the effect estimate relating baseline periodontitis to prediabetes/diabetes development, the biomarker could be playing a role in mediating the association.
RESULTS
The median follow-up time for this cohort was 2.96 years (interquartile range 2.88–3.01), and 941 participants with complete follow-up were included. Over the 3-year follow-up, 69 (7.3%) of the 941 individuals developed type 2 diabetes, and 142 (34.9%) of the 407 with normal glycemia at baseline developed prediabetes. Table 1 shows that baseline characteristics are similar between participants retained for these analyses versus the overall baseline participants. Individuals show higher sleep breathing disorders and hypertension at follow-up, but improved physical activity, CRP, BOP and periodontitis. Table 2 shows baseline descriptive statistics by baseline periodontal status. Participants with higher mean CAL (above the median) were slightly older, had a greater percent of males, smoked more, had higher alcohol intake, were less educated, exercised less, and had lower income. They had fewer dental visits in the past 12 months, and had lower frequency of brushing, flossing, or using mouthwash. They had higher plaque index and BOP, fewer teeth, and more hypertension.
Table 1.
Descriptive statistics comparing all baseline participants (baseline characteristics)with participants retained for these analyses (baseline and follow-up characteristics), reported as %, mean ± SD, or median with 25th and 75th percentiles
| All baseline (N=1,206a) |
Retained in analysis Baseline (N=941a) |
Follow-up Characteristics (N=941a) |
|
|---|---|---|---|
| Age (years) | 50.4 ± 6.8 | 50.6 ± 6.8 | 53.5 ± 6.8 |
| Male | 27.3 | 25.8 | 25.8 |
| Current smoker | 19.2 | 18.3 | 19.1 |
| Annual income (<$20,000) | 56.1 | 54.1 | 55.5 |
| Education | |||
| Less than high school | 12.2 | 10.8 | - |
| High school diploma | 43.0 | 42.8 | - |
| More than high school | 44.8 | 46.5 | - |
| Physical activity (METs) | 7.9 (0, 26.7) | 7.9 (0, 26.9) | 10 (0.3, 32.6) |
| Obese | 63.7 | 63.7 | 65.4 |
| Waist circumference (cm) | 106.3 ± 14.4 | 106.0 ± 14.2 | 105.7 ± 15.0 |
| Alcohol (g/day) | 2.3 ± 5.8 | 2.2 ± 5.7 | 2.3 ± 5.5 |
| Sleep breathing disorderb | 15.6 | 16.8 | 20.1 |
| Hypertension statusc | |||
| Elevated Blood Pressure | 31.8 | 31.1 | 15.0 |
| Hypertension | 45.2 | 46.3 | 56.9 |
| Pre-diabetes | 57.5 | 56.7 | 52.0 |
| Diabetes | Excluded | Excluded | 6.0 |
| High-sensitivity C-reactive protein (mmol/L) |
5.7 ± 6.3 | 5.5 ± 6.2 | 5.1 ± 5.9 |
| HOMA-IR | 2.5 ± 1.7 | 2.5 ± 1.7 | 2.9 ± 2.4 |
| Plaque score | 0.8 ± 0.6 | 0.8 ± 0.6 | 0.8 ± 0.6 |
| Number of surfaces bleeding on probing | 13.0 ± 12.3 | 12.6 ± 12.0 | 10.6 ± 11.0 |
| Periodontitisd (moderate/severe) | 65.8 | 63.1 | 58.6 |
| Mean CAL (mm) | 2.0±1.3 | 1.9 ± 1.2 | 1.9 ± 1.2 |
| Number of teeth | 23.3 ± 4.6 | 23.6 ± 4.4 | 23.0 ± 4.8 |
Sample size reduces for some variables as per the available data.
Sleep breathing disorder was defined as apnea or report of quitting breathing while sleeping.
Hypertension if reported physician diagnosis of hypertension, and/or high blood pressure with systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 80 mm Hg; elevated blood pressure if no hypertension and systolic blood pressure between 120 and 130 mm Hg and diastolic blood pressure < 80 mm Hg.
Periodontal disease was classified using the CDC-AAP definition.
Table 2.
Baseline descriptive statistics for groups with high or low mean CAL categorized by median (1.61 mm at baseline using interproximal sites only), reported as %, mean ± SD, or median (25th, 75th percentiles)
| Mean CAL ≤ Median (N=471) |
Mean CAL > Median (N=470) |
|
|---|---|---|
| Age (years) | 49.8 ± 6.7 | 51.4 ± 6.9 |
| Male | 21.7 | 30.0 |
| < High school education | 6.8 | 14.5 |
| Annual income < $20,000 | 45.9 | 62.1 |
| Smoking status | ||
| Never | 67.7 | 60.2 |
| Former | 18.5 | 17.0 |
| Current | 13.8 | 22.8 |
| Number of cigarettes/week | 50.0 ± 44.8 | 74.4 ± 67.4 |
| Alcohol intake (g/day) | 2.0 ± 5.9 | 2.5 ± 5.4 |
| Physical activity (METS/week) | 10 (0, 27.7) | 7.9 (0, 24.3) |
| Fruit and vegetable intake (servings/week) | 7.4 ± 4.1 | 7.1 ± 4.0 |
| BMI (kg/m2) | 33.2 ± 6.2 | 33.3 ± 6.2 |
| Waist circumference (cm) | 105.3 ± 13.8 | 106.8 ± 14.6 |
| Hypertension | 44.2 | 50.2 |
| Bleeding on Probing > 30% surfaces | 18.9 | 51.1 |
| Mean number of teeth | 24.5 ± 3.8 | 22.6 ± 4.8 |
| Number of teeth (%) | ||
| 25–28 | 59.2 | 42.3 |
| 17–24 | 36.9 | 46.6 |
| 11–16 | 2.6 | 8.1 |
| 4–10 | 1.3 | 3.0 |
| Dental visits in last 12 months | 70.1 | 54.0 |
| Tooth brushing less than twice a day | 7.4 | 10.0 |
| Dental flossing less than once per day | 53.5 | 61.7 |
| Mouthwash use at least twice per day | 78.6 | 77.9 |
| Plaque score | 0.6 ± 0.4 | 1.0 ± 0.7 |
| HOMA-IR | 2.4 ± 1.6 | 2.5 ± 1.7 |
| Fasting glucose (mg/dl) | 91.8 ± 9.0 | 93.0 ± 8.8 |
| 1-hour glucose (mg/dl) | 151.1 ± 40.3 | 153.8 ± 40.9 |
| 2-hour glucose (mg/dl) | 114.6 ± 29.6 | 114.6 ± 29.9 |
| HbA1c (%) | 5.7 ± 0.3 | 5.7 ± 0.3 |
| HDL-C (mg/dl) | 47.8 ± 12.0 | 48.7 ± 13.2 |
| Triglycerides (mg/dL) | 144.2 ± 77.9 | 146.8 ± 81.4 |
| hs-C reactive protein > 3.0 (mg/l) | 57.7 | 59.5 |
| IL-6 (ng/mL) | 1.0 ± 0.9 | 1.1 ± 1.0 |
| TNF-α (ng/mL) | 2.4 ± 0.9 | 2.6 ± 2.5 |
| ICAM-1 (ng/mL) | 524.4 ± 128.3 | 561.9 ± 171.9 |
| VCAM (ng/mL) | 578.2 ± 138.9 | 624.2 ± 187.5 |
| Adiponectin (ng/mL) | 9.3 ± 4.6 | 8.9 ± 4.6 |
Table 3 shows results from Poisson regression models relating baseline periodontitis measures with development of prediabetes/diabetes. Models controlled for age, gender, smoking status, family history of diabetes, education, waist circumference, alcohol consumption, and physical activity (METs). High BOP was associated with reduced development of diabetes and progression of HOMA-IR. Using the 4 categories of the CDC/AAP definition (none, mild, moderate, and severe), mild periodontitis was associated with a non-significant higher risk of developing prediabetes/diabetes over the follow-up (IRR=1.40; 95% CI: 0.98–2.02) compared to none. However, when periodontitis was categorized in 2 groups, moderate/severe periodontitis was associated with a lower risk of developing prediabetes/diabetes compared to none/mild periodontitis. Higher baseline mean PD (IRR=0.81 per mm; 95% CI: 0.67–0.99) and mean CAL (IRR=0.86 per mm; 95% CI: 0.74–0.99) were associated with significantly lower risks of developing prediabetes/diabetes. On the other hand, change in mean CAL was associated with increased prediabetes/diabetes development (IRR=1.25; 95% CI: 1.09–1.42). Also, an increase in pocket depth was associated with >20% fasting glucose increase (multivariate IRR=1.43; 95% CI: 1.14–1.79; data not shown). Mild, moderate, and severe periodontitis, mean PD, and mean CAL showed inverse associations with > 20% progression of 1-hour glucose. CDC/AAP defined severe periodontitis was significantly associated with lower progression of the 2-hour glucose over follow-up (IRR=0.62; 95% CI: 0.46–0.85).
Table 3.
Longitudinal multivariate incidence rate ratios (IRR) relating oral health measures with pre-diabetes/diabetes and HOMA progression using Poisson regression (only interproximal sites)
| Pre-diabetes/ Diabetes progression | HOMA-IR increase > 20% | |
|---|---|---|
| IRRa (95% CI) | IRRa (95% CI) | |
| BOP ≥ 30% vs < 30% surfaces | 0.65 (0.50–0.86)* | 0.81 (0.70–0.95)* |
| CDC/AAP periodontitis measure | ||
| None | 1.00 | 1.00 |
| Mild | 1.40 (0.98–2.02) | 0.93 (0. 72–1.21) |
| Severe | 0.85 (0.63–1.15) | 0.96 (0.81–1.13) |
| Moderate | 0.80 (0.56–1.16) | 0.94 (0.78–1.15) |
| CDC/AAP periodontitis measure | ||
| None/Mild | 1.00 | 1.00 |
| Moderate/Severe | 0.75 (0.59–0.96)* | 0.97 (0.84–1.12) |
| Mean PD | 0.81 (0.67–0.99)* | 0.88 (0.79–0.98)* |
| Mean CAL | 0.86 (0.74–0.99)* | 0.96 (0.90–1.02) |
| ≥1 tooth with CAL ≥ 7 vs. none | 1.01 (0.75–1.35) | 1.01 (0.85–1.19) |
| Number of teeth at baseline | ||
| 25–28 | 1.00 | 1.00 |
| 4–10 | 1.48 (0.76–2.89) | 1.37(1.00–1.89)* |
| ≥1 site with CAL ≥ 7 and/or < 10 teeth vs. none | 1.08 (0.81–1.43) | 1.04 (0.89–1.22) |
| Change in mean CAL (follow-up minus baseline) | 1.25 (1.09–1.42)* | 0.99 (0.92–1.07) |
| ≥1 tooth lost over follow-up vs none | 0.86 (0.58–1.26) | 1.06 (0.83–1.35) |
Adjusted for age, gender, smoking status, family history of diabetes, education, waist circumference, alcohol consumption, and physical activity (METs).
Statistically significant at p ≤ 0.05
People with 4–10 teeth had a borderline significantly higher risk of progression of HOMA (IRR=1.37; 95% CI: 1.00–1.89) compared to people with 25–28 teeth, and elevated risk for development of prediabetes/diabetes which was not significant. Loss of one or more teeth over the follow-up did not show any association with pre-diabetes/diabetes. None of these factors changed the estimate relating the primary exposure and outcome (mean CAL and development to prediabetes/diabetes) beyond 10% and hence, were not included in the final model.
Further adjustment for baseline factors, including plaque, gingivitis (BOP), number of teeth, visits to a dentist/hygienist and reasons for the visits, frequency of brushing, flossing, mouthwash use, income, HOMA-IR, HDL-C, triglycerides, fasting or 2-hr glucose, hypertension status, intake of sugary drinks, or high fiber food (whole bread, beans, vegetables, and fruits), and periodontal treatment during follow-up, did not change the IRR (change in estimate less than 10% for all the associations). Hence, these variables were not included in the final model. Only 14 participants reported aspirin use at baseline. Hence, aspirin use is unlikely to confound the results in this study. In any case, we conducted sensitivity analysis excluding these participants, and the results remained unchanged.
We also conducted subgroup analyses by number of teeth for prediabetes/diabetes development using the CAL variables as exposure (Table 4). We also evaluated the association among people with ≥10 teeth and the results were very similar. Change in mean CAL was associated with increased risk of diabetes development, while mean CAL and severe CAL at baseline were inversely associated with diabetes development. The directions of these associations were consistent across the teeth categories and there was no clear effect modification, but the associations were stronger among people with 24–27 teeth. To explore dose response, we also evaluated quartiles of mean CAL at baseline. There was some evidence of a dose-response relationship for prediabetes/diabetes development and progression of fasting glucose (IRR for 2nd, 3rd, and 4th quartiles were 0.63 and 0.62, 0.51 and 0.87, 0.72, and 0.62 respectively). There was no dose-response relationship for the other outcomes.
Table 4.
Longitudinal multivariate Poisson analyses relating clinical attachment loss measures (using only interproximal sites) and pre-diabetes/diabetes progression stratified by baseline number of teeth
| Baseline number of teeth | ||||
|---|---|---|---|---|
| 28 teeth (n=174) |
24–27 teeth (n=406) |
21–23 teeth (n=178) |
4–20 teeth (n=183) |
|
| IRRa (95% CI) | IRRa (95% CI) | IRRa (95% CI) | IRRa (95% CI) | |
| Mean CAL | 0.88 (0.57–1.35) | 0.78 (0.52–0.99)* | 0.96 (0.76–1.21) | 0.84 (0.58–1.22) |
| ≥1 tooth with CAL ≥ 7 vs. none | 0.92 (0.48–1.76) | 0.69 (0.47–1.01) | 0.85 (0.50–1.44) | 0.80 (0.42–1.53) |
| Change in mean CAL (follow-up minus baseline) | 1.32 (0.92–1.90) | 1.35 (1.08–1.69)* | 1.27 (0.92–1.74) | 1.34 (1.01–1.78)* |
Adjusted for age, gender, smoking status, family history of diabetes, education, waist circumference, alcohol consumption, and physical activity (METs)
Statistically significant at p ≤ 0.05
To evaluate potential mediators for the associations where oral health measures were significantly associated with increased HOMA-IR or diabetes development, we added the mediators of dyslipidemia, inflammation, endothelial dysfunction, and adiponectin to the final model. The effect estimates were essentially similar and there was no evidence of mediation by these factors. Only the association of number of teeth (4–10 vs. 25–28) and HOMA-IR increase > 20% was slightly mediated by adiponectin, Il-6, TNF-α, ICAM, and VCAM with 9.5%, 10.0%, 10.1%, 10.2%, and 11.0% change in estimate, respectively.
DISCUSSION
Previous longitudinal studies showed associations between baseline periodontitis and diabetes incidence [14–17] whereas one showed no association [11]. Few cross-sectional studies showed periodontitis to be associated with precursors of diabetes [18–22]. SOALS is among the first longitudinal study evaluating standard measures of gingivitis, pocket depth, attachment loss, and tooth loss as potential risk factors for development of diabetes and its precursors. Since the CDC/AAP definition is commonly used but was designed for surveys and surveillance rather than such etiologic studies, we evaluated that as well as additional measures of periodontitis. However, we did not see a consistent association, and some associations were in the opposite direction to the findings from the literature. This may be because earlier studies evaluating prediabetes used non-standard measures of periodontitis and/or prediabetes. The direction of associations in our study is still surprising as significant positive associations between periodontitis and prediabetes were seen cross-sectionally in SOALS, and in analyses relating changes in periodontitis measures with changes in glycaemia. The participants that completed the follow-up and were included in the analysis are similar to the baseline sample, hence the inconsistent results between the cross-sectional and longitudinal analyses cannot be explained by selection bias. Our findings were also independent of high BOP progression by major risk factors for diabetes, other than genetics which is a limitation in most studies. Since baseline characteristics vary on lifestyle factors between people with and without periodontitis, we cannot exclude residual confounding (although most confounders would likely bias the results in the opposite direction). We had high quality data with standard NHANES methods for oral health assessment and ADA cutoffs for prediabetes and diabetes assessment using a combination of fasting glucose, 2-hour post load glucose, and HbA1c.
Previously published work ascertaining whether periodontitis was associated with increased risk of developing prediabetes and/or diabetes among people without diabetes is sparse and most longitudinal studies are limited by the diagnostic criterion used to ascertain periodontal disease. Periodontitis criteria in studies showing associations ranged from use of Russell’s Periodontal Index (PI) to the Community Periodontal Index (CPI) to the Centers for Disease Control and Prevention and American Academy of Periodontology (CDC/AAP) criteria for periodontitis. All three methodological approaches rely on differing concepts on how to measure PD and CAL, and how to use the assessed clinical measures of PD and CAL, including information from other factors, to construct the diagnostic criteria for periodontal disease.
Demmer et al. assessed whether periodontitis predicts incident diabetes over a 17-year follow up among 7,168 individuals from NHANES-I [17]. Incident diabetes was defined by death certificate, self-reported diabetes diagnosis (limited to individuals requiring medications), or hospital stay with diabetes diagnosis at discharge. Periodontitis was assessed using a PI in which gingivitis, pocket depth, and tissue loss was aggregated into a single score. The overall PI score was calculated and individuals were categorized into healthy, 1 of 5 PI quintiles, and edentulous. Associations were seen with diabetes incidence as follows: PI quintile 3: OR=2.26 (95% CI: 1.56–3.27), PI quintile 4: OR=1.71 (95% CI: 1.00–2.69), PI quintile 5: 0R=1.50 (95% CI: 0.99–2.27), and edentulous: 0R=1.30 (95% CI: 1.00–1.70). In a five-year follow-up study in Taiwan with 5,885 men and women aged 35–44, the CPI was associated with increased risk of prediabetes/diabetes. Diabetes status was ascertained by fasting glucose only. The CPI was categorized as 0 for healthy periodontium, 1 for gingival bleeding, 2 for calculus, 3 for a 4–5mm periodontal pocket, and 4 for a > 6 mm periodontal pocket 1 to 3 (healthy, inflammation-free gingiva, and periodontium) to 4 (most severe form of periodontitis with loss of function of the teeth). A CPI > 3 showed a multivariate hazard ratio (HR) of 1.33 (95% CI: 1.09–1.63) for prediabetes/diabetes incidence [16]. In another recent five-year follow-up study among 2,469 Japanese men aged 36–55 [15], self-reported gingival bleeding did not show a significant association (RR=1.32, 95% CI: 0.95–1.85), whereas, tooth loosening showed a significant association (RR=1.73, 95% CI: 1.14–2.64) with prediabetes/diabetes incidence. Diabetes was self-reported or assessed using only fasting glucose (HbA1c was available only in a subgroup). Another longitudinal study found that, using the CDC/AAP criteria, moderate to severe periodontitis (compared to no/mild) was associated with significantly increased risk of incident diabetes (HR=1.69, 95% CI: 1.06–2.69) among 1,331 dentate diabetes free men, 58–72 years, in Northern Ireland in a 7.8 year follow-up [14]. In another longitudinal study conducted among 1,023 Japanese men and women who had no components of metabolic syndrome present, periodontitis at baseline was associated with the positive conversion of each metabolic-syndrome component over a 4-year follow-up [12]. Periodontitis was assessed as CPI codes < 2 (without a periodontal pocket) versus at least one sextant with a CPI code > 3 (periodontal pocket > 4 mm). There was a non-significant association between periodontitis and development of hyperglycemia (OR= 1.4, 95% CI: 1.0–2.1), and the association was null for one or more missing teeth. Another analysis among 572 workers followed for 9 years showed a significant association between baseline periodontitis and hyperglycemia. Periodontitis was defined as a cumulative duration of periodontal pockets for ≥ 6 years and compared to participants without pockets or participants with a cumulative duration of periodontal pockets for ≤ 5 years [13]. A very recent study from Pomerania evaluated periodontitis as a risk factor for incident diabetes among 2047 subjects aged 20–81years [11]. Baseline periodontitis was not associated with diabetes incidence over the 11-year follow-up (comparing fourth versus first quartile: mean CAL, IRR=0.82, 95% CI: 0.49–1.37; and mean PD, IRR=1.27, 95% CI: 0.78–2.07). Diabetes was assessed from selfreported physician diagnoses, antidiabetic medication use, HbA1c ≥ 6.5% or non-fasting blood glucose levels (glucose from fasting or OGTT samples was not available).
The CDC/AAP case definition for moderate and severe periodontitis is based on interproximal sites. Periodontal disease usually begins and is most severe in interproximal sites, in contrast to buccal or lingual sites. In addition, dropping the buccal or lingual sites minimizes the effects of gingival recession (which is more related to chemical or mechanical oral hygiene procedures) on the accuracy of the PD measurements. Hence, we computed all summary exposure variables limiting to only the four interproximal sites. We evaluated different periodontitis measures in relation to tooth loss, and the CAL measures were clearly superior, with mean CAL and percent of sites with CAL ≥ 7 mm showing higher correlation coefficients with tooth loss compared to their PD counterparts. Also in logistic regression models predicting any tooth loss compared to none, models using mean CAL showed higher fit compared to mean PD.
Earlier longitudinal studies showing associations only evaluated prediabetes/diabetes or impaired fasting glucose as the outcome, did not use classic measures of CAL, and/or clinically assessed comprehensive standard measures of diabetes, limiting direct comparisons. However, some longitudinal studies showed associations between periodontal disease and incident prediabetes and/or diabetes, even while using different periodontitis measures ranging from selfreported tooth loosening to PI and CPI to CDC/AAP definition based on PD and CAL [12–17]. In Demmer et al.’s study, Russell’s PI was used, which is much more subjective compared to measures like periodontal PD and CAL [17]. The PI does not use a periodontal probe, hence in many ways the PI is more like an indirect “visual” assessment. The Taiwan study [16] used the CPI, which does not assess recession or CAL. It is designed to only assess pocket depth at two different thresholds. A Japanese study showed associations for self-reported tooth loosening, but not for gingival bleeding [15]. Also, the CDC/AAP definitions were not designed for etiologic studies. The studies evaluating impaired fasting glucose assessed periodontitis as CPI or duration of periodontal pockets for > 6 years. Hence, it is hard to compare and determine whether our results are consistent with these prior longitudinal studies, but are consistent with the Pomeranian study. It is also possible that due to publication bias, studies showing positive associations were more likely to be published.
Potential limitations of the study include the short follow-up of 3 years. In addition, only 211 people developed pre-diabetes/diabetes during the follow-up; hence power was limited despite the large sample size and high retention. Another possible explanation for the unexpected results could be that those participants who had periodontitis at baseline were made aware (through their participation in SOALS) of its potential association to the development of prediabetes/diabetes, and it may have motivated healthy oral/general lifestyle changes. When we evaluated the data, people with baseline moderate/severe periodontal disease did not show more frequent brushing or flossing, more dental visits, or more periodontal treatment compared to people with none/mild periodontitis. However, participants with baseline moderate/severe periodontitis did show higher reduction in plaque scores and non-significantly higher increase in physical activity over the follow-up compared to participants with none/mild periodontitis at baseline, which could reduce the risk of periodontitis and prediabetes/diabetes. We also did sensitivity analysis additionally controlling for change in plaque scores and change in physical activity (METs/wk.), and the estimates remained similar.
Contrary to our longitudinal associations, and consistent with our cross-sectional associations reported earlier [23], the change in mean CAL from baseline was associated with increased diabetes/prediabetes development. Several biological pathways have been proposed for such associations. One alternative interpretation of this positive association between worsening of periodontitis and increased development to prediabetes/diabetes is that health conditions tend to get worse over time, i.e., periodontitis gets worse and diabetes gets worse, and some people may get worse at higher rates than others for reasons that are not accounted for in the model. Also, the time sequence is unclear when both changes are measured simultaneously, hence, it is hard to distinguish whether change in periodontitis is a cause or consequence of the change in glycemia. Similarly, fewer teeth at baseline, which partly reflects teeth lost due to severe periodontitis, is significantly associated with increased progression of insulin resistance and shows a tendency to be associated with prediabetes/diabetes development.
In summary, this is the first study to evaluate longitudinally the associations between standard periodontitis and prediabetes measures. Baseline periodontitis seems to show an inverse association with progression of insulin resistance or development of prediabetes/diabetes. On the other hand, positive associations are seen cross-sectionally and when relating changes in exposures with changes in outcomes, and fewer baseline teeth is also associated with increased development of prediabetes/diabetes. Additional large well conducted longitudinal studies are needed to better understand these associations.
ACKNOWLEDGEMENTS
Dr. Kaumudi Joshipura is the guarantor for this work and takes responsibility for the contents of the article. The authors acknowledge Dr. Oelisoa M. Andriankaja, Dr. Maribel Campos, Ms. Tania Ginebra, Ms. Carla León, Ms. Yashira Maldonado, Dr. Sasha Martínez, Dr. Ashwinkumar Modi, Ms. Xiomara O’Farrill, Ms. Samantha Ordaz, Ms. Elaine Rodríguez, Ms. Rosalyn Román, Mr. Rafael Ruiz, Ms. Yadiris Santaella, Ms. Grace Vélez, Mr. José Vergara, Ms. Lay Wah, Mr. Jeanpaul Fernández, and PRCTRC laboratory personnel (Ms. Aracelis Arroyo and Ms. Nilda González) who contributed to the conduct/oversight/planning of data collection and their help with the study. The authors have no conflicts of interest. Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research Grant R01DE020111, the National Institute on Minority Health and Health Disparities Grants 2U54MD007587 and S21MD001830 of the National Institutes of Health. Part of this work was presented as an abstract in the IADR 2017 General Session and Exhibition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Collin HL, Uusitupa M, Niskanen L, Kontturi-Narhi V, Markkanen H, Koivisto AM, et al. Periodontal findings in elderly patients with non-insulin dependent diabetes mellitus. Journal of periodontology. 1998;69:962–6. [DOI] [PubMed] [Google Scholar]
- [2].Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, et al. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. Journal of periodontology. 1996;67:1085–93. [DOI] [PubMed] [Google Scholar]
- [3].Teshome A, Yitayeh A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC oral health. 2016;17:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. The Cochrane database of systematic reviews. 2015:CD004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li Q, Hao S, Fang J, Xie J, Kong XH, Yang JX. Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: a meta-analysis of randomized controlled trials. Trials. 2015;16:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International diabetes Federation and the European Federation of Periodontology. Diabetes Res Clin Pract. 2017. [DOI] [PubMed] [Google Scholar]
- [7].Wang X, Han X, Guo X, Luo X, Wang D. The effect of periodontal treatment on hemoglobin a1c levels of diabetic patients: a systematic review and meta-analysis. PLoS One. 2014;9:e108412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Madianos PN, Koromantzos PA. An update of the evidence on the potential impact of periodontal therapy on diabetes outcomes. J Clin Periodontol. 2018;45:188–95. [DOI] [PubMed] [Google Scholar]
- [9].Engebretson SP, Hyman LG, Michalowicz BS, Schoenfeld ER, Gelato MC, Hou W, et al. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA. 2013;310:2523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34:47–52. [DOI] [PubMed] [Google Scholar]
- [11].Kebede TG, Pink C, Rathmann W, Kowall B, Volzke H, Petersmann A, et al. Does periodontitis affect diabetes incidence and haemoglobin A1c change? An 11-year follow-up study. Diabetes Metab. 2017. [DOI] [PubMed] [Google Scholar]
- [12].Morita T, Yamazaki Y, Mita A, Takada K, Seto M, Nishinoue N, et al. A cohort study on the association between periodontal disease and the development of metabolic syndrome. J Periodontol. 2010;81:512–9. [DOI] [PubMed] [Google Scholar]
- [13].Morita T, Yamazaki Y, Fujiharu C, Ishii T, Seto M, Nishinoue N, et al. Association Between the Duration of Periodontitis and Increased Cardiometabolic Risk Factors: A 9-Year Cohort Study. Metab Syndr Relat Disord. 2016;14:475–82. [DOI] [PubMed] [Google Scholar]
- [14].Winning L, Patterson CC, Neville CE, Kee F, Linden GJ. Periodontitis and incident type 2 diabetes: a prospective cohort study. Journal of clinical periodontology. 2017;44:266–74. [DOI] [PubMed] [Google Scholar]
- [15].Miyawaki A, Toyokawa S, Inoue K, Miyoshi Y, Kobayashi Y. Self-Reported Periodontitis and Incident Type 2 Diabetes among Male Workers from a 5-Year Follow-Up to MY Health Up Study. PLoS One. 2016;11:e0153464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chiu SY, Lai H, Yen AM, Fann JC, Chen LS, Chen HH. Temporal sequence of the bidirectional relationship between hyperglycemia and periodontal disease: a community-based study of 5,885 Taiwanese aged 35–44 years (KCIS No. 32). Acta Diabetol. 2015;52:123–31. [DOI] [PubMed] [Google Scholar]
- [17].Demmer RT, Jacobs DR Jr., Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 2008;31:1373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Benguigui C, Bongard V, Ruidavets JB, Chamontin B, Sixou M, Ferrieres J, et al. Metabolic syndrome, insulin resistance, and periodontitis: a cross-sectional study in a middle-aged French population. Journal of clinical periodontology. 2010;37:601–8. [DOI] [PubMed] [Google Scholar]
- [19].Demmer RT, Squillaro A, Papapanou PN, Rosenbaum M, Friedewald WT, Jacobs DR Jr., et al. Periodontal infection, systemic inflammation, and insulin resistance: results from the continuous National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Care. 2012;35:2235– 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Islam SK, Seo M, Lee YS, Moon SS. Association of periodontitis with insulin resistance, beta-cell function, and impaired fasting glucose before onset of diabetes. Endocrine journal. 2015;62:981–9. [DOI] [PubMed] [Google Scholar]
- [21].Song IS, Han K, Park YM, Ji S, Jun SH, Ryu JJ, et al. Severe Periodontitis Is Associated with Insulin Resistance in Non-abdominal Obese Adults. The Journal of clinical endocrinology and metabolism. 2016;101:4251–9. [DOI] [PubMed] [Google Scholar]
- [22].Timonen P, Suominen-Taipale L, Jula A, Niskanen M, Knuuttila M, Ylostalo P. Insulin sensitivity and periodontal infection in a non-diabetic, non-smoking adult population. Journal of clinical periodontology. 2011;38:17–24. [DOI] [PubMed] [Google Scholar]
- [23].Perez CM, Munoz F, Andriankaja OM, Ritchie CS, Martinez S, Vergara J, et al. Cross-sectional associations of impaired glucose metabolism measures with bleeding on probing and periodontitis. J Clin Periodontol. 2017;44:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].ADA. Standards of medical care in diabetes−−2011. Diabetes Care. 2011;34 Suppl 1:S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].CDC. National Health and Nutrition Examination Survey (NHANES). Oral Health Examiners Manual, 2009–2010.
- [26].Dye BA, Li X, Lewis BG, Iafolla T, Beltran-Aguilar ED, Eke PI. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2009–2010. J Public Health Dent. 2014;74:248–56. [DOI] [PubMed] [Google Scholar]
- [27].Chaves ES, Wood RC, Jones AA, Newbold DA, Manwell MA, Kornman KS. Relationship of “bleeding on probing” and “gingival index bleeding” as clinical parameters of gingival inflammation. Journal of clinical periodontology. 1993;20:139–43. [DOI] [PubMed] [Google Scholar]
- [28].Joss A, Adler R, Lang NP. Bleeding on probing. A parameter for monitoring periodontal conditions in clinical practice. J Clin Periodontol. 1994;21:402–8. [DOI] [PubMed] [Google Scholar]
- [29].Claffey N, Nylund K, Kiger R, Garrett S, Egelberg J. Diagnostic predictability of scores of plaque, bleeding, suppuration and probing depth for probing attachment loss. 3 1/2 years of observation following initial periodontal therapy. J Clin Periodontol. 1990;17:108–14. [DOI] [PubMed] [Google Scholar]
- [30].Badersten A, Nilveus R, Egelberg J. Scores of plaque, bleeding, suppuration and probing depth to predict probing attachment loss. 5 years of observation following nonsurgical periodontal therapy. J Clin Periodontol. 1990;17:102–7. [DOI] [PubMed] [Google Scholar]
- [31].Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. Journal of periodontology. 2007;78:1387–99. [DOI] [PubMed] [Google Scholar]
- [32].Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. Journal of periodontology. 2012;83:1449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Silness J, Loe H. Periodontal Disease in Pregnancy. Ii. Correlation between Oral Hygiene and Periodontal Condtion. Acta odontologica Scandinavica. 1964;22:121–35. [DOI] [PubMed] [Google Scholar]
- [34].CDC. Third national health and nutrition examination survey anthropometric procedures video: Centers for Disease Control and Prevention. National Center for Health Statistics.
- [35].Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697– 716. [DOI] [PubMed] [Google Scholar]
- [36].Cameron AC, Trivedi PK. Microeconometrics using Stata. College Station, Tex.: Stata Press; 2009. [Google Scholar]
- [37].Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- [38].Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Statistical methods in medical research. 2013;22:661–70. [DOI] [PubMed] [Google Scholar]
- [39].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22:719–48. [PubMed] [Google Scholar]
- [40].Rothman KJ, Boice John D. Epidemiologic analysis with a programmable calculator. Bethesda, Md. Washington: U.S. Dept. of Health, Education, and Welfare, Public Health Service for sale by the Supt. of Docs; 1979. [Google Scholar]
- [41].WHO. Global Recommendations on Physical Activity for Health. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]


